- Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Jiangsu, China
Cortactin, a member of the actin-binding protein family, plays an important role in cell movement involving the cytoskeleton, as cell movement mediated by cortactin may induce the epithelial–mesenchymal transition. Cortactin participates in tumor proliferation, migration, and invasion and other related disease processes by binding to different proteins and participating in different pathways and mechanisms that induce the occurrence of these disease processes. Therefore, this article reviews the correlations between cortactin, the actin cytoskeleton, and the epithelial–mesenchymal transition and discusses its clinical importance in tumor therapy.
Introduction
The internal cytoskeleton of eukaryotic cells is composed of actin filaments, microtubules, and intermediate filaments (Remedios et al., 2003). As the main component of microfilaments, actin forms actin filaments that interact with numerous accessory proteins to generate the actin cytoskeleton (Svitkina, 2018). Actin filaments are formed by the polymerization of spherical monomeric actin (G-actin) with a polar structure into the double helical structure of filamentous actin (F-actin) with two different ends (Feldt et al., 2019). The actin cytoskeleton is the primary cellular machinery that generates force. It produces pushing, pulling, and resistance forces. These forces are produced by the coordinated polymerization of various actin filaments, sliding of bipolar filaments of myosin II along actin filaments, and the formation of a cross-linked membrane-related filament array, respectively (Svitkina, 2018). The force produced by the actin cytoskeleton is important for many cell movements, the structure and mechanical energy of the cytoplasmic matrix (Pollard and Cooper, 1986), and facilitates embryonic development, immune defenses, and wound healing (Kirkbride et al., 2011). The movement of the actin cytoskeleton in cells is mainly promoted by the binding of actin and corresponding proteins (Feldt et al., 2019). These proteins are called actin-binding proteins (ABPs). To date, 162 ABPs have been discovered. ABPs include membrane-associated proteins, membrane receptors, and ion transporters. These proteins are involved in the cross-linking of actin filaments, mediate the interactions of microfilaments with other cytoskeletal components, and promote the polymerization and depolymerization of filaments (Qu et al., 2007).
Cortactin, a member of the ABP family, plays an important role in cell movement involving the cytoskeleton (Zhang et al., 2009). Cell movement mediated by cortactin induces the epithelial–mesenchymal transition (EMT) and then participates in relevant disease processes, such as tumor proliferation, migration, and invasion (Karamanou et al., 2020). The inhibition of cortactin blocks the EMT, thereby preventing the proliferation, migration, and invasion of cancer cells (Huang et al., 2019). From a clinical perspective, these results support the application of cortactin as a promising therapeutic target for diseases such as cancer (Yilmaz and Christofori, 2009; Yin et al., 2017). Therefore, this article reviews the correlations between cortactin, the actin cytoskeleton, and the EMT and discusses its clinical value in tumor therapy.
Cortactin
Cortactin is an F-actin-binding protein that regulates cell movement and adhesion junction assembly (Zhang et al., 2009). Cortactin is located on chromosome 11q13, is composed of 550 amino acids, and has a molecular weight of 61.582 kDa (Schuuring et al., 1993). According to the amino acid sequence of cortactin, it contains three important structural domains: an N-terminal acidic region (NTA), a 6.5 F-actin repeat structural domain, and a C-terminal SH3 structural domain. An α-helix and a proline-rich region are located in the central part of the protein between the F-actin repeat structural domain and the SH3 structural domain (Figure 1).
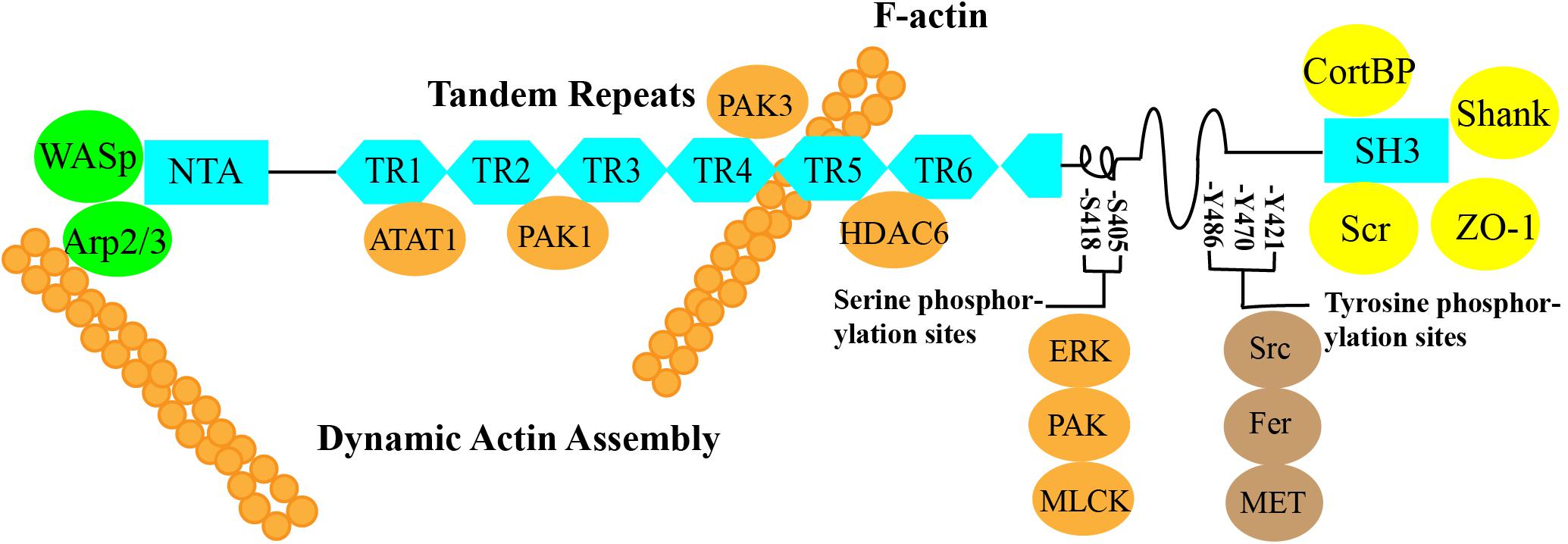
Figure 1. ➀ N-terminal acidic region (NTA): The NTA of cortactin binds to Arp2/3 alone or cooperates with Wiskott–Aldrich syndrome protein (WASP) to bind Arp2/3 to regulate branched actin assembly and regulate F-actin polymerization and contraction (Weed et al., 2000; Takehito et al., 2001; Weaver et al., 2002; Pant et al., 2006). ➁ Cortactin function can be regulated by posttranslational modifications of the 6.5 F-actin repeat domain, which contains a repeating 37-amino acid sequence. These modifications include phosphorylation and acetylation by PAK1, PAK3, ATAT1, and HDAC6 (Ohoka and Takai, 1998; van Rossum et al., 2003, 2005; Katsube et al., 2004; Zhang et al., 2007; Castro-Castro et al., 2012; Hayes et al., 2013; Li et al., 2017). ➂ SH3: The C-terminal domain allows actin to function as a scaffold protein because many cytoskeletal, membrane transport, and signaling proteins are bound to the C-terminal SH3 domain, such as ZO-1, CortBP, and Shank (Weed et al., 1998; Weed and Parsons, 2001; Burkhardt et al., 2008). ➃ The central part of the protein between the F-actin repeat domain and the SH3 domain contains an α-helix and a proline-rich region that includes three tyrosine phosphorylation sites, Y421, Y470, and Y486 (human), which are phosphorylated by Src, Fer, and c-Met. The two serine phosphorylation sites, S405 and S418, are phosphorylated by ERK, PAK, MLCK, and other kinases (Beaty et al., 2013; Rosse et al., 2014; Moshfegh et al., 2015).
Cortactin and the Actin Cytoskeleton
Cortactin is a primary regulator of the actin cytoskeleton. At a specific time and in discrete places within the cell, cortactin binding to F-actin regulates the structure of the actin cytoskeleton, thereby altering the morphology and function of the cell (Stossel et al., 1985). It also regulates the formation of corpuscles and lamellipodia, integrin signaling, axon guidance, and extracellular matrix degradation (Yamaguchi and Condeelis, 2007; Weaver, 2008; Marioni et al., 2018). The expression and cytoplasmic localization of cortactin are also crucial for maintaining the structure of the actin cytoskeleton (Motonishi et al., 2015). Overexpression of cortactin increases cell viability (Hill et al., 2006; Rothschild et al., 2006), mainly because of its functions in the assembly of the actin cytoskeleton and in promoting the persistence of lamellar protrusions (Bryce et al., 2005). When cortactin is located at the margin of the cell, it regulates the structure of the actin cytoskeleton and promotes the formation of an invasive pseudopod, which plays a complex role in the EMT, promotes the in situ polymerization of actin, and regulates autocrine signaling (Wang et al., 2013). Therefore, all cell activities in which cortactin is involved, including cell migration, invasion, and localization, require the actin cytoskeleton (Weed et al., 2000; Uruno et al., 2001; Katsube et al., 2004; Ayala et al., 2008).
The EMT and the Actin Cytoskeleton
The EMT was first discovered in embryonic cells (Hugo et al., 2007). According to a recent study, the EMT occurs naturally in numerous tissue types and various stages of development (Zhang and Weinberg, 2018). The EMT is crucial for normal development and tissue remodeling and contributes to disease progression, e.g., fibrosis and cancer metastasis (Kalluri and Neilson, 2003; Borok et al., 2011; Rana et al., 2018). The mechanism of the EMT is to convert epithelial cells into cells with a mesenchymal phenotype that are arranged along the epithelial and mesenchymal axes (Zhang and Weinberg, 2018). Epithelial cells exhibit interepithelial cell connections and apical bases, whereas mesenchymal cells exhibit increased motility and invasiveness and lack a spindle-like morphology and basic polarity (Nieto et al., 2016). Therefore, the occurrence of the EMT plays an important role in cell transformation. The EMT is the initial stage and necessary step of the transfer cascade reaction and is characterized by the loss of root tip polarity and intercellular adhesion, and the morphology and movement of mesenchymal cells occur through cytoskeletal reconstruction (Yilmaz and Christofori, 2009; Tsai et al., 2012; Nieto, 2013; Ye et al., 2016). Cytoskeletal reconstruction is based on the balance and control of the extent of the local assembly and disassembly of actin filaments (Yilmaz and Christofori, 2009). During the EMT, the cytoskeleton must be reshaped at the leading edge to form pseudopodia and allow the cell to move in the surrounding environment (Huang et al., 2019). Therefore, cytoskeletal reconstruction is crucial in the process of EMT-induced cell transformation.
Proteins That Interact With Cortactin
Cortactin, a key regulator of actin cytoskeletal assembly and remodeling, is mainly distributed in structures required for cell movement, such as lamellipodia and filopodia. Cortactin-induced dynamic reconstruction of the actin cytoskeleton provides the motile force that promotes the occurrence of the EMT (Huang et al., 2019). Cortactin regulated the occurrence of the EMT through various mechanisms, such as synergy with E-cadherin to induce the occurrence of the EMT (Yilmaz and Christofori, 2009), a change in cortactin expression that reshapes the actin cytoskeleton and induces the invasion of single cells and groups of cells (Yamaguchi and Condeelis, 2007), and the mutual effects of cortactin and site-specific binding partners on inducing different cellular activities, including the EMT (Helwani et al., 2004; Kirkbride et al., 2011) (Figure 2).
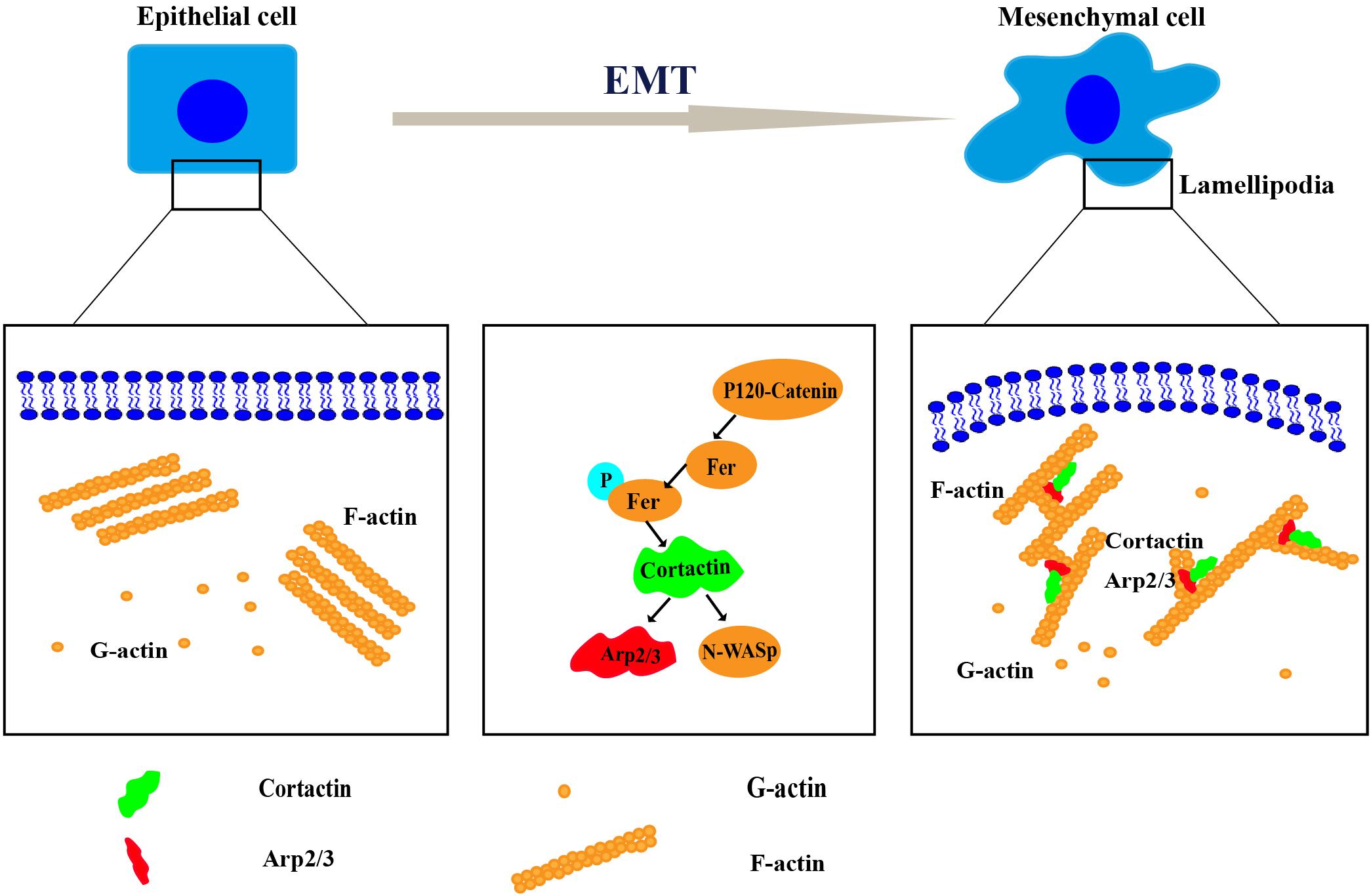
Figure 2. The mechanism of cortactin-induced cytoskeletal remodeling during the epithelial–mesenchymal transition (EMT). Remodeling of the cytoskeleton requires the coordination of several processes, including the protrusion of a lamellipodium at the leading edge, adhesion, contraction of the actin bundle, and retraction of the trailing edge of the cell. Cortactin regulates cytoskeletal remodeling by interacting with Arp2/3, neural Wiskott–Aldrich syndrome protein (N-WASP), and other proteins. Then, it promotes the EMT.
Cortactin and Arp2/3
The Arp2/3 complex is the primary molecular regulator of actin polymerization and is essential for the nucleation and formation of branched actin filament networks in cells (Pollard et al., 2000; Luo, 2002). The branched actin network provides the cellular structure and facilitates processes involving the plasma membrane, such as the formation of the cell–cell connection formation (Johnston et al., 2008), the motility of pathogens, the transport of vesicles, and the formation of cell–cell junctions (Goley and Welch, 2006; Garcia-Ponce et al., 2015). Cellular signals activate the inactive Arp2/3 complex (Higgs and Pollard, 1999). As a scaffold protein and activator of the Arp2/3 complex, cortactin interacts with and activates the Arp2/3 complex through its NTA (Higgs et al., 1999; Machesky et al., 1999; Yin et al., 2017). The cortactin and Arp2/3 complex is colocalized on dynamic particle structures rich in actin filaments. Through this biological process, actin filaments are assembled and extend to the nucleus to form a protein filament network, where the actin filament branch is stimulated (Takehito et al., 2001; Weaver et al., 2001). Cortactin mainly promotes the formation of the shape of the branched actin network by the Arp2/3 complex through two mechanisms. First, cortactin activates Arp2/3 separately and interacts with N-Wiskott–Aldrich syndrome protein (WASP), after which cortactin inhibits the disintegration of the preformed Arp2/3-core filament network (Weaver et al., 2001). The activated Arp2/3 complex produces unbranched actin filaments and branched actin filaments to induce the EMT (Rana et al., 2018).
Cortactin and N-WASP
Members of the WASP family, such as N-WASP, participate in the mechanism regulating the reorganization of the actin cytoskeletal and induce the occurrence of the EMT (Salvi and Thanabalu, 2017). The EMT induced by N-WASP mainly results in the reconstruction of the actin cytoskeleton through the activation of the Arp2/3 complex. However, N-WASP and cortactin activate the Arp2/3 complex alone or in combination (Helgeson et al., 2014). Both cortactin and N-WASP contain an acidic structural domain, which is necessary for binding to the Arp2/3 complex (Weaver et al., 2002). Phosphorylation at specific sites regulates the activities of cortactin and N-WASP, which are two important regulators of actin nucleation (Uzair et al., 2019). When cortactin is phosphorylated and overexpressed in cells, it interacts with N-WASP to facilitate actin polymerization (Wu and Parsons, 1993; Luttrell et al., 1994; Artym et al., 2006; Tehrani et al., 2007; Oser et al., 2009). For instance, the phosphorylation of cortactin by the serine/threonine kinase extracellular regulatory kinase 1/2 and p21-activated kinase 1 enhances the interaction of N-WASP with cortactin (Martinez-Quiles et al., 2004; Grassart et al., 2010) and promotes its phosphorylation of the Arp2/3 complex and transport to the plasmalemma to induce the EMT (Uzair et al., 2019).
Cortactin and Ezrin
Ezrin is a crosslinker of the actin skeleton and cell membrane, and a member of the ezrin–radixin–moesin family (He et al., 2017). Ezrin interacts with cortactin to induce various cellular processes, such as the regulation of the assembly of branched actin filaments, cell–cell adhesion, membrane transport, and ECM degradation (Sung et al., 2011). Both ezrin and cortactin are closely related to cell migration, which is an important component of the EMT process (He et al., 2017). Moreover, phosphorylation has been shown to enhance the function of cortactin by changing the complement of cortactin-bound proteins during migration and invasion (Lapetina et al., 2009; Oser et al., 2009, 2010; Kelley et al., 2010), thereby promoting the invasion and metastasis of various tumors (Weaver, 2008; Ni et al., 2015; Li et al., 2016). The interaction of ezrin with cortactin is a new mechanism involved in the EMT during the tumor metastasis process (He et al., 2017).
Cortactin and Snail1
Snail1 and cortactin are the key factors among all EMT-related proteins (Wu et al., 2015). Cortactin plays important roles in cellular migration and endocytosis, and Snail1 is a potential EMT activator that directly inhibits E-cadherin. Snail1 regulates proteins containing an E-box motif, including E-cadherin, in various tumors as a transcriptional repressor (Cano et al., 2000). This Snail1-mediated inhibition of E-cadherin is generally considered one of the signs of the EMT. During tumor metastasis, the EMT transforms epithelial cells into active and aggressive mesenchymal cells (Villarejo et al., 2014). Snail1 and cortactin both promote the EMT; however, recent reports have identified a negative regulatory effect of Snail1 on cortactin (Lee et al., 2014). For instance, during cultivation in a 3D collagen gel, the exposure of MDA-MB-231 cells to different environmental stimuli increases Snail1 expression and reduces cortactin expression. Inhibition of the JNK signaling pathway increases the expression of Snail1, which subsequently inhibits cortactin, thereby regulating the occurrence of the EMT (Lee et al., 2014).
Participating Pathways
Cortactin is the main organizer of membranous and invasive protrusions and is a presynaptic regulator of rapid activity-dependent signaling in synaptic structures. It induces the EMT by participating in multiple pathways. The level of cortactin at the membrane of the stimulated synapse is increased, which requires neuronal activity, de novo transcription, and Wg/Wnt-dependent expression (Alicea et al., 2017). In melanoma, RNF128 interferes with the ubiquitination and degradation of CD44 and cortactin proteins, activates the Wnt pathway, and promotes the cellular EMT and stem cell development (Wei et al., 2019). Cortactin also induces the EMT by participating in the pathway regulated by the Rho GTPase Rac1. Local actin polymerization is induced by mechanical stimuli and N-cadherin, therefore ensuring the integrity of the adhesion complex. The Rho GTPase Rac1 is activated by N-cadherin, which recruits cortactin to the N-cadherin adhesion complex (El Sayegh et al., 2005). In this process, Fer, a non-receptor tyrosine kinase, binds to N-cadherin through a mechanism mediated by p120-catenin (Kim and Wong, 1995). The phosphorylation of Fer activates cortactin, which induces the reorganization of the actin cytoskeleton, increases the movement of N-cadherin, extends the adhesion area, and then promotes the formation of a stable cell adhesion to ultimately regulate the shape of the cell. In addition to the aforementioned pathways, cortactin also participates in other pathways to induce the EMT. The related pathways and upstream and downstream molecules are shown in Table 1.
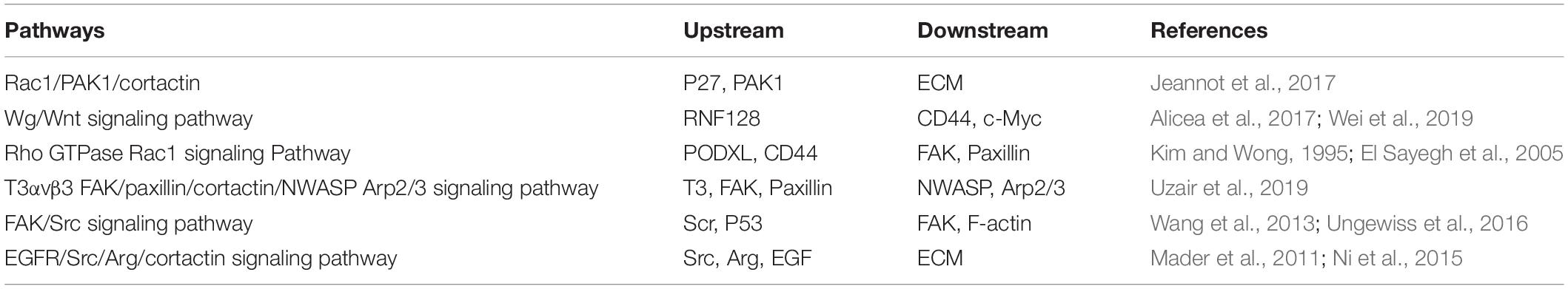
Table 1. Correlated signaling pathways and upstream and downstream molecules related to cortactin-induced epithelial–mesenchymal transition (EMT).
Cortactin in Cancer
Cortactin is overexpressed in many epithelial cancers (Marioni et al., 2017). In human tumors, cortactin overexpression leads to increased cell migration, invasion, and metastasis (Clark and Weaver, 2008). The transformation of breast cancer is related to the EMT. During this process, actin-rich protrusions on the serosa form invasive pseudopodia, which promote protein degradation in the extracellular matrix and tumor invasion. The formation and main function of invasive pseudopodia are controlled by cortactin (Karamanou et al., 2020). The polymerization of phosphorylated cortactin and actin at aggressive pseudopodia increases the invasiveness of human breast cancer cells and subsequently induces matrix degradation and aggressive behavior (Mader et al., 2011). In pancreatic ductal adenocarcinoma, the overexpression and phosphorylation of cortactin promotes the occurrence of the EMT, followed by the metastasis and migration of pancreatic ductal carcinoma (Stock et al., 2019). Cortactin is mainly phosphorylated at Y421 (Head et al., 2003), resulting in increased recruitment of proteins containing an SH2 domain, activation of the Arp2/3 complex, and increased stability and conversion of focal adhesions (Okamura and Resh, 1995; Tehrani et al., 2007). The tyrosine phosphorylation of cortactin is often used as an EMT marker due to its relationship with the protease activity necessarily for matrix degradation and cell invasion (Bowden et al., 2006). Cortactin induces the EMT in other types of cancer and promotes tumorigenesis (Table 2).
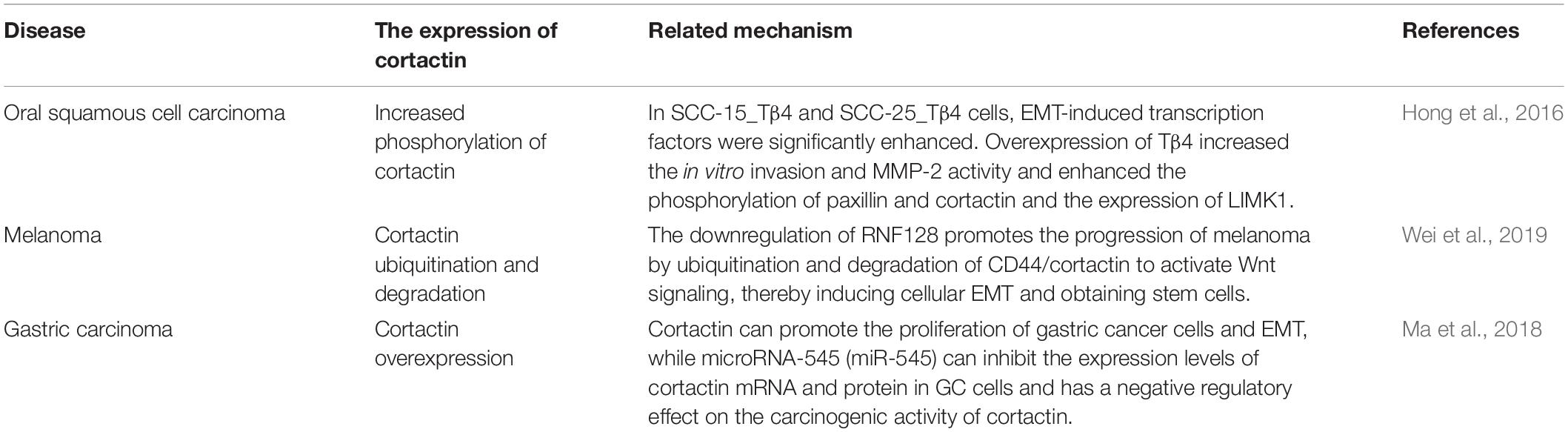
Table 2. Cortactin induces EMT in other cancer syndromes and promotes the development of cancer syndromes.
Conclusion and Prospects
Based on accumulating evidence from recent studies, cortactin is an important ABP. Its binding to F-actin regulates cell movement and adhesion. Cortactin also regulates the structure of the actin cytoskeleton by binding to various protein, thereby inducing the occurrence of the EMT. The role of the EMT in the occurrence and development of diseases, particularly its important role in tumor metastasis, has gradually attracted attention. Therefore, this article discusses the role of cortactin in the induction of the EMT by binding to different proteins and participating in different pathways and mechanisms to induce the occurrence of the EMT. However, other related binding proteins and mechanisms of cortactin in the EMT remain to be discovered. Perhaps we will be able to prevent the development of diseases by regulating the activity of cortactin-related proteins and pathways to control the EMT. As researchers are increasingly focusing on the role of cortactin complexed with different proteins in diseases, additional regulatory mechanisms will be discovered. The mechanisms will provide new insights for studies of related diseases and the development of new drugs and treatment methods and will provide additional evidence for the clinical application of cortactin in the future.
Author Contributions
L-QH designed the work. RJ and X-JZ wrote the manuscript and prepared the figures. Z-RW drafted and revised the manuscript. All the authors contributed to manuscript revision, read and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (No. 2019YFC1709704), Suzhou Youth Science and Technology Project (No. KJXW2019058), and Zhangjiagang Youth Science and Technology Project (No. ZJGQNKJ201808).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alicea, D., Perez, M., Maldonado, C., Dominicci-Cotto, C., and Marie, B. (2017). Cortactin Is a Regulator of Activity-Dependent Synaptic Plasticity Controlled by Wingless. J. Neurosci. 37, 2203–2215. doi: 10.1523/JNEUROSCI.1375-16.2017
Artym, V. V., Zhang, Y., Seillier-Moiseiwitsch, F., Yamada, K. M., and Mueller, S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034–3043. doi: 10.1158/0008-5472.CAN-05-2177
Ayala, I., Baldassarre, M., Giacchetti, G., Caldieri, G., Tete, S., Luini, A., et al. (2008). Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J. Cell Sci. 121(Pt 3), 369–378. doi: 10.1242/jcs.008037
Beaty, B. T., Sharma, V. P., Bravo-Cordero, J. J., Simpson, M. A., Eddy, R. J., Koleske, A. J., et al. (2013). beta1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol. Biol. Cell 24, 1661–1675. doi: 10.1091/mbc.E12-12-0908
Borok, Z., Whitsett, J. A., Bitterman, P. B., Thannickal, V. J., Kotton, D. N., Reynolds, S. D., et al. (2011). Cell plasticity in lung injury and repair: report from an NHLBI workshop. April 19-20, 2010. Proc. Am. Thorac. Soc. 8, 215–222. doi: 10.1513/pats.201012-067CB
Bowden, E. T., Onikoyi, E., Slack, R., Myoui, A., Yoneda, T., Yamada, K. M., et al. (2006). Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp. Cell Res. 312, 1240–1253. doi: 10.1016/j.yexcr.2005.12.012
Bryce, N. S., Clark, E. S., Leysath, J. L., Currie, J. D., Webb, D. J., and Weaver, A. M. (2005). Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15, 1276–1285. doi: 10.1016/j.cub.2005.06.043
Burkhardt, J. K., Carrizosa, E., and Shaffer, M. H. (2008). The actin cytoskeleton in T cell activation. Annu. Rev. Immunol. 26, 233–259. doi: 10.1146/annurev.immunol.26.021607.090347
Cano, A., Perez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., del Barrio, M. G., et al. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83. doi: 10.1038/35000025
Castro-Castro, A., Janke, C., Montagnac, G., Paul-Gilloteaux, P., and Chavrier, P. (2012). ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur. J. Cell Biol. 91, 950–960. doi: 10.1016/j.ejcb.2012.07.001
Clark, E. S., and Weaver, A. M. (2008). A new role for cortactin in invadopodia: regulation of protease secretion. Eur. J. Cell Biol. 87, 581–590. doi: 10.1016/j.ejcb.2008.01.008
El Sayegh, T. Y., Arora, P. D., Fan, L., Laschinger, C. A., Greer, P. A., McCulloch, C. A., et al. (2005). Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol. Biol. Cell 16, 5514–5527. doi: 10.1091/mbc.e05-05-0410
Feldt, J., Schicht, M., Garreis, F., Welss, J., Schneider, U. W., and Paulsen, F. (2019). Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 20:e7. doi: 10.1017/erm.2018.7
Garcia-Ponce, A., Citalan-Madrid, A. F., Velazquez-Avila, M., Vargas-Robles, H., and Schnoor, M. (2015). The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb. Haemost. 113, 20–36. doi: 10.1160/TH14-04-0298
Goley, E. D., and Welch, M. D. (2006). The ARP2/3 compound: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713–726. doi: 10.1038/nrm2026
Grassart, A., Meas-Yedid, V., Dufour, A., Olivo-Marin, J. C., Dautry-Varsat, A., and Sauvonnet, N. (2010). Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic 11, 1079–1091. doi: 10.1111/j.1600-0854.2010.01075.x
Hayes, K. E., Walk, E. L., Ammer, A. G., Kelley, L. C., Martin, K. H., and Weed, S. A. (2013). Ableson kinases negatively regulate invadopodia function and invasion in head and neck squamous cell carcinoma by inhibiting an HB-EGF autocrine loop. Oncogene 32, 4766–4777. doi: 10.1038/onc.2012.513
He, J., Ma, G., Qian, J., Zhu, Y., Liang, M., Yao, N., et al. (2017). Interaction between Ezrin and cortactin in promoting epithelial to Mesenchymal transition in breast cancer cells. Med. Sci. Monit. 23, 1583–1596. doi: 10.12659/msm.904124
Head, J. A., Jiang, D., Li, M., Zorn, L. J., Schaefer, E. M., Parsons, J. T., et al. (2003). Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol. Biol. Cell 14, 3216–3229. doi: 10.1091/mbc.e02-11-0753
Helgeson, L. A., Prendergast, J. G., Wagner, A. R., Rodnick-Smith, M., and Nolen, B. J. (2014). Interactions with actin monomers, actin filaments, and Arp2/3 compound define the roles of WASP family proteins and cortactin in coordinately regulating branched actin networks. J. Biol. Chem. 289, 28856–28869. doi: 10.1074/jbc.M114.587527
Helwani, F. M., Kovacs, E. M., Paterson, A. D., Verma, S., Ali, R. G., Fanning, A. S., et al. (2004). Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 164, 899–910. doi: 10.1083/jcb.200309034
Higgs, H. N., Blanchoin, L., and Pollard, T. D. (1999). Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 compound on actin polymerization. Biochemistry 38, 15212–15222. doi: 10.1021/bi991843+
Higgs, H. N., and Pollard, T. D. (1999). Regulation of actin polymerization by Arp2/3 compound and WASp/Scar proteins. J. Biol. Chem. 274, 32531–32534. doi: 10.1074/jbc.274.46.32531
Hill, A., McFarlane, S., Mulligan, K., Gillespie, H., Draffin, J. E., Trimble, A., et al. (2006). Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene 25, 6079–6091. doi: 10.1038/sj.onc.1209628
Hong, K. O., Lee, J. I., Hong, S. P., and Hong, S. D. (2016). Thymosin beta4 induces proliferation, invasion, and epithelial-to-mesenchymal transition of oral squamous cell carcinoma. Amino Acids 48, 117–127. doi: 10.1007/s00726-015-2070-6
Huang, D., Cao, L., Xiao, L., Song, J. X., Zhang, Y. J., Zheng, P., et al. (2019). Hypoxia induces actin cytoskeleton remodeling by regulating the binding of CAPZA1 to F-actin via PIP2 to drive EMT in hepatocellular carcinoma. Cancer Lett. 448, 117–127. doi: 10.1016/j.canlet.2019.01.042
Hugo, H., Ackland, M. L., Blick, T., Lawrence, M. G., Clements, J. A., Williams, E. D., et al. (2007). Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J. Cell. Physiol. 213, 374–383. doi: 10.1002/jcp.21223
Jeannot, P., Nowosad, A., Perchey, R. T., Callot, C., Bennana, E., Katsube, T., et al. (2017). p27(Kip1) promotes invadopodia turnover and invasion through the regulation of the PAK1/Cortactin pathway. eLife 6:e22207. doi: 10.7554/eLife.22207
Johnston, S. A., Bramble, J. P., Yeung, C. L., Mendes, P. M., and Machesky, L. M. (2008). Arp2/3 compound activity in filopodia of spreading cells. BMC Cell Biol. 9:65. doi: 10.1186/1471-2121-9-65
Kalluri, R., and Neilson, E. G. (2003). Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112, 1776–1784. doi: 10.1172/JCI20530
Karamanou, K., Franchi, M., Vynios, D., and Brezillon, S. (2020). Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Semin. Cancer Biol. 62, 125–133. doi: 10.1016/j.semcancer.2019.08.003
Katsube, T., Togashi, S., Hashimoto, N., Ogiu, T., and Tsuji, H. (2004). Filamentous actin binding ability of cortactin isoforms is responsible for their cell-cell junctional localization in epithelial cells. Arch. Biochem. Biophys. 427, 79–90. doi: 10.1016/j.abb.2004.04.015
Kelley, L. C., Hayes, K. E., Ammer, A. G., Martin, K. H., and Weed, S. A. (2010). Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS One 5:e13847. doi: 10.1371/journal.pone.0013847
Kim, L., and Wong, T. W. (1995). The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol. Cell. Biol. 15:4553.
Kirkbride, K. C., Sung, B. H., Sinha, S., and Weaver, A. M. (2011). Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh. Migr. 5, 187–198. doi: 10.4161/cam.5.2.14773
Lapetina, S., Mader, C. C., Machida, K., Mayer, B. J., and Koleske, A. J. (2009). Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J. Cell Biol. 185, 503–519. doi: 10.1083/jcb.200809085
Lee, M. S., Kim, S., Kim, B. G., Won, C., Nam, S. H., Kang, S., et al. (2014). Snail1 induced in breast cancer cells in 3D collagen I gel environment suppresses cortactin and impairs effective invadopodia formation. Biochim. Biophys. Acta 1843, 2037–2054. doi: 10.1016/j.bbamcr.2014.05.007
Li, A., Zhang, L., Zhang, X., Jin, W., and Ren, Y. (2016). Expression and clinical significance of cortactin protein in ovarian neoplasms. Clin. Transl. Oncol. 18, 220–227. doi: 10.1007/s12094-015-1360-5
Li, X., Tao, Y., Murphy, J. W., Scherer, A. N., Lam, T. T., Marshall, A. G., et al. (2017). The repeat region of cortactin is intrinsically disordered in solution. Sci. Rep. 7:16696. doi: 10.1038/s41598-017-16959-1
Luo, L. (2002). Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18, 601–635. doi: 10.1146/annurev.cellbio.18.031802.150501
Luttrell, D. K., Lee, A., Lansing, T. J., Crosby, R. M., Jung, K. D., Willard, D., et al. (1994). Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc. Natl. Acad. Sci. U.S.A. 91, 83–87. doi: 10.1073/pnas.91.1.83
Ma, M., Zhao, J., Wu, Q., Xiao, K., Li, S., Zhu, H., et al. (2018). MiRNA-545 negatively regulates the oncogenic activity of EMS1 in gastric cancer. Cancer Med. 7, 2452–2462. doi: 10.1002/cam4.1520
Machesky, L. M., Mullins, R. D., Higgs, H. N., Kaiser, D. A., Blanchoin, L., May, R. C., et al. (1999). Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 compound. Proc. Natl. Acad. Sci. U.S.A. 96, 3739–3744. doi: 10.1073/pnas.96.7.3739
Mader, C. C., Oser, M., Magalhaes, M. A. O., Bravo-Cordero, J. J., Condeelis, J., Koleske, A. J., et al. (2011). An EGFR–Src–Arg–Cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 71, 1730–1741. doi: 10.1158/0008-5472.Can-10-1432
Marioni, G., Lionello, M., Marchese-Ragona, R., Fasanaro, E., Valentini, E., Zanoletti, E., et al. (2018). Cortactin and phosphorylated cortactin tyr(421) and tyr(466) expression in supraglottic laryngeal carcinomas and lymph node metastases. Int. J. Biol. Markers 33, 79–86. doi: 10.5301/ijbm.5000297
Marioni, G., Zanoletti, E., Mazzoni, A., Gianatti, A., Valentini, E., Girasoli, L., et al. (2017). Cortactin and phosphorylated cortactin tyr(466) expression in temporal bone carcinoma. Am. J. Otolaryngol. 38, 208–212. doi: 10.1016/j.amjoto.2017.01.012
Martinez-Quiles, N., Ho, H. Y., Kirschner, M. W., Ramesh, N., and Geha, R. S. (2004). Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell. Biol. 24, 5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004
Moshfegh, Y., Bravo-Cordero, J. J., Miskolci, V., Condeelis, J., and Hodgson, L. (2015). A Trio-Rac1-Pak1 signalling axis drives invadopodia disassembly. Nat. Cell Biol. 17:350. doi: 10.1038/ncb3123
Motonishi, S., Nangaku, M., Wada, T., Ishimoto, Y., Ohse, T., Matsusaka, T., et al. (2015). Sirtuin1 maintains actin Cytoskeleton by Deacetylation of cortactin in injured podocytes. J. Am. Soc. Nephrol. 26, 1939–1959. doi: 10.1681/ASN.2014030289
Ni, Q. F., Yu, J. W., Qian, F., Sun, N. Z., Xiao, J. J., and Zhu, J. W. (2015). Cortactin promotes colon cancer progression by regulating ERK pathway. Int. J. Oncol. 47, 1034–1042. doi: 10.3892/ijo.2015.3072
Nieto, M. A. (2013). Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342:1234850. doi: 10.1126/science.1234850
Nieto, M. A., Huang, R. Y., Jackson, R. A., and Thiery, J. P. (2016). Emt: 2016. Cell 166, 21–45. doi: 10.1016/j.cell.2016.06.028
Ohoka, Y., and Takai, Y. (1998). Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes Cells 3, 603–612. doi: 10.1046/j.1365-2443.1998.00216.x
Okamura, H., and Resh, M. D. (1995). p80/85 Cortactin Associates with the Src SH2 Domain and Colocalizes with v-Src in Transformed Cells. J. Biol. Chem. 270, 26613–26618.
Oser, M., Mader, C. C., Gil-Henn, H., Magalhaes, M., Bravo-Cordero, J. J., Koleske, A. J., et al. (2010). Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J. Cell Sci. 123(Pt 21), 3662–3673. doi: 10.1242/jcs.068163
Oser, M., Yamaguchi, H., Mader, C. C., Bravo-Cordero, J. J., Arias, M., Chen, X., et al. (2009). Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571–587. doi: 10.1083/jcb.200812176
Pant, K., Chereau, D., Hatch, V., Dominguez, R., and Lehman, W. (2006). Cortactin binding to F-actin revealed by electron microscopy and 3D reconstruction. J. Mol. Biol. 359, 840–847. doi: 10.1016/j.jmb.2006.03.065
Pollard, T. D., Blanchoin, L., and Mullins, R. D. (2000). Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29, 545–576. doi: 10.1146/annurev.biophys.29.1.545
Pollard, T. D., and Cooper, J. A. (1986). Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu. Rev. Biochem. 55, 987–1035. doi: 10.1146/annurev.bi.55.070186.005011
Rana, M. K., Aloisio, F. M., Choi, C., and Barber, D. L. (2018). Formin-dependent TGF-β signaling for epithelial to mesenchymal transition. Mol. Biol. Cell 29:mbcE17050325. doi: 10.1091/mbc.E17-05-0325
Remedios, C. G. D., Chhabra, D., Kekic, M., Dedova, I. V., Tsubakihara, M., Berry, D. A., et al. (2003). Actin Binding Proteins Regulation of Cytoskeletal Microfilaments. Physiol. Rev. 83, 433–473. doi: 10.1152/physrev.00026.2002
Rosse, C., Lodillinsky, C., Fuhrmann, L., Nourieh, M., Monteiro, P., Irondelle, M., et al. (2014). Control of MT1-MMP transport by atypical PKC during breast-cancer progression. Proc. Natl. Acad. Sci. U.S.A. 111, E1872–E1879. doi: 10.1073/pnas.1400749111
Rothschild, B. L., Shim, A. H., Ammer, A. G., Kelley, L. C., Irby, K. B., Head, J. A., et al. (2006). Cortactin overexpression regulates actin-related protein 2/3 compound activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 66, 8017–8025. doi: 10.1158/0008-5472.CAN-05-4490
Salvi, A., and Thanabalu, T. (2017). WIP promotes in-vitro invasion ability, anchorage independent growth and EMT progression of A549 lung adenocarcinoma cells by regulating RhoA levels. Biochem. Biophys. Res. Commun. 482, 1353–1359. doi: 10.1016/j.bbrc.2016.12.040
Schuuring, E., Verhoeven, E., Litvinov, S., and Michalides, R. J. (1993). The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol. Cell. Biol. 13, 2891–2898. doi: 10.1128/mcb.13.5.2891
Stock, K., Borrink, R., Mikesch, J. H., Hansmeier, A., Rehkamper, J., Trautmann, M., et al. (2019). Overexpression and Tyr421-phosphorylation of cortactin is induced by three-dimensional spheroid culturing and contributes to migration and invasion of pancreatic ductal adenocarcinoma (PDAC) cells. Cancer Cell Int. 19:77. doi: 10.1186/s12935-019-0798-x
Stossel, T. P., Chaponnier, C., Ezzell, R. M., Hartwig, J. H., Janmey, P. A., Kwiatkowski, D. J., et al. (1985). Nonmuscle actin-binding proteins. Ann. Rev. Cell Bio. 1, 353–402. doi: 10.1146/annurev.cb.01.110185.002033
Sung, B. H., Zhu, X., Kaverina, I., and Weaver, A. M. (2011). Cortactin controls cell motility and lamellipodial dynamics by regulating ECM secretion. Curr. Biol. 21, 1460–1469. doi: 10.1016/j.cub.2011.06.065
Svitkina, T. M. (2018). Ultrastructure of the actin cytoskeleton. Curr. Opin. Cell Biol. 54, 1–8. doi: 10.1016/j.ceb.2018.02.007
Takehito, U., Jiali, L., Peijun, Z., Ying-xin, F., and Coumaran, E. (2001). Activation of Arp2/3 compoundmediated actin polymerization. Nat. Cell Biol. 3, 259–266. doi: 10.1038/35060051
Tehrani, S., Tomasevic, N., Weed, S., Sakowicz, R., and Cooper, J. A. (2007). Src phosphorylation of cortactin enhances actin assembly. Proc. Natl. Acad. Sci. U.S.A. 104, 11933–11938. doi: 10.1073/pnas.0701077104
Tsai, J. H., Donaher, J. L., Murphy, D. A., Chau, S., and Yang, J. (2012). Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736. doi: 10.1016/j.ccr.2012.09.022
Ungewiss, C., Rizvi, Z. H., Roybal, J. D., Peng, D. H., Gold, K. A., Shin, D. H., et al. (2016). The microRNA-200/Zeb1 axis regulates ECM-dependent beta1-integrin/FAK signaling, cancer cell invasion and metastasis through CRKL. Sci. Rep. 6:18652. doi: 10.1038/srep18652
Uruno, T., Liu, J., Zhang, P., Fan, Y., Egile, C., Li, R., et al. (2001). Activation of Arp2/3 compound-mediated actin polymerization by cortactin. Nat. Cell Biol. 3, 259–266. doi: 10.1038/35060051
Uzair, I. D., Conte Grand, J., Flamini, M. I., and Sanchez, A. M. (2019). Molecular actions of thyroid hormone on breast cancer cell migration and invasion via Cortactin/N-WASP. Front. Endocrinol. 10:139. doi: 10.3389/fendo.2019.00139
van Rossum, A. G., de Graaf, J. H., Schuuring-Scholtes, E., Kluin, P. M., Fan, Y. X., Zhan, X., et al. (2003). Alternative splicing of the actin binding domain of human cortactin affects cell migration. J. Biol. Chem. 278, 45672–45679. doi: 10.1074/jbc.M306688200
van Rossum, A. G., Schuuring-Scholtes, E., van Buuren-van Seggelen, V., Kluin, P. M., and Schuuring, E. (2005). Comparative genome analysis of cortactin and HS1: the significance of the F-actin binding repeat domain. BMC Genomics 6:15. doi: 10.1186/1471-2164-6-15
Villarejo, A., Cortes-Cabrera, A., Molina-Ortiz, P., Portillo, F., and Cano, A. (2014). Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition. J. Biol. Chem. 289, 930–941. doi: 10.1074/jbc.M113.528026
Wang, Y., Zhang, Y. X., Kong, C. Z., Zhang, Z., and Zhu, Y. Y. (2013). Loss of P53 facilitates invasion and metastasis of prostate cancer cells. Mol. Cell. Biochem. 384, 121–127. doi: 10.1007/s11010-013-1789-1
Weaver, A. M. (2008). Cortactin in tumor invasiveness. Cancer Lett. 265, 157–166. doi: 10.1016/j.canlet.2008.02.066
Weaver, A. M., Heuser, J. E., Karginov, A. V., Lee, W. L., Parsons, J. T., and Cooper, J. A. (2002). Interaction of cortactin and N-WASp with Arp2/3 compound. Curr. Biol. 12, 1270–1278. doi: 10.1016/s0960-9822(02)01035-7
Weaver, A. M., Karginov, A. V., Kinley, A. W., Weed, S. A., Li, Y., Parsons, J. T., et al. (2001). Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11, 370–374. doi: 10.1016/s0960-9822(01)00098-7
Weed, S. A., Du, Y., and Parsons, J. T. (1998). Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 111(Pt 16), 2433–2443.
Weed, S. A., Karginov, A. V., Schafer, D. A., Weaver, A. M., Kinley, A. W., Cooper, J. A., et al. (2000). Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 compound. J. Cell Biol. 151, 29–40. doi: 10.1083/jcb.151.1.29
Weed, S. A., and Parsons, J. T. (2001). Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20, 6418–6434. doi: 10.1038/sj.onc.1204783
Wei, C. Y., Zhu, M. X., Yang, Y. W., Zhang, P. F., Yang, X., Peng, R., et al. (2019). Downregulation of RNF128 activates Wnt/beta-catenin signaling to induce cellular EMT and stemness via CD44 and CTTN ubiquitination in melanoma. J. Hematol. Oncol. 12:21. doi: 10.1186/s13045-019-0711-z
Wu, H., and Parsons, J. T. (1993). Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 120, 1417–1426. doi: 10.1083/jcb.120.6.1417
Wu, W., Ding, H., Cao, J., and Zhang, W. (2015). FBXL5 inhibits metastasis of gastric cancer through suppressing Snail1. Cell Physiol. Biochem. 35, 1764–1772. doi: 10.1159/000373988
Yamaguchi, H., and Condeelis, J. (2007). Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773, 642–652. doi: 10.1016/j.bbamcr.2006.07.001
Ye, L. Y., Chen, W., Bai, X. L., Xu, X. Y., Zhang, Q., Xia, X. F., et al. (2016). Hypoxia-Induced Epithelial-to-Mesenchymal Transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 76, 818–830. doi: 10.1158/0008-5472.CAN-15-0977
Yilmaz, M., and Christofori, G. (2009). EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 28, 15–33. doi: 10.1007/s10555-008-9169-0
Yin, M., Ma, W., and An, L. (2017). Cortactin in cancer cell migration and invasion. Oncotarget 8, 88232–88243. doi: 10.18632/oncotarget.21088
Zhang, K., Wang, D., and Song, J. (2009). Cortactin is involved in transforming growth factor-beta1-induced epithelial-mesenchymal transition in AML-12 cells. Acta Biochim. Biophys. Sin. 41, 839–845. doi: 10.1093/abbs/gmp070
Zhang, X., Yuan, Z., Zhang, Y., Yong, S., Salas-Burgos, A., Koomen, J., et al. (2007). HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell. 27, 197–213. doi: 10.1016/j.molcel.2007.05.033
Keywords: actin cytoskeleton, cortactin, epithelial-mesenchymal transition (EMT), Arp2/3, N-WASP, ezrin, Snail1, cancer
Citation: Ji R, Zhu X-J, Wang Z-R and Huang L-Q (2020) Cortactin in Epithelial–Mesenchymal Transition. Front. Cell Dev. Biol. 8:585619. doi: 10.3389/fcell.2020.585619
Received: 21 July 2020; Accepted: 24 September 2020;
Published: 20 October 2020.
Edited by:
Chang-Duk Jun, Gwangju Institute of Science and Technology, South KoreaReviewed by:
Shanming Ruan, Zhejiang Chinese Medical University, ChinaYong-Nyun Kim, National Cancer Center, South Korea
Copyright © 2020 Ji, Zhu, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Ji, cm95amlyb25nMUAxNjMuY29t; Li-Qiang Huang, MTkwNDI2MDc4QHFxLmNvbQ==
†These authors have contributed equally to this work
 Rong Ji
Rong Ji Xiao-Juan Zhu†
Xiao-Juan Zhu†