
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 02 September 2020
Sec. Cellular Biochemistry
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.571237
This article is part of the Research Topic Targeting Lipid Rafts as a Strategy against Infection and Cancer View all 6 articles
Modulation of membrane lipid composition and organization is currently developing as an effective therapeutic strategy against a wide range of diseases, including cancer. This field, known as membrane-lipid therapy, has risen from new discoveries on the complex organization of lipids and between lipids and proteins in the plasma membranes. Membrane microdomains present in the membrane of all eukaryotic cells, known as lipid rafts, have been recognized as an important concentrating platform for protein receptors involved in the regulation of intracellular signaling, apoptosis, redox balance and immune response. The difference in lipid composition between the cellular membranes of healthy cells and tumor cells allows for the development of novel therapies based on targeting membrane lipids in cancer cells to increase sensitivity to chemotherapeutic agents and consequently defeat multidrug resistance. In the current manuscript strategies based on influencing cholesterol/sphingolipids content will be presented together with innovative ones, more focused in changing biophysical properties of the membrane bilayer without affecting the composition of its constituents.
Lipid-driven membrane organization is essential for the physiological functions of eukaryotic cells since it regulates a multitude of processes including intracellular signaling, redox balance and cell death (Muro et al., 2014; Santos and Preta, 2018). Behind these regulatory properties, there is the lipids capacity to laterally aggregate, forming highly dynamic and heterogeneous regions, referred to as lipid rafts. Lipid rafts are nanoscale membrane microdomains (<200 nm), particularly enriched in cholesterol and sphingolipids, that selectively recruit certain protein receptors (Simons and Toomre, 2000; Sezgin et al., 2017). Lipid rafts form microscopic domains (>300 nm) upon clustering induced by protein-protein or protein-lipid interaction. The raft model was supported by observation on artificial membrane models, demonstrating that certain lipids specifically tend to interact with others to generate large scale lateral domains (Simons and Vaz, 2004; Kaiser et al., 2009). The presence of plasma membrane specific organization has been observed across different organisms, ranging from bacteria to yeasts, providing further support for their biological significance (Kaiser et al., 2009; Henderson and Block, 2014; Lopez, 2015). Changes in the composition and organization of lipids have several effects on cellular functions, influencing signal transduction, membrane plasticity, and membrane trafficking. Plasma membrane cholesterol is one of the most important regulators of lipid organization, representing the majority (up to 90%) of the total cellular cholesterol and its levels in the cells are tightly regulated (de Duve, 1971; Lange et al., 1989). According to a recent study there are three pools of cholesterol in the plasma membrane: a labile pool, depleted by cholesterol-targeting agents, a sphingomyelin-bound pool and an essential pool, necessary for cell viability (Das et al., 2014). Only the cholesterol not sequestered by proteins or lipids can be transported in the endoplasmic reticulum (ER) where it binds to specific sensors, shutting down cholesterol synthesis and uptake (Infante and Radhakrishnan, 2017). The pathway between cholesterol removal from plasma membrane and its subsequent transport to the ER represents a field of extensive investigation aimed to identify specific transporters involved in the regulation of cholesterol homeostasis. Recent studies identified Aster/GRAMD1 as essential transporters of cholesterol into ER and regulating the cellular uptake of HDL-derived cholesterol (Sandhu et al., 2018; Naito et al., 2019). ORP2 protein was also identified as a unique transporter of cholesterol from ER to the plasma membrane (Wang et al., 2019). There is no doubt that this recent progress in understanding cholesterol homeostasis and metabolism set the basis for the development of current therapies based on cholesterol and lipids targeting. A decrease in membrane cholesterol has been observed to have beneficial effects against different pathological condition including cancer and neurodegenerative diseases (Simons et al., 1998; Canevari and Clark, 2007; Guardia-Laguarta et al., 2009; Barros et al., 2018; Chen et al., 2018; Gu et al., 2019). Cholesterol-targeting can be achieved via cholesterol depletion, sequestration or inhibition of synthesis. The first effect is observed using cyclodextrins, a group of chemical compounds extracting cholesterol from the plasma membrane and widely used in the biomedical field in different experimental settings (Zidovetzki and Levitan, 2007; Lopez et al., 2013; Mahammad and Parmryd, 2015). Cholesterol sequestration is the mechanism used by different pore forming agents, by the antibiotic filipin, amphotericin, and nystatin (Bittman and Fischkoff, 1972; Silva et al., 2006; Kaminski, 2014). Cholesterol sequestration also effectively reduces the ability of cholesterol to interact with other membrane constituents. Statins, a widely used class of lipid-lowering medications are the best representatives of the inhibitors of cholesterol synthesis (Stancu and Sima, 2001; Kuipers and van den Elsen, 2007). These include other compounds like bisphosphonates or zaragozic acid acting at different levels of the mevalonate pathway (Amin et al., 1992; Griffin et al., 2017).
It is relevant to underline that few chemical compounds can affect the lipid membranes by different mechanisms. The dynamin inhibitor Dynasore has been shown to influence both cholesterol transport on the cell membrane and cholesterol concentration (Girard et al., 2011; Preta et al., 2015a,b). Beyond the cholesterol-lowering effects of statins, cholesterol-independent or pleiotropic effects are reported, including the capacity to modify plasma-membrane organization and structure (Wang et al., 2008; Penkauskas et al., 2020). Studies using artificial model membranes showed that statins alter the nanomechanical stability of the bilayers, intercalating the lipid-water interface and increasing membrane heterogeneity (Redondo-Morata et al., 2016; Galiullina et al., 2019). A better understanding of how therapeutic agents affect the membrane organization and composition, led in the last years to the development of a new field, named membrane-lipid therapy (MLT). MLT involves the identification and optimization of drugs capable to modify membrane lipid structures for pharmaceutical applications (Escriba, 2006; Escriba et al., 2015). Due to the essential role of the plasma membrane in many physiological processes, it is expected that MLT will provide new treatments for a wide range of diseases, including oncological disorders, neurodegenerative diseases, diabetes and stroke (Escriba, 2017).
One of the hallmarks of cancer is the resistance to apoptosis and, more in general, the higher rate of proliferation versus death (Hanahan and Weinberg, 2000, 2011). The dynamicity of cell membranes plays an essential role in the regulation of cell surviving, through all the phases of a cell: lipid flexibility contributes to an increase in the mechanical stability during division and to a decrease of shear force during cell separation (Patra, 2008). To adapt rapidly, cancer cells re-organize their plasma membranes to preserve proliferation, escape apoptosis and resist to anticancer drugs treatment (Bernardes and Fialho, 2018). The latter is a crucial problem in anticancer therapy and often leads to multidrug resistance (MDR). Among many others, one of the causes of MDR is the decreased free diffusion of anticancer drugs through the plasma membrane. Therefore, the study and development of anticancer drugs capable to exert therapeutic effect by modulating the properties of tumor membranes, is constantly increasing (Rios-Marco et al., 2017; Zalba and Ten Hagen, 2017; Kopecka et al., 2020). The rationale for MLT is that there are fundamental differences in composition between normal and MDR cancer cells (Figure 1). MDR cells possess higher levels of total cholesterol as a result of an increased activity of HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis (Kawata et al., 1990; Harwood et al., 1991). Additional studies reported an increase in mevalonate levels and in the expression of the low-density lipoprotein receptor compared to normal cells (Duncan et al., 2004; Kopecka et al., 2011). The observed higher amount of membrane cholesterol is responsible of a more rigid and less permeable membrane (Peetla et al., 2013; Niero et al., 2014). Moreover, MDR cells keep low ceramide levels by increasing sphingomyelin (SM) synthesis: this is an important anti-apoptotic strategy since implies a decrease in ceramide-enriched lipid rafts involved in the induction of cell death. Furthermore, phosphatidylserine (PS) and phosphatidylethanolamine (PE) which, under physiological conditions exist mainly in the inner leaflet of cell membranes have increased surface expressions on the outer membrane of tumor cells (Tan et al., 2017). The asymmetrical distribution of PS, maintained by a group of amino-phospholipid translocases that use ATP hydrolysis to flip PS from the external to the cytosolic leaflet, is also lost during the apoptotic process. The loss of PS asymmetry in cancer cells may be related to a reduced activity of these ATP-dependent phospholipid translocases or to an elevated activity of phospholipid scramblase, due to high levels of intracellular calcium (Ca2+i) (Chen et al., 1999). PS on the outer membrane of tumor cells can be used as an effective target for cancer therapy (Ran et al., 2002; Riedl et al., 2011; Davis et al., 2019). The PS-targeting antibody bavituximab (Chalasani et al., 2015; Gerber et al., 2015; Grilley-Olson et al., 2018) and the PS-binding peptide/peptoid hybrid PPS1D1 (Desai et al., 2016; Desai and Udugamasooriya, 2017) have shown significant cytotoxic effects in cancer cells. Another strategy largely used in anticancer therapy is to entrap the drug in a specific carrier, which held the tumor-targeting property (Dass and Choong, 2006; Fanciullino and Ciccolini, 2009). For example, a cationic liposomal carrier, phosphatidylcholine-stearylamine (PC-SA), strongly binds and kills cancer cells through direct interaction with negatively charged surface-exposed PS (De et al., 2018). The anticancer properties of drugs like camptothecin and doxorubicin entrapped in PC-SA liposomes was demonstrated on cancer cell lines, both in vitro and in different mice models (De et al., 2018, 2020). These and other studies showed the potential use in MLT of PS-targeting vesicle alone or in combination with anticancer drugs (Blanco et al., 2014; Ayesa et al., 2017). PE represents another chemotherapeutic target on the membrane surface of cancer cells. Duramycin is a small tetracyclic peptide produced by the bacterium Streptoverticillium cinnamoneus and is closely related to cinnamycin produced by Streptomyces sp. (Iwamoto et al., 2007; Hullin-Matsuda et al., 2016). Both duramycin and cinnamycin are capable to bind to PE specifically into areas of membrane with high curvature, inducing trans-bilayer phospholipid movements that lead to cell death (Makino et al., 2003; Iwamoto et al., 2007; Hullin-Matsuda et al., 2016). Another group of interesting molecules are cyclotides, cyclic peptides which exert their biological activities by acting on cell membrane, binding to phospholipids containing PE headgroups. This binding is followed by an insertion that subsequently leads to membrane disruption and cell death as a result of pore formation (Wang et al., 2012). The increased levels of exposed PE on the outer membrane of cancer cell allow those membrane-active peptides to exert their cytotoxic effects without harming healthy cells. A third target for MLT is ceramide. Ceramide is present in small amounts in cell membranes, as intermediate in the metabolism of sphingolipids or as a result of sphingomyelinase activity, which produces ceramide from SM (Kartal Yandim et al., 2013; Peetla et al., 2013). Altered ceramide metabolism in cancer has been described as an effective drug resistance mechanism: tumors have low levels of ceramide by increasing SM synthesis or by preventing its degradation (Senchenkov et al., 2001; Lewis et al., 2018). One possible strategy is to increase ceramide membrane levels using short chain ceramide and use lipid rafts as platforms to enhance apoptosis, since in presence of an excess of ceramide, cholesterol is displaced from lipid rafts, inducing activation of Fas/CD95 pathway (Selzner et al., 2001; Stover and Kester, 2003; Stover et al., 2005; Chiantia et al., 2007). Ceramide levels can also be increased by inhibiting the enzyme ceramidase (using the ceramide analogs B13, LCL-464 and KPB-27) or sphingosine kinase inhibitors (like N, N-dimethylsphingosine) (Bhabak and Arenz, 2012; Bhabak et al., 2013; Chen et al., 2014). Few reviews provide a complete list of compounds used in MLT based on regulation of ceramide levels (Lin et al., 2006; Kartal Yandim et al., 2013; Liu et al., 2013).
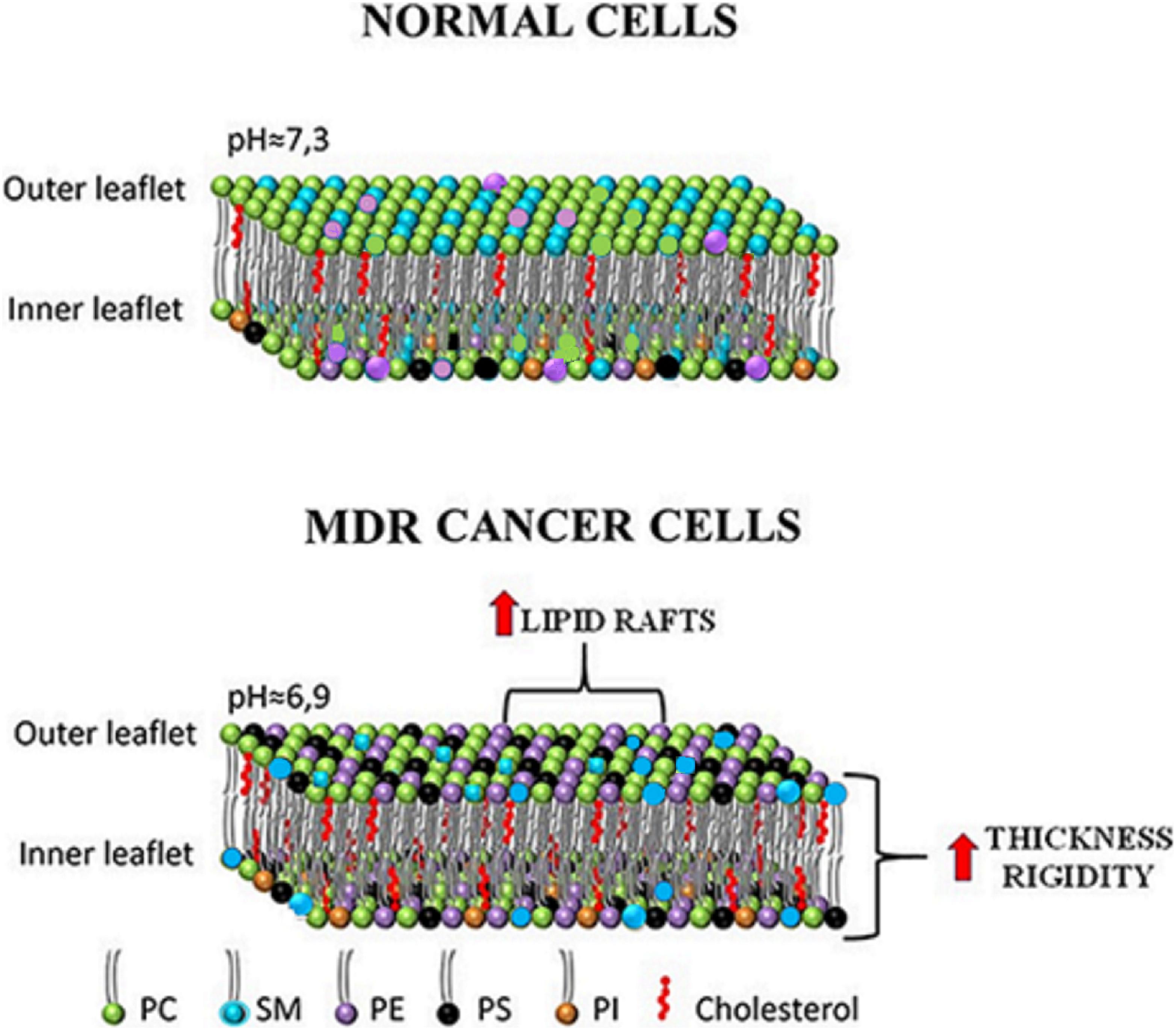
Figure 1. Differences between membrane lipid composition and organization in normal vs MDR cancer cells. In cancer cells PS and PE, mainly confined in the inner leaflet of the membranes, are present in high concentrations in the outer leaflet. Cancer cells have also higher concentrations of cholesterol and consequently an increase in membrane thickness and rigidity is observed. Increased levels of saturated fatty acyl chains in membrane lipids have been associated to the presence of more lipid rafts while low amount of ceramide in MDR cells is a consequence of the low activity of SMase or of the increased SM levels. Changes in lipid composition of the outer membrane of cancer cells are also correlated to a more acidic extracellular pH.
Activation of the Fas pathway is the target of treatment with different anticancer agents including Edelfosine, Miltefosine and Perifosine, lipid clustering agents promoting apoptosis (Gajate and Mollinedo, 2007; Gomide et al., 2013). Resveratrol, a common constituent of red wine, has been shown to have anti-tumor activity for its tendency to accumulate in lipid rafts and is mainly used in combination with death receptor agonists (Delmas et al., 2013). Azurin is a membrane-associated protein from Pseudomonas aeruginosa. Azurin and its derived peptide p28 have been intensively studied as an anticancer protein, down-regulating fundamental signaling pathways downstream of membrane receptors and affecting processes such as adhesion and invasiveness (Gao et al., 2017; Bernardes and Fialho, 2018). These effects are dependent on the caveolin 1 and ganglioside 1-mediated uptake of azurin, leading to alteration of lipid rafts; decrease in plasma membrane stiffness and in the number of ordered domains (Bernardes and Fialho, 2018). The increased sensitivity of cancer cells to chemotherapeutic agents like paclitaxel and doxorubicin in combination with azurin confirms that part of the anticancer effect of azurin occurs by altering the membrane properties and increasing the membrane permeability to anticancer drugs (Bernardes et al., 2018). However, the use of these peptides in MLT, has some limitations: they require further optimization to enhance their selectivity toward cancer cells and to decrease toxicity; in few cases their use alone or in combination with chemotherapeutic agents did not show any beneficial effect in clinical trials (Planting et al., 1993; Gills and Dennis, 2009; Cho et al., 2012; Gerber et al., 2018).
Changing the membrane bilayer properties such as intrinsic curvature, elasticity and fluidity is a characteristic of several amphiphilic molecules. Statins, beyond the classical cholesterol lowering effects have been shown to alter lipid organization of artificial membranes and cell membranes in a cholesterol-independent way (Redondo-Morata et al., 2016; Penkauskas et al., 2020). This property can be included among the pleiotropic effects of statins, which are behind many benefits observed during statin therapy (Banfi et al., 2017; Oesterle et al., 2017). According to an established hypothesis, the biological properties of statins depend on their localization in the cellular membrane due to their amphiphilic properties (Mason et al., 2005; Galiullina et al., 2017). Several recent studies investigated the interactions of different statins with phospholipid membranes and their influence on the membrane structure (Sahu et al., 2019; Sariisik et al., 2019; Penkauskas et al., 2020). Statins seem to bind and influence lipid membranes, possessing different average location into the bilayer (Galiullina et al., 2019). However, a clear connection between a determined statin and the capacity to interact and alter membrane bilayer properties cannot be fully established, mainly due to the different membrane models and experimental settings used. Clinical studies in cancer patients have suggested lower cancer mortality and less side effects with lipophilic statins compared to hydrophilic ones (Ahern et al., 2011; Ahmadi et al., 2018; Beckwitt et al., 2018). In the last years the anti-tumor activity of statins was remarkably improved by using statins formulated in different drug delivery systems (Coimbra et al., 2010; Alupei et al., 2015; Safwat et al., 2017a; Matusewicz et al., 2019). In many cases the drug delivery system includes the statin in combination with a chemotherapeutic agent as doxorubicin (Pinzon-Daza et al., 2012). Indeed, the incorporation of statins in nanoparticulate drug delivery systems not only increased statins cytotoxicity but also overcame the resistance of cancer cells against common chemotherapeutic agents (Safwat et al., 2017b). This field is continuously developing, trying to identify the best carrier capable to enhance drug loading capacity, stability and therapeutic activity. According to this point of view, chitosan nanoparticles (CSNPs) are an optimal choice since they possess low toxicity and immunogenicity and good levels of biodegradability (Prabaharan, 2015). In Figure 2 are presented the different rationales behind the use of statins in cancer therapy for modulation of membrane lipids. An innovative strategy for treatment of oncological disorders is the use of amphiphilic drug-drug conjugates (ADDC), where an amphiphilic molecule, with high capacity to interact with and penetrate the lipid bilayer, is created by combining an hydrophilic anticancer drug with a hydrophobic one (Huang et al., 2014; Gao et al., 2018). Most of the times, this strategy overcomes the necessity to use a proper delivery system and since these two drugs have different pharmacokinetics, it is possible that these molecules induce synergistic pharmacological effects improving the therapeutic efficacy both in vitro and in vivo. However, ADDC is another example where the overall effect achieved in cancer therapy should not be only reconnected to the sum of the individual ones. For example, camptothecin-classical anticancer activity is related to binding to the topoisomerase-1 and DNA complex, while floxuridine, a derivative of 5-fluorouracil, is known for its high antitumor activity against cancer metastases. The combination of the hydrophobic camptothecin and hydrophilic floxuridine, used to enhance apoptosis in colon cancer cell lines creates amphiphilic molecules capable to alter the lipid bilayer properties (Hu et al., 2015). Additionally, changes in bilayer physical properties regulate membrane protein functions including the ones involved in the regulation of apoptosis (Lundbaek et al., 2010). Therefore, behind the individual molecular target of each chemotherapeutic agents, the potential effect on membrane bilayers, derived by the creation of amphiphilic molecules should be evaluated (Bruno et al., 2013; Kumar et al., 2015). A better understanding of the biological effects of chemotherapeutic drugs on lipid membranes is essential to overcome MDR since, as mentioned before, cancer cells rearrange lipid composition and organization to avoid apoptosis and resist anticancer drugs (Bernardes and Fialho, 2018; Rivel et al., 2019).
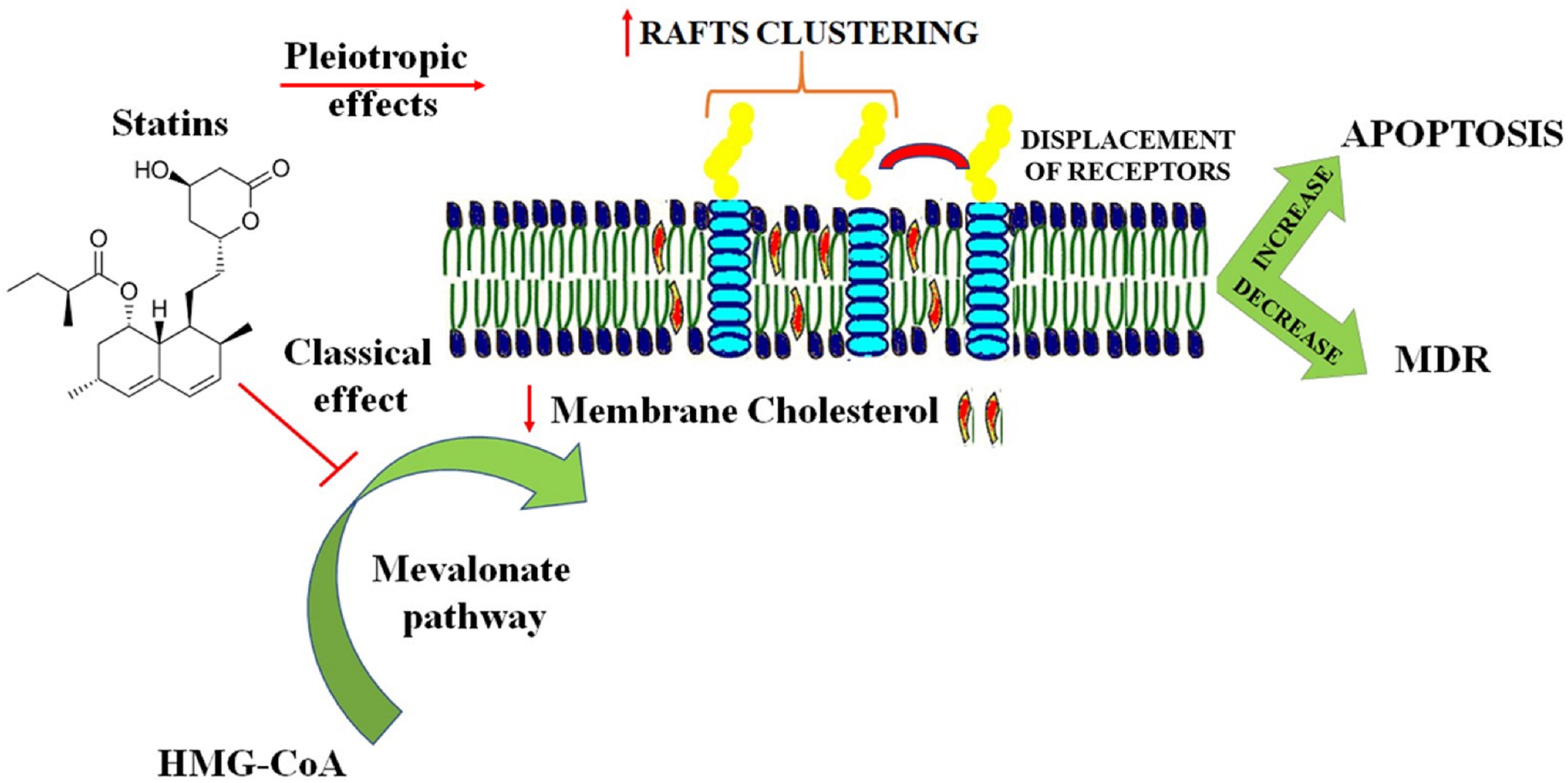
Figure 2. Different rationales behind the use of statins in cancer therapy for modulation of membrane lipids. The beneficial effects of statins in cancer therapy are achieved by a combination of classical and pleiotropic effects. The classical cholesterol lowering activity decreases the cholesterol concentration in the plasma membrane influencing membrane fluidity and thickness. Pleiotropic effects include lipid rafts clustering and displacement of receptors in non-raft domains. All these effects contribute to an increase in apoptosis and in decrease in resistance of cancer cells to chemotherapeutic agents.
Several clinical studies have strongly indicated a role for fish oil and polyunsaturated fatty acids (PUFA) in cancer prevention (Caygill et al., 1996; Azrad et al., 2013). One of the main lipids present in fish oil, docosahexaenoic acid (DHA), has been shown to alter plasma membrane properties including membrane fluidity, phase behavior and permeability (Yang et al., 2011; Levental et al., 2016; Bie et al., 2020). Moreover, different studies shown that DHA can influence lipid rafts composition, altering their size or clustering capacities and consequently affecting lipid raft-regulated signaling (Shaikh et al., 2009; Turk and Chapkin, 2013; Wassall et al., 2018). Studies in mice fed with a PUFA-enriched diet shown that the molecular targets of DHA are cholesterol and sphingomyelin, two essentials building blocks of lipid rafts (Fan et al., 2003, 2004). These properties can have beneficial effects in anti-cancer therapy since behind the modification of membrane lipids, there is also a regulation of the protein receptors enriched in these membrane microdomains. For example, in breast cancer cell lines, DHA was found to influence epidermal growth factors receptors function and to enhance chemotherapy efficacy, by inducing CD95 translocation into lipid rafts, while in colon cancer cell lines it was responsible for an increase in oxidative stress and in TRAIL-induced apoptosis (Ewaschuk et al., 2012; Skender et al., 2014; Pettersen et al., 2016). These and many similar studies demonstrated two bullet-points: (1) lipid rafts play a functional role during tumorigenesis of different types of cancer (2) a therapeutic role for PUFA, since these fatty acids alter lipid raft structure/organization/function. The role for PUFA in prevention and treatment of cancer is wide and well documented, but the real efficacy of PUFA is still debated. Indeed, it is not fully established whether dietary PUFAs are integrated into raft lipids or whether their low affinity to cholesterol causes phase separation from rafts and, consequently, displacement of raft proteins (Yaqoob and Shaikh, 2010). Currently, they are mainly used in combination with different cytotoxic drugs to enhance chemotherapy efficiency (Granci et al., 2013; Newell et al., 2019). A compound reported to induce lipid rafts clustering is epigallocatechin-3-gallate (EGCG) the major polyphenol of green tea with chemo-preventives and chemo-therapeutic activities (Surh, 2003; Yang et al., 2009). However, the overall effect seems to be dependent by the tumor type since this polyphenol was observed to induce apoptosis in multiple myeloma cells (Tsukamoto et al., 2012; Huang et al., 2015), while in colon adenocarcinoma cells it increased cell viability and proliferation (Pajak et al., 2011). This discrepancy is probably related to the fact that EGCG modulates a wide spectrum of molecular targets including epidermal growth factor receptor, mitogen activated protein kinase and cyclin-dependent kinases (Khan et al., 2006; Ma et al., 2014; Fang et al., 2015). Therefore, there is not always a unique pattern of response to disruption of lipid rafts or to depletion of cholesterol from the membrane and each treatment should be evaluated in the context of the particular type of cancer and also of the specific therapeutic strategy adopted. In the last years, many scientists became interested in the evaluation of the synergistic effects of the combination of EGCG and anticancer compounds. For example, Fujiki et al. (2015) showed that the combinations of EGCG or other green tea catechins and 46 anticancer drugs synergistically induced in vitro anticancer effects in 58 different human cancer cell lines. Therefore, EGCG is a natural compound with proven beneficial effects both in cancer prevention and cancer therapy in combination with anticancer compounds (Fujiki and Suganuma, 2012; Fujiki et al., 2015). In Table 1 is presented the list of compounds described in this manuscript with the main mechanism of actions for MLT.
The better understanding of membrane lipid composition and organization gained in the last years, together with the lipidic alterations reported in tumor membranes, provides a big opportunity for cancer prevention and treatment. Nowadays, the strategy to modify membrane cholesterol/sphingolipids content is gradually replaced by a more focused approach on the modulation of membrane bilayer properties, including fluidity and elasticity, by inducing changes in the organization of lipid rafts. Rafts proteins have also an essential role in regulating lipid properties and a future field of study in MLT could be the investigation of how changes in the structural composition of raft proteins influence lipid microdomains organization. The lack of attention toward targeting these proteins as a strategy for MLT is quite surprising and it is related to the consideration of these membrane proteins as merely guests rather than as active components of lipid rafts. Further studies on these protein-lipid interactions may lead to a better understanding of the molecular mechanism of raft domains organization and may provide new strategies for their manipulation. The final aim of this modulation in cancer therapy is to increase the overall efficiency of chemotherapeutic agents, achieving a synergistic effect and defeating MDR. Studying and testing membrane-lipid targeting agents in combination with chemotherapeutic agents is a promising and innovative approach for the development of new therapeutic strategies.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
GP’s research was supported by the Research Council of Lithuania.
Ahern, T. P., Pedersen, L., Tarp, M., Cronin-Fenton, D. P., Garne, J. P., Silliman, R. A., et al. (2011). Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J. Natl. Cancer Inst. 103, 1461–1468. doi: 10.1093/jnci/djr291
Ahmadi, Y., Karimian, R., and Panahi, Y. (2018). Effects of statins on the chemoresistance-The antagonistic drug-drug interactions versus the anti-cancer effects. Biomed. Pharmacother. 108, 1856–1865. doi: 10.1016/j.biopha.2018.09.122
Alupei, M. C., Licarete, E., Patras, L., and Banciu, M. (2015). Liposomal simvastatin inhibits tumor growth via targeting tumor-associated macrophages-mediated oxidative stress. Cancer Lett. 356(2 Pt B), 946–952. doi: 10.1016/j.canlet.2014.11.010
Amin, D., Cornell, S. A., Gustafson, S. K., Needle, S. J., Ullrich, J. W., Bilder, G. E., et al. (1992). Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J. Lipid Res. 33, 1657–1663.
Ayesa, U., Gray, B. D., Pak, K. Y., and Chong, P. L. (2017). Liposomes containing lipid-soluble Zn(II)-Bis-dipicolylamine derivatives show potential to be targeted to phosphatidylserine on the surface of cancer cells. Mol. Pharm. 14, 147–156. doi: 10.1021/acs.molpharmaceut.6b00760
Azrad, M., Turgeon, C., and Demark-Wahnefried, W. (2013). Current evidence linking polyunsaturated Fatty acids with cancer risk and progression. Front. Oncol. 3:224. doi: 10.3389/fonc.2013.00224
Banfi, C., Baetta, R., Gianazza, E., and Tremoli, E. (2017). Technological advances and proteomic applications in drug discovery and target deconvolution: identification of the pleiotropic effects of statins. Drug Discov. Today 22, 848–869. doi: 10.1016/j.drudis.2017.03.001
Barros, M. C. F., Ribeiro, A. C. F., and Esteso, M. A. (2018). Cyclodextrins in Parkinson’s Disease. Biomolecules 9:3.
Beckwitt, C. H., Shiraha, K., and Wells, A. (2018). Lipophilic statins limit cancer cell growth and survival, via involvement of Akt signaling. PLoS One 13:e0197422. doi: 10.1371/journal.pone.0197422
Bernardes, N., and Fialho, A. M. (2018). Perturbing the dynamics and organization of cell membrane components: a new paradigm for cancer-targeted therapies. Int. J. Mol. Sci. 19:3871. doi: 10.3390/ijms19123871
Bernardes, N., Garizo, A. R., Pinto, S. N., Canico, B., Perdigao, C., Fernandes, F., et al. (2018). Azurin interaction with the lipid raft components ganglioside GM-1 and caveolin-1 increases membrane fluidity and sensitivity to anti-cancer drugs. Cell Cycle 17, 1649–1666. doi: 10.1080/15384101.2018.1489178
Bhabak, K. P., and Arenz, C. (2012). Novel amide- and sulfonamide-based aromatic ethanolamines: effects of various substituents on the inhibition of acid and neutral ceramidases. Bioorg. Med. Chem. 20, 6162–6170. doi: 10.1016/j.bmc.2012.08.031
Bhabak, K. P., Kleuser, B., Huwiler, A., and Arenz, C. (2013). Effective inhibition of acid and neutral ceramidases by novel B-13 and LCL-464 analogues. Bioorg. Med, Chem. 21, 874–882. doi: 10.1016/j.bmc.2012.12.014
Bie, N., Han, L., Meng, M., Yan, Z., and Wang, C. (2020). The immunomodulatory effect of docosahexaenoic acid (DHA) on the RAW264.7 cells by modification of the membrane structure and function. Food Funct. 11, 2603–2616. doi: 10.1039/c9fo02618e
Bittman, R., and Fischkoff, S. A. (1972). Fluorescence studies of the binding of the polyene antibiotics filipin 3, amphotericin B, nystatin, and lagosin to cholesterol. Proc. Natl. Acad. Sci. U.S.A. 69, 3795–3799. doi: 10.1073/pnas.69.12.3795
Blanco, V. M., Chu, Z., Vallabhapurapu, S. D., Sulaiman, M. K., Kendler, A., Rixe, O., et al. (2014). Phosphatidylserine-selective targeting and anticancer effects of SapC-DOPS nanovesicles on brain tumors. Oncotarget 5, 7105–7118. doi: 10.18632/oncotarget.2214
Bruno, M. J., Rusinova, R., Gleason, N. J., Koeppe, R. E. II, and Andersen, O. S. (2013). Interactions of drugs and amphiphiles with membranes: modulation of lipid bilayer elastic properties by changes in acyl chain unsaturation and protonation. Faraday Discuss 161, 461–480. discussion 563–89 doi: 10.1039/c2fd20092a
Canevari, L., and Clark, J. B. (2007). Alzheimer’s disease and cholesterol: the fat connection. Neurochem. Res. 32, 739–750. doi: 10.1007/s11064-006-9200-1
Caygill, C. P., Charlett, A., and Hill, M. J. (1996). Fat, fish, fish oil and cancer. Br. J. Cancer 74, 159–164. doi: 10.1038/bjc.1996.332
Chalasani, P., Marron, M., Roe, D., Clarke, K., Iannone, M., Livingston, R. B., et al. (2015). A phase I clinical trial of bavituximab and paclitaxel in patients with HER2 negative metastatic breast cancer. Cancer Med. 4, 1051–1059. doi: 10.1002/cam4.447
Chen, C. Y., Ingram, M. F., Rosal, P. H., and Graham, T. R. (1999). Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 147, 1223–1236. doi: 10.1083/jcb.147.6.1223
Chen, K., Pan, Q., Gao, Y., Yang, X., Wang, S., Peppelenbosch, M. P., et al. (2014). DMS triggers apoptosis associated with the inhibition of SPHK1/NF-kappaB activation and increase in intracellular Ca2+ concentration in human cancer cells. Int. J. Mol. Med. 33, 17–24. doi: 10.3892/ijmm.2013.1541
Chen, Q., Pan, Z., Zhao, M., Wang, Q., Qiao, C., Miao, L., et al. (2018). High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J. Cell Physiol. 233, 6722–6732. doi: 10.1002/jcp.26351
Chiantia, S., Kahya, N., and Schwille, P. (2007). Raft domain reorganization driven by short- and long-chain ceramide: a combined AFM and FCS study. Langmuir 23, 7659–7665. doi: 10.1021/la7010919
Cho, D. C., Hutson, T. E., Samlowski, W., Sportelli, P., Somer, B., Richards, P., et al. (2012). Two phase 2 trials of the novel Akt inhibitor perifosine in patients with advanced renal cell carcinoma after progression on vascular endothelial growth factor-targeted therapy. Cancer 118, 6055–6062. doi: 10.1002/cncr.27668
Coimbra, M., Banciu, M., Fens, M. H., de Smet, L., Cabaj, M., Metselaar, J. M., et al. (2010). Liposomal pravastatin inhibits tumor growth by targeting cancer-related inflammation. J. Control Release 148, 303–310. doi: 10.1016/j.jconrel.2010.09.011
Das, A., Brown, M. S., Anderson, D. D., Goldstein, J. L., and Radhakrishnan, A. (2014). Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife 3:e02882. doi: 10.7554/eLife.02882
Dass, C. R., and Choong, P. F. (2006). Targeting of small molecule anticancer drugs to the tumour and its vasculature using cationic liposomes: lessons from gene therapy. Cancer Cell Int. 6:17.
Davis, H. W., Vallabhapurapu, S. D., Chu, Z., Vallabhapurapu, S. L., Franco, R. S., Mierzwa, M., et al. (2019). Enhanced phosphatidylserine-selective cancer therapy with irradiation and SapC-DOPS nanovesicles. Oncotarget 10, 856–868. doi: 10.18632/oncotarget.26615
De, M., Ghosh, S., Asad, M., Banerjee, I., and Ali, N. (2020). Combining doxorubicin with stearylamine-bearing liposomes elicits Th1 cytokine responses and cures metastasis in a mouse model. Cancer Immunol. Immunother. 69, 1725–1735. doi: 10.1007/s00262-020-02578-9
De, M., Ghosh, S., Sen, T., Shadab, M., Banerjee, I., Basu, S., et al. (2018). A novel therapeutic strategy for cancer using phosphatidylserine targeting stearylamine-bearing cationic liposomes. Mol. Ther. Nucleic Acids 10, 9–27. doi: 10.1016/j.omtn.2017.10.019
Delmas, D., Aires, V., Colin, D. J., Limagne, E., Scagliarini, A., Cotte, A. K., et al. (2013). Importance of lipid microdomains, rafts, in absorption, delivery, and biological effects of resveratrol. Ann. N. Y. Acad. Sci. 1290, 90–97. doi: 10.1111/nyas.12177
Desai, T. J., Toombs, J. E., Minna, J. D., Brekken, R. A., and Udugamasooriya, D. G. (2016). Identification of lipid-phosphatidylserine (PS) as the target of unbiasedly selected cancer specific peptide-peptoid hybrid PPS1. Oncotarget 7, 30678–30690. doi: 10.18632/oncotarget.8929
Desai, T. J., and Udugamasooriya, D. G. (2017). A comprehensive lipid binding and activity validation of a cancer-specific peptide-peptoid hybrid PPS1. Biochem. Biophys. Res. Commun. 486, 545–550. doi: 10.1016/j.bbrc.2017.03.083
Duncan, R. E., El-Sohemy, A., and Archer, M. C. (2004). Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J. Biol. Chem. 279, 33079–33084. doi: 10.1074/jbc.m400732200
Escriba, P. V. (2006). Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol. Med. 12, 34–43. doi: 10.1016/j.molmed.2005.11.004
Escriba, P. V. (2017). Membrane-lipid therapy: a historical perspective of membrane-targeted therapies - From lipid bilayer structure to the pathophysiological regulation of cells. Biochim. Biophys. Acta Biomembr. 1859(9 Pt B), 1493–1506. doi: 10.1016/j.bbamem.2017.05.017
Escriba, P. V., Busquets, X., Inokuchi, J., Balogh, G., Torok, Z., Horvath, I., et al. (2015). Membrane lipid therapy: modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 59, 38–53. doi: 10.1016/j.plipres.2015.04.003
Ewaschuk, J. B., Newell, M., and Field, C. J. (2012). Docosahexanoic acid improves chemotherapy efficacy by inducing CD95 translocation to lipid rafts in ER(-) breast cancer cells. Lipids 47, 1019–1030. doi: 10.1007/s11745-012-3717-7
Fan, Y. Y., Ly, L. H., Barhoumi, R., McMurray, D. N., and Chapkin, R. S. (2004). Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J. Immunol. 173, 6151–6160. doi: 10.4049/jimmunol.173.10.6151
Fan, Y. Y., McMurray, D. N., Ly, L. H., and Chapkin, R. S. (2003). Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J. Nutr. 133, 1913–1920. doi: 10.1093/jn/133.6.1913
Fanciullino, R., and Ciccolini, J. (2009). Liposome-encapsulated anticancer drugs: still waiting for the magic bullet? Curr. Med. Chem. 16, 4361–4371. doi: 10.2174/092986709789712916
Fang, C. Y., Wu, C. C., Hsu, H. Y., Chuang, H. Y., Huang, S. Y., Tsai, C. H., et al. (2015). EGCG inhibits proliferation, invasiveness and tumor growth by up-regulation of adhesion molecules, suppression of gelatinases activity, and induction of apoptosis in nasopharyngeal carcinoma cells. Int. J. Mol. Sci. 16, 2530–2558. doi: 10.3390/ijms16022530
Fujiki, H., Sueoka, E., Watanabe, T., and Suganuma, M. (2015). Primary cancer prevention by green tea, and tertiary cancer prevention by the combination of green tea catechins and anticancer compounds. J. Cancer Prev. 20, 1–4. doi: 10.15430/jcp.2015.20.1.1
Fujiki, H., and Suganuma, M. (2012). Green tea: an effective synergist with anticancer drugs for tertiary cancer prevention. Cancer Lett. 324, 119–125. doi: 10.1016/j.canlet.2012.05.012
Gajate, C., and Mollinedo, F. (2007). Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood 109, 711–719. doi: 10.1182/blood-2006-04-016824
Galiullina, L. F., Aganova, O. V., Latfullin, I. A., Musabirova, G. S., Aganov, A. V., and Klochkov, V. V. (2017). Interaction of different statins with model membranes by NMR data. Biochim. Biophys. Acta Biomembr. 1859, 295–300. doi: 10.1016/j.bbamem.2016.12.006
Galiullina, L. F., Scheidt, H. A., Huster, D., Aganov, A., and Klochkov, V. (2019). Interaction of statins with phospholipid bilayers studied by solid-state NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 1861, 584–593. doi: 10.1016/j.bbamem.2018.12.013
Gao, C., Bhattarai, P., Chen, M., Zhang, N., Hameed, S., Yue, X., et al. (2018). Amphiphilic drug conjugates as nanomedicines for combined cancer therapy. Bioconjug. Chem. 29, 3967–3981. doi: 10.1021/acs.bioconjchem.8b00692
Gao, M., Zhou, J., Su, Z., and Huang, Y. (2017). Bacterial cupredoxin azurin hijacks cellular signaling networks: protein-protein interactions and cancer therapy. Protein. Sci. 26, 2334–2341. doi: 10.1002/pro.3310
Gerber, D. E., Hao, G., Watkins, L., Stafford, J. H., Anderson, J., Holbein, B., et al. (2015). Tumor-specific targeting by Bavituximab, a phosphatidylserine-targeting monoclonal antibody with vascular targeting and immune modulating properties, in lung cancer xenografts. Am. J. Nucl. Med. Mol. Imaging 5, 493–503.
Gerber, D. E., Horn, L., Boyer, M., Sanborn, R., Natale, R., Palmero, R., et al. (2018). Randomized phase III study of docetaxel plus bavituximab in previously treated advanced non-squamous non-small-cell lung cancer. Ann. Oncol. 29, 1548–1553. doi: 10.1093/annonc/mdy177
Gills, J. J., and Dennis, P. A. (2009). Perifosine: update on a novel Akt inhibitor. Curr. Oncol. Rep. 11, 102–110. doi: 10.1007/s11912-009-0016-4
Girard, E., Paul, J. L., Fournier, N., Beaune, P., Johannes, L., Lamaze, C., et al. (2011). The dynamin chemical inhibitor dynasore impairs cholesterol trafficking and sterol-sensitive genes transcription in human HeLa cells and macrophages. PLoS One 6:e29042. doi: 10.1371/journal.pone.0029042
Gomide, A. B., Thome, C. H., dos Santos, G. A., Ferreira, G. A., Faca, V. M., Rego, E. M., et al. (2013). Disrupting membrane raft domains by alkylphospholipids. Biochim. Biophys. Acta 1828, 1384–1389. doi: 10.1016/j.bbamem.2013.01.017
Granci, V., Cai, F., Lecumberri, E., Clerc, A., Dupertuis, Y. M., and Pichard, C. (2013). Colon cancer cell chemosensitisation by fish oil emulsion involves apoptotic mitochondria pathway. Br. J. Nutr. 109, 1188–1195. doi: 10.1017/s000711451200308x
Griffin, S., Preta, G., and Sheldon, I. M. (2017). Inhibiting mevalonate pathway enzymes increases stromal cell resilience to a cholesterol-dependent cytolysin. Sci. Rep. 7:17050.
Grilley-Olson, J. E., Weiss, J., Ivanova, A., Villaruz, L. C., Moore, D. T., Stinchcombe, T. E., et al. (2018). Phase Ib study of bavituximab with carboplatin and pemetrexed in chemotherapy-naive advanced nonsquamous non-small-cell lung cancer. Clin. Lung. Cancer 19, e481–e487.
Gu, L., Saha, S. T., Thomas, J., and Kaur, M. (2019). Targeting cellular cholesterol for anticancer therapy. FEBS J. 286, 4192–4208. doi: 10.1111/febs.15018
Guardia-Laguarta, C., Coma, M., Pera, M., Clarimon, J., Sereno, L., Agullo, J. M., et al. (2009). Mild cholesterol depletion reduces amyloid-beta production by impairing APP trafficking to the cell surface. J. Neurochem. 110, 220–230. doi: 10.1111/j.1471-4159.2009.06126.x
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Harwood, H. J. Jr., Alvarez, I. M., Noyes, W. D., and Stacpoole, P. W. (1991). In vivo regulation of human leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase: increased enzyme protein concentration and catalytic efficiency in human leukemia and lymphoma. J. Lipid Res. 32, 1237–1252.
Henderson, C. M., and Block, D. E. (2014). Examining the role of membrane lipid composition in determining the ethanol tolerance of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 80, 2966–2972. doi: 10.1128/aem.04151-13
Hu, M., Huang, P., Wang, Y., Su, Y., Zhou, L., Zhu, X., et al. (2015). Synergistic combination chemotherapy of camptothecin and floxuridine through self-assembly of amphiphilic drug-drug conjugate. Bioconjug Chem. 26, 2497–2506. doi: 10.1021/acs.bioconjchem.5b00513
Huang, P., Wang, D., Su, Y., Huang, W., Zhou, Y., Cui, D., et al. (2014). Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 136, 11748–11756. doi: 10.1021/ja505212y
Huang, Y., Kumazoe, M., Bae, J., Yamada, S., Takai, M., Hidaka, S., et al. (2015). Green tea polyphenol epigallocatechin-O-gallate induces cell death by acid sphingomyelinase activation in chronic myeloid leukemia cells. Oncol. Rep. 34, 1162–1168. doi: 10.3892/or.2015.4086
Hullin-Matsuda, F., Makino, A., Murate, M., and Kobayashi, T. (2016). Probing phosphoethanolamine-containing lipids in membranes with duramycin/cinnamycin and aegerolysin proteins. Biochimie 130, 81–90. doi: 10.1016/j.biochi.2016.09.020
Infante, R. E., and Radhakrishnan, A. (2017). Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife 6:e25466.
Iwamoto, K., Hayakawa, T., Murate, M., Makino, A., Ito, K., Fujisawa, T., et al. (2007). Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophys. J. 93, 1608–1619. doi: 10.1529/biophysj.106.101584
Kaiser, H. J., Lingwood, D., Levental, I., Sampaio, J. L., Kalvodova, L., Rajendran, L., et al. (2009). Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 106, 16645–16650. doi: 10.1073/pnas.0908987106
Kaminski, D. M. (2014). Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 43, 453–467. doi: 10.1007/s00249-014-0983-8
Kartal Yandim, M., Apohan, E., and Baran, Y. (2013). Therapeutic potential of targeting ceramide/glucosylceramide pathway in cancer. Cancer Chemother. Pharmacol. 71, 13–20. doi: 10.1007/s00280-012-1984-x
Kawata, S., Takaishi, K., Nagase, T., Ito, N., Matsuda, Y., Tamura, S., et al. (1990). Increase in the active form of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human hepatocellular carcinoma: possible mechanism for alteration of cholesterol biosynthesis. Cancer Res. 50, 3270–3273.
Khan, N., Afaq, F., Saleem, M., Ahmad, N., and Mukhtar, H. (2006). Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 66, 2500–2505. doi: 10.1158/0008-5472.can-05-3636
Kopecka, J., Campia, I., Olivero, P., Pescarmona, G., Ghigo, D., Bosia, A., et al. (2011). A LDL-masked liposomal-doxorubicin reverses drug resistance in human cancer cells. J. Control Release 149, 196–205. doi: 10.1016/j.jconrel.2010.10.003
Kopecka, J., Trouillas, P., Gasparovic, A. C., Gazzano, E., Assaraf, Y. G., and Riganti, C. (2020). Phospholipids and cholesterol: inducers of cancer multidrug resistance and therapeutic targets. Drug Resist. Updat 49:100670. doi: 10.1016/j.drup.2019.100670
Kuipers, H. F., and van den Elsen, P. J. (2007). Immunomodulation by statins: inhibition of cholesterol vs. isoprenoid biosynthesis. Biomed. Pharmacother. 61, 400–407. doi: 10.1016/j.biopha.2007.06.005
Kumar, S., Bhargava, P., Sreekanth, V., and Bajaj, A. (2015). Design, synthesis, and physico-chemical interactions of bile acid derived dimeric phospholipid amphiphiles with model membranes. J. Colloid Interface Sci. 448, 398–406. doi: 10.1016/j.jcis.2015.01.069
Lange, Y., Swaisgood, M. H., Ramos, B. V., and Steck, T. L. (1989). Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J. Biol. Chem. 264, 3786–3793.
Levental, K. R., Lorent, J. H., Lin, X., Skinkle, A. D., Surma, M. A., Stockenbojer, E. A., et al. (2016). Polyunsaturated lipids regulate membrane domain stability by tuning membrane order. Biophys. J. 110, 1800–1810. doi: 10.1016/j.bpj.2016.03.012
Lewis, A. C., Wallington-Beddoe, C. T., Powell, J. A., and Pitson, S. M. (2018). Targeting sphingolipid metabolism as an approach for combination therapies in haematological malignancies. Cell Death Discov. 4:4.
Lin, C. F., Chen, C. L., and Lin, Y. S. (2006). Ceramide in apoptotic signaling and anticancer therapy. Curr. Med. Chem. 13, 1609–1616. doi: 10.2174/092986706777441986
Liu, J., Beckman, B. S., and Foroozesh, M. (2013). A review of ceramide analogs as potential anticancer agents. Future Med. Chem. 5, 1405–1421. doi: 10.4155/fmc.13.107
Lopez, C. A., de Vries, A. H., and Marrink, S. J. (2013). Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci. Rep. 3:2071.
Lopez, D. (2015). Molecular composition of functional microdomains in bacterial membranes. Chem. Phys. Lipids 192, 3–11. doi: 10.1016/j.chemphyslip.2015.08.015
Lundbaek, J. A., Collingwood, S. A., Ingolfsson, H. I., Kapoor, R., and Andersen, O. S. (2010). Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J. R. Soc. Interface 7, 373–395. doi: 10.1098/rsif.2009.0443
Ma, Y. C., Li, C., Gao, F., Xu, Y., Jiang, Z. B., Liu, J. X., et al. (2014). Epigallocatechin gallate inhibits the growth of human lung cancer by directly targeting the EGFR signaling pathway. Oncol. Rep. 31, 1343–1349. doi: 10.3892/or.2013.2933
Mahammad, S., and Parmryd, I. (2015). Cholesterol depletion using methyl-beta-cyclodextrin. Methods Mol. Biol. 1232, 91–102. doi: 10.1007/978-1-4939-1752-5_8
Makino, A., Baba, T., Fujimoto, K., Iwamoto, K., Yano, Y., Terada, N., et al. (2003). Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement. J. Biol. Chem. 278, 3204–3209. doi: 10.1074/jbc.m210347200
Mason, R. P., Walter, M. F., Day, C. A., and Jacob, R. F. (2005). Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am. J. Cardiol. 96, 11F–23F.
Matusewicz, L., Filip-Psurska, B., Psurski, M., Tabaczar, S., Podkalicka, J., Wietrzyk, J., et al. (2019). EGFR-targeted immunoliposomes as a selective delivery system of simvastatin, with potential use in treatment of triple-negative breast cancers. Int. J. Pharm. 569:118605. doi: 10.1016/j.ijpharm.2019.118605
Muro, E., Atilla-Gokcumen, G. E., and Eggert, U. S. (2014). Lipids in cell biology: how can we understand them better? Mol. Biol. Cell 25, 1819–1823. doi: 10.1091/mbc.e13-09-0516
Naito, T., Ercan, B., Krshnan, L., Triebl, A., Koh, D. H. Z., Wei, F. Y., et al. (2019). Movement of accessible plasma membrane cholesterol by the GRAMD1 lipid transfer protein complex. eLife 8:e51401. doi: 10.7554/eLife.51401
Newell, M., Brun, M., and Field, C. J. (2019). Treatment with dha modifies the response of MDA-MB-231 breast cancer cells and tumors from nu/nu mice to doxorubicin through apoptosis and cell cycle arrest. J. Nutr. 149, 46–56. doi: 10.1093/jn/nxy224
Niero, E. L., Rocha-Sales, B., Lauand, C., Cortez, B. A., de Souza, M. M., Rezende-Teixeira, P., et al. (2014). The multiple facets of drug resistance: one history, different approaches. J. Exp. Clin. Cancer Res. 33:37.
Oesterle, A., Laufs, U., and Liao, J. K. (2017). Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 120, 229–243. doi: 10.1161/circresaha.116.308537
Pajak, B., Kania, E., Gajkowska, B., and Orzechowski, A. (2011). Lipid rafts mediate epigallocatechin-3-gallate- and green tea extract-dependent viability of human colon adenocarcinoma COLO 205 cells; clusterin affects lipid rafts-associated signaling pathways. J. Physiol. Pharmacol. 62, 449–459.
Patra, S. K. (2008). Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim. Biophys. Acta 1785, 182–206. doi: 10.1016/j.bbcan.2007.11.002
Peetla, C., Vijayaraghavalu, S., and Labhasetwar, V. (2013). Biophysics of cell membrane lipids in cancer drug resistance: implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 65, 1686–1698. doi: 10.1016/j.addr.2013.09.004
Penkauskas, T., Zentelyte, A., Ganpule, S., Valincius, G., and Preta, G. (2020). Pleiotropic effects of statins via interaction with the lipid bilayer: a combined approach. Biochim. Biophys. Acta Biomembr. 1862:183306. doi: 10.1016/j.bbamem.2020.183306
Pettersen, K., Monsen, V. T., Hakvag Pettersen, C. H., Overland, H. B., Pettersen, G., Samdal, H., et al. (2016). DHA-induced stress response in human colon cancer cells - Focus on oxidative stress and autophagy. Free Radic. Biol. Med. 90, 158–172. doi: 10.1016/j.freeradbiomed.2015.11.018
Pinzon-Daza, M., Garzon, R., Couraud, P., Romero, I., Weksler, B., Ghigo, D., et al. (2012). The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood-brain barrier cells. Br. J. Pharmacol. 167, 1431–1447. doi: 10.1111/j.1476-5381.2012.02103.x
Planting, A. S., Stoter, G., and Verweij, J. (1993). Phase II study of daily oral miltefosine (hexadecylphosphocholine) in advanced colorectal cancer. Eur. J. Cancer 29, 518–519. doi: 10.1016/s0959-8049(05)80142-x
Prabaharan, M. (2015). Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 72, 1313–1322. doi: 10.1016/j.ijbiomac.2014.10.052
Preta, G., Cronin, J. G., and Sheldon, I. M. (2015a). Dynasore - not just a dynamin inhibitor. Cell Commun. Signal. 13:24.
Preta, G., Lotti, V., Cronin, J. G., and Sheldon, I. M. (2015b). Protective role of the dynamin inhibitor Dynasore against the cholesterol-dependent cytolysin of Trueperella pyogenes. FASEB J. 29, 1516–1528. doi: 10.1096/fj.14-265207
Ran, S., Downes, A., and Thorpe, P. E. (2002). Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 62, 6132–6140.
Redondo-Morata, L., Lea Sanford, R., Andersen, O. S., and Scheuring, S. (2016). Effect of statins on the nanomechanical properties of supported lipid bilayers. Biophys. J. 111, 363–372. doi: 10.1016/j.bpj.2016.06.016
Riedl, S., Rinner, B., Asslaber, M., Schaider, H., Walzer, S., Novak, A., et al. (2011). In search of a novel target - phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim. Biophys. Acta 1808, 2638–2645. doi: 10.1016/j.bbamem.2011.07.026
Rios-Marco, P., Marco, C., Galvez, X., Jimenez-Lopez, J. M., and Carrasco, M. P. (2017). Alkylphospholipids: an update on molecular mechanisms and clinical relevance. Biochim. Biophys. Acta Biomembr. 1859(9 Pt B), 1657–1667. doi: 10.1016/j.bbamem.2017.02.016
Rivel, T., Ramseyer, C., and Yesylevskyy, S. (2019). The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 9:5627.
Safwat, S., Hathout, R. M., Ishak, R. A., and Mortada, N. D. (2017a). Augmented simvastatin cytotoxicity using optimized lipid nanocapsules: a potential for breast cancer treatment. J. Liposome Res. 27, 1–10. doi: 10.3109/08982104.2015.1137313
Safwat, S., Ishak, R. A., Hathout, R. M., and Mortada, N. D. (2017b). Statins anticancer targeted delivery systems: re-purposing an old molecule. J. Pharm. Pharmacol. 69, 613–624. doi: 10.1111/jphp.12707
Sahu, S. S., Sarkar, P., Shrivastava, S., and Chattopadhyay, A. (2019). Differential effects of simvastatin on membrane organization and dynamics in varying phases. Chem. Phys. Lipids 225:104831. doi: 10.1016/j.chemphyslip.2019.104831
Sandhu, J., Li, S., Fairall, L., Pfisterer, S. G., Gurnett, J. E., Xiao, X., et al. (2018). Aster proteins facilitate nonvesicular plasma membrane to ER cholesterol transport in mammalian cells. Cell 175, 514.e20–529.e20.
Santos, A. L., and Preta, G. (2018). Lipids in the cell: organisation regulates function. Cell Mol. Life Sci. 75, 1909–1927. doi: 10.1007/s00018-018-2765-4
Sariisik, E., Kocak, M., Kucuk Baloglu, F., and Severcan, F. (2019). Interaction of the cholesterol reducing agent simvastatin with zwitterionic DPPC and charged DPPG phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1861, 810–818. doi: 10.1016/j.bbamem.2019.01.014
Selzner, M., Bielawska, A., Morse, M. A., Rudiger, H. A., Sindram, D., Hannun, Y. A., et al. (2001). Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 61, 1233–1240.
Senchenkov, A., Litvak, D. A., and Cabot, M. C. (2001). Targeting ceramide metabolism–a strategy for overcoming drug resistance. J. Natl. Cancer Inst. 93, 347–357. doi: 10.1093/jnci/93.5.347
Sezgin, E., Levental, I., Mayor, S., and Eggeling, C. (2017). The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374. doi: 10.1038/nrm.2017.16
Shaikh, S. R., Rockett, B. D., Salameh, M., and Carraway, K. (2009). Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 139, 1632–1639. doi: 10.3945/jn.109.108720
Silva, L., Coutinho, A., Fedorov, A., and Prieto, M. (2006). Competitive binding of cholesterol and ergosterol to the polyene antibiotic nystatin. A fluorescence study. Biophys. J. 90, 3625–3631. doi: 10.1529/biophysj.105.075408
Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39.
Simons, K., and Vaz, W. L. (2004). Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33, 269–295. doi: 10.1146/annurev.biophys.32.110601.141803
Simons, M., Keller, P., De Strooper, B., Beyreuther, K., Dotti, C. G., and Simons, K. (1998). Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 95, 6460–6464. doi: 10.1073/pnas.95.11.6460
Skender, B., Hofmanova, J., Slavik, J., Jelinkova, I., Machala, M., Moyer, M. P., et al. (2014). DHA-mediated enhancement of TRAIL-induced apoptosis in colon cancer cells is associated with engagement of mitochondria and specific alterations in sphingolipid metabolism. Biochim. Biophys. Acta 1841, 1308–1317. doi: 10.1016/j.bbalip.2014.06.005
Stancu, C., and Sima, A. (2001). Statins: mechanism of action and effects. J. Cell Mol. Med. 5, 378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x
Stover, T., and Kester, M. (2003). Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J. Pharmacol. Exp. Ther. 307, 468–475. doi: 10.1124/jpet.103.054056
Stover, T. C., Sharma, A., Robertson, G. P., and Kester, M. (2005). Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin. Cancer Res. 11, 3465–3474. doi: 10.1158/1078-0432.ccr-04-1770
Surh, Y. J. (2003). Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 3, 768–780. doi: 10.1038/nrc1189
Tan, L. T., Chan, K. G., Pusparajah, P., Lee, W. L., Chuah, L. H., Khan, T. M., et al. (2017). Targeting membrane lipid a potential cancer cure? Front. Pharmacol. 8:12. doi: 10.3389/fphar.2017.00012
Tsukamoto, S., Hirotsu, K., Kumazoe, M., Goto, Y., Sugihara, K., Suda, T., et al. (2012). Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cdelta and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 443, 525–534. doi: 10.1042/bj20111837
Turk, H. F., and Chapkin, R. S. (2013). Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 88, 43–47. doi: 10.1016/j.plefa.2012.03.008
Wang, C. K., Wacklin, H. P., and Craik, D. J. (2012). Cyclotides insert into lipid bilayers to form membrane pores and destabilize the membrane through hydrophobic and phosphoethanolamine-specific interactions. J. Biol. Chem. 287, 43884–43898. doi: 10.1074/jbc.m112.421198
Wang, C. Y., Liu, P. Y., and Liao, J. K. (2008). Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol. Med. 14, 37–44. doi: 10.1016/j.molmed.2007.11.004
Wang, H., Ma, Q., Qi, Y., Dong, J., Du, X., Rae, J., et al. (2019). ORP2 delivers cholesterol to the plasma membrane in exchange for Phosphatidylinositol 4, 5-Bisphosphate (PI(4,5)P2). Mol. Cell 73, 458.e7–473.e7.
Wassall, S. R., Leng, X., Canner, S. W., Pennington, E. R., Kinnun, J. J., Cavazos, A. T., et al. (2018). Docosahexaenoic acid regulates the formation of lipid rafts: a unified view from experiment and simulation. Biochim. Biophys. Acta Biomembr. 1860, 1985–1993. doi: 10.1016/j.bbamem.2018.04.016
Yang, C. S., Wang, X., Lu, G., and Picinich, S. C. (2009). Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 9, 429–439. doi: 10.1038/nrc2641
Yang, X., Sheng, W., Sun, G. Y., and Lee, J. C. (2011). Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 58, 321–329. doi: 10.1016/j.neuint.2010.12.004
Yaqoob, P., and Shaikh, S. R. (2010). The nutritional and clinical significance of lipid rafts. Curr. Opin. Clin. Nutr. Metab. Care 13, 156–166. doi: 10.1097/mco.0b013e328335725b
Zalba, S., and Ten Hagen, T. L. (2017). Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 52, 48–57. doi: 10.1016/j.ctrv.2016.10.008
Keywords: lipid rafts, membrane fluidity, cell signaling, amphiphilic molecules, protein receptors
Citation: Preta G (2020) New Insights Into Targeting Membrane Lipids for Cancer Therapy. Front. Cell Dev. Biol. 8:571237. doi: 10.3389/fcell.2020.571237
Received: 10 June 2020; Accepted: 13 August 2020;
Published: 02 September 2020.
Edited by:
Congbao Kang, Experimental Drug Development Centre (EDDC), SingaporeReviewed by:
Yasunori Saheki, Nanyang Technological University, SingaporeCopyright © 2020 Preta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulio Preta, Z2l1bGlvLnByZXRhQGJjaGkudnUubHQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.