
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol., 02 July 2020
Sec. Stem Cell Research
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.00548
This article is part of the Research TopicContext-Dependent Regulation of Neurogenesis: Common Themes and Unique Features of the Neurogenic Process in Different Model SystemsView all 21 articles
In the mammalian adult hippocampus, new neurons are continuously generated throughout life in the subgranular zone of the dentate gyrus. Increasing evidence point out the contribution of adult-born hippocampal granule cells (GCs) to cognitive processes such as learning and memory, indicating the relevance of understanding the molecular mechanisms that control the development of these new neurons in the preexisting hippocampal circuits. Cell proliferation and functional integration of adult-born GCs is a process highly regulated by different intrinsic and extrinsic factors. In this review, we discuss recent advances related with cellular components and extrinsic signals of the hippocampal neurogenic niche that support and modulate neurogenesis under physiological conditions.
Several studies provide evidences indicating that hippocampal neurogenesis is needed for the integration of new information into pre-existing context promoting flexible learning and adaptive behaviors. Physiological experiences such as learning, physical exercise and exposition to enrich environment (EEs) have been associated with an increase in survival, proliferation and differentiation of adult-born hippocampal cells. Moreover, others pathophysiological conditions, such as aging, stress, and degenerative disorders (like Alzheimer disease, AD) have been described to impair and decrease adult neurogenesis (Goncalves et al., 2016; Toda et al., 2019). These effects are modulated through different signaling molecules produced in the adult hippocampal neurogenic niche.
Understanding the signals derived from this specific microenvironment results essential to enhance the process of neuronal integration in the aged and diseased brain. In this minireview, we focus our attention in the complexity of the adult hippocampal neurogenic niche, which provides multiple signals that are integrated by the neural stem cells (NSCs) and the newborn neurons to respond adequately in different circumstances.
Adult hippocampal neurogenesis has been confirmed in the majority of mammals, but whether it is present in humans has been the issue of an intense recent debate (Boldrini et al., 2018; Sorrells et al., 2018). Methodological factors seem to contribute to the discrepancies between studies that describe the presence or absence of neurogenesis in the human adult dentate gyrus (DG). Future research using different approaches will be needed to understand how adult-born granule cells (GCs) are generated. Recent studies describe that human hippocampal neurogenesis persists through the ninth decade of life and is associated with cognitive status in patients with AD, providing evidence of the potential relevance of this process for many human disorders (Moreno-Jimenez et al., 2019; Tobin et al., 2019).
The general pattern of hippocampal neurogenesis is conserved across different mammalian species. Hippocampal NSCs give rise to GCs throughout a highly regulated process, which involves the exit of the quiescence state, posterior divisions, specification to a neuronal fate, neuronal differentiation, and the physiological integration in the preexisting hippocampal circuits. Along this period morphological, intrinsic electrical properties and synaptic connections evolve in parallel toward a mature neuronal phenotype. All the process is tightly controlled by physiological stimuli, that modify the hippocampal niche (Toni and Schinder, 2015; Toda et al., 2019).
The adult hippocampal neurogenic niche is a specialized and dynamic microenvironment, which involves both cellular and non-cellular components of the DG. Altogether, cells and the signals produced by them can regulate the neurogenic process acting at different levels from proliferation to functional integration (Figure 1).
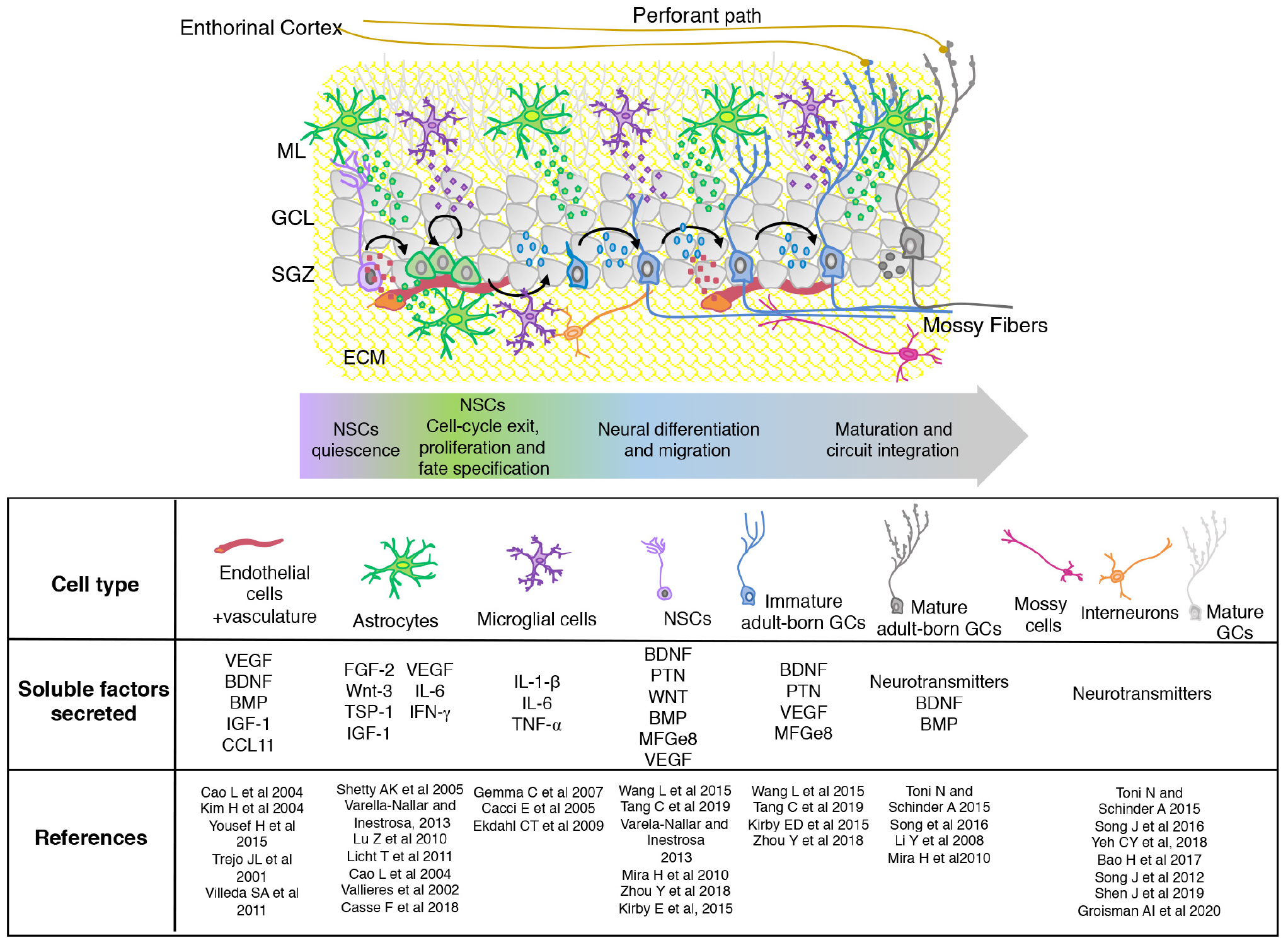
Figure 1. Scheme showing the organization and composition of the adult hippocampal neurogenic niche. The different stages of adult born GCs maturation are shown with neuronal and non-neuronal (astrocytes, microglia, and vascular cells) components. The extracellular matrix (ECM) is indicated in yellow. Soluble diffusible signaling molecules produced by the different cellular components of the SGZ niche are mentioned in the table. SGZ, subgranular zone; GCL, granular cell layer; ML, molecular layer.
Astrocytes represent one of the main modulators of the neurogenic niche (Song et al., 2002). They control cell proliferation, migration, differentiation and synaptic integration of newborn GCs through membrane-associated molecules and by secreting soluble signals like fibroblast growth factor-2 (FGF-2), WNT (Wingless) ligands, thrombospondin-1 (TSP-1), cytokines, and extracellular matrix (ECM) proteins among others (Trejo et al., 2001; Shetty et al., 2005; Lu and Kipnis, 2010; Casse et al., 2018). They also control the availability of neurotransmitters in the synaptic cleft. The relevance of astrocytes in the maturation of adult-born GCs was evidenced using transgenic approaches to block vesicular release. This strategy resulted in both reduced glutamatergic synaptic input and dendritic spine density that was accompanied by a reduction in cell survival and functional integration of adult-born, but not of mature DG neurons (Sultan et al., 2015). Astrocytes can affect positively or negatively neurogenesis, depending on their metabolic state. While in normal physiological conditions astrocytes produce molecules that positively regulate this process, in pathological situations, they suffer modifications in their transcriptome and secretome that may contribute to impairment of neurogenesis and cognitive deficits. Thus, cytokines such as IL-6, TNF-α, and IFN-γ are produced by astrocytes in inflammatory processes (Vallieres et al., 2002; Liddelow and Barres, 2017; Casse et al., 2018).
Several studies have shown the relevance of microglia in adult hippocampal neurogenesis. They are involved in phagocytosis of apoptotic adult-born GCs (Sierra et al., 2010). Therefore, ablation of microglia in the adult DG results in decreased number of neuroblasts (Kreisel et al., 2019). Interestingly, a recent report has described that phagocytic microglia act as a sensor of local cell death and modulate the balance between cell proliferation and cell survival in the neurogenic niche (Diaz-Aparicio et al., 2020). Microglial cells regulate neurogenesis through both cell-cell interaction mechanisms and secreted factors. Thus, animals lacking CX3CR1 microglial receptor, involved in microglial-neuronal interaction, resulted in impaired morphology and deficient synaptic integration of adult-born GCs in the DG (Bolos et al., 2018). Microglial activation by pro-inflammatory molecules results in defects in different steps of adult neurogenesis. Cytokines secreted by microglia in the context of inflammation include: IL-6, IL-1β, and tumor necrosis factor-α (TNF-α; Cacci et al., 2005; Gemma et al., 2007; Ekdahl et al., 2009).
A growing body of data indicates that blood vessels are essential components of hippocampal NSC niches. Vascular cells can impact neurogenesis directly by producing neurogenic factors or indirectly, transporting neurogenic substances produced by other cells. Many studies indicate that endothelial cells secrete different trophic factors such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and chemokines such as CCL11, which affect NSCs proliferation and maturation of these cells (Cao et al., 2004; Kim et al., 2004; Licht et al., 2011; Villeda et al., 2011; Licht and Keshet, 2015). A recent study indicates that endothelial cells, through the expression of the monocarboxylic acid transporter 1 (MCT1), contribute to the maintenance of lactate homeostasis promoting neurogenesis and cognitive functions (Wang et al., 2019). Another important source of neurogenic signals comes from the brain vasculature which provide signaling molecules secreted by local or distal sources. These include trophic factors, hormones, lipids and exosomes (Batiz et al., 2015; Licht and Keshet, 2015).
Increasing evidence shows an important role for NSCs as regulators of their own niche, influencing the development of their progeny at different neurogenic stages. VEGF, neurotrophin-3 (NT3), Pleiotrophin (PTN), and BDNF are some of the factors released by the NSCs (Vicidomini et al., 2020).
Neuronal activity regulates multiple stages of adult neurogenesis from proliferation, survival, neuronal maturation, and synaptic integration. Local interneurons, hilar inhibitory neurons, mossy glutamatergic neurons and mature GCs from the DG control different stages of newborn GCs integration. Extensive literature has demonstrated an essential role of neurotransmitters locally released by DG neurons or by axons arising from projecting neurons in the modulation of adult-born GCs development (Song et al., 2012, 2016; Toni and Schinder, 2015; Bao et al., 2017; Yeh et al., 2018; Groisman et al., 2020). This topic will not be discussed in the present revision.
The cellular components of the subgranular zone (SGZ) provide a complex regulatory architecture that allow the correct development of the adult-born GCs, promoting their correct integration in the preexisting hippocampal circuits. Neural activity triggered by physiological experiences is essential to govern the interaction between the different cellular components that control the neurogenic process by secreting specific signals. An interesting example of the signal integration in the hippocampal neurogenic niche was evidenced in a recent study which shows that hippocampus-associated behaviors increase microvascular blood-flow velocity in the DG and enhance hippocampal neurogenesis. The authors proved that this effect is mediated by parvalbumin-expressing neurons which increase blood flow via nitric-oxide signaling. This increase in the microvascular hemodynamics enhances IGF-1 signaling promoting the newborn cell survival (Shen et al., 2019).
The different cellular components of the neurogenic niche can modulate neurogenesis by multiple signaling mechanisms (Figure 2). Here we describe different types of signals produced by the SGZ niche focusing in the new advances and novel factors that has been described during the last years.
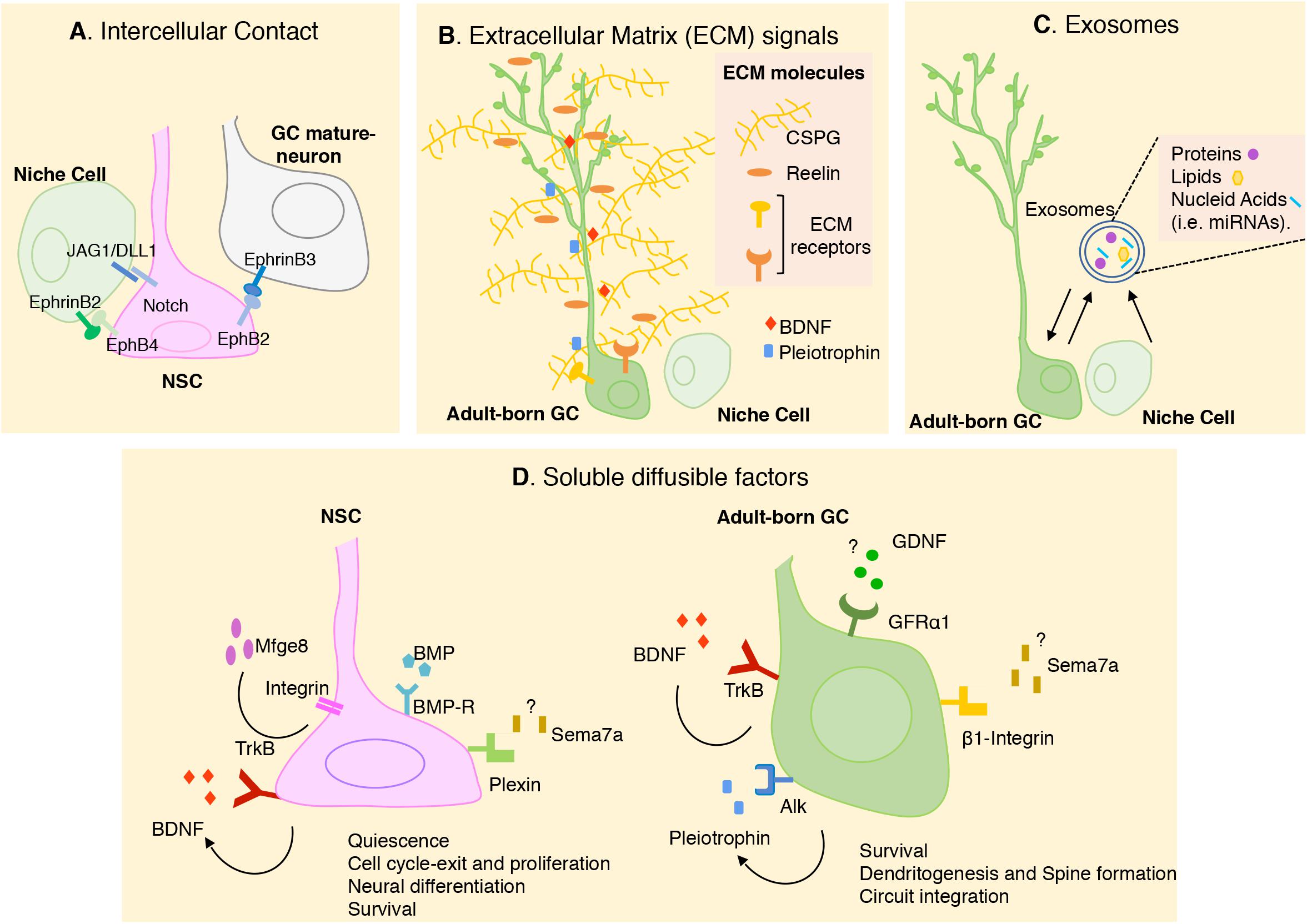
Figure 2. Schematic representation of the different cellular and molecular mechanisms that modulate adult neurogenesis in SGZ. In the figure, we summarize the novel signals most recently described. (A) Intercellular contacts including Notch/JAG1/DLL1 and Eph/ephrines between NSCs and adjacent cells. (B) Extracellular matrix (ECM) molecules contributes to the preservation of stem cells pool and the morphological differentiation of adult born-GCs. ECM can also modulate the availability of soluble factors present in the SGZ niche, like Pleiotrophin (PTN) and BDNF. (C) Exosomes has recently been proposed to have a key role in cell-cell communication in SGZ niche. (D) Soluble diffusible factors have been described to have multiple roles in regulating adult hippocampal neurogenesis. Some external signals, their receptors and their biological action were indicating. Question mark indicates that the source of the ligand is still unknown. Arrow indicates autocrine signaling. More studies are needed to understand the interaction between these signals. GC, granular cell; NSC, neural stem cell; CSPG, chondroitin sulfate proteoglycan.
Direct cell–cell interaction is critical in stem cell maintenance. A known membrane molecule, Notch, and its ligands can mediate direct interaction between NSCs and neighboring cells, and thus play an important role in neurogenesis. Ablation of Notch in hippocampal NSCs during adulthood promotes cell cycle exit and neuronal fate determination (Breunig et al., 2007; Ables et al., 2011). The importance of Notch signaling in the maintenance of NSC quiescence in SGZ has been also demonstrated by ablation of the Notch ligands DELTA1 (DLL1) and JAGGED1 (JAG1) in DG stem cells (Ehm et al., 2010; Imayoshi et al., 2010; Kawaguchi et al., 2013; Lavado and Oliver, 2014). Notch ligands are also expressed by astrocytes from the adult DG and reduction in the levels of JAG1 results in a reduction in Notch signaling and increase in neuronal differentiation (Wilhelmsson et al., 2012).
Eprhrin/Eph signaling has also been involved as important players regulating stem cell behavior. Initial studies showed that Ephrin-B2 presented by astrocytes interacts with EphB4 receptors on NSCs, promoting neuronal differentiation (Ashton et al., 2012). A recent study indicates that the intercellular signaling between mature GCs and NSCs regulates the transition of quiescent NSCs to newborn neurons. During running, membrane-bound ligand, Ephrin-B3 on mature GCs acts as a negative regulator for activation of adjacent NSCs expressing EphB2 receptor (Dong et al., 2019).
All cell types in the SGZ niche are in contact with the ECM, a complex and dynamic network of macromolecules with different physical and biochemical properties. The ECM acts providing a physical supportive structure and also molecular signals to regulate NSC development. The contribution of the ECM molecules to the modulation of hippocampal neurogenesis is complex, as they can act by interacting directly with cellular receptors or indirectly as modulators of the availability of soluble factors present in the neurogenic niche (Figure 2B). Among ECM molecules that have been involved in hippocampal neurogenesis is the extracellular glycoprotein Reelin, which promotes NSC proliferation and also dendritic maturation (Won et al., 2006; Teixeira et al., 2012). During the last years proteoglycans have emerged as important cues for the proliferation and differentiation of new neurons in the SGZ. Thus, pharmacological depletion of chondroitin sulfate proteoglycan (CSPG) in the DG reduces the densities of newborn GCs. The dendritic arborization of these neurons was also reduced by CSPG digestion, and behavioral analysis of these animals revealed cognitive memory impairments. Interestingly, the ability of EE to promote GC production and improve cognitive behaviors was impaired in mice that lacked a key enzyme for CSPG synthesis indicating that the extracellular CSPGs participate in the pro-neurogenic effects of the EE (Yamada et al., 2018). Another major constituent of the forebrain ECM is the glycosaminoglycan hyaluronan (Hyaluronic acid, HA), which is present in the SGZ. Mice lacking the HA transmembrane receptor, CD44, which is expressed by NSC, show an increase in stem cell proliferation, suggesting a role of this molecule in NSC quiescence. The fact that HA is synthesized by NSC and increases in the SGZ with aging suggest that HA accumulation may contribute to the reduced neurogenesis observed in aged animals (Su et al., 2017).
The different cells that constitute the DG neurogenic niche regulate stem cell activity by secreting diffusible signaling molecules, which represent the majority of extracellular cues that regulate neurogenesis (Figure 2D). Among them, the role of bone morphogenetic proteins (BMPs) and WNT signaling has been well established. Thus, WNT signaling produced by NSCs and astrocytes in the SGZ can regulate different stages of adult neurogenesis. It is well-known that WNT signaling promotes proliferation and NSC self-renewal, while, endogenous WNT signaling inhibitors, such as sFRP3 and Dkk1, promote stem cell quiescence and controls the timing of newborn granule neuron maturation (Lie et al., 2005; Bowman et al., 2013; Jang et al., 2013; Seib et al., 2013; Varela-Nallar and Inestrosa, 2013). Different members of the WNT family have also been associated to the promotion of dendrite development of adult born GCs (Arredondo et al., 2020). Regarding to the BMPs, they have emerged as critical inducers of NSC quiescence and long-term maintenance in SGZ (Gobeske et al., 2009; Mira et al., 2010; Yousef et al., 2015). The soluble factor Sonic Hedgehog (Shh), which is critical at early stages of embryonic brain development, has also been involved in adult hippocampal neurogenesis promoting the proliferation SGZ NSCs before they become quiescent (Han et al., 2008; Noguchi et al., 2019).
Trophic factors, such as IGF-1 and VEGF are relevant players involved in adult neurogenesis at different developmental stages that have previously been deeply analyzed (Cheng et al., 2001; Lichtenwalner et al., 2001; Fournier and Duman, 2012; Kirby et al., 2015; Nieto-Estevez et al., 2016; Mir et al., 2017).
During the last years new soluble molecules known for other functions, have emerged as modulators of the neurogenic process. Thus, the Globule-epidermal growth factor (EGF) 8 (MFGe8), a molecule involved in the phagocytosis of apoptotic cells, was found to be expressed by quiescent NSCs and astrocytes in the SGZ. Recently, it was shown that adult specific deletion of MFGe8 in NSCs promotes the increase in NSC proliferation and depletion of the neurogenic pool causing a decreased neurogenesis at later developmental stages (Zhou et al., 2018). Another soluble protein, Semaphorin7a (Sema7a), which has been previously described as a guidance molecule, has emerged as a novel key factor in the control of adult hippocampal neurogenesis. Interestingly, Sema7a regulates different stages of adult neurogenesis via two, stage-specific different receptors. Thus, Sema7a inhibits progenitor proliferation by acting though Plexin, in early neural progenitors and subsequently, during differentiation, Sema7a promotes dendrite maturation and spine development acting through β1-integrin receptors (Jongbloets et al., 2017).
The role of the neurotrophins in hippocampal adult neurogenesis is well documented. Particularly, BDNF is expressed in SGZ by NSCs, mature DG granule neurons and also by non-neuronal cells, while its receptor, TrkB, is broadly expressed by NSCs at different developmental stages (Vilar and Mira, 2016). Brain-derived neurotrophic factor acting through TrkB has been associated to survival, proliferation and maturation of adult-born GCs (Scharfman et al., 2005; Li et al., 2008; Taliaz et al., 2010). Dendrite development, spine growth and synapse formation were markedly impaired in adult-born GCs from TrkB-deficient mice in which the receptor was conditionally deleted in NSC and in animals in which BDNF was ablated in the entire forebrain (Bergami et al., 2008). Interestingly, conditional deletion of BDNF in NSCs resulted in a similar impairment in dendrite growth indicating that the effect of BDNF on dendrite maturation is mainly autocrine. In support of an autocrine role of BDNF, its deletion in NSC abolished the promotion of dendritic growth induced by running (Wang et al., 2015).
Other member of the neurotrophin family, NT-3 is highly expressed in the adult DG. Conditional ablation of NT-3 in the brain throughout development shows normal proliferation in the SGZ, a reduction in the number of newly generated granule neurons and an increase in the proportion of cells that do not express differentiation markers, indicating a role of NT3 in maturation of neural progenitor cells (Shimazu et al., 2006).
A more recent work has demonstrated that the protein PTN secreted by hippocampal NSCs from the SGZ niche is important for the correct development and integration of the new neurons in the DG. Ablation of PTN leads to defects in neuronal integration and synaptic activity of the newborn neurons in the hippocampus without affecting the production or survival of them. This effect is mediated by one of the PTN receptors, ALK, which is expressed by NSCs. Interestingly, this study showed that the expression of PTN is reduced with aging but that the administration of PTN is able to ameliorate the age-induced defects of hippocampal neurogenesis (Tang et al., 2019).
Recently, glial-derived neurotrophic factor (GDNF), a neurotrophic factor initially described for its potent effect on the survival of dopaminergic nigrostriatal neurons was described as a novel regulator of newborn GCs integration (Paratcha and Ledda, 2008; Bonafina et al., 2019). The receptor of GDNF, the GPI-linked protein GFRα1, is expressed by immature and mature adult-born GCs. Conditional ablation of GFRα1 in NSCs indicated that GDNF/GFRα1 complex is required for proper maturation and integration of adult-born GCs into preexisting hippocampal circuits. Conditional knockout mice for GFRα1 showed impairment in behavioral pattern separation, which has been associated to deficits in adult neurogenesis. This study shows that voluntary physical exercise promotes GDNF expression in the DG and dendritic development. However, the deletion of GFRα1 in the newborn GCs abolishes the increase in dendrite complexity induced by running, revealing that the effect of running on dendrite development depends partially on GDNF expression (Bonafina et al., 2019).
As growth factors involved in hippocampal neurogenesis acts through different receptors triggering specific downstream signaling pathways, the remaining question is how newborn neurons integrate this information. One possibility is that the same cell expresses all the receptors but need to integrate the different signals in order to respond appropriately. A second possibility is the existence of subpopulations of adult-born GCs each of which respond to different growth factors expressing specific receptor repertoires. Moreover, the presence and the abundance of receptors and the downstream signaling partners can be modified during the maturation process. Thus, the expression of different arrays of trophic factor receptors in the adult-born GCs deserve further analysis.
These small membrane extracellular vesicles have emerged as one of the major mediators of intercellular communication (Figure 2C). Diverse array of proteins, lipids, mRNAs and miRNAs have been identified in exosomes from different cell types found in the SGZ niche. Although the role of exosomes in the adult neurogenic niches is still unclear, growing indirect evidence suggest that exosomes might play a critical role in cell-cell communication in neurogenic niches (Batiz et al., 2015). Some of the molecules expressed in the neurogenic environments have been reported to be present in exosomes. Recently, a study has shown that injection of purified exosomes derived from neural cultures in postnatal mouse brains increases SGZ neurogenesis indicating that exosomes contain molecular cargo that regulates this process (Sharma et al., 2019).
Over the last years, lipids have gained attention in the regulation of adult neurogenesis (Knobloch, 2017). Lipids can be taken up from circulation or synthetized de novo by NSCs. Cholesterol-carrying lipoproteins receptor, LDL-r, has been associated to adult hippocampal neurogenesis. Ablation of LDL-r in mice results in a reduction of the proliferation of NSCs and also a decline in the number of newborn GCs. These results were confirmed by in vitro experiments in which NPCs exposed to high concentration of plasma LDL results in a decreased proliferation and reduced differentiation toward a neuronal lineage (Engel et al., 2019). Although several studies indicate the relevance of lipids in neurogenesis, how lipids affect this process needs to be addressed in more detail.
The large literature about the different cells and the nature of signals which modulate adult hippocampal neurogenesis indicates that extrinsic control of this process is much more complex than previously envisioned. The distribution of different factors in the neurogenic niche, the precise signaling pathways that they trigger, the interaction with other intrinsic and extrinsic signals and their function in pathological process deserves further investigation.
The great diversity of signals present in the niche should be appropriately integrated by the adult-born GCs to promote the proper maturation and integration of them into preexisting circuits. The different factors derived from the microenvironment induce specific transcriptional programs that drive the maturation of the new cells and determine the morphological and physiological properties of GCs at the different stages during neuronal development and their response to external stimuli.
In parallel to the great diversity of signals that have been described as modulators of the hippocampal neurogenesis, different studies pointed out to the heterogeneity of NSCs. This idea indicates that not all NSCs or immature GCs respond similarly to the different extracellular signals that are present in the niche (Shin et al., 2015). Cellular heterogeneity in these neurons may result in some populations being more responsive to the variety of factors present in the niche and also being more susceptible to different pathologies.
The identification of the array of factors present in the SGZ niche during neurogenesis represents a crucial knowledge because it opens the possibility to combine them in order to improve the development of adult-born neurons in physiopathological conditions. In this context a recent study reported that mimicking the beneficial effects of exercise by pharmacological induction of neurogenesis, combined with elevation of BDNF levels in the DG revert the negative effects of Alzheimer’s disease on newborn hippocampal neurons in a mouse model of the disease (Choi et al., 2018).
Thus, understanding the complexity of the SGZ neurogenic niche becomes essential for the development of novel therapeutic strategies for the treatment of cognitive impairments associated with aging and brain disorders in which adult hippocampal neurogenesis is affected.
All authors contributed equally to this mini-review.
This work is funded by grants from the Argentine Agency for Promotion of Science and Technology (Fondo para la Investigación Científica y Tecnológica; ANPCyT) PICT-2015-3814, PICT-2017-4597, and PICT-2017-4513. AB was supported by a posdoctoral fellowship from ANPCyT. GP and FL are supported by an independent research career position from the Argentine Medical Research Council (CONICET), Argentina.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ables, J. L., Breunig, J. J., Eisch, A. J., and Rakic, P. (2011). Not(ch) just development: notch signalling in the adult brain. Nat. Rev. Neurosci. 12, 269–283. doi: 10.1038/nrn3024
Arredondo, S. B., Guerrero, F. G., Herrera-Soto, A., Jensen-Flores, J., Bustamante, D. B., Onate-Ponce, A., et al. (2020). Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem Cells 38, 422–436. doi: 10.1002/stem.3121
Ashton, R. S., Conway, A., Pangarkar, C., Bergen, J., Lim, K. I., Shah, P., et al. (2012). Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 15, 1399–1406. doi: 10.1038/nn.3212
Bao, H., Asrican, B., Li, W., Gu, B., Wen, Z., Lim, S. A., et al. (2017). Long-range GABAergic inputs regulate neural stem cell quiescence and control adult hippocampal neurogenesis. Cell Stem Cell 21, 604–617.e5. doi: 10.1016/j.stem.2017.10.003
Batiz, L. F., Castro, M. A., Burgos, P. V., Velasquez, Z. D., Munoz, R. I., Lafourcade, C. A., et al. (2015). Exosomes as novel regulators of adult neurogenic niches. Front. Cell. Neurosci. 9:501. doi: 10.3389/fncel.2015.00501
Bergami, M., Rimondini, R., Santi, S., Blum, R., Gotz, M., and Canossa, M. (2008). Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. U.S.A. 105, 15570–15575. doi: 10.1073/pnas.0803702105
Boldrini, M., Fulmore, C. A., Tartt, A. N., Simeon, L. R., Pavlova, I., Poposka, V., et al. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589–599.e5. doi: 10.1016/j.stem.2018.03.015
Bolos, M., Perea, J. R., Terreros-Roncal, J., Pallas-Bazarra, N., Jurado-Arjona, J., Avila, J., et al. (2018). Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain Behav. Immun. 68, 76–89. doi: 10.1016/j.bbi.2017.10.002
Bonafina, A., Trinchero, M. F., Rios, A. S., Bekinschtein, P., Schinder, A. F., Paratcha, G., et al. (2019). GDNF and GFRalpha1 are required for proper integration of adult-born hippocampal neurons. Cell Rep. 29, 4308–4319.e4. doi: 10.1016/j.celrep.2019.11.100
Bowman, A. N., van Amerongen, R., Palmer, T. D., and Nusse, R. (2013). Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/beta-catenin-responsive neural stem cells. Proc. Natl. Acad. Sci. U.S.A. 110, 7324–7329. doi: 10.1073/pnas.1305411110
Breunig, J. J., Silbereis, J., Vaccarino, F. M., Sestan, N., and Rakic, P. (2007). Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 104, 20558–20563. doi: 10.1073/pnas.0710156104
Cacci, E., Claasen, J. H., and Kokaia, Z. (2005). Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J. Neurosci. Res. 80, 789–797. doi: 10.1002/jnr.20531
Cao, L., Jiao, X., Zuzga, D. S., Liu, Y., Fong, D. M., Young, D., et al. (2004). VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 36, 827–835. doi: 10.1038/ng1395
Casse, F., Richetin, K., and Toni, N. (2018). Astrocytes’ contribution to adult neurogenesis in physiology and Alzheimer’s disease. Front. Cell. Neurosci. 12:432. doi: 10.3389/fncel.2018.00432
Cheng, C. M., Cohen, M., Tseng, V., and Bondy, C. A. (2001). Endogenous IGF1 enhances cell survival in the postnatal dentate gyrus. J. Neurosci. Res. 64, 341–347. doi: 10.1002/jnr.1084
Choi, S. H., Bylykbashi, E., Chatila, Z. K., Lee, S. W., Pulli, B., Clemenson, G. D., et al. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361:eaan8821. doi: 10.1126/science.aan8821
Diaz-Aparicio, I., Paris, I., Sierra-Torre, V., Plaza-Zabala, A., Rodriguez-Iglesias, N., Marquez-Ropero, M., et al. (2020). Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J. Neurosci. 40, 1453–1482. doi: 10.1523/JNEUROSCI.0993-19.2019
Dong, J., Pan, Y. B., Wu, X. R., He, L. N., Liu, X. D., Feng, D. F., et al. (2019). A neuronal molecular switch through cell-cell contact that regulates quiescent neural stem cells. Sci. Adv. 5:eaav4416. doi: 10.1126/sciadv.aav4416
Ehm, O., Goritz, C., Covic, M., Schaffner, I., Schwarz, T. J., Karaca, E., et al. (2010). RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J. Neurosci. 30, 13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010
Ekdahl, C. T., Kokaia, Z., and Lindvall, O. (2009). Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158, 1021–1029. doi: 10.1016/j.neuroscience.2008.06.052
Engel, D. F., Grzyb, A. N., de Oliveira, J., Potzsch, A., Walker, T. L., Brocardo, P. S., et al. (2019). Impaired adult hippocampal neurogenesis in a mouse model of familial hypercholesterolemia: a role for the LDL receptor and cholesterol metabolism in adult neural precursor cells. Mol. Metab. 30, 1–15. doi: 10.1016/j.molmet.2019.09.002
Fournier, N. M., and Duman, R. S. (2012). Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression. Behav. Brain Res. 227, 440–449. doi: 10.1016/j.bbr.2011.04.022
Gemma, C., Bachstetter, A. D., Cole, M. J., Fister, M., Hudson, C., and Bickford, P. C. (2007). Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur. J. Neurosci. 26, 2795–2803. doi: 10.1111/j.1460-9568.2007.05875.x
Gobeske, K. T., Das, S., Bonaguidi, M. A., Weiss, C., Radulovic, J., Disterhoft, J. F., et al. (2009). BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One 4:e7506. doi: 10.1371/journal.pone.0007506
Goncalves, J. T., Schafer, S. T., and Gage, F. H. (2016). Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167, 897–914. doi: 10.1016/j.cell.2016.10.021
Groisman, A. I., Yang, S. M., and Schinder, A. F. (2020). Differential coupling of adult-born granule cells to parvalbumin and somatostatin interneurons. Cell Rep. 30, 202–214.e4. doi: 10.1016/j.celrep.2019.12.005
Han, Y. G., Spassky, N., Romaguera-Ros, M., Garcia-Verdugo, J. M., Aguilar, A., Schneider-Maunoury, S., et al. (2008). Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 11, 277–284. doi: 10.1038/nn2059
Imayoshi, I., Sakamoto, M., Yamaguchi, M., Mori, K., and Kageyama, R. (2010). Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 30, 3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010
Jang, M. H., Bonaguidi, M. A., Kitabatake, Y., Sun, J., Song, J., Kang, E., et al. (2013). Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12, 215–223. doi: 10.1016/j.stem.2012.11.021
Jongbloets, B. C., Lemstra, S., Schellino, R., Broekhoven, M. H., Parkash, J., Hellemons, A. J., et al. (2017). Stage-specific functions of Semaphorin7A during adult hippocampal neurogenesis rely on distinct receptors. Nat. Commun. 8:14666. doi: 10.1038/ncomms14666
Kawaguchi, D., Furutachi, S., Kawai, H., Hozumi, K., and Gotoh, Y. (2013). Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat. Commun. 4:1880. doi: 10.1038/ncomms2895
Kim, H., Li, Q., Hempstead, B. L., and Madri, J. A. (2004). Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J. Biol. Chem. 279, 33538–33546. doi: 10.1074/jbc.M404115200
Kirby, E. D., Kuwahara, A. A., Messer, R. L., and Wyss-Coray, T. (2015). Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc. Natl. Acad. Sci. U.S.A. 112, 4128–4133. doi: 10.1073/pnas.1422448112
Knobloch, M. (2017). The role of lipid metabolism for neural stem cell regulation. Brain Plast. 3, 61–71. doi: 10.3233/BPL-160035
Kreisel, T., Wolf, B., Keshet, E., and Licht, T. (2019). Unique role for dentate gyrus microglia in neuroblast survival and in VEGF-induced activation. Glia 67, 594–618. doi: 10.1002/glia.23505
Lavado, A., and Oliver, G. (2014). Jagged1 is necessary for postnatal and adult neurogenesis in the dentate gyrus. Dev. Biol. 388, 11–21. doi: 10.1016/j.ydbio.2014.02.004
Li, Y., Luikart, B. W., Birnbaum, S., Chen, J., Kwon, C. H., Kernie, S. G., et al. (2008). TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412. doi: 10.1016/j.neuron.2008.06.023
Licht, T., Goshen, I., Avital, A., Kreisel, T., Zubedat, S., Eavri, R., et al. (2011). Reversible modulations of neuronal plasticity by VEGF. Proc. Natl. Acad. Sci. U.S.A. 108, 5081–5086. doi: 10.1073/pnas.1007640108
Licht, T., and Keshet, E. (2015). The vascular niche in adult neurogenesis. Mech. Dev. 138(Pt 1), 56–62. doi: 10.1016/j.mod.2015.06.001
Lichtenwalner, R. J., Forbes, M. E., Bennett, S. A., Lynch, C. D., Sonntag, W. E., and Riddle, D. R. (2001). Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience 107, 603–613. doi: 10.1016/s0306-4522(01)00378-5
Liddelow, S. A., and Barres, B. A. (2017). Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967. doi: 10.1016/j.immuni.2017.06.006
Lie, D. C., Colamarino, S. A., Song, H. J., Desire, L., Mira, H., Consiglio, A., et al. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375. doi: 10.1038/nature04108
Lu, Z., and Kipnis, J. (2010). Thrombospondin 1–a key astrocyte-derived neurogenic factor. FASEB J. 24, 1925–1934. doi: 10.1096/fj.09-150573
Mir, S., Cai, W., Carlson, S. W., Saatman, K. E., and Andres, D. A. (2017). IGF-1 mediated neurogenesis involves a novel RIT1/Akt/Sox2 cascade. Sci. Rep. 7:3283.
Mira, H., Andreu, Z., Suh, H., Lie, D. C., Jessberger, S., Consiglio, A., et al. (2010). Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78–89. doi: 10.1016/j.stem.2010.04.016
Moreno-Jimenez, E. P., Flor-Garcia, M., Terreros-Roncal, J., Rabano, A., Cafini, F., Pallas-Bazarra, N., et al. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560. doi: 10.1038/s41591-019-0375-9
Nieto-Estevez, V., Defterali, C., and Vicario-Abejon, C. (2016). IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 10:52. doi: 10.3389/fnins.2016.00052
Noguchi, H., Castillo, J. G., Nakashima, K., and Pleasure, S. J. (2019). Suppressor of fused controls perinatal expansion and quiescence of future dentate adult neural stem cells. eLife 8:e42918. doi: 10.7554/eLife.42918
Paratcha, G., and Ledda, F. (2008). GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 31, 384–391. doi: 10.1016/j.tins.2008.05.003
Scharfman, H., Goodman, J., Macleod, A., Phani, S., Antonelli, C., and Croll, S. (2005). Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 192, 348–356. doi: 10.1016/j.expneurol.2004.11.016
Seib, D. R., Corsini, N. S., Ellwanger, K., Plaas, C., Mateos, A., Pitzer, C., et al. (2013). Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 12, 204–214. doi: 10.1016/j.stem.2012.11.010
Sharma, P., Mesci, P., Carromeu, C., McClatchy, D. R., Schiapparelli, L., Yates, J. R., et al. (2019). Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. U.S.A. 116, 16086–16094. doi: 10.1073/pnas.1902513116
Shen, J., Wang, D., Wang, X., Gupta, S., Ayloo, B., Wu, S., et al. (2019). Neurovascular coupling in the dentate gyrus regulates adult hippocampal neurogenesis. Neuron 103, 878–890.e3. doi: 10.1016/j.neuron.2019.05.045
Shetty, A. K., Hattiangady, B., and Shetty, G. A. (2005). Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia 51, 173–186. doi: 10.1002/glia.20187
Shimazu, K., Zhao, M., Sakata, K., Akbarian, S., Bates, B., Jaenisch, R., et al. (2006). NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn. Mem. 13, 307–315. doi: 10.1101/lm.76006
Shin, J., Berg, D. A., Zhu, Y., Shin, J. Y., Song, J., Bonaguidi, M. A., et al. (2015). Single-cell RNA-seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell 17, 360–372. doi: 10.1016/j.stem.2015.07.013
Sierra, A., Encinas, J. M., Deudero, J. J., Chancey, J. H., Enikolopov, G., Overstreet-Wadiche, L. S., et al. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495. doi: 10.1016/j.stem.2010.08.014
Song, H., Stevens, C. F., and Gage, F. H. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44. doi: 10.1038/417039a
Song, J., Olsen, R. H., Sun, J., Ming, G. L., and Song, H. (2016). Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb. Perspect. Biol. 8:a018937. doi: 10.1101/cshperspect.a018937
Song, J., Zhong, C., Bonaguidi, M. A., Sun, G. J., Hsu, D., Gu, Y., et al. (2012). Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489, 150–154. doi: 10.1038/nature11306
Sorrells, S. F., Paredes, M. F., Cebrian-Silla, A., Sandoval, K., Qi, D., Kelley, K. W., et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. doi: 10.1038/nature25975
Su, W., Foster, S. C., Xing, R., Feistel, K., Olsen, R. H., Acevedo, S. F., et al. (2017). CD44 transmembrane receptor and hyaluronan regulate adult hippocampal neural stem cell quiescence and differentiation. J. Biol. Chem. 292, 4434–4445. doi: 10.1074/jbc.M116.774109
Sultan, S., Li, L., Moss, J., Petrelli, F., Casse, F., Gebara, E., et al. (2015). Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88, 957–972. doi: 10.1016/j.neuron.2015.10.037
Taliaz, D., Stall, N., Dar, D. E., and Zangen, A. (2010). Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry 15, 80–92. doi: 10.1038/mp.2009.67
Tang, C., Wang, M., Wang, P., Wang, L., Wu, Q., and Guo, W. (2019). Neural stem cells behave as a functional niche for the maturation of newborn neurons through the secretion of PTN. Neuron 101, 32–44.e6. doi: 10.1016/j.neuron.2018.10.051
Teixeira, C. M., Kron, M. M., Masachs, N., Zhang, H., Lagace, D. C., Martinez, A., et al. (2012). Cell-autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. J. Neurosci. 32, 12051–12065. doi: 10.1523/JNEUROSCI.1857-12.2012
Tobin, M. K., Musaraca, K., Disouky, A., Shetti, A., Bheri, A., Honer, W. G., et al. (2019). Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 24, 974–982.e3. doi: 10.1016/j.stem.2019.05.003
Toda, T., Parylak, S. L., Linker, S. B., and Gage, F. H. (2019). The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 24, 67–87. doi: 10.1038/s41380-018-0036-2
Toni, N., and Schinder, A. F. (2015). Maturation and functional integration of new granule cells into the adult hippocampus. Cold Spring Harb. Perspect. Biol. 8:a018903. doi: 10.1101/cshperspect.a018903
Trejo, J. L., Carro, E., and Torres-Aleman, I. (2001). Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634. doi: 10.1523/jneurosci.21-05-01628.2001
Vallieres, L., Campbell, I. L., Gage, F. H., and Sawchenko, P. E. (2002). Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 22, 486–492. doi: 10.1523/jneurosci.22-02-00486.2002
Varela-Nallar, L., and Inestrosa, N. C. (2013). Wnt signaling in the regulation of adult hippocampal neurogenesis. Front. Cell. Neurosci. 7:100. doi: 10.3389/fncel.2013.00100
Vicidomini, C., Guo, N., and Sahay, A. (2020). Communication, cross talk, and signal integration in the adult hippocampal neurogenic niche. Neuron 105, 220–235. doi: 10.1016/j.neuron.2019.11.029
Vilar, M., and Mira, H. (2016). Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front. Neurosci. 10:26. doi: 10.3389/fnins.2016.00026
Villeda, S. A., Luo, J., Mosher, K. I., Zou, B., Britschgi, M., Bieri, G., et al. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. doi: 10.1038/nature10357
Wang, J., Cui, Y., Yu, Z., Wang, W., Cheng, X., Ji, W., et al. (2019). Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell 25, 754–767.e9. doi: 10.1016/j.stem.2019.09.009
Wang, L., Chang, X., She, L., Xu, D., Huang, W., and Poo, M. M. (2015). Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J. Neurosci. 35, 8384–8393. doi: 10.1523/JNEUROSCI.4682-14.2015
Wilhelmsson, U., Faiz, M., de Pablo, Y., Sjoqvist, M., Andersson, D., Widestrand, A., et al. (2012). Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 30, 2320–2329. doi: 10.1002/stem.1196
Won, S. J., Kim, S. H., Xie, L., Wang, Y., Mao, X. O., Jin, K., et al. (2006). Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp. Neurol. 198, 250–259. doi: 10.1016/j.expneurol.2005.12.008
Yamada, J., Nadanaka, S., Kitagawa, H., Takeuchi, K., and Jinno, S. (2018). Increased synthesis of chondroitin sulfate proteoglycan promotes adult hippocampal neurogenesis in response to enriched environment. J. Neurosci. 38, 8496–8513. doi: 10.1523/JNEUROSCI.0632-18.2018
Yeh, C. Y., Asrican, B., Moss, J., Quintanilla, L. J., He, T., Mao, X., et al. (2018). Mossy cells control adult neural stem cell quiescence and maintenance through a dynamic balance between direct and indirect pathways. Neuron 99, 493–510.e4. doi: 10.1016/j.neuron.2018.07.010
Yousef, H., Morgenthaler, A., Schlesinger, C., Bugaj, L., Conboy, I. M., and Schaffer, D. V. (2015). Age-associated increase in BMP signaling inhibits hippocampal neurogenesis. Stem Cells 33, 1577–1588. doi: 10.1002/stem.1943
Keywords: neural stem cells, adult hippocampal neurogenesis, niche signals, adult born granule cells, granule cell integration
Citation: Bonafina A, Paratcha G and Ledda F (2020) Deciphering New Players in the Neurogenic Adult Hippocampal Niche. Front. Cell Dev. Biol. 8:548. doi: 10.3389/fcell.2020.00548
Received: 06 April 2020; Accepted: 10 June 2020;
Published: 02 July 2020.
Edited by:
Flavio Zolessi, Universidad de la República, UruguayReviewed by:
Dan Lindholm, University of Helsinki, FinlandCopyright © 2020 Bonafina, Paratcha and Ledda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernanda Ledda, ZmxlZGRhQGxlbG9pci5vcmcuYXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.