- 1Department of Anesthesiology, Shanghai Eastern Hepatobiliary Surgery Hospital, Naval Medical University, Shanghai, China
- 2Department of Anesthesiology, The 988th Hospital of Joint Logistic Support Force of PLA, Zhengzhou, China
- 3Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 4Department of Anesthesiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5Department of Anesthesiology, Zhejiang Province Zhoushan Hospital, Zhoushan, China
- 6Department of Critical Care Medicine, Shanghai Eastern Hepatobiliary Surgery Hospital, Naval Medical University, Shanghai, China
Background: Cholestasis patients often suffer from pain desensitization, resulting in serious complications in perioperative period. This study was aim to investigate the mechanism of bilirubin in cholestasis mediating pain desensitization through 5-hydroxytryptamine 3A (5-HT3A) receptor activation in spinal dorsal horn (SDH).
Methods: A cholestasis model was established by bile duct ligation (BDL) in rats. Pain thresholds of rats were measured after BDL or intrathecally injecting bilirubin in the presence or absence of agonist (mCPBG) and antagonists (ondansetron, bicuculline, or CGP55845). Expression of 5-HT3 receptors, and the affinity and binding mode of bilirubin to 5-HT3A receptor were determined. Effects of bilirubin on γ-aminobutyric acid (GABA) pathway and the interactions with 5-HT3A receptor were tested.
Results: Bilirubin was elevated significantly in both serum and CSF in BDL rats, accompanied with the up-regulation of pain thresholds. Both of 5-HT3A receptor and GABAA receptor antagonists could reverse the increased pain threshold in BDL rats. Further, 5-HT3A and GABAA receptor expressions were increased in BDL rats or intervention with bilirubin. Molecular docking suggested that bilirubin entered the hydrophobic pocket pre-formed in 5-HT3A receptor with potential hydrogen bonding. Bilirubin also increased GABA concentrations in CSF and GABAergic spontaneous inhibitory postsynaptic current in spinal cord, and directly induced inward currents in HEK293 cells which were overexpressed 5-HT3A receptor by lentivirus.
Conclusion: In conclusion, bilirubin induced pain desensitization in cholestasis by activating 5-HT3A receptor in spinal cord. The activation of 5-HT3A receptor might regulate pain threshold by acting on the GABA pathway.
Introduction
Cholestasis is characterized by the obstruction of intrahepatic/extrahepatic bile ducts by stones, tumors, or pancreatic diseases (Hegade et al., 2015). Patients with cholestasis show insensitivity to nociceptive stimulation, which often leads to the concealment of symptoms and delayed treatment (Tian et al., 2016). Moreover, respiratory depression and delayed recovery occur more frequently in cholestasis patients, resulting in an increased risk of death in the perioperative period. Thus, exploration of the mechanism of pain desensitization in cholestasis can provide theoretical basis and treatment targets for cholestasis.
Multiple substances such as 5-hydroxytryptamine (5-HT), endogenous opioids, and cholecystokinin participate in pain transmission in cholestasis (Hasanein and Javanmardi, 2008). The 5-HT pathway is regarded to mediate various physiologic activities by seven receptors, and the 5-HT3 receptors are the only ligand-gated ion channel in the 5-HT receptor family (Hannon and Hoyer, 2008). The 5-HT3 receptors are widely distributed in the central nervous system (CNS), especially the spinal dorsal horn (SDH). Activation of 5-HT3 receptors in SDH produces an anti-nociceptive response, whereas the antagonists of 5-HT3 receptors can reverse this effect. Blockers of the γ-aminobutyric acid (GABA) pathway can also reverse the anti-nociceptive effect caused by the 5-HT3 receptors, suggesting that the 5-HT3 receptors in SDH could regulate pain threshold through GABA pathway (Pelissier et al., 1996; Iwasaki et al., 2013; Roczniak et al., 2013; Bobinski et al., 2015). It has also been confirmed that morphine regulated pain transmission by combining with the 5-HT3A receptor (Schaaf et al., 2014).
Bilirubin, with a molecular weight of 584, derives from senescent erythrocyte, hemoglobin, and ineffective hematopoiesis. Free bilirubin that does not bind to glucuronic acid, can damage the blood-brain barrier (BBB) and increase its permeability directly. As a small molecule, bilirubin can pass the BBB readily and be toxic to gliocyte and neurons. Bilirubin can also induce inflammation, neurodegeneration and apoptosis, which can affect cognition, memory, pain sensation, and can even cause kernicterus in newborns.
In patients with cholestasis, concentrations of bilirubin usually increase significantly in serum and cerebrospinal fluid (CSF) because indirect bilirubin (IB, also characterized by unconjugated bilirubin) can pass through the damaged BBB into the CNS (Cardoso et al., 2012; Xu et al., 2016). Admittedly, morphine can exert an analgesic role clinically by activating opioids receptors. Additionally, morphine and bilirubin also share similar spatial structure. Recent studies have suggested that morphine and bilirubin can both induce pain regulation, withdrawal reaction, and chronic itching (Meixiong et al., 2019; Shah and John, 2020). Therefore, we hypothesized that elevated bilirubin in the CNS might mediate pain desensitization in cholestasis by activating the 5-HT3A receptor and GABA pathway in the spinal cord.
Materials and Methods
Animals and Drugs
This study was carried out in accordance with the International Association for the Study of Pain guidelines and the Ethics Committee of the Second Military Medical University (Shanghai, China). Adult male Sprague–Dawley rats (200–220 g) were provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). Rats were housed in a pathogen-free environment (room temperature of 24°C and 50% humidity) under a 12 h light-dark cycle, with food and water ad libitum. Animals were sacrificed before pentobarbital sodium 30 mg/kg intraperitoneal anesthetization.
Drugs used in this study were intrathecally injected through PE-10 catheter implantation (Njoo et al., 2014) in 10 μL normal saline (0.9%) mixed with: bilirubin (Sigma-Aldrich, Saint Louis, MO, United States) 250, 500, or 1000 μM; 1-(m-Chlorophenyl)-biguanide (mCPBG, 5-HT3A receptor agonist; Sigma-Aldrich) 10 μg; ondansetron (5-HT3A receptor antagonist; Sigma-Aldrich) 10, 30, or 50 μg; bicuculline (GABAA receptor antagonist; Abcam, Cambridge, United Kingdom) 1 μg; and CGP55845 (GABAB receptor antagonist; Abcam) 0.5 μg. An unconjugated bilirubin stock solution (10 mM) was prepared in 0.1 M NaOH immediately before use and the pH of the incubation medium was restored to 7.4 by addition of equal amounts of 0.1 M HCl.
Grouping
Rats were randomly divided into sham and bile duct ligation (BDL)−1, −3, −5, and −7 groups to test changes in pain thresholds depending on the days after BDL (n = 6). The intrathecally administered bilirubin and that with mCPBG groups were subdivided into pre-drugs and post-drugs groups to test pain thresholds with or without ondansetron, bicuculline, or CGP55845 pretreatment for 1 h (n = 6). Further, the functions of the 5-HT3A receptor and the GABA receptors were evaluated. Rats were divided into saline, ondansetron (30 μg), bicuculline (1 μg), and CGP55845 (0.5 μg) groups to measure changes of pain thresholds after intrathecal administration of antagonists only (n = 6). Western blot was performed in the BDL group, the intrathecally administered bilirubin group, and the spinal dorsal horn neurons (SDHNs) cultured with bilirubin group (n = 6) to detect changes in expression of the 5-HT3A receptors and GABA receptors. GABA concentrations of CSF were detected in the BDL group and intrathecally administered bilirubin group (n = 6). Changes of GABAergic spontaneous inhibitory postsynaptic current (sIPSC) were recorded in the ACSF group, the mCPBG group, bilirubin group, the mCPBG + ondansetron group, and bilirubin + ondansetron group (n = 4).
Creation of Cholestasis Rat Model
The model of cholestasis was established by BDL (Yang et al., 2015). Rats were anesthetized by administering 30 mg/kg of pentobarbital sodium intraperitoneally. After opening the abdominal cavity, the bile duct was ligated at a point proximal to the hilus and a point immediately distal to the entry of the bile duct, and transected between two ligatures. Sham operation was carried out in a similar manner without ligation or transection. Cholestasis was confirmed by serum biochemical analysis and proximal dilation of the bile duct.
Biochemical Analyses of Liver Function Indices in Serum and CSF
The blood and CSF samples of rats were placed and centrifuged for 10 min at room temperature for 10 min, then the supernate was drawn to detect liver function indexes immediately under light protection. The automatic biochemical analyzer (Hitachi 7000) was used to detect [alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), direct bilirubin (DB), IB, and total bile acids (TBAs)] in the sample.
Measurement of Mechanical and Thermal Pain Thresholds
Mechanical and thermal pain thresholds were measured in a quiet room between 09:00 and 11:00. Rats were allowed to acclimatize to surroundings for 2 h in a set of PlexiglasTM cages with wire mesh floor. Von Frey filaments (North Coast Medical, Gilroy, CA, United States) of increasing stiffness were applied to six sites distributed across the plantar surface of the rat hindpaw until the filaments bent slightly. A positive withdrawal was scored if rat showed a brisk withdrawal. Eight von Frey monofilaments (0.5, 1, 2, 4, 6, 8, 10, and 12 g) were used in testing. Pain threshold was defined as the force corresponding to a 50% withdrawal, and was determined by a Hill equation linear fitting [Origin v6.0 (MicroCal, Studio City, CA, United States)] (Xiang et al., 2008). Thermal threshold was undertaken in individual cages with a transparent acrylic floor. After acclimatization, the heating device was placed under the hindpaw at certain heat intensity and a cut-off time of 30 s to avoid tissue damage. The withdrawal latency of the hindpaw was detected thrice for each rat at an interval of 5 min, and the mean value was taken as the thermal threshold.
Western Blotting
L4-5 vertebral segments and cultured SDHNs were collected to test the expression of related proteins. Tissues and cultured SDHNs were sonicated in ice-cold RIPA lysis buffer. Protein concentrations were measured using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, United States). Proteins were denatured by heating at 99°C for 10 min, followed by separation using 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, United States). PVDF membranes were blocked using 5% bovine serum albumin for 2 h, followed by incubation with primary antibodies at 4°C overnight. Finally, they were incubated with secondary antibodies for 2 h. The primary antibodies used were listed as follows: goat 5-HT3A antibody (1:1000; Abcam), rabbit 5-HT3B antibody (1:1000; Abcam), rabbit 5-HT3C antibody (1:1000; Abcam), rabbit 5-HT3D antibody (1:1000; Affinity Biologicals), rabbit 5-HT3E antibody (1:1000; Abcam), rabbit GABAA receptor antibody (1:1000; Affinity Biologicals), rabbit GABAB receptor antibody (1:1000; Affinity Biologicals), mouse GAPDH antibody (1:2000; Proteintech). Proteins were detected using enhanced chemiluminescence, and densitometry was performed using Image J software.
Immunofluorescence
L4-5 vertebral segments and cultured SDHNs were prepared for immunofluorescence to observe neuron activation and 5-HT3A receptor expression. L4-5 vertebral segments were post-fixed in 4% paraformaldehyde and dehydrated in 30% sucrose solution. Next, transverse frozen tissue sections (thickness, 20 μm) were cut using a freezing microtome (Leica, Wetzlar, Germany). Similarly, SDHNs cultured on cover glasses were post-fixed in 4% paraformaldehyde for 30 min. Then, spinal-cord slices and cultured SDHNs were incubated with 5% goat serum for 2 h, followed by incubation with primary antibodies at 4°C. Finally, they were incubated with secondary antibodies for 2 h. The primary antibodies were listed as follows: mouse glutamic acid decarboxylase (GAD) 65 antibody (1:200; Abcam), mouse microtubule-associated protein 2 (MAP2) antibody (1:200; Santa Cruz Biotechnology), and mouse C-fos antibody (1:200; Santa Cruz Biotechnology). Digital images were captured with a high-resolution fluorescence microscope and merged by PhotoshopTM (Adobe, San Jose, CA, United States).
Enzyme-Linked Immunosorbent Assay
GABA concentrations in CSF were tested by enzyme-linked immunosorbent assay (ELISA) to observe the effect of bilirubin on GABA release. CSF samples of rats were acquired by puncture of the occipital fonticulus. 50 μL of standards and samples were prepared in the sample diluent and eight standards (0, 1.56, 3.12, 6.25, 12.5, 25, 50, and 100 ng/mL) were established. Briefly, 50 μL of Detection A Working Solution was added immediately and incubated for 1 h at 37°C. Being aspirated and washed, each well added 100 μL of Detection B Working Solution, followed by incubation for 45min at 37°C. After aspirating and washing again, each well added 90 μL of Substrate Solution, followed by incubation for 20 min in dark at 37°C. Then, 50 μL of Stop Solution was added to each well. Finally, the optical density of each well was determined simultaneously using PlateReader AF2000 (Eppendorf, Hamburg, Germany) at 450 nm.
Culture of SDHNs
Spinal dorsal horn neurons were taken from fetuses of late-pregnancy rats and cultured with bilirubin. Briefly, fetuses in uteruses of 20-day pregnant rats were removed into Hanks buffer after deep anesthesia (3% desflurane inhalation). Under dissecting microscope, the spinal cord was split down the midline, and each half divided longitudinally into dorsal and ventral strips. The dorsal strips of all fetuses were dissociated by trituration and placed in a tube along with 0.25% trypsin for 15 min digestion at 37°C. Then the mixture was centrifuged at 200 g for 4 min and rinsed several times with Hanks buffer. Cells were plated onto six-well plates coated with poly-L-lysine and cultured in NeurobasalTM Growth Medium maintained at 37°C in the humidified incubator at an atmosphere of 5% CO2 (Langlois et al., 2010).
Electrophysiological Testing of Spinal Cord Slices
The effect of bilirubin on GABAergic sIPSC in SDH was recorded by patch clamping. Lumbar enlargements of spinal cords were moved into ice-cold ACSF whose composition was (in mM): NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25, and glucose 11 with 95% O2 + 5% CO2 air and cut transversely at 400 μm. The slices were incubated for recovery at 33°C and transferred to a recording chamber with ACSF perfusion. The patch electrode, filled with pipette solution whose composition was (in mM): Cs2SO4 110, CaCl2 0.5, MgCl2 2, EGTA 5, HEPES 5, TEA 5, and ATP-Mg 5, was advanced onto the surface of neurons from substantia gelatinosa, followed by cytomembrane being sucked in. sIPSC was recorded in voltage-clamp mode using MultiClampTM 700B amplifier (Molecular Devices, Silicon Valley, CA, United States) and pClamp10.2 (Axon Instruments, Union City, CA, United States) data-acquisition software under perfusion with 10 μM bilirubin, 30 μM mCPBG, or 50 μM ondansetron.
Human 5-HT3A Receptor Overexpression and Electrophysiological Testing of Human Embryonic Kidney 293 Cells
Lentivirus with plasmids carrying the target gene (human 5-HT3A receptor) were prepared and integrated into host cells to acquire highly efficient human embryonic kidney (HEK293) cell lines overexpressing the human 5-HT3A receptor (HEK293-5-HT3A cells). HEK293 cells were divided into: the blank group without lentivirus transfection; the control group with blank lentivirus transfection; and the plasmid group with successful 5-HT3A-lentivirus transfection. Currents induced by bilirubin on HEK293-5-HT3A cells were recorded. HEK293 cells were transferred to round glass plates and cultured for 24 h with individual density. Then, the glass plates were placed in a recording groove with extracellular fluid perfusion whose composition was (in mM): NaCl 140, KCl 4.7, CaCl2 2, MgCl2 1, HEPES 10, and glucose 11. An electrode containing pipette solution, whose composition was (in mM): CsCl 110, CaCl2 1, MgCl2 1, EGTA 10, HEPES 10, TEA-Cl 25, and ATP-Na2 2, was moved to the cell surface, followed by cytomembrane being sucked in. The inward currents generated by drug perfusion were recorded at −50 mV using the MultiClamp 700B amplifier and pClamp10.2 data-acquisition software.
Radioligand Binding Assay
The affinity of bilirubin and the 5-HT3A receptor was measured by radioligand binding assay. 5-HT (non-specific agonist of 5-HT3A receptor) was used as a positive control and 8-OH-DPAT (5-HT1A receptor agonist) as a negative control. Glycodesoxycholic acid (GDCA, one kind of bile acid), which increased in cholestasis, was also tested. HEK293-5-HT3A cells were cultured, trypsinized, and collected. After centrifugation, supernatants were collected and protein concentrations of membrane samples were determined by bicinchoninic acid protein assay kit. Membrane samples were diluted to 10 doses at five-fold serial dilution in dimethylsulfoxide (DMSO). Then, [3H]-Granisetron was transferred into wells. Meanwhile, the filters within Unifilter-96 GF/C filter plates (PerkinElmer, Waltham, MA, United States) were soaked in 0.5% polyethyleneimine for 30 min. When binding assays were completed, the contents of the binding assay were vacuumed through the filters. After being washed and dried, the bottom of the filter-plate wells was sealed using Unifilter-96 Backing Seal Tape (PerkinElmer), followed by the addition of 50 μL of MicroscintTM 20 Cocktail (PerkinElmer) and sealing of the top of the filter plates with TopSeal-A Sealing Film (PerkinElmer). Finally, [3H] trapped on filters was counted using a TopCount NXT HTS Reader (PerkinElmer). Data were analyzed using GraphPad Prism 5 (San Diego, CA, United States). Inhibition (%) was calculated using the following equation:
The half-maximal inhibitory concentration (IC50) was determined using four-parameter logistic non-linear regression analysis employing the following equation:
where Y is the inhibition rate and X is the log concentration of the sample.
Homologous Modeling
Homologous modeling uses large numbers of known three-dimensional protein structures to predict unknown protein structures. Proteins are characterized to belong to the same family if they share more than 40% similarity in homology. Proteins with known structures can be searched as candidate templates if they possess a mostly similar sequence to the target protein. Then, alignment is undertaken between the target sequence and candidate template. Multiple template combinations are selected as the final template depending on the sequence identity. The atoms that are exactly the same as the template sequence are copied completely, whereas only skeleton atoms are copied from the similar template. Side-chain coordinates and missing sequences are supplemented in a loop database. Finally, energy optimization under specific potential field parameters is done to obtain the final model.
Molecular Docking
Molecular docking is based on the lock-key relationship to simulate the interaction between ligands and receptors. Interactions of bilirubin and the 5-HT3A receptor were established and optimized by AutoDockTM. The structure of bilirubin ligand was drawn and energy was optimized using Chenmidraw 11.0 software, and AutodockTM 4.0 was used to conduct the hydrogenation, calculate the charge, and combine non-polar hydrogen. The result acquired from homologous modeling was also used to conduct the hydrogenation, calculate the charge, and combine non-polar hydrogen in AutodockTM 4.0. The ligand and receptor files were opened in Autogrid software, and a 60 × 60 × 60 box centered on the active sites of the protein was established. Finally, the ligand and receptor files were calculated in AutodockTM 4.0 software for 30 times in a flexible molecular docking method to acquire the final results.
Statistical Analyses
Statistical analyses were carried out using the IBM SPSS Statistics 23.0 (SPSS Inc., Armonk, NY, United States). Quantitative data are presented as the mean ± SD. Two-way repeated-measure ANOVA was used to compare withdrawal thresholds among different groups and at different time points. The relative gray value of western blotting and immunofluorescence was tested using one-way ANOVA followed by Dunnett’s t-test. Electrophysiological results were analyzed using Clampfit (Axon Instruments). P-values < 0.05 were considered statistically significant.
Results
Bilirubin Increased Pain Threshold of Cholestasis Rats by Activating the 5-HT3A Receptor
Biochemical analyses of serum showed that liver function indices (ALT, AST, TB, DB, IB, and TBAs) increased significantly in a time-dependent manner (Supplementary Table 1), suggesting that cholestasis model was created successfully. However, only IB levels in CSF increased significantly 3 days after BDL and was maintained stably (1.15 ± 0.3 μmol/L) without changes in TBAs (Supplementary Table 2), suggesting that indirect bilirubin could pass the BBB in BDL rats. Then, by testing pain thresholds, we found that mechanical and thermal pain thresholds increased significantly in BDL rats on days 3, 5, and 7 compared with the sham group (P < 0.05, Figures 1A,B). For further detecting the effects of bilirubin on pain thresholds in the spinal cord, different concentrations of bilirubin were intrathecally administrated into normal rats. Results showed that indirect bilirubin levels in CSF increased significantly 20 min after intrathecal administration (Figure 1C). At the same time, mechanical and thermal thresholds also increased significantly than the sham group (Figures 1D,E).
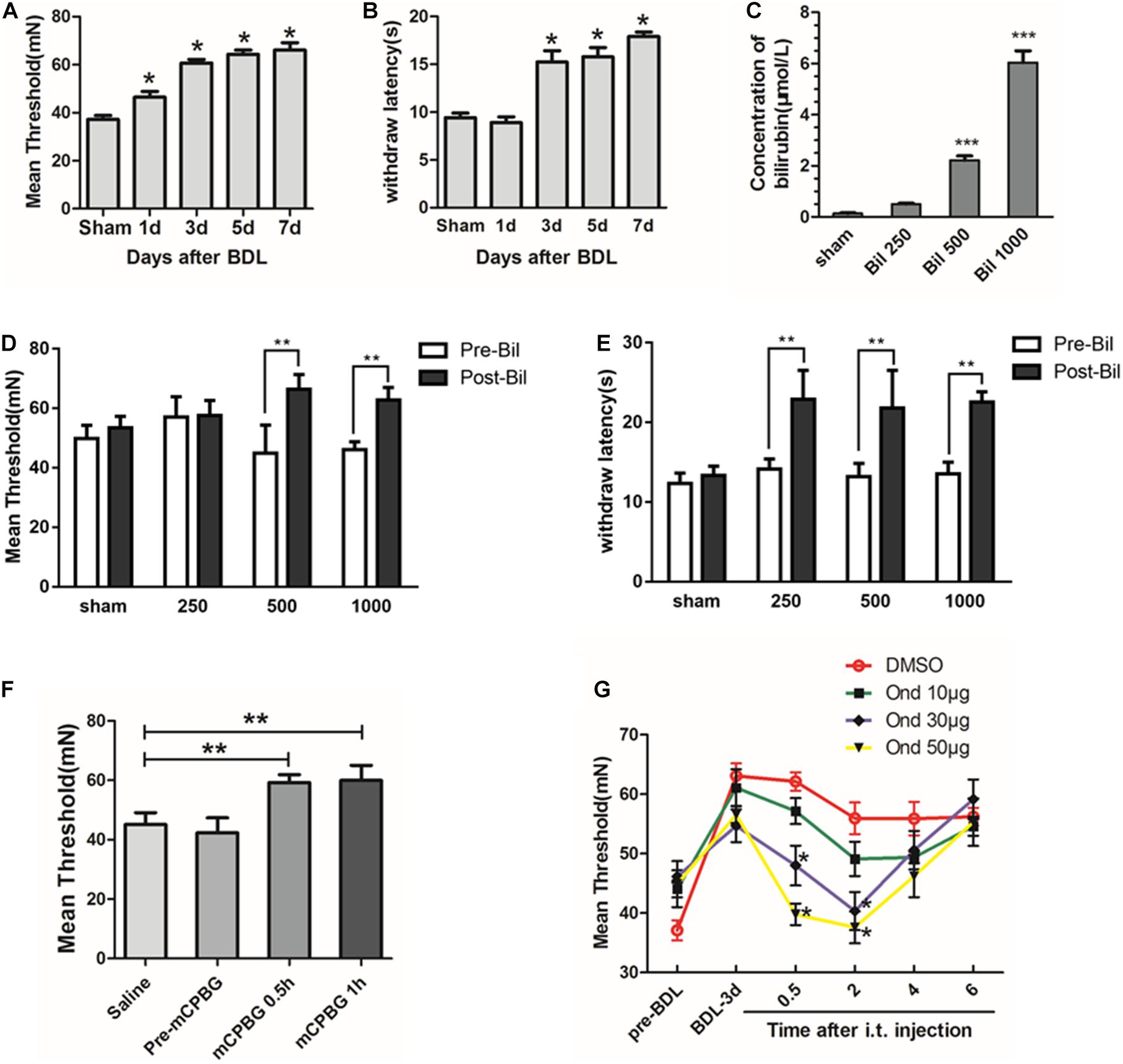
Figure 1. Bilirubin increased pain threshold of cholestasis rats by activating the 5-HT3A receptor. (A) Mechanical pain threshold increased significantly in BDL rats on days 1, 3, 5, and 7 compared with sham group. (B) Thermal pain threshold increased significantly in BDL rats on days 3, 5, and 7 compared with sham group. (C) Indirect bilirubin level in CSF increased 20 min after intrathecal administration of bilirubin. (D) Mechanical pain threshold increased by 48 and 36% at 500 and 1000 μM after administrating bilirubin intrathecally, respectively. (E) Intrathecal administration of bilirubin increased the thermal withdrawal latency by 62, 65.2, and 66.9% at 250, 500, and 1000 μM, respectively. (F) Intrathecal administration of mCPBG—5-HT3A receptor agonist—increased mechanical threshold in normal rats compared with intrathecal administration of saline. (G) Intrathecal administration of ondansetron—a 5-HT3A receptor antagonist—reversed the increase of mechanical pain threshold in BDL-3-day rats in a dose-dependent manner. *P < 0.05; **P < 0.01; ***P < 0.001.
To explore whether the 5-HT3A receptor participates in the regulation of pain thresholds in the spinal cord, mCPBG (5-HT3A receptor agonist) was intrathecally administrated into normal rats. Results suggested that the mechanical pain threshold was significantly increased after mCPBG administration (Figure 1F). Then, ondansetron (5-HT3A receptor antagonist) was used in BDL rats, and the results showed that ondansetron could reverse the up-regulation of pain threshold in BDL-3-day rats in a dose-dependent manner (Figure 1G). It suggested that bilirubin might induce pain desensitization by activating the 5-HT3A receptor in cholestasis rats.
Bilirubin Increased 5-HT3A Receptor Expression and Neuron Activities in the Spinal Cord
Expressions of 5-HT3 receptors in the spinal cords of BDL rats were determined by western blotting, and the results showed that only the 5-HT3A receptor expression increased significantly, especially in BDL-3d and BDL-5d, whereas expressions of 5-HT3B, 5-HT3C, 5-HT3D, and 5-HT3E receptors did not change (Figures 2A,B). In addition, the up-regulation of 5-HT3A receptor was observed in normal rats by intrathecally administrating bilirubin without changes in other subtypes (Figures 2C,D). Ondansetron could reverse the increasing of 5-HT3A receptor expression induced by bilirubin-treated rats. Similarly, SDHNs cultured in different concentrations of bilirubin could also increase 5-HT3A receptor expression, but not other subtypes (Figures 2E,F).
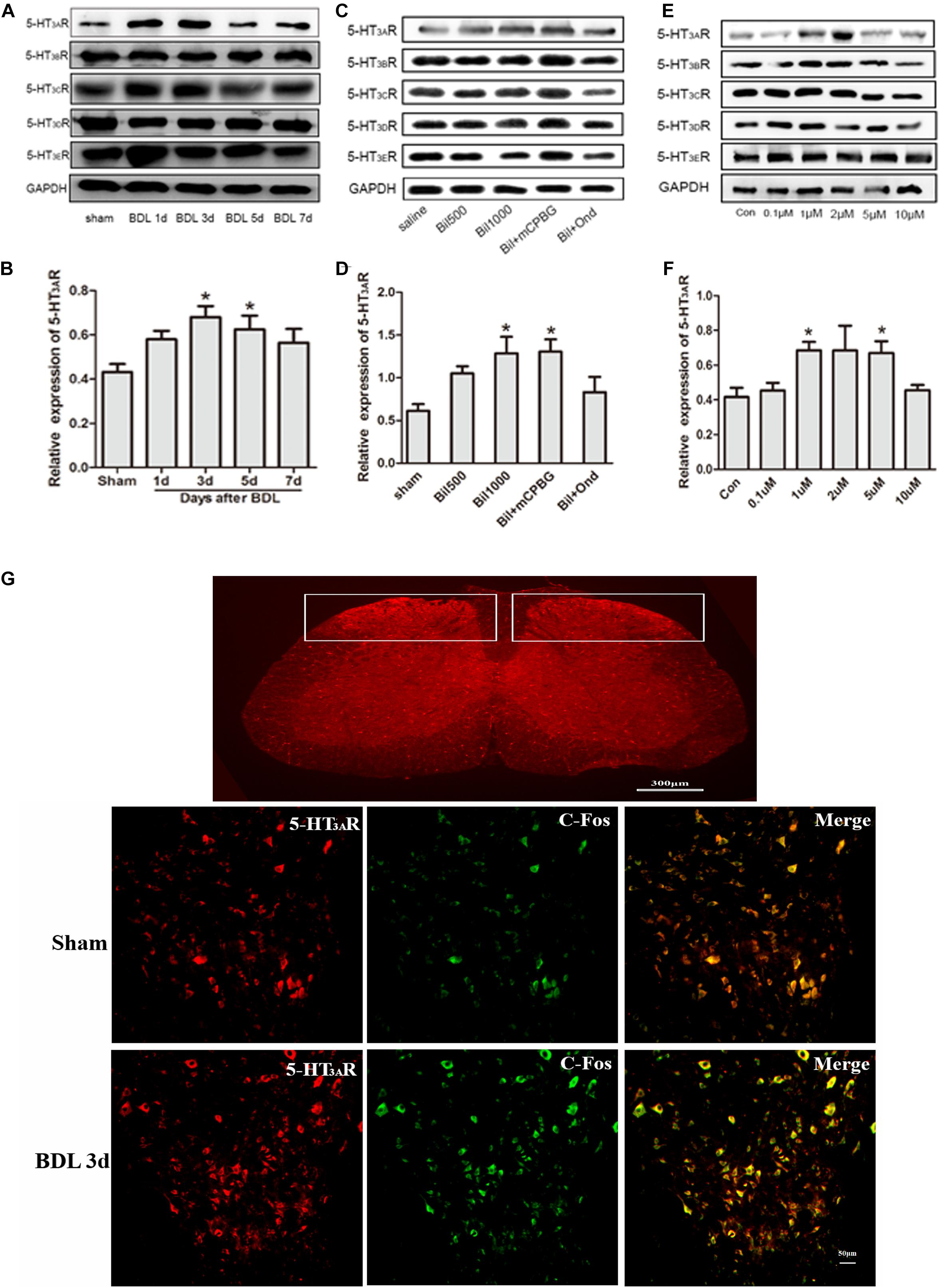
Figure 2. Bilirubin increased 5-HT3A receptor expression and neuron activities in the spinal cord. (A) 5-HT3A receptor expression increased gradually in spinal cord enlargement of BDL rats, whereas expression of 5-HT3B, 5-HT3C, 5-HT3D, and 5-HT3E receptors did not change significantly. (B) The quantitative analysis of 5-HT3A receptor expression in BDL rats. (C) Intrathecal administration of bilirubin increased 5-HT3A receptor expression without changing other subtypes. (D) The quantitative analysis of 5-HT3A receptor expression after intrathecal administration of bilirubin in normal rats. (E) SDHNs cultured with bilirubin showed increased 5-HT3A receptor expression without changing other subtypes. (F) The quantitative analysis of 5-HT3A receptor expression in SDHNs. (G) Immunofluorescence showed that 5-HT3A receptor expression increased in laminas I and II in the spinal cords of BDL rats. The increased C-Fos protein (marker of neuron activity) expression indicated that neuron activities were also enhanced in the spinal cords of BDL rats. *P < 0.05.
To understand the interaction between the activation of the 5-HT3A receptor and neuron activity in the spinal cord, immunofluorescence of SDH was carried out. Results showed that the expression of the 5-HT3A receptor increased in laminae I and II of the SDH in BDL rats (Figure 2G). Additionally, increased C-Fos protein (marker of neuron activity) expression in SDH indicated that more neurons were activated in BDL rats (Figure 2G). It suggested that the up-regulation of the 5-HT3A receptor might play a key role in the enhancement of neuron activity in BDL rats.
Bilirubin Directly Activated 5-HT3A Receptor With Low Affinity by the Formation of Hydrogen Bonds
To further find the direct evidence that bilirubin could activate the 5-HT3A receptor, we overexpressed the human 5-HT3A receptor in HEK293 cells by lentivirus. Western blotting demonstrated that transfected HEK293 cells successfully overexpressed the 5-HT3A receptor (Figure 3A), and both mCPBG and bilirubin (10 μM) could successfully induce inward currents on HEK293 cells (Figure 3B). It provided evidence that bilirubin activated the 5-HT3A receptor directly, and the normalized intensity of inward currents induced by bilirubin was approximately equivalent to 40% inward currents induced by mCPBG (Figure 3C). By radioligand binding assay, the dose-response curves (Figure 3D and Supplementary Table 3) showed that 5-HT (positive control) had a strong affinity for the 5-HT3A receptor, and that the affinity of 8-OH-DPAT (negative control) for the 5-HT3A receptor was rather low. Nevertheless, the affinity of bilirubin for the 5-HT3A receptor fell in between 5-HT and 8-OH-DPAT, suggesting that bilirubin interacted with the 5-HT3A receptor via weak combination bonds rather than ionic or covalent bonds. Moreover, GDCA also had a rather low affinity for the 5-HT3A receptor. Homologous modeling and molecular docking were used to predict the possible combination modes of bilirubin and the 5-HT3A receptor. Bilirubin entered the hydrophobic gaps formed by the subunits of the 5-HT3A receptor with benign complementarity (Figures 4A,B). Calculations showed that bilirubin formed hydrogen bonds with multiple amino acid residues of the 5-HT3A receptor. The binding sites were MET661, LYS 119, TYR118, ILE86, TYR38, and ASN103. Hydrogen bonds were formed between TYR38, LYS119, and two carbonyl groups of bilirubin (Figure 4C).
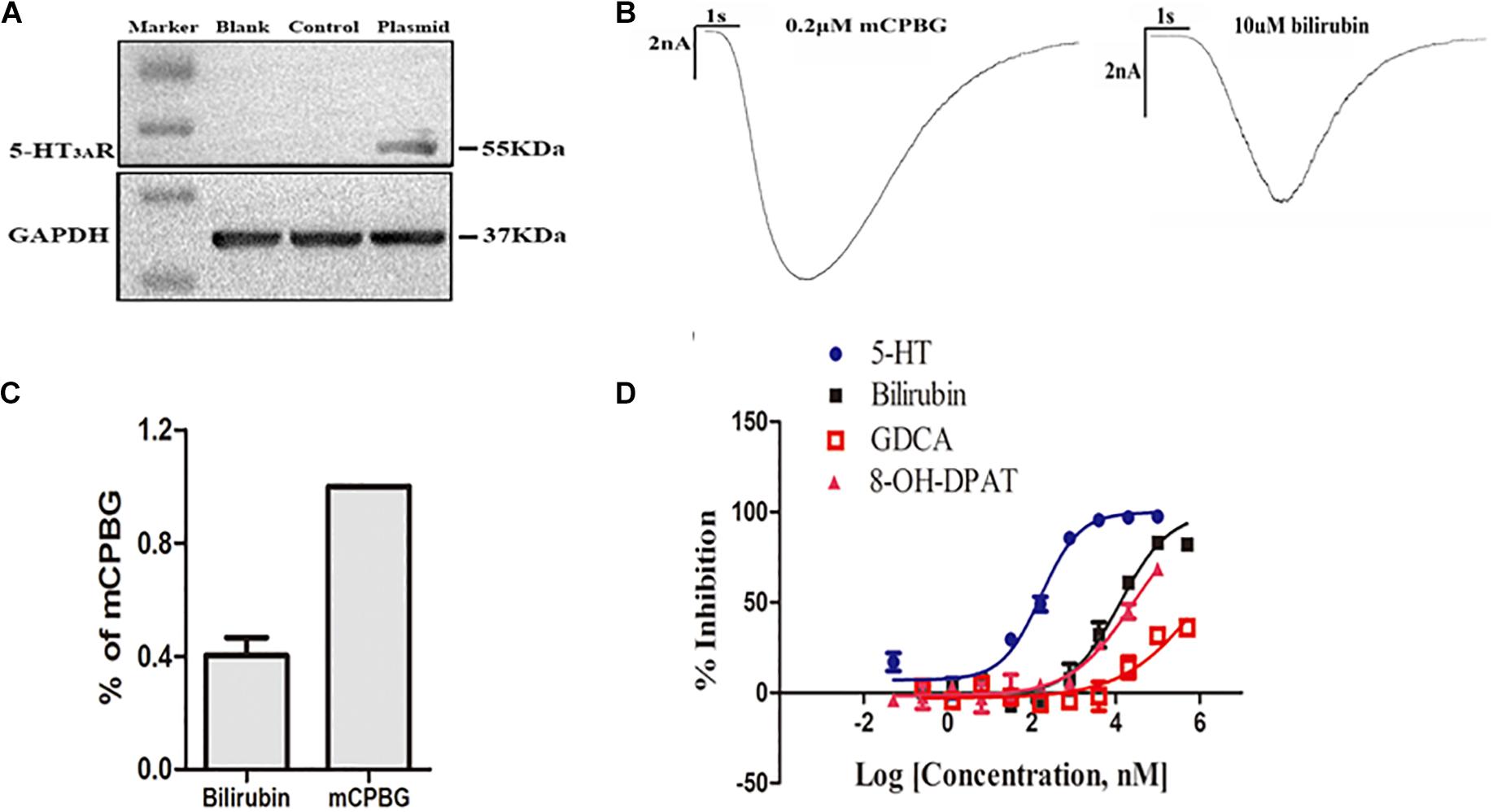
Figure 3. Bilirubin directly activated the 5-HT3A receptor with low affinity. (A) Western blot analysis showed the establishment of HEK293 cells overexpressing the human 5-HT3A receptor was successful. (B) Both mCPBG and bilirubin could induce obvious inward currents in HEK293 cells. (C) Normalized current intensity of HEK293 cells induced by bilirubin and mCPBG. (D) The dose-response curve showed that the affinity of bilirubin for the 5-HT3A receptor fell in between 5-HT and 8-OH-DPAT.
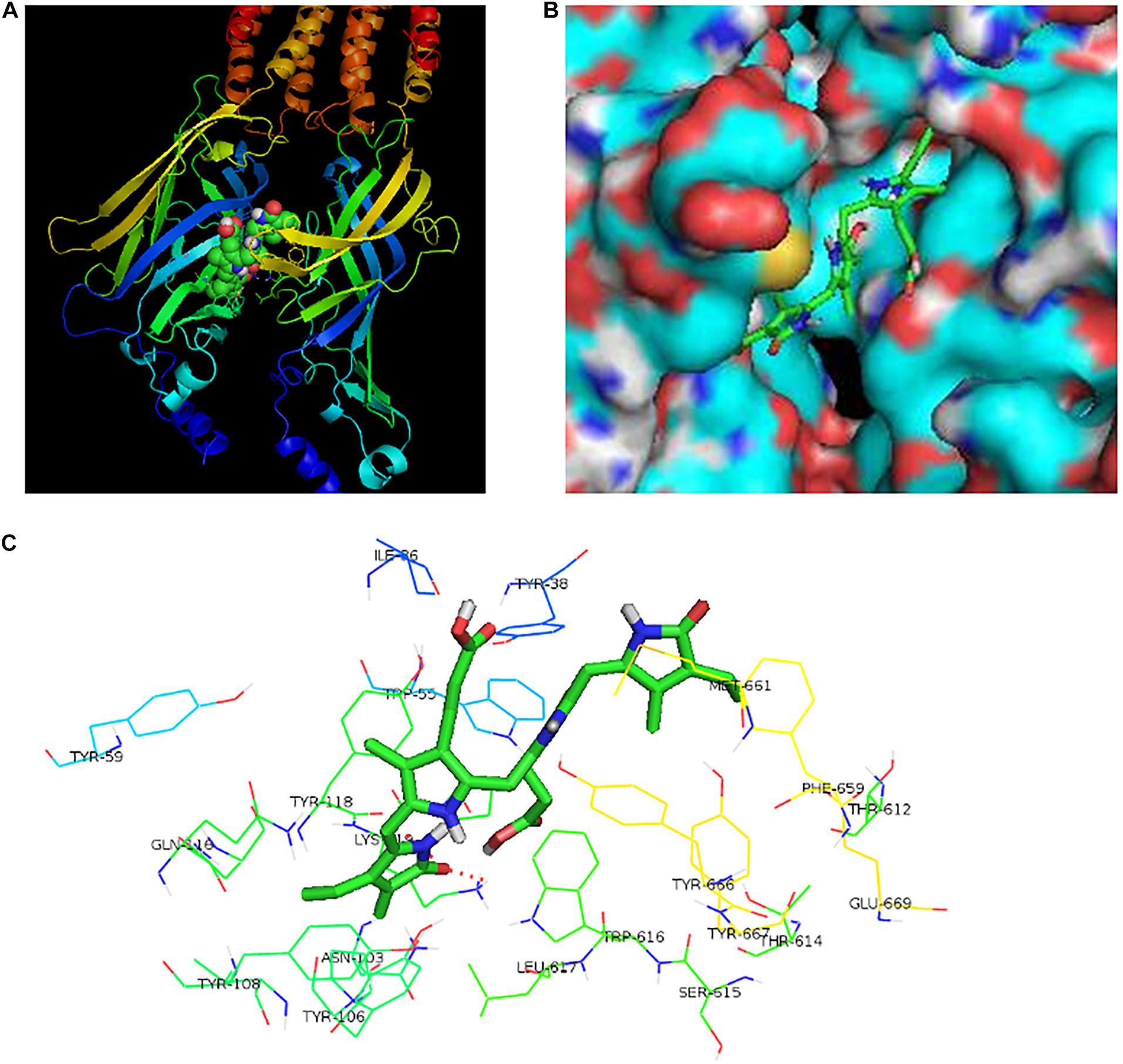
Figure 4. The potential binding sites of bilirubin and the 5-HT3A receptor. (A) Bilirubin combined with the spatial structure of 5-HT3A receptor. (B) Bilirubin entered the hydrophobic gap formed by 5-HT3A receptor subunits with benign complementarity. (C) Through calculation, bilirubin was able to bond to multiple amino acid residues of the 5-HT3A receptor with hydrogen bond formation. The binding sites of bilirubin and the 5-HT3A receptor were MET661, LYS 119, TYR118, ILE86, TYR38, and ASN103. Hydrogen bonds were found between TYR38, LYS119 and two carbonyls of bilirubin.
The 5-HT3A Receptor Could Regulate Pain Thresholds Through GABA Pathway in Cholestasis Rats
To explore the potential mechanism of regulating pain thresholds by the 5-HT3A receptor, ondansetron (5-HT3A receptor antagonist), bicuculline (GABAA receptor antagonist), and CGP55845 (GABAB receptor antagonist) were intrathecally administrated into normal or BDL rats, respectively. In normal rats, bicuculline but not CGP55845 could significantly decrease the mechanical threshold (Figure 5A). In BDL rats, both ondansetron and bicuculline could reverse the up-regulation of pain thresholds while CGP55845 had no effects on it (Figure 5B). Moreover, ondansetron exerted stronger inhibition ratio than bicuculline while no difference was observed between CGP55845 and saline in BDL rats (Figure 5C). These results suggested that GABAA receptor rather than GABAB receptor participated in the regulation of pain thresholds.
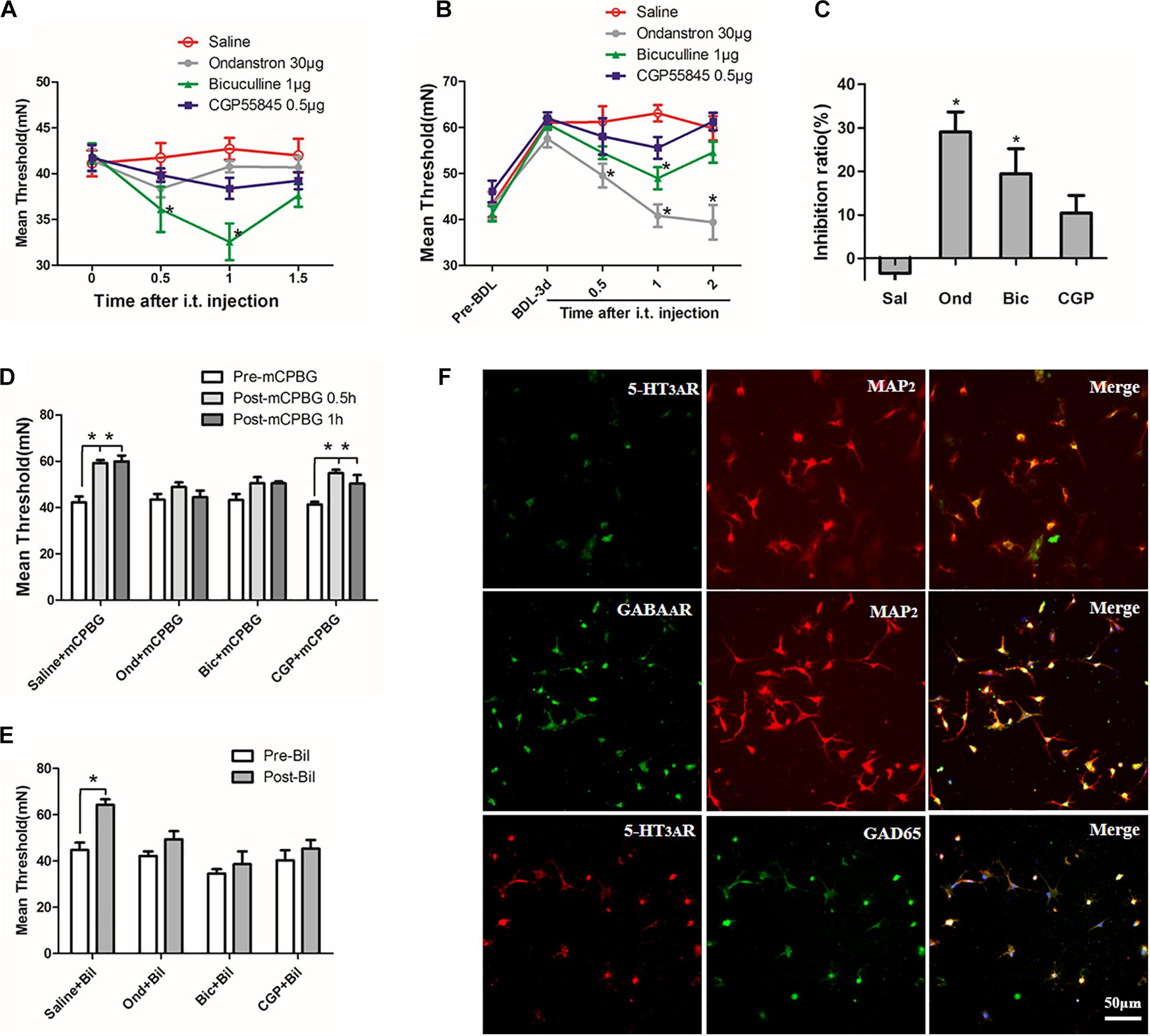
Figure 5. The 5-HT3A receptor regulated pain thresholds through GABA pathway in cholestasis rats. (A) In normal rats, bicuculline reduced mechanical threshold significantly by intrathecal injection 30 min later. (B) In BDL rats, both ondansetron and bicuculline reversed the increased pain thresholds. (C) Comparison of inhibition ratios among three antagonists. Results showed that ondansetron exerted stronger inhibition ratio than bicuculline and CGP55845 in BDL rats. (D) Both ondansetron and bicuculline, but not CGP55845, could reverse the up-regulation of pain threshold induced by mCPBG in normal rats. (E) Ondansetron, bicuculline, and CGP55845 could reverse the effect induced by intrathecally administrating bilirubin in normal rats. (F) Expression of the 5-HT3A receptor on GABAergic neurons in cultured SDHNs. Most of the cultured neurons (MAP2 is regarded as neuron marker) expressed the 5-HT3A receptor and GAD65 (marker of GABAergic neuron). The merged images showed co-expression of the 5-HT3A receptor and GAD65, suggesting that the majority of GABAergic neurons expressed the 5-HT3A receptor in SDH. *P < 0.05.
Then, mCPBG (5-HT3A receptor agonist), ondansetron, CGP55845, and bicuculline were used to further detect the interaction between the 5-HT3A receptor and GABAA receptor. Results showed that both ondansetron and bicuculline, but not CGP55845, could reverse the effect induced by mCPBG in normal rats (Figure 5D). Similarly, ondansetron, bicuculline, and CGP55845 could also reverse the effect induced by intrathecally administrating bilirubin in normal rats (Figure 5E). These results suggested that bilirubin could increase pain thresholds by activating the 5-HT3A receptor and GABAA pathway. Further, immunofluorescence on SDHNs showed that the majority of cultured neurons [microtubule-associated protein 2 (MAP2) was considered as neuron marker] expressed 5-HT3A receptor and glutamic acid decarboxylase (GAD) 65 (marker of GABAergic neuron). Merged images showed co-expression of the 5-HT3A receptor and GAD65, suggesting that most GABAergic neurons expressed the 5-HT3A receptor in the SDH (Figure 5F).
Bilirubin Increased GABAA Receptor Expression and GABA Concentration of CSF
Results indicated that GABAA receptor expression in the spinal cord enlargement of BDL rats increased gradually and was reduced by ondansetron, whereas GABAB receptor expression did not change significantly (Figures 6A,B). Intrathecal administration of bilirubin also increased GABAA receptor expression, and co-administration of ondansetron blocked the effect of bilirubin without changing GABAB receptor expression (Figures 6C,D). Similarly, SDHNs cultured with different concentrations of bilirubin also increased GABAA receptor expression, whereas ondansetron reversed it (Figures 6E,F). Moreover, results showed that GABA concentrations in CSF were significantly higher in BDL group (Figure 6G) and in the intrathecally administered bilirubin (1000 μM) group (Figure 6H) than that in control group, and ondansetron could reverse the increase of GABA concentrations.
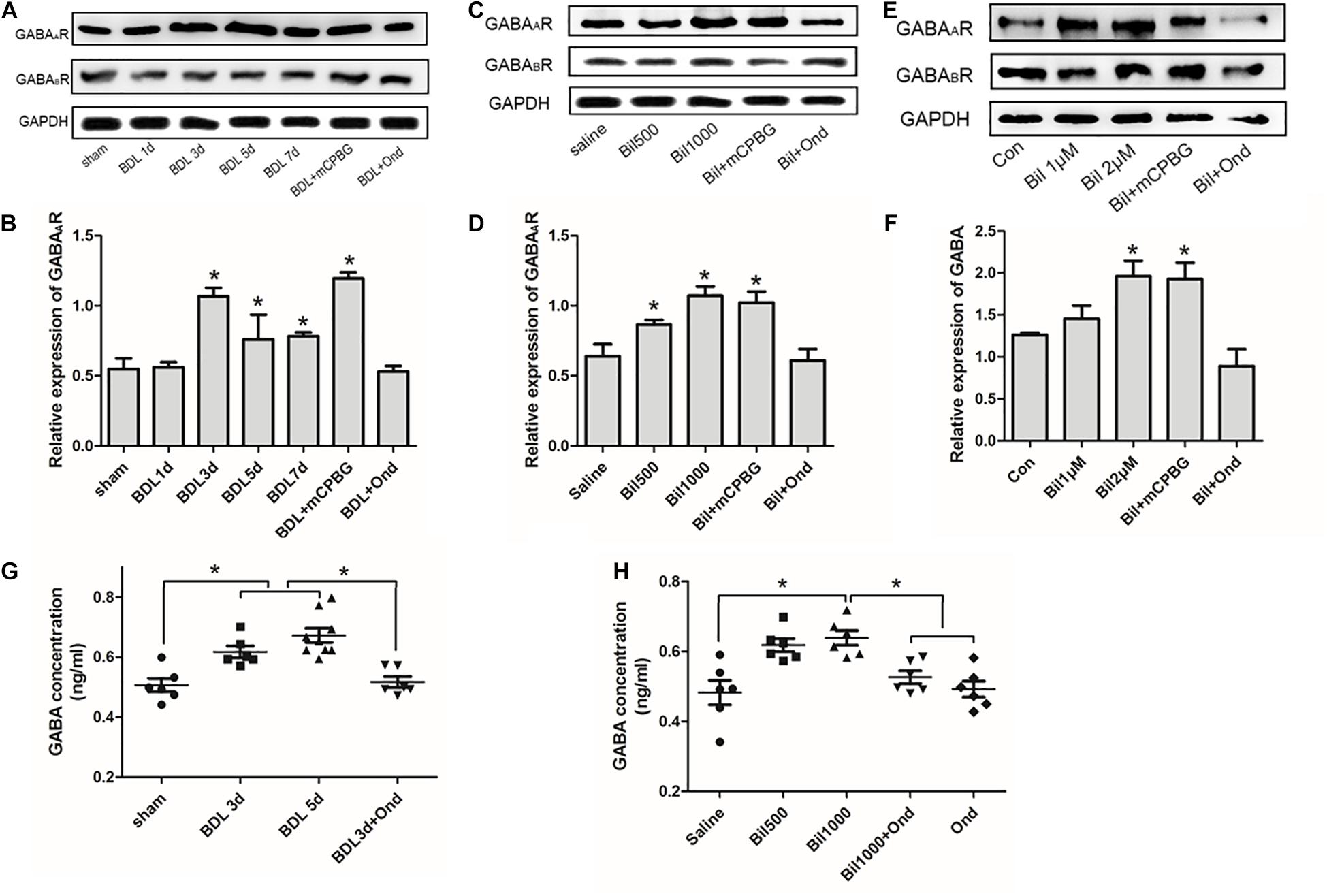
Figure 6. Bilirubin increased GABAA receptor expression, GABA concentration, and GABAergic sIPSC in spinal cords. (A) GABAA receptor expression increased gradually in the spinal cord enlargement of BDL rats without changing GABAB receptor expression, and ondansetron reduced GABAA receptor expression. (B) The quantitative analysis of GABAA receptor expression in the BDL rats. (C) Intrathecal administration of bilirubin increased GABAA receptor expression, and co-administration of bilirubin and ondansetron decreased it without changing GABAB receptor expression. (D) The quantitative analysis of GABAA receptor expression after intrathecal administration of bilirubin in normal rats. (E) SDHNs cultured with bilirubin showed increased GABAA receptor expression without changing GABAB receptor expression, ondansetron reversed GABAA receptor expression. (F) The quantitative analysis of GABAA receptor expression in SDHNs. (G) GABA concentration in CSF of BDL group was significantly higher than sham group, and ondansetron intervention reversed this change. (H) Intrathecal administration of bilirubin (1000 μM) increased GABA concentration in CSF significantly, whereas ondansetron could reverse it. *P < 0.05.
Bilirubin Increased the Activity of sIPSC of GABAergic Neurons
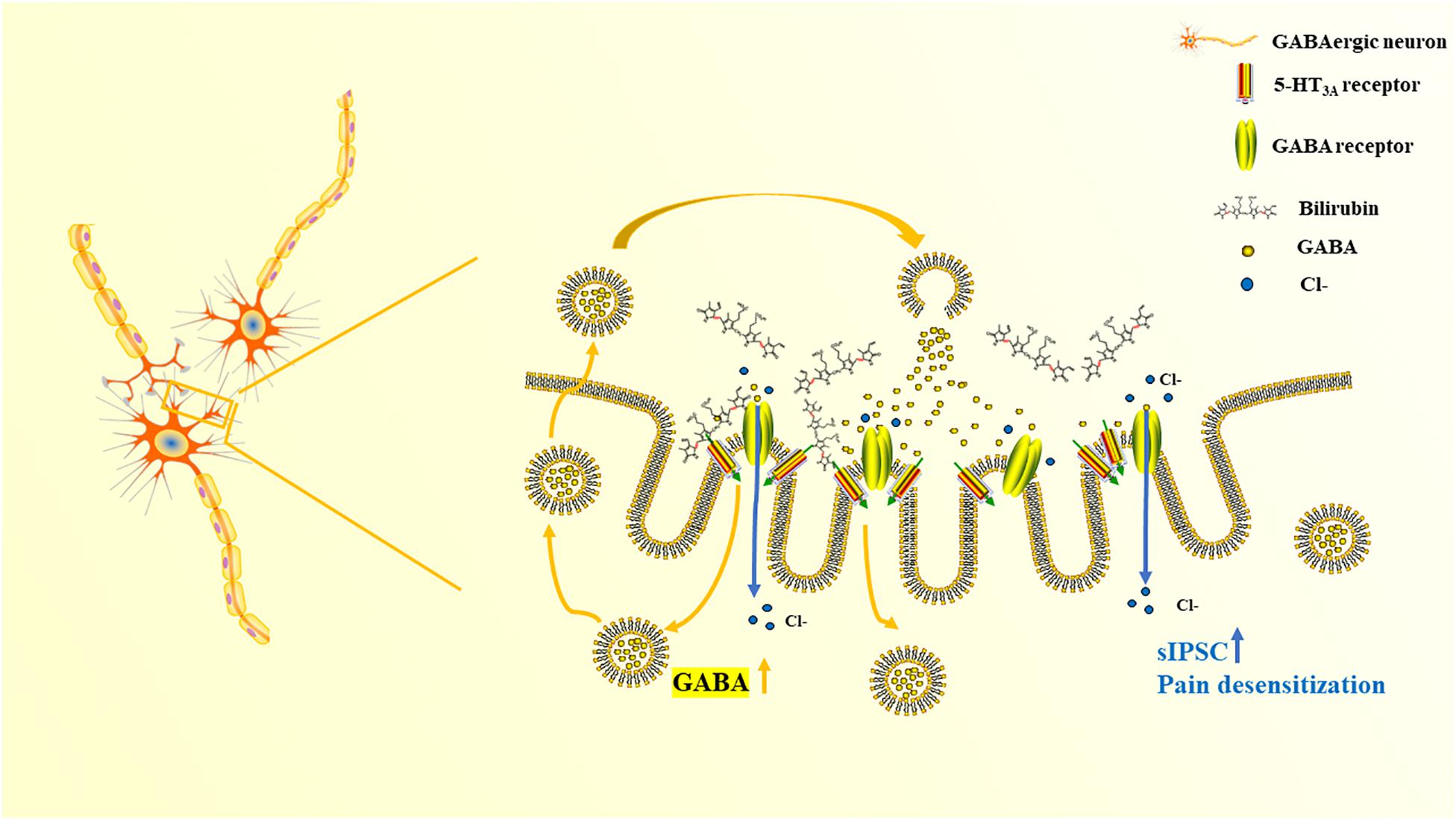
Both mCPBG (0.2 μM) and bilirubin (10 μM) enhanced the amplitude of sIPSC of GABAergic neurons significantly (Figures 7A,B). No differences were observed by mCPBG or bilirubin intervention in the presence of ondansetron perfusion in GABAergic sIPSC (Figure 7C). In addition, mCPBG could increase the frequency of sIPSC, whereas bilirubin had no effect on it (Figure 7D). These results suggested that by activating the 5-HT3A receptor, bilirubin might influence the amplitude of sIPSC of GABAergic neurons. The mechanism map showed the potential interactions among bilirubin, the 5-HT3A receptor, the GABA receptor, and GABA, as shown in our studies (Figure 8).
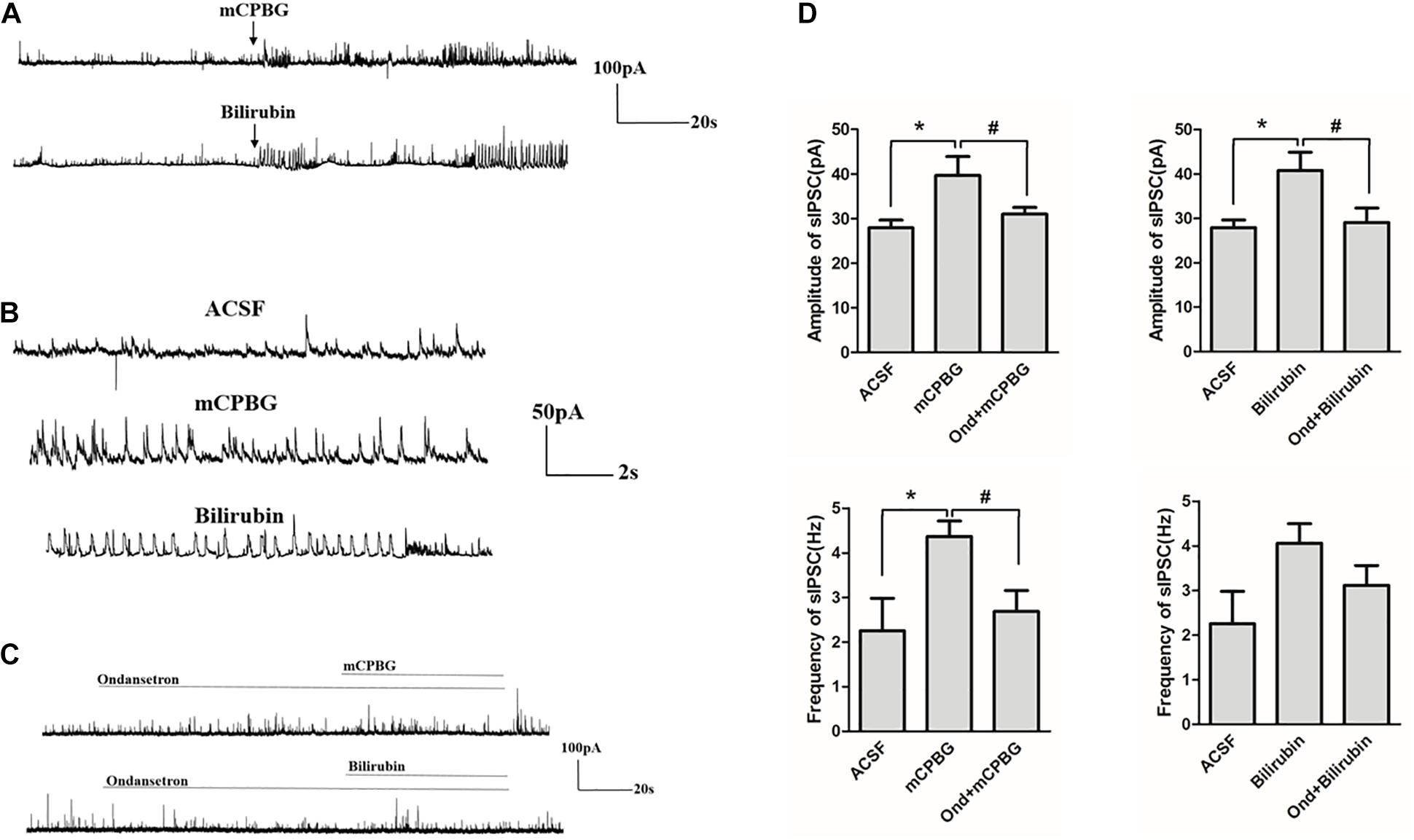
Figure 7. Bilirubin increased the activity of sIPSC of GABAergic neurons. (A) Both 0.2 μM of mCPBG and 10 μM of bilirubin enhanced the activity of GABAergic sIPSC significantly. (B) The enlargement of currents by ACSF, mCPBG, and bilirubin perfusion. (C) No differences were observed by mCPBG or bilirubin intervention in the presence of ondansetron perfusion in GABAergic sIPSC. (D) Histogram of the frequency and amplitude of GABAergic sIPSC changed by mCPBG and bilirubin with or without ondansetron intervention. *P < 0.05; #P < 0.05.
Discussion
Cholestasis, characterized by obstruction of intrahepatic and extrahepatic bile ducts, can damage liver and kidney functions because of the accumulation of bilirubin, bile acid, opioids, and other substances (Wang et al., 2013). Cholestasis patients tend to be insensitive to stimulation, as well as showing concealment of symptoms, recovery delay, and pain desensitization. Morphine consumption in cholestasis decreases remarkably by 33% (Tao et al., 2014). Although studies have shown that bile acids can affect the pain sensation by activating peripheral G protein-coupled bile acid receptor 1 (Dawson and Karpen, 2014), it is difficult for bile acids to pass through the BBB. However, as a small molecule, unconjugated bilirubin can pass the BBB readily (Palmela et al., 2012) and be toxic to gliocyte and neurons. Bilirubin can also induce inflammation, neurodegeneration, and apoptosis, which can affect cognition, memory, pain sensation, and even cause kernicterus in newborns (Erlinger et al., 2014). In our study, we found that pain thresholds increased significantly in BDL rats and normal rats which were administered bilirubin intrathecally, suggesting that bilirubin may contribute to pain sensitization in cholestasis.
As the main relay station, SDH regulates the integration of pain signals with various interneurons, and transmits signals to the brain (Gupta and Deshpande, 2008). As the major inhibitory system involved in pain regulation in SDH, the GABAergic pathway plays important roles in neuropathic pain and other types of pain sensation (Kami et al., 2016). GABAergic neurons take up extracellular glutamate and convert it to GABA by GAD. In synaptic clefts, GABA not only inhibits the release of excitatory neurotransmitters directly by activating presynaptic GABA receptors but also induces inhibitory postsynaptic currents by acting on postsynaptic receptors (Tremblay et al., 2016).
As members of the 5-HT receptors family, the 5-HT3 receptors are the only non-selective ligand-gated ion channels and are distributed mainly in the cerebral cortex, hippocampus, and amygdala (Wang et al., 2017). An electrical stimulus on the periaqueductal gray can activate 5-HT neurons in the rostral ventromedial medulla and inhibit the excitability of pain-sensitive interneurons, which can be antagonized by 5-HT3 receptors antagonists in the spinal cord (Peng et al., 1996). It suggested that the 5-HT3 receptors in the spinal cord was involved in regulating interneuron activities. In the spinal cord, 5-HT3 receptors are expressed mainly in the postsynaptic membrane of GABAergic neurons in laminae I and II (Lee et al., 2010) and can mediate GABA release in a calcium-dependent manner or enhance sIPSC (Pandhare et al., 2017). Therefore, the 5-HT3 receptors exerted important roles in the integration of pain signals.
Morphological studies have shown that most GABAergic neurons in the SDH can express the 5-HT3A receptor (Huang et al., 2008). Kawamata et al. (2003) found that intrathecal administration of 5-HT3A receptor agonists could increase GABA release while other neurotransmitters such as glutamate and glycine did not change. It suggested that the 5-HT3A receptor was associated with GABAergic activities. According to the latest studies, two potential pathways involved were as follows: (1) By activating the 5-HT3A receptor in primary afferent nerves, excitatory neurotransmitters are released and act on GABAergic neurons to strength GABA synthesis in the spinal cord (Huang et al., 2008). (2) The 5-HT3A receptor could enhance the activities of GABAergic and glycinergic neurons by activating capsaicin receptors and inducing glutamate release in interneurons (Nakatsuka and Gu, 2001). We noticed the response discrepancy to CGP55845 between mCPBG injected group and bilirubin injected group in Figures 4D,E. mCPBG is the specific 5-HT3A receptor agonist, while the specific role of bilirubin on 5-HT3A receptor is unclear. In Figure 4D, mCPBG intrathecally induced significant increase of pain threshold in the presence of CGP55845, suggesting inhibition of GABAB receptor showed little effect on mCPBG-mediated 5-HT3A pathway, and GABAA receptor may be the vital downstream molecule. In Figure 4E, bilirubin intrathecally did not induce significant increase of pain threshold in the presence of CGP55845, suggesting inhibition of GABAB receptor showed effect on bilirubin-mediated 5-HT3A pathway, and GABAA and GABAB receptor both participated in the signal pathway of bilirubin. In our study, we found that the activation of the 5-HT3A receptor induced by bilirubin could excite the activity of GABAergic neurons, and it might attribute to the increase of GABA in CSF. Some limitations still need improvement in our study. First, the interaction between bilirubin and GABA receptors was unclear, and further studies were needed to explore if bilirubin could bind with GABA receptors. Second, mechanisms of the increase of GABA in CSF were unclear. The activation of the 5-HT3A receptor induced by bilirubin might influence the generation, release, and degradation of GABA in GABAergic neurons. More studies are needed to detect relevant pathways in the future.
Conclusion
Our study found that elevated bilirubin in cholestasis can enhance the activity of the GABAergic pathway by binding with 5-HT3A receptors in SDH. It increased the understandings of the pain desensitization phenomenon in cholestasis and supplied new targets for clinical treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of the Naval Medical University.
Author Contributions
YS and FW: conceptualization and methodology. XW and XF: software. FW: validation and supervision, project administration, and funding acquisition. EK, HW, and XW: formal analysis and writing—original draft preparation. YZ: investigation. JZ: resources. WY: data curation. YS, FW, and XW: writing—review and editing. EK and XF: visualization. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (81671082); Medical Science and Technology Research Program of Henan Province (SBGJ202003056) and (LHGJ20200781); Department of Health of Zhejiang Province Foundation (2017KY681); and Department of Science and Technology of Zhejiang Province (2018C37134).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.605855/full#supplementary-material
References
Bobinski, F., Ferreira, T. A. A., Cordova, M. M., Dombrowski, P. A., da Cunha, C., Santo, C., et al. (2015). Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain 156, 2595–2606. doi: 10.1097/j.pain.0000000000000372
Cardoso, F. L., Kittel, A., and Veszelka, S. (2012). Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS One. 7:e35919. doi: 10.1371/journal.pone.0035919
Dawson, P. A., and Karpen, S. J. (2014). Bile acids reach out to the spinal cord: new insights to the pathogenesis of itch and analgesia in cholestatic liver disease. Hepatology (Baltimore, Md) 59, 1638–1641. doi: 10.1002/hep.26786
Erlinger, S., Arias, I. M., and Dhumeaux, D. (2014). Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology 146, 1625–1638. doi: 10.1053/j.gastro.2014.03.047
Gupta, R., and Deshpande, S. B. (2008). 3-Nitropropionic acid depresses spinal reflexes involving GABAergic and glycinergic transmission in neonatal rat spinal cord in vitro. Life Sci. 83, 756–760. doi: 10.1016/j.lfs.2008.09.017
Hannon, J., and Hoyer, D. (2008). Molecular biology of 5-HT receptors. Behav. Brain Res. 195, 198–213. doi: 10.1016/j.bbr.2008.03.020
Hasanein, P., and Javanmardi, K. (2008). A potent and selective inhibitor of endocannabinoid uptake, UCM707, potentiates antinociception induced by cholestasis. Fundament. Clin. Pharmacol. 22, 517–522. doi: 10.1111/j.1472-8206.2008.00626.x
Hegade, V. S., Kendrick, S. F., and Jones, D. E. (2015). Drug treatment of pruritus in liver diseases. Clin. Med. (Lond.) 15, 351–357. doi: 10.7861/clinmedicine.15-4-351
Huang, J., Wang, Y. Y., Wang, W., Li, Y. Q., Tamamaki, N., and Wu, S. X. (2008). 5-HT(3A) receptor subunit is expressed in a subpopulation of GABAergic and enkephalinergic neurons in the mouse dorsal spinal cord. Neurosci. Lett. 441, 1–6. doi: 10.1016/j.neulet.2008.04.105
Iwasaki, T., Otsuguro, K., Kobayashi, T., Ohta, T., and Ito, S. (2013). Endogenously released 5-HT inhibits A and C fiber-evoked synaptic transmission in the rat spinal cord by the facilitation of GABA/glycine and 5-HT release via 5-HT(2A) and 5-HT(3) receptors. Eur. J. Pharmacol. 702, 149–157. doi: 10.1016/j.ejphar.2013.01.058
Kami, K., Taguchi Ms, S., Tajima, F., and Senba, E. (2016). Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol. Pain 12:1744806916629059.
Kawamata, T., Omote, K., Toriyabe, M., Yamamoto, H., and Namiki, A. (2003). The activation of 5-HT(3) receptors evokes GABA release in the spinal cord. Brain Res. 978, 250–255. doi: 10.1016/s0006-8993(03)02952-4
Langlois, S. D., Morin, S., Yam, P. T., and Charron, F. (2010). Dissection and culture of commissural neurons from embryonic spinal cord. J. Vis. Exp. 25:1773.
Lee, S., Hjerling-Leffler, J., Zagha, E., Fishell, G., and Rudy, B. (2010). The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. : Official J. Soc. Neurosci. 30, 16796–16808. doi: 10.1523/jneurosci.1869-10.2010
Meixiong, J., Vasavda, C., and Green, D. (2019). Identification of a bilirubin receptor that may mediate a component of cholestatic itch. Elife 8:e44116.
Nakatsuka, T., and Gu, J. G. (2001). ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J. Neurosci. : Official J. Soc. Neurosci. 21, 6522–6531. doi: 10.1523/jneurosci.21-17-06522.2001
Njoo, C., Heinl, C., and Kuner, R. (2014). In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J. Vis. Exp. 22:51229.
Palmela, I., Sasaki, H., Cardoso, F. L., Moutinho, M., Kim, K. S., Brites, D., et al. (2012). Time-dependent dual effects of high levels of unconjugated bilirubin on the human blood-brain barrier lining. Front. Cell. Neurosci. 6:22. doi: 10.3389/fncel.2012.00022
Pandhare, A., Pappu, A. S., Wilms, H., Blanton, M. P., and Jansen, M. (2017). The antidepressant bupropion is a negative allosteric modulator of serotonin type 3A receptors. Neuropharmacology 113(Pt A), 89–99. doi: 10.1016/j.neuropharm.2016.09.021
Pelissier, T., Alloui, A., Caussade, F., Dubray, C., Cloarec, A., Lavarenne, J., et al. (1996). Paracetamol exerts a spinal antinociceptive effect involving an indirect interaction with 5-hydroxytryptamine3 receptors: in vivo and in vitro evidence. J. Pharmacol. Exp. Therapeutics. 278, 8–14.
Peng, Y. B., Lin, Q., and Willis, W. D. (1996). The role of 5-HT3 receptors in periaqueductal gray-induced inhibition of nociceptive dorsal horn neurons in rats. J. Pharmacol. Exp. Ther. 276, 116–124.
Roczniak, W., Wrobel, J., Dolczak, L., and Nowak, P. (2013). Influence of central noradrenergic system lesion on the serotoninergic 5-HT3 receptor mediated analgesia in rats. Adv. Clin. Exp. Med. : Official Organ Wrocław Med. Univ. 22, 629–638.
Schaaf, T., Lyutenska, M., Urban, B. W., and Wittmann, M. (2014). Direct effects of morphine but not of fentanyl-type opioids on human 5-HT3A receptors in outside-out patch-clamp studies. Eur. J. Pain. 18, 1165–1172. doi: 10.1002/j.1532-2149.2014.00463.x
Shah, R., and John, S. (2020). Cholestatic Jaundice (Cholestasis, Cholestatic Hepatitis). In: StatPearls. Treasure Island (FL): StatPearls Publishing.
Tao, K. M., Tao, Y., Chen, C. Y., Yang, L. Q., Lu, Z. J., Sun, Y. M., et al. (2014). Proteinase-activated receptor 1 contributed to up-regulation of enkephalin in keratinocytes of patients with obstructive jaundice. Anesthesiology 121, 127–139. doi: 10.1097/aln.0000000000000210
Tian, B., Wang, X. L., and Huang, Y. (2016). Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci. Rep. 6:36286.
Tremblay, R., Lee, S., and Rudy, B. (2016). GABAergic interneurons in the Neocortex: from cellular properties to circuits. Neuron 91, 260–292. doi: 10.1016/j.neuron.2016.06.033
Wang, C. T., Mao, C. J., Zhang, X. Q., Zhang, C. Y., Lv, D. J., Yang, Y. P., et al. (2017). Attenuation of hyperalgesia responses via the modulation of 5-hydroxytryptamine signalings in the rostral ventromedial medulla and spinal cord in a 6-hydroxydopamine-induced rat model of Parkinson’s disease. Mol. Pain 13:1744806917691525.
Wang, Z. M., Zhang, P., Lin, M. J., Tan, B., Qiu, H. B., and Yu, W. F. (2013). Influence of obstructive jaundice on pharmacodynamics of rocuronium. PLoS One. 8:e78052. doi: 10.1371/journal.pone.0078052
Xiang, Z., Xiong, Y., Yan, N., Li, X., Mao, Y., Ni, X., et al. (2008). Functional up-regulation of P2X 3 receptors in the chronically compressed dorsal root ganglion. Pain 140, 23–34. doi: 10.1016/j.pain.2008.07.006
Xu, P., Ling, Z. L., and Zhang, J. (2016). Unconjugated bilirubin elevation impairs the function and expression of breast cancer resistance protein (BCRP) at the blood-brain barrier in bile duct-ligated rats. Acta Pharmacol. Sin. 37, 1129–1140. doi: 10.1038/aps.2016.25
Keywords: bilirubin, cholestasis, 5-HT3A receptor, pain desensitization, GABA
Citation: Kong E, Wang H, Wang X, Zhang Y, Zhang J, Yu W, Feng X, Sun Y and Wu F (2021) Bilirubin Induces Pain Desensitization in Cholestasis by Activating 5-Hydroxytryptamine 3A Receptor in Spinal Cord. Front. Cell Dev. Biol. 9:605855. doi: 10.3389/fcell.2021.605855
Received: 13 September 2020; Accepted: 15 March 2021;
Published: 01 April 2021.
Edited by:
Catherine Ketcham, United States and Canadian Academy of Pathology (USCAP), United StatesReviewed by:
Jean-Paul Desaulniers, Ontario Tech University, CanadaWangyuan Zou, Central South University, China
Copyright © 2021 Kong, Wang, Wang, Zhang, Zhang, Yu, Feng, Sun and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Yu, eXdmODA4QHllYWgubmV0; Yuming Sun, MTMzMDE4MzY5MzBAMTYzLmNvbQ==; Feixiang Wu, ZmVpeGlhbmd3dUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Erliang Kong1,2†
Erliang Kong1,2† Xiaoqiang Wang
Xiaoqiang Wang Weifeng Yu
Weifeng Yu Feixiang Wu
Feixiang Wu