- 1Department of Neurosurgery and Institute for Functional Brain Disorders, Tangdu Hospital, Fourth Military Medical University, Xi’an, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 3Department of Radiology and Functional and Molecular Imaging Key Lab of Shaanxi Province, Tangdu Hospital, Fourth Military Medical University, Xi’an, China
There has been an increased interest for observational studies or randomized controlled trials exploring the impact of calcium intake on cardiovascular diseases (CVD) including coronary artery disease (CAD) and ischemic stroke (IS). However, a direct relationship between total calcium intake and CVD has not been well established and remains controversial. Mendelian randomization (MR) studies have been performed to evaluate the causal association between serum calcium levels and CAD risk and found that increased serum calcium levels could increase the risk of CAD. However, MR analysis found no significant association between genetically higher serum calcium levels and IS as well as its subtypes. Hence, three MR studies reported inconsistent effects of serum calcium levels on CAD and IS. Here, we performed an updated MR study to investigate the association of serum calcium levels with the risk of IS using large-scale genome-wide association study (GWAS) datasets. We selected 14 independent genetic variants as the potential instrumental variables from a large-scale serum calcium GWAS dataset and extracted summary statistics corresponding to the 14 serum calcium genetic variants from the MEGASTROKE Consortium IS GWAS dataset. Interestingly, we found a significant association between serum calcium levels and IS risk using the robust inverse-variance weighted (IVW) and penalized robust IVW methods, with β = 0.243 and P = 0.002. Importantly, the MR results from the robust MR-Egger and penalized robust MR-Egger methods further supported the causal association between serum calcium levels and IS risk, with β = 0.256 and P = 0.005. Meanwhile, the estimates from other MR methods are also consistent with the above findings.
Introduction
In recent years, there has been an increased interest for observational studies or randomized controlled trials exploring the impact of calcium intake on cardiovascular diseases (CVD) including coronary artery disease (CAD) and ischemic stroke (IS; Heaney et al., 2012; Anderson et al., 2016; Tankeu et al., 2017). In fact, a significant number of studies have reported an association between calcium intake and adverse CVD (Bolland et al., 2008, 2010, 2011; Tankeu et al., 2017). However, these conclusions have been widely questioned by a number of experts who have raised concerns about the methodology, potential biases, and confounders (Lappe and Heaney, 2008; Puccetti, 2008; Ramlackhansingh, 2008; Grove and Cook, 2010; Heiss et al., 2010; Black, 2011). Until now, a direct relationship between total calcium intake and CVD has not been well established and remains controversial, as described in two recent reviews (Heaney et al., 2012; Tankeu et al., 2017).
Until recently, Mendelian randomization (MR) studies have been performed to evaluate the causal association between increased serum calcium levels and CAD risk (Xu et al., 2017; Larsson et al., 2017a). Xu et al. (2017) selected four independent variants for the main analysis and 13 correlated variants for a sensitivity analysis as the included instrumental variables. All these genetic variants could influence the serum calcium levels, with the genome-wide significance (P < 5.00E-08) from a recent genome-wide association study (GWAS) including 20,611 individuals of European ancestry (O’Seaghdha et al., 2010). Larsson et al. (2017a) selected seven independent genetic variants influencing serum calcium levels, with the genome-wide significance (P < 5.00E-08) from a recent GWAS including 61,079 individuals of European ancestry (O’Seaghdha et al., 2013), as the instrumental variables. Both Xu et al. (2017) and Larsson et al. (2017a) identified that increased serum calcium levels could increase the risk of CAD.
Importantly, MR analysis has also been performed to evaluate the causal association between increased serum calcium levels and IS risk (Larsson et al., 2019). Larsson et al. (2019) selected seven independent genetic variants influencing serum calcium levels and a large-scale IS dataset from the MEGASTROKE Consortium (34,217 cases and 404,630 controls). However, they found no significant association between genetically higher serum calcium levels and IS as well as its subtypes (Larsson et al., 2019). Hence, these three MR studies reported inconsistent effects of serum calcium levels on CAD and IS (Xu et al., 2017; Larsson et al., 2017a, 2019). Here, we performed an updated MR study to investigate the association of serum calcium levels with the risk of stroke using large-scale serum calcium GWAS dataset and IS GWAS dataset.
Materials and Methods
Study Design
The MR study design has been well established in recent studies (Cheng et al., 2018; Zhuang et al., 2019a,b; Sun et al., 2020). In brief, we only selected the GWAS summary datasets about serum calcium levels and IS (O’Seaghdha et al., 2013; Malik et al., 2018). Informed consent was provided by all participants in all these corresponding original studies (O’Seaghdha et al., 2013; Malik et al., 2018).
Serum Calcium Genetic Variants
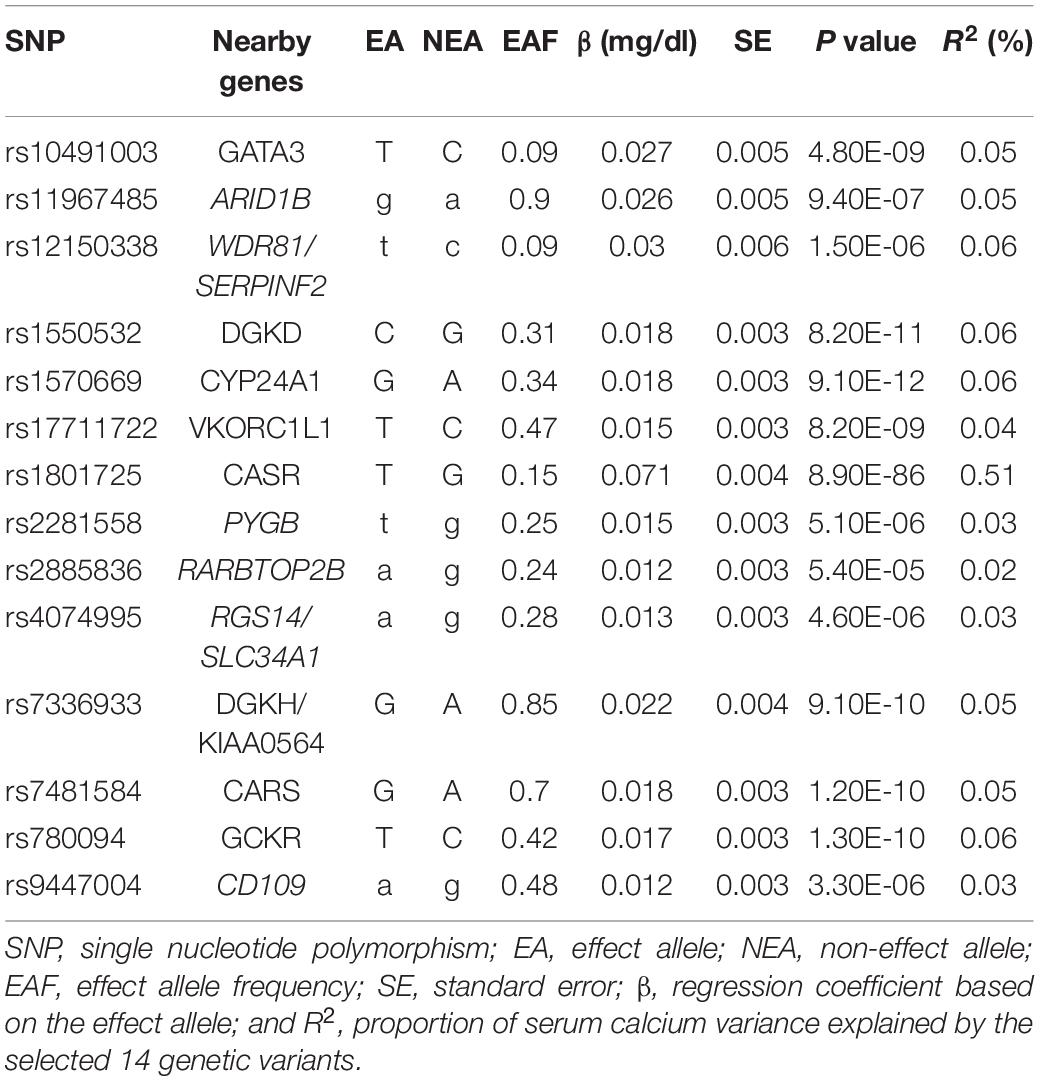
We selected 14 independent genetic variants as the potential instrumental variables from a large-scale serum calcium GWAS dataset (O’Seaghdha et al., 2013). In brief, this dataset consisted of 61,079 individuals of European descent, including 39,400 individuals in the discovery stage and 21,679 individuals in the replication stage (O’Seaghdha et al., 2013). A linear regression method was used to evaluate the association of each genetic variant with serum calcium level using an additive genetic effect by adjusting some key covariates including age, sex, principal components, and study center (O’Seaghdha et al., 2013). Of these 14 genetic variants, eight were associated with serum calcium levels with P < 5.00E-08 and six genetic variants were associated with serum calcium levels with P < 1.00E-04 (O’Seaghdha et al., 2013). We provide more detailed information about these 14 variants in Table 1. Recent studies have provided more detailed information about this dataset (O’Seaghdha et al., 2013; Xu et al., 2017; Larsson et al., 2017a, 2019).
IS GWAS Dataset
The IS GWAS dataset is from a large-scale multi-ancestry stroke GWAS of 67,162 cases and 454,450 controls from the MEGASTROKE Consortium (Malik et al., 2018). The participants are of multiple ancestries including European, African, South Asian, mixed Asian, and Latin American (Malik et al., 2018). Before the normal GWAS analysis, the MEGASTROKE Consortium conducted genotyping, imputation, and quality control analyses (Malik et al., 2018). They further conducted a fixed-effects inverse-variance weighted (IVW) meta-analysis using METAL in each ancestral group and then performed a meta-analysis of the ancestry-specific meta-analysis results (Malik et al., 2018). In order to be consistent with the serum calcium GWAS dataset, we limit our follow-up analysis to samples of European ancestry, including 34,217 IS cases and 406,111 controls (Malik et al., 2018). More detailed information about the MEGASTROKE dataset has been widely described in the original study (Malik et al., 2018) and in the recent MR study (Larsson et al., 2019).
Pleiotropy Analysis
Using the summary results of these 14 genetic variants in both serum calcium levels and IS GWAS datasets, we first conducted a pleiotropy analysis using two statistical methods including the MR-Egger intercept test and the MR pleiotropy residual sum and outlier (MR-PRESSO) test (Verbanck et al., 2018). Both methods have been widely used in recent MR studies (Liu et al., 2018; Larsson et al., 2019; He et al., 2020; Wang et al., 2020). The statistical significance for evidence of pleiotropy is P < 0.05.
MR Analysis
For MR analysis, we selected 11 MR methods including simple median, weighted median, penalized weighted median, IVW, penalized IVW, robust IVW, penalized robust IVW, MR-Egger, penalized MR-Egger, robust MR-Egger, and penalized robust MR-Egger (Larsson et al., 2019; He et al., 2020). IVW is a standard MR analysis method. For multiple independent genetic variants, IVW could weigh the average of these single causal estimates using the inverse of their approximate variances as weights (Burgess and Thompson, 2017; Larsson et al., 2019; He et al., 2020). The causal estimate from the MR-Egger is obtained using the same regression model as the weighted linear regression described above, but allowing the intercept to be estimated as part of the analysis (Burgess and Thompson, 2017). The simple median estimator is calculated as the median of the Wald ratio estimates [ratio of single nucleotide polymorphism (SNP) on outcome to SNP on calcium]. A weighted median of these causal estimates could be considered to account for differences in the precision of estimates and could provide consistent estimates even if 50% of the instrumental variables are invalid (Burgess et al., 2017). The robust option, penalized option, and the penalized option of the weighted median, IVW, and MR-Egger are all improved MR methods (Yavorska and Burgess, 2017).
Statistical Analysis
The odds ratio (OR) as well as the 95% confidence interval (CI) of IS correspond to per 0.5 mg/dl increase [about 1 standard deviation (SD)] in serum calcium levels. The significance threshold was P < 0.05. All statistical tests were completed by R (version 3.5.1) and R package “MendelianRandomization” (Yavorska and Burgess, 2017).
Power Analysis
The proportion of serum calcium variance explained by the selected 14 genetic variants could be estimated using R2.
where β is the effect size (beta coefficient) between SNPi and the serum calcium levels, MAFSNP_i is the minor allele frequency for SNPi, and SD = 0.5 mg/dl (Larsson et al., 2017a; He et al., 2020). Using R2 and other necessary information, we performed a power analysis using mRnd: Power calculations for MR1 (Brion et al., 2013). The input variables include the sample size, type I error rate, proportion of cases in the study, true odds ratio of the outcome variable per standard deviation of the exposure variable, and the proportion of variance explained for the association between the SNP and the exposure variable (Brion et al., 2013).
Results
Association of 14 Serum Calcium Variants With IS
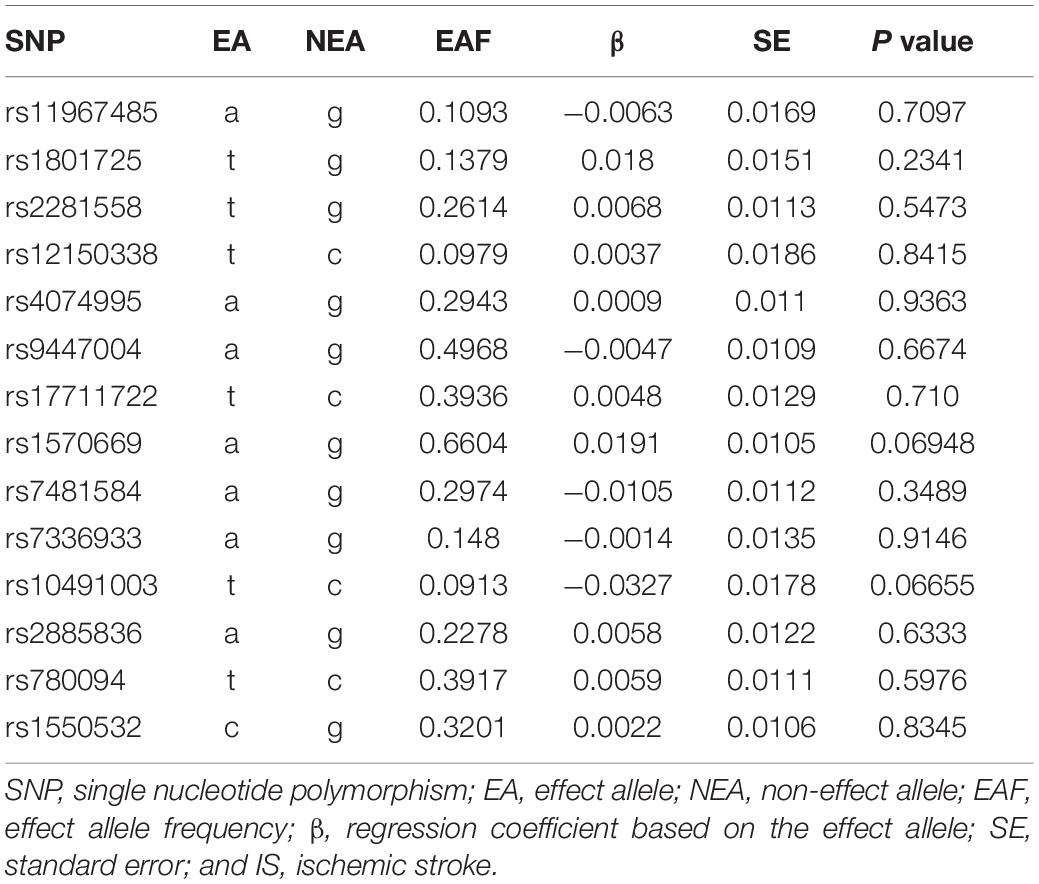
In the IS GWAS dataset from the MEGASTROKE Consortium, we extracted the summary statistics corresponding to the 14 serum calcium genetic variants. The results indicated that none of these 14 variants was significantly associated with IS (P < 0.05; Table 2).
Statistical Analysis
The pleiotropy analysis using the MR-Egger intercept test indicated no significant pleiotropy, with intercept = −0.004 and P = 0.574. The pleiotropy analysis using the MR-PRESSO test further indicated no significant pleiotropy, with P = 0.669. Hence, all these 14 variants are valid instrumental variables and could be used in the MR analysis.
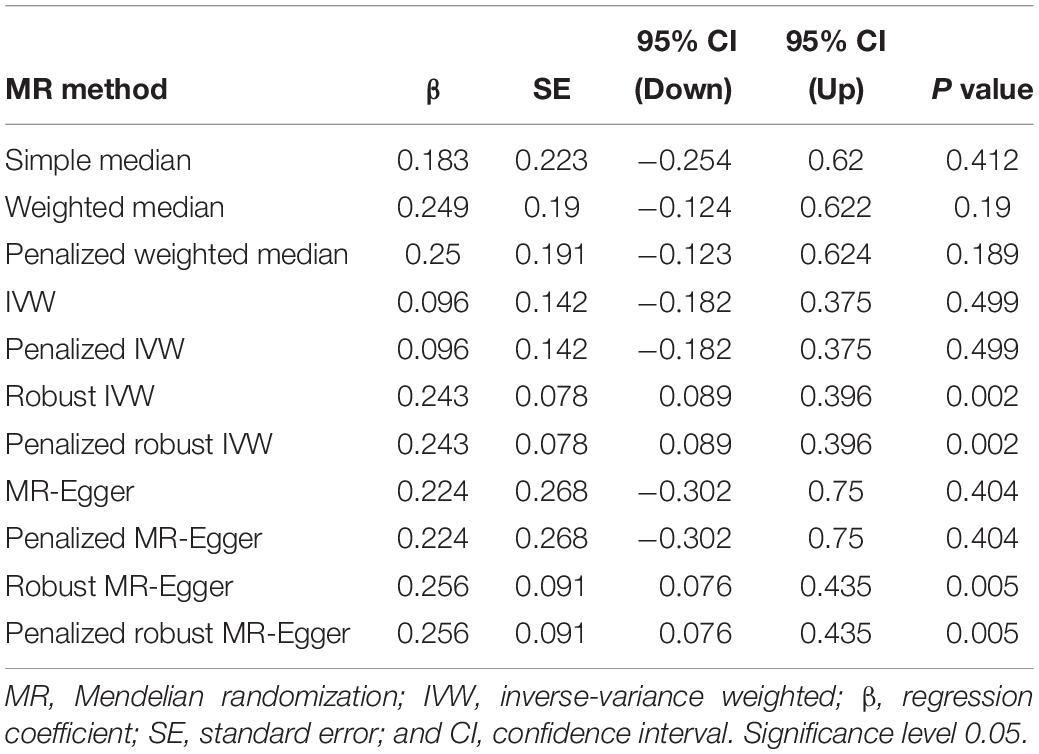
Inverse-variance weighted indicated no significant association between serum calcium levels and IS risk, with β = 0.096 and P = 0.499. Interestingly, we found a significant association between serum calcium levels and IS risk using the robust IVW and penalized robust IVW methods, with β = 0.243 and P = 0.002 (Table 3). Importantly, the MR results from the robust MR-Egger and penalized robust MR-Egger methods further supported the causal association between serum calcium levels and IS risk, with β = 0.256 and P = 0.005 (Table 3). Meanwhile, the estimates from the other MR methods are also consistent with the above findings, as provided in Table 3. We also provided the single MR estimates from each of the 14 genetic variants using 11 MR methods, as described in Figure 1.
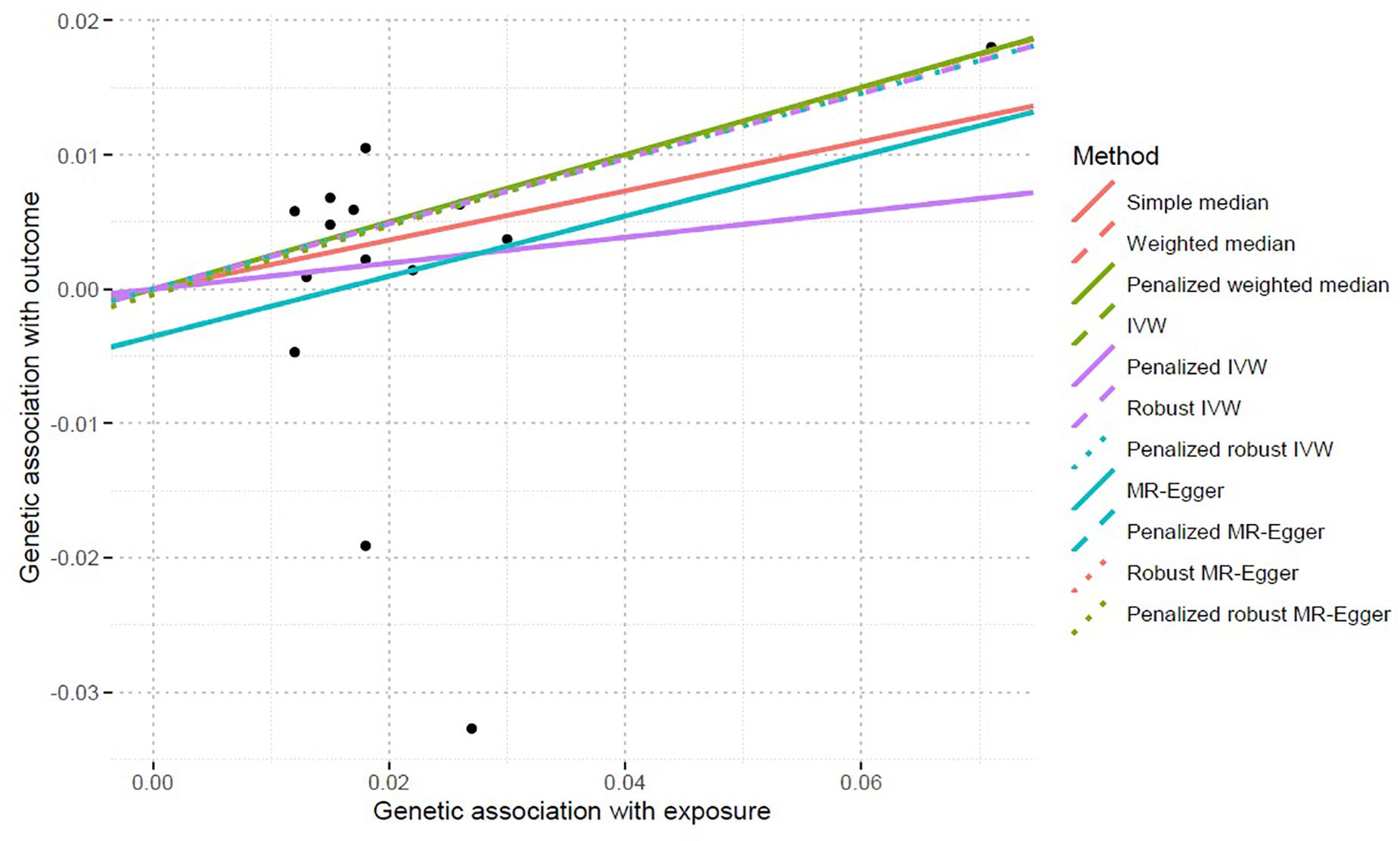
Figure 1. Single MR estimates from each of the 14 genetic variants using 11 MR methods. MR, Mendelian randomization; IVW, inverse-variance weighted. The black scatter plots indicate single causal estimates from each of the 14 genetic variants associated with serum calcium levels on the x-axis and IS risk on the y-axis. The continuous line represents the causal effect of serum calcium levels on IS risk.
Statistical Power
These 14 genetic variants could explain 1.10% of the serum calcium variance (R2 = 1.10%). Power analysis using mRnd indicated that our MR study had 80% power to detect effect sizes of moderate magnitude, with ORs as low as 0.83 and as high as 1.15 per SD (0.5 mg/dl) increase in serum calcium levels for IS. The power is 100% to detect the causal association, with OR = 1.28 (β = 0.243).
Discussion
Until now, meta-analyses of randomized controlled trials have not demonstrated convincing evidence that calcium intake (diet and supplements) could improve stroke (Heaney et al., 2012; Tankeu et al., 2017). Some studies even reported an association between calcium intake and adverse stroke outcomes (Bolland et al., 2008, 2010, 2011). In 2018, Jenkins et al. (2018) performed a meta-analysis of individual randomized controlled trials from previous meta-analyses and additional searches. They found that calcium supplements showed no consistent benefit for the prevention of stroke, nor was there a benefit for all-cause mortality to support their continued use (Jenkins et al., 2018).
Until recently, MR studies have been performed to evaluate the causal effects of high serum calcium levels on the risk of CAD and IS. However, these MR studies reported inconsistent findings. Some studies indicated that high serum calcium levels contributed to an increased risk of CAD (Xu et al., 2017; Larsson et al., 2017a). One MR study indicated no significant association between high serum calcium levels and IS as well as its subtypes (Larsson et al., 2019). Hence, these inconsistent findings drove us to conduct an updated MR analysis, and we found a significant association between high serum calcium levels and increased IS risk.
There are two main differences between our MR study and previous MR studies (Xu et al., 2017; Larsson et al., 2017a, 2019). Firstly, we selected 14 genetic variants associated with serum calcium levels as the potential instrumental variables, as these 14 genetic variants could explain 1.10% of the serum calcium variance. In 2017, Larsson et al. (2017a) selected seven genetic variants and excluded the rs780094 variant to evaluate the causal association between serum calcium levels and CAD, as it had a pleiotropic association with cardiometabolic risk factors. The remaining six genetic variants only explained about 0.8% of the variation in serum calcium levels (Larsson et al., 2017a). In 2019, Larsson et al. (2019) selected seven genetic variants to evaluate the causal association between serum calcium levels and IS risk, which could explain 0.9% of the variance in serum calcium levels. In 2017, Xu et al. (2017) only selected four variants influencing serum calcium levels as the instrumental variables. Hence, our MR study has the largest explained variation in serum calcium levels, which may further contribute to identify positive findings.
Secondly, we selected more MR methods than did the previous MR studies (Xu et al., 2017; Larsson et al., 2017a, 2019). In our MR study, we selected a total of 11 MR methods including simple median, weighted median, penalized weighted median, IVW, penalized IVW, robust IVW, penalized robust IVW, MR-Egger, penalized MR-Egger, robust MR-Egger, and penalized robust MR-Egger. Importantly, all these MR estimates were consistent with each other in terms of direction and magnitude, as provided in Table 3. In 2017, Larsson et al. (2017a) selected three MR methods including IVW, weighted median, and MR-Egger. In 2017, Xu et al. (2017) selected four MR methods including weighted generalized linear regression, IVW, weighted median, and MR-Egger regression. In 2019, Larsson et al. (2019) selected five MR methods including IVW, weighted median, heterogeneity-penalized model averaging method, MR-Egger, and MR-PRESSO.
Hence, our findings are consistent with recent findings from MR analysis in CAD (Xu et al., 2017; Larsson et al., 2017a). Meanwhile, our findings are consistent with previous observational studies (Chung et al., 2015). Chung et al. measured the levels of serum calcium and albumin-corrected calcium in 1,915 participants (Chung et al., 2015). They found that the serum calcium level was significantly associated with increased modified Rankin scale (MRS) [1.19 (1.03–1.38)]. The albumin-corrected calcium level was also significantly associated with increased MRS [1.21 (1.01–1.44)] (Chung et al., 2015). The authors further identified that a high albumin-corrected serum calcium level could cause a poorer short-term outcome and increase the long-term mortality in acute IS (Chung et al., 2015). In 2017, de Abajo et al. (2017) performed a nested case–control study to evaluate the risk of IS with calcium supplement using 2,690 IS cases and 19,538 controls. Their results showed that calcium supplement was associated with an increased trend of IS risk in the whole population (OR = 1.18, 95% CI = 0.86–1.61, and P = 0.31).
Our MR study also has some limitations. One limitation is that not all of the selected 14 genetic variants are associated with serum calcium levels with genome-wide significance threshold P < 5.00E-08. Only eight genetic variants were associated with serum calcium levels with P < 5.00E-08 and six genetic variants were associated with serum calcium levels with P < 1.00E-04 (O’Seaghdha et al., 2013). The other limitation is that we only selected two statistical methods—the MR-Egger intercept test and the MR-PRESSO test—to conduct a pleiotropy analysis and test the confounders. Interestingly, we did not find any significant pleiotropy using both methods. However, the statistical methods could not completely exclude all confounders. It is a general challenge in current MR studies (Emdin et al., 2017; Larsson et al., 2017a, 2019; He et al., 2020). Hence, follow-up studies are necessary to replicate our findings.
In summary, our MR study provides genetic evidence that high serum calcium levels are significantly associated with an increased risk of IS in the general population.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
QC and DF designed the project. QM, LH, and KT analyzed the data. All authors wrote and approved the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (grant nos. 81500909, 81971129, and 81771245) and the National Natural Science Foundation of Shaanxi Province (grant no. 2018JM7019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Anderson, J. J., Kruszka, B., Delaney, J. A., He, K., Burke, G. L., Alonso, A., et al. (2016). Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J. Am. Heart Assoc. 5:e003815.
Black, S. L. (2011). Analysis of absolute incidence tells different story. BMJ 342:d3530. doi: 10.1136/bmj.d3530
Bolland, M. J., Avenell, A., Baron, J. A., Grey, A., Maclennan, G. S., Gamble, G. D., et al. (2010). Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 341:c3691. doi: 10.1136/bmj.c3691
Bolland, M. J., Barber, P. A., Doughty, R. N., Mason, B., Horne, A., Ames, R., et al. (2008). Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 336, 262–266. doi: 10.1136/bmj.39440.525752.be
Bolland, M. J., Grey, A., Avenell, A., Gamble, G. D., and Reid, I. R. (2011). Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 342:d2040. doi: 10.1136/bmj.d2040
Brion, M. J., Shakhbazov, K., and Visscher, P. M. (2013). Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 42, 1497–1501. doi: 10.1093/ije/dyt179
Burgess, S., Bowden, J., Dudbridge, F., and Thompson, S. G. (2017). Robust instrumental variable methods using multiple candidate instruments with application to Mendelian randomization. arXiv [Preprint]. Available online at: https://arxiv.org/abs/1606.03729 (accessed May 15, 2020).
Burgess, S., and Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32, 377–389. doi: 10.1007/s10654-017-0255-x
Cheng, L., Zhuang, H., Yang, S., Jiang, H., Wang, S., and Zhang, J. (2018). Exposing the causal effect of C-reactive protein on the risk of type 2 diabetes mellitus: a mendelian randomization study. Front. Genet. 9:657. doi: 10.3389/fgene.2018.00657
Chung, J. W., Ryu, W. S., Kim, B. J., and Yoon, B. W. (2015). Elevated calcium after acute ischemic stroke: association with a poor short-term outcome and long-term mortality. J. Stroke 17, 54–59. doi: 10.5853/jos.2015.17.1.54
de Abajo, F. J., Rodriguez-Martin, S., Rodriguez-Miguel, A., and Gil, M. J. (2017). Risk of ischemic stroke associated with calcium supplements with or without vitamin d: a nested case-control Study. J. Am. Heart Assoc. 6:e005795.
Emdin, C. A., Khera, A. V., Natarajan, P., Klarin, D., Zekavat, S. M., Hsiao, A. J., et al. (2017). Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 317, 626–634. doi: 10.1001/jama.2016.21042
Grove, M. L., and Cook, D. (2010). Calcium and heart attacks. Doesn’t apply to most calcium prescriptions. BMJ 341:c5003. doi: 10.1136/bmj.c5003
He, Y., Zhang, H., Wang, T., Han, Z., Ni, Q. B., Wang, K., et al. (2020). Impact of serum calcium levels on Alzheimer’s disease: a mendelian randomization study. J. Alzheimers Dis. 76, 713–724.
Heaney, R. P., Kopecky, S., Maki, K. C., Hathcock, J., Mackay, D., and Wallace, T. C. (2012). A review of calcium supplements and cardiovascular disease risk. Adv. Nutr. 3, 763–771. doi: 10.3945/an.112.002899
Heiss, G., Hsia, J., Pettinger, M., Howard, B. V., and Anderson, G. (2010). Calcium and heart attacks. No evidence for increased risk. BMJ 341:c4995.
Jenkins, D. J. A., Spence, J. D., Giovannucci, E. L., Kim, Y.-I., Josse, R., Vieth, R., et al. (2018). Supplemental vitamins and minerals for CVD prevention and treatment. J. Am. College Cardiol. 71, 2570–2584.
Lappe, J. M., and Heaney, R. P. (2008). Calcium supplementation: results may not be generalisable. BMJ 336, 403–404.
Larsson, S. C., Burgess, S., and Michaelsson, K. (2017a). Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA 318, 371–380. doi: 10.1001/jama.2017.8981
Larsson, S. C., Traylor, M., Burgess, S., Boncoraglio, G. B., Jern, C., Michaelsson, K., et al. (2019). Serum magnesium and calcium levels in relation to ischemic stroke: mendelian randomization study. Neurology 92, e944–e950.
Larsson, S. C., Traylor, M., Malik, R., Dichgans, M., Burgess, S., and Markus, H. S. (2017b). Modifiable pathways in Alzheimer’s disease: mendelian randomisation analysis. BMJ 359:j5375. doi: 10.1136/bmj.j5375
Liu, G., Zhao, Y., Jin, S., Hu, Y., Wang, T., Tian, R., et al. (2018). Circulating vitamin E levels and Alzheimer’s disease: a Mendelian randomization study 9. Neurobiol. Aging 72, e181–e189.
Malik, R., Chauhan, G., Traylor, M., Sargurupremraj, M., Okada, Y., Mishra, A., et al. (2018). Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 50, 524–537.
O’Seaghdha, C. M., Wu, H., Yang, Q., Kapur, K., Guessous, I., Zuber, A. M., et al. (2013). Meta-analysis of genome-wide association studies identifies six new Loci for serum calcium concentrations. PLoS Genet. 9:e1003796. doi: 10.1371/journal.pgen.1003796
O’Seaghdha, C. M., Yang, Q., Glazer, N. L., Leak, T. S., Dehghan, A., Smith, A. V., et al. (2010). Common variants in the calcium-sensing receptor gene are associated with total serum calcium levels. Hum. Mol. Genet. 19, 4296–4303. doi: 10.1093/hmg/ddq342
Sun, W., Han, Y., Yang, S., Zhuang, H., Zhang, J., Cheng, L., et al. (2020). The assessment of interleukin-18 on the risk of coronary heart disease. Med. Chem. 16, 626–634.
Tankeu, A. T., Ndip Agbor, V., and Noubiap, J. J. (2017). Calcium supplementation and cardiovascular risk: a rising concern. J. Clin. Hypertens. 19, 640–646. doi: 10.1111/jch.13010
Verbanck, M., Chen, C. Y., Neale, B., and Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. doi: 10.1038/s41588-018-0099-7
Wang, L., Qiao, Y., Zhang, H., Zhang, Y., Hua, J., Jin, S., et al. (2020). Circulating Vitamin D levels and Alzheimer’s disease: a mendelian randomization study in the IGAP and UK biobank. J. Alzheimers Dis. 73, 609–618. doi: 10.3233/jad-190713
Xu, L., Lin, S. L., and Schooling, C. M. (2017). A Mendelian randomization study of the effect of calcium on coronary artery disease, myocardial infarction and their risk factors. Sci. Rep. 7:42691.
Yavorska, O. O., and Burgess, S. (2017). MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46, 1734–1739. doi: 10.1093/ije/dyx034
Zhuang, H., Han, J., Cheng, L., and Liu, S. L. (2019a). A positive causal influence of IL-18 levels on the risk of T2DM: a mendelian randomization study. Front. Genet. 10:295. doi: 10.3389/fgene.2019.00295
Keywords: serum calcium, ischaemic stroke, Mendelian randomization, genome-wide association study, coronary artery disease
Citation: Meng Q, Huang L, Tao K, Liu Y, Jing J, Wang W, Qin H, Feng D and Cai Q (2020) Integrated Genetics and Micronutrient Data to Inform the Causal Association Between Serum Calcium Levels and Ischemic Stroke. Front. Cell Dev. Biol. 8:590903. doi: 10.3389/fcell.2020.590903
Received: 03 August 2020; Accepted: 07 October 2020;
Published: 11 November 2020.
Edited by:
Liang Cheng, Harbin Medical University, ChinaReviewed by:
Guiyou Liu, Tianjin Institute of Industrial Biotechnology (CAS), ChinaJing Gao, University of Helsinki, Finland
Copyright © 2020 Meng, Huang, Tao, Liu, Jing, Wang, Qin, Feng and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dayun Feng, dGRmZW5nZHlAMTYzLmNvbQ==; Qing Cai, c3hjYWlxaW5nQDEyNi5jb20=
†These authors share first authorship
 Qiang Meng1,2†
Qiang Meng1,2† Dayun Feng
Dayun Feng