
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell Dev. Biol. , 29 May 2020
Sec. Molecular and Cellular Pathology
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.00336
This article is part of the Research Topic Molecular Mechanisms in Chronic Kidney Disease View all 20 articles
 Giuseppe Coppolino*
Giuseppe Coppolino* Nicola Comi
Nicola Comi Davide Bolignano
Davide Bolignano Gemma Patella
Gemma Patella Alessandro Comi
Alessandro Comi Michele Provenzano
Michele Provenzano Laura Rivoli
Laura Rivoli Michele Andreucci
Michele Andreucci Giorgio Fuiano
Giorgio FuianoObjective: Available biomarkers for monitoring primary glomerulonephritides (GNs), often lack the ability to assess longitudinal changes and have great variability with poor sensitivity. Accruing evidence has demonstrated that Neutrophil Gelatinase-Associated Lipocalin (NGAL), holds promising capacities in predicting renal function worsening in various renal diseases. We aimed at analyzing urinary NGAL (uNGAL) levels in a cohort of individuals with biopsy-proven GNs in order to evaluate its ability to reflect the entity of renal damage and to predict disease evolution overtime.
Methods: We enrolled 61 consecutive GNs patients still naïve to pathogenic therapy. uNGAL levels were measured at baseline and patients prospectively followed until the manifestation of a combined outcome of doubling of baseline serum creatinine and/or end-stage kidney disease requiring permanent dialysis support.
Results: Median uNGAL levels were 107[35–312] ng/mL. At univariate and multivariate analyses an inverse correlation was found between eGFR and uNGAL levels (p = 0.001). Progressor subjects showed exceedingly increased baseline uNGAL values as compared with non-progressors (p < 0.001). Twenty-one patients (34%) reached the composite renal endpoint. Subjects with uNGAL values above the optimal, ROC-derived, cut-off of 107 ng/mL experienced a more rapid progression to the renal endpoint (p < 0.001; HR: 5.47; 95% CI 2.31–12.95) with a mean follow-up time to progression of 73.4 vs 83.5 months.
Conclusion: In patients affected by primary glomerulonephritides, uNGAL may represent a real-time indicator of renal damage and an independent predictor of renal disease progression. Further studies on larger populations are warranted to confirm these findings.
The clinical management of primary glomerulonephritides (GNs) remains a major challenge for nephrologists. This particularly concerns their early identification, but also the choice of the optimal therapeutic pathway to procrastinate the evolution toward end-stage kidney disease (ESKD) (Floege and Amann, 2016). Renal biopsy represents the key-approach for diagnosis, risk stratification and therapeutic management of patients affected by GNs (Fuiano et al., 2000). However, its invasive nature prevents a routinely use, particularly to monitor disease evolution and therapy success over short time periods.
The measurement of proteinuria has been proved to be a good marker in such regard. However, it often lacks of sensitivity, even if stratified for glomerular filtration rate (Provenzano et al., 2020a), and it is far from being an early indicator of renal impairment, as it usually reflects an already evident damage of the glomerular barrier (Simeoni et al., 2016; Provenzano et al., 2020b).
Hence, the search for alternative, surrogate markers able to predict the tendency of renal disease worsening in a non-invasive and easily reproducible manner remains an important research priority in renal medicine.
Biomarkers, which have been tested so far for monitoring GNs, often lack in the ability to assess longitudinal changes and have great variability with poor sensitivity, mostly due to the influence of concurrent conditions (Bolignano and Coppolino, 2014; Caliskan and Kiryluk, 2014; Rysz et al., 2017; Coppolino et al., 2018). To overcome this important limitation, a focus on markers specifically correlated to kidney disease pathology is desirable (Wasung et al., 2015).
During the last years, accruing evidence has demonstrated that Neutrophil Gelatinase-Associated Lipocalin (NGAL), a small 25-kD protein released in blood and urine from injured renal tubular cells, holds interesting capacities in anticipating renal function worsening in patients affected by chronic kidney disease (CKD) (Bolignano et al., 2008c). Interestingly, this capacity remained evident even after adjustment for a variety of potential confounders, including the severity of renal impairment itself (Bolignano et al., 2009). Furthermore, other reports evidenced the ability of urinary NGAL (uNGAL) to predict successful response to treatment in idiopathic membranous nephropathy, a frequent form of glomerular disease (Yavas et al., 2013).
With this background in mind, we aimed at analyzing uNGAL levels in a small cohort of individuals with biopsy-proven primitive glomerulonephritides still naïve to immunosuppressive therapy, in order to evaluate its ability to reflect the entity of renal damage and to predict disease evolution overtime.
We enrolled 61 consecutive patients with a biopsy-proven glomerulonephritis, who were referred to Renal Unit of the University-Hospital “Magna Graecia” of Catanzaro, Italy. The local Ethic Committee approved the study and all patients gave a written informed consent to participate. Inclusion criteria were the following: the presence of non-advanced renal impairment (intended as normal renal function or early renal insufficiency stages 1–3) and a stable renal function with no documented transitory or permanent doubling in serum creatinine levels over the last 6 months before starting the study. All patients were naïve to steroid or immunosuppressive therapy. Patients with active or previous history of malignancy, liver, inflammatory or infectious diseases, as well as those with altered blood glucose levels or active alterations in leucocyte count or formula, were excluded from the study. Patients’ history and drug assumption were carefully collected by interview and confirmed by checking patients’ record. Blood pressure was measured three times and the average value was recorded for analysis.
Blood samples were collected in the morning after an overnight fast, together with a second urine collection of the same day. Ten milliliters of fresh urine were then immediately mixed with 1 mL of 10 mM tris buffer, pH 8.6 with 0.05% Tween 20 and 0.01% of NaN3 containing protease inhibitors (10 mM benzamidine, 10 mM aminocaproic acid, 20 mM ethylenediaminetetracetate and aprotinin). This mixture was centrifuged at 3000 rpm for 8 min at room temperature and then stored at −80°C until assayed.
Common laboratory parameters, including creatinine, uric acid, electrolytes, serum lipids, albumin, hemoglobin, 24-h proteinuria, fibrinogen and C-Reactive protein (CRP) were measured at baseline in all participants, according to routine standard methods. GFR was estimated by the Modification of Diet in Renal Disease formula, Eq. 7. Urinary NGAL levels were measured using an ABBOTT ARCHITECT® instrument for the rapid quantitative determination of Neutrophil Gelatinase-Associated Lipocalin (NGAL) according to the manufacturer’s instructions. All specimens were often diluted to obtain the optimal density for analysis. The enzymatic reactions were quantified in an automatic microplate photometer. All measurements were made in a blinded, duplicate manner. NGAL levels were expressed as ng per mL. In order to minimize the potential influence of urine volume, all data analyses were checked by normalizing uNGAL for urinary creatinine.
After the baseline measurements, patients were prospectively followed until the established end of the observation period or the occurrence of CKD progression, as defined by a combined outcome of doubling of baseline serum creatinine and/or the end-stage kidney disease (ESKD) requiring permanent dialysis support. Patients were directly contacted in case they missed any appointment and at study conclusion, in order to minimize loss to follow-up.
Statistical analysis was performed with SPSS for Windows (version 24.0) and MedCalc (version 12.0). Estimating a 30% occurrence of the endpoint of CKD progression over 5 years in a population with non-advanced CKD and a correlation between uNGAL and the outcome of around 0.25, we computed a sample size of at least 54 participants to give approximately 80% power (alpha = 0.05, two-tailed) to reject the null hypothesis. Data were presented as mean ± SD, median (IQ range) or frequency as appropriate. Differences between groups were established by unpaired t-test for normally distributed values and by Kruskal-Wallis analysis followed by Dunn’s test for non-parametric values. Dichotomized values were compared using the X2 test. Pearson or Spearman correlation coefficients were employed to test correlations between variables with all non-normally distributed values being log-transformed to better approximate normal distributions. Variables incorporated into the MDRD formula for GFR estimation were excluded from analysis. Receiver Operating Characteristics (ROC) analyses were performed to estimate the area under the curve (AUC) for uNGAL and to find the best NGAL cut-off values able to identify individuals who progressed to the endpoint. Kaplan-Meier curves were employed to evaluate renal survival in subjects with urinary NGAL values above and below the optimal ROC-calculated cut-off level. Adjusted risk estimates for progression endpoint were calculated using univariate Cox proportional hazard regression analysis including all variables which resulted significantly different at baseline between “CKD-progressor” and “non-CKD progressor” subjects. Variables significantly associated with the endpoint were then tested in a multivariate Cox model. All results were considered significant if p value was <0.05.
Mean age of patients was 53 ± 17 yrs and 35 (57%) of them were male. Mean serum creatinine was 1.23 ± 0.6 mg/dL with a mean estimated GFR of 75.8 ± 22.1 mL/min/1.73 m2. Median 24 h proteinuria levels were 3.3[1.1–7.2] g/24 h. Median Urinary NGAL levels were 107 [35–312] ng/mL. According to biopsy results, IgA nephropathy was the leading glomerular disease (25 pts, 41.0%), while three (4.9%) patients had minimal change disease, 20 (32.8%) had membranous nephropathy, 12 (19.7%) had focal glomerulosclerosis and only one subject (1.6%) had a membranoproliferative disease. There was no difference in uNGAL levels across different types of glomerulonephritis’s (data not shown). Table 1 summarizes the main baseline data of the study cohort.
At univariate analyses, fibrinogen levels were directly correlated to eGFR (R: 0.25; p = 0.05) while an inverse correlation was found between eGFR and age (R: −0.28; p = 0.05), systolic blood pressure (R: −0.39; p = 0.006), diastolic blood pressure (R: −0.22; p = 0.03) and, particularly, uNGAL levels (R: −0.45; p = 0.001). In multivariate model including all significant predictors at univariate analyses, only fibrinogen (β: 0.35; p = 0.005), systolic BP (β:−0.32; p = 0.05) and uNGAL (β:−0.48; p < 0.001) remained significantly associated to eGFR. The model explained about 46% of the total variance of eGFR. Supplementary Table 1 summarizes univariate and multivariate associations of baseline eGFR.
Twenty-one patients (34%) reached the composite renal endpoint over a mean follow-up of 83.1 ± 24.5 mo. There was no regression of serum creatinine to baseline levels in any of the individuals who progressed to the endpoint, therefore excluding a misleading manifestation of acute kidney injury instead of CKD progression.
The remaining 40 patients (66%) not experiencing a worsening in renal function completed the whole observational period (96 mo). At baseline, CKD-progressor subjects were significantly older and showed increased serum creatinine, systolic blood pressure and fibrinogen levels and lower eGFR values. On the contrary, they did not differ for other parameters such as gender, proteinuria, serum lipids (triglycerides and cholesterol), hemoglobin, CRP, electrolytes, uric acid, diastolic blood pressure and glomerulonephritis diagnosis. The main data and differences between patients experiencing or not-experiencing CKD progression are reported in Table 1.
Progressor subjects showed exceedingly increased baseline uNGAL values as compared with non-progressors (253 [150–432] vs 118 [75–318] ng/mL; p < 0.001). At ROC analyses (Figure 1), the Area Under the Curve (AUC) for uNGAL to identifying progressor subjects was 0.76 (95% CI 0.63–0.86). Of note, this AUC was virtually superimposable to that of eGFR (data not shown).
The optimal uNGAL cut-off value was 107 ng/mL (Sens. 80.9%, Spec. 67.5%). Interestingly, this coincided with the median value of uNGAL in the whole cohort.
Kaplan-Meier survival curves in individuals with uNGAL levels above or below this ROC-derived threshold are presented in Figure 2. Subjects with uNGAL values above 107 ng/mL experienced a more rapid progression to the renal endpoint (p < 0.001; HR: 5.47; 95% CI 2.31–12.95) with a mean follow-up time to progression of 73.4 mo (95% CI 62.8–84.6) vs 83.1 mo (95% CI 83.1–93.6).
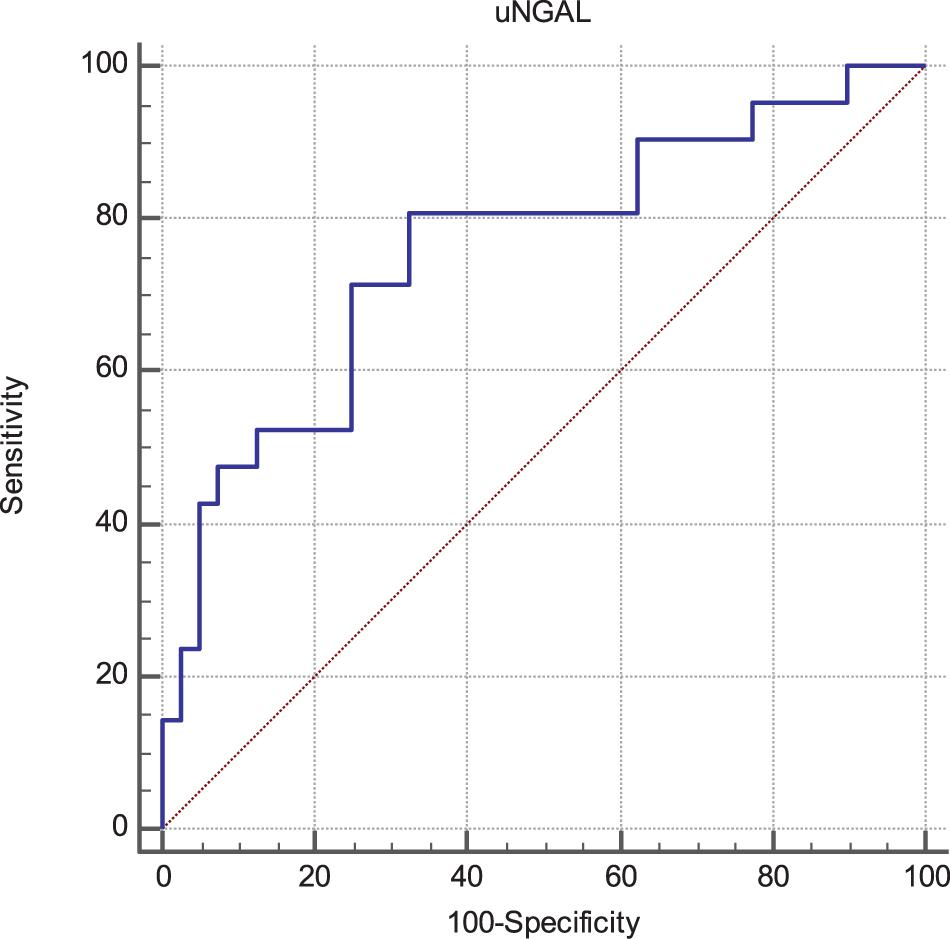
Figure 1. Receiver Operating Characteristics (ROC) curves of uNGAL considering progression of CKD as status variable. The Area Under the Curve (AUC) was 0.76 (95% CI 0.63–0.86). The best cut-off values able to predict the progression of CKD was found to be 107 ng/mL with a sensibility of 80.9 (95% CI 58.1–94.6) and a specificity of 67.5 (95% CI 50.9–81.4).
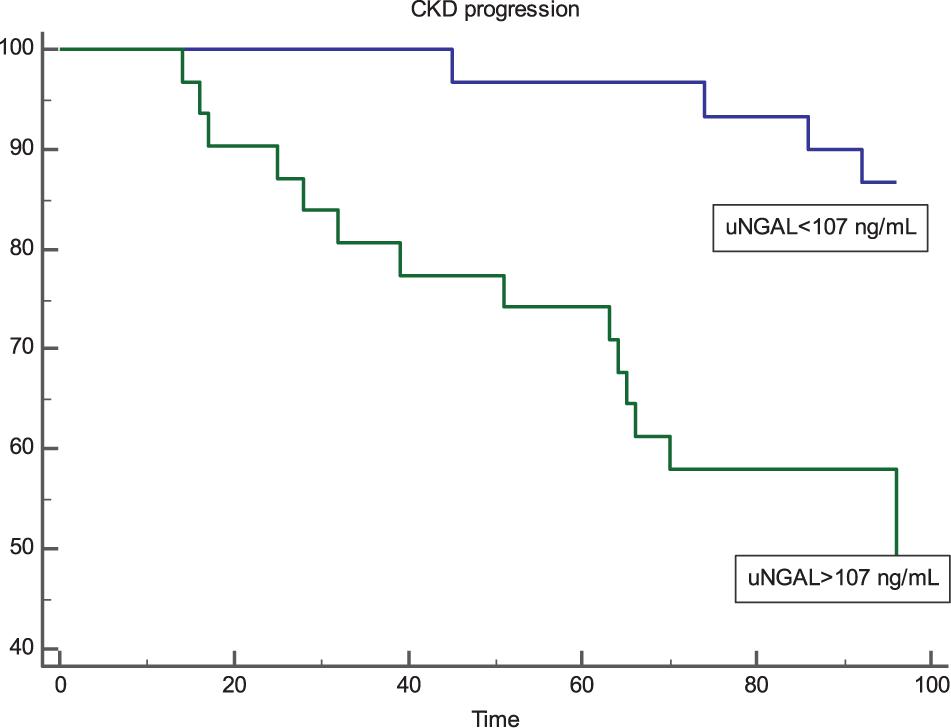
Figure 2. Kaplan-Meier survival curves of renal end-point in patients with uNGAL levels above and below the optimal ROC cut-off level of 107 ng/mL. Patients with uNGAL >107 ng/mL showed a significantly faster progression to endpoint (p < 0.001; Log-Rank Test) with a Hazard Ratio of 5.47 (95% CI 2.31–12.95).
To detect possible risk factors associated with the renal outcome, we tested in a Cox regression analysis all variables that were different at baseline between CKD-progressors and non-progressors (age, systolic BP, eGFR, fibrinogen and uNGAL; Supplementary Table 1). Univariate analysis showed that only age (HR 0.99; 95% CI 0.68–0.986; p = 0.05), eGFR (HR 0.97; 95% CI 0.95–0.99; p = 0.03) and uNGAL (HR 1.01; 95% CI 1.01–1.07; p = 0.004) were significantly associated to the endpoint, while systolic BP and fibrinogen failed to reach statistical significance.
However, in a multiple Cox regression model including these three variables, only eGFR and uNGAL remained significantly associated to CKD progression while age apparently lost its predictive power. More in detail, an increase of 10 ng/mL of uNGAL was associated with a 4% increased risk of CKD progression (HR 1.04; 95% CI 1.01–1.08, p = 0.03), whereas an increase of 10 mL/min/1.73m2 in eGFR reduced this risk by 3% (HR 0.97; 95% CI 0.96–0.99, p = 0.04). Supplementary Table 1 summarizes data from univariate and multivariate Cox-regression analyses Tables 2, 3.
The main aim of our study was to test the ability of urinary NGAL to predict progression of renal disease over a long follow-up period in a selected cohort of patients affected by primitive glomerulonephritides. Indeed, in this regard, we found a significant diagnostic capacity of NGAL which was independent from other important confounders, such as the type of glomerular disease and the baseline GFR itself. After diagnosis, besides the evaluation of proteinuria and GFR slopes overtime, only invasive procedures like renal biopsy are available to follow the natural history of GNs but routinely execution of biopsy is risky and unfeasible in common clinical practice. Hence, the search for early, non-invasive, alternative biomarkers able to stratify the risk to ESKD in this particular population setting remains a demanding priority. Similarly, the ability of these biomarkers to predict risk of relapses and response to different therapies would deserve targeted explorations. Unfortunately, the present study was not sufficiently powered to analyze also this aspect due to the relatively small cohort and the large variability of patients’ diagnosis and characteristics.
In two recent studies, Bennett et al. (2012, 2017) demonstrated the capacity of NGAL to predict the degree of response to steroid therapy in children with idiopathic nephrotic syndrome, allowing to discriminate between steroid-sensitive and steroid-resistant individuals.
In our cohort, the majority of participants was affected by IgA nephropathy (41%). Hence, our findings were mostly in accordance with Ding et al. (2007), who also demonstrated that uNGAL is a powerful predictor of disease progression in patients with IgA nephropathy with tubulointerstitial injury. In contrast with these results, Neuhaus et al. (2018) failed to predict the progression of disease with a panel of urinary biomarkers including NGAL in a sub-cohort of the STOP-IgAN trial, although their first purpose was to identify subjects who could benefit from immunosuppression as compared to a supportive therapy alone. An et al. (2019) found higher urinary NGAL levels in patients with nephrotic range proteinuria as compared to those with sub-nephrotic range proteinuria. In our study, we did not find any correlation between uNGAL and proteinuria, perhaps due to the relatively limited sample size and the high heterogeneity of case-mix of our cohort. This also apparently contradicts findings from another previous paper, in which increased urinary NGAL levels resulted highly correlated with proteinuria in a larger cohort of proteinuric patients (Bolignano et al., 2008b). According to current knowledge, high urinary NGAL found in renal diseases is not merely an expression of circulating serum NGAL that is passively loss through the damaged glomerular membrane, but also the active response of tubular cells to a non-specific damage. From this point of view, in our study, the elevated levels of urinary NGAL would therefore represent a “real-time” indicator of how much damage and active suffering is present within the chronic renal impairment (Mori and Nakao, 2007; Bolignano et al., 2008a; Kuwabara et al., 2009). The measurement of urinary NGAL was demonstrated to be an early indicator of renal damage that anticipate common markers used in clinical practice like serum creatinine or cystatin C (George and Gounden, 2019). These common markers are practical and economically sustainable, but far from being a gold standard in terms of prognostic capacity. In fact, their implementation in determined algorithms allows the stadial categorization of renal impairment progression, but has the limit to hide the first phases of organ dysfunction. In fact, until more of the 50% of renal structures are destroyed, the estimated renal function may be normal or paradoxically increased due to hyperfiltration of the remnants glomeruli (Hostetter, 1995). Stressful activation of this functional compensatory effort is itself at the basis of pathological development of lesions, leading to accelerated worsening of renal function (Kliem et al., 1996). Our study has some strengths and limits that deserve mentioning. The main strength was the very long follow-up and the robust statistical analysis implant in which urinary NGAL remained an independent predictor of CKD progression even in a Cox proportional hazards model adjustment. Furthermore, all patients were still naïve to targeted drug approaches, therefore limiting the potential influence of pre-existing immunosuppressive therapy. Main limits are probably represented by the small, single-center evaluation and by the heterogeneity of GNs, which could prevent application of findings to specific GN subpopulations.
We demonstrated that urinary NGAL represents a real-time indicator of renal damage and an independent predictor of renal disease progression in patients affected by primary glomerulonephritides. Future studies would be desirable to ascertain whether this biomarker could also be useful to guide therapeutic management of patients affected by GNs.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by University Magna Graecia of Catanzaro. The patients/participants provided their written informed consent to participate in this study.
GC, GF, NC, MA, and DB contributed to the research idea. DB and GC wrote the manuscript. GP, AC, LR, and MP collected the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2020.00336/full#supplementary-material
An, C., Akankwasa, G., Liu, J., Wang, D., Cheng, G., Zhang, J., et al. (2019). Urine markers of renal tubular injury in idiopathic membranous nephropathy: a cross sectional study. Clin. Chim. Acta 492, 7–11. doi: 10.1016/j.cca.2019.01.015
Bennett, M. R., Piyaphanee, N., Czech, K., Mitsnefes, M., and Devarajan, P. (2012). NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatr. Nephrol. 27, 807–812. doi: 10.1007/s00467-011-2075-7
Bennett, M. R., Pleasant, L., Haffner, C., Ma, Q., Haffey, W. D., Ying, J., et al. (2017). A novel biomarker panel to identify steroid resistance in childhood idiopathic nephrotic syndrome. Biomark. Insights 12:1177271917695832. doi: 10.1177/1177271917695832
Bolignano, D., and Coppolino, G. (2014). Biomarkers of cardio-renal damage in chronic kidney disease: one size cannot fit all. Crit. Care 18:134. doi: 10.1186/cc13834
Bolignano, D., Coppolino, G., Aloisi, C., Romeo, A., Nicocia, G., and Buemi, M. (2008a). Effect of a single intravenous immunoglobulin infusion on neutrophil gelatinase-associated lipocalin levels in proteinuric patients with normal renal function. J. Investig. Med. 56, 997–1003. doi: 10.2310/JIM.0b013e31818e7e95
Bolignano, D., Coppolino, G., Campo, S., Aloisi, C., Nicocia, G., Frisina, N., et al. (2008b). Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol. Dial Transpl. 23, 414–416. doi: 10.1093/ndt/gfm541
Bolignano, D., Donato, V., Coppolino, G., Campo, S., Buemi, A., Lacquaniti, A., et al. (2008c). Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 52, 595–605. doi: 10.1053/j.ajkd.2008.01.020
Bolignano, D., Lacquaniti, A., Coppolino, G., Donato, V., Campo, S., Fazio, M. R., et al. (2009). Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 4, 337–344. doi: 10.2215/CJN.03530708
Caliskan, Y., and Kiryluk, K. (2014). Novel biomarkers in glomerular disease. Adv. Chron. Kidney Dis. 21, 205–216. doi: 10.1053/j.ackd.2013.12.002
Coppolino, G., Leporini, C., Rivoli, L., Ursini, F., di Paola, E. D., Cernaro, V., et al. (2018). Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol. Res. 129, 274–294. doi: 10.1016/j.phrs.2017.12.001
Ding, H., He, Y., Li, K., Yang, J., Li, X., Lu, R., et al. (2007). Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin. Immunol. 123, 227–234. doi: 10.1016/j.clim.2007.01.010
Floege, J., and Amann, K. (2016). Primary glomerulonephritides. Lancet 387, 2036–2048. doi: 10.1016/S0140-6736(16)00272-5
Fuiano, G., Mazza, G., Comi, N., Caglioti, A., De Nicola, L., Iodice, C., et al. (2000). Current indications for renal biopsy: a questionnaire-based survey. Am. J. Kidney Dis. 35, 448–457. doi: 10.1016/s0272-6386(00)70197-1
George, J. A., and Gounden, V. (2019). Novel glomerular filtration markers. Adv. Clin. Chem. 88, 91–119.
Hostetter, T. H. (1995). Progression of renal disease and renal hypertrophy. Annu. Rev. Physiol. 57, 263–278. doi: 10.1146/annurev.ph.57.030195.001403
Kliem, V., Johnson, R. J., Alpers, C. E., Yoshimura, A., Couser, W. G., Koch, K. M., et al. (1996). Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 49, 666–678. doi: 10.1038/ki.1996.95
Kuwabara, T., Mori, K., Mukoyama, M., Kasahara, M., Yokoi, H., Saito, Y., et al. (2009). Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 75, 285–294. doi: 10.1038/ki.2008.499
Mori, K., and Nakao, K. (2007). Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 71, 967–970. doi: 10.1038/sj.ki.5002165
Neuhaus, J., Bauer, F., Fitzner, C., Hilgers, R. D., Seibert, F., Babel, N., et al. (2018). Urinary biomarkers in the prediction of prognosis and treatment response in IgA nephropathy. Kidney Blood Press Res. 43, 1563–1572. doi: 10.1159/000494442
Provenzano, M., Chiodini, P., Minutolo, R., Zoccali, C., Bellizzi, V., Conte, G., et al. (2020a). Reclassification of chronic kidney disease patients for end-stage renal disease risk by proteinuria indexed to estimated glomerular filtration rate: multicentre prospective study in nephrology clinics. Nephrol. Dial Transplant 35, 138–147. doi: 10.1093/ndt/gfy217
Provenzano, M., Garofalo, C., Chiodini, P., Mancuso, C., Barbato, E., De Nicola, L., et al. (2020b). Role of proteinuria in clinical research: for each old-answer, a new key-question. Recenti Progressi Med. 111, 74–81. doi: 10.1701/3309.32797
Rysz, J., Gluba-Brzozka, A., Franczyk, B., Jablonowski, Z., and Cialkowska-Rysz, A. (2017). Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int. J. Mol. Sci. 18:1702. doi: 10.3390/ijms18081702
Simeoni, M., Nicotera, R., Colao, M., Citraro, M. L., Pelagi, E., Cerantonio, A., et al. (2016). Direct inhibition of plasmatic renin activity with aliskiren: a promising but under-investigated therapeutic option for non-diabetic glomerulonephritis. Int. Urol. Nephrol. 48, 229–237. doi: 10.1007/s11255-015-1128-4
Wasung, M. E., Chawla, L. S., and Madero, M. (2015). Biomarkers of renal function, which and when? Clin. Chim. Acta 438, 350–357. doi: 10.1016/j.cca.2014.08.039
Keywords: glomerulonefritis, CKD – chronic kidney disease, prediction, urinary NGAL, renal function
Citation: Coppolino G, Comi N, Bolignano D, Patella G, Comi A, Provenzano M, Rivoli L, Andreucci M and Fuiano G (2020) Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) Predicts Renal Function Decline in Patients With Glomerular Diseases. Front. Cell Dev. Biol. 8:336. doi: 10.3389/fcell.2020.00336
Received: 05 March 2020; Accepted: 17 April 2020;
Published: 29 May 2020.
Edited by:
Claudia Torino, Italian National Research Council, ItalyReviewed by:
Francesco Pesce, University of Bari Medical School, ItalyCopyright © 2020 Coppolino, Comi, Bolignano, Patella, Comi, Provenzano, Rivoli, Andreucci and Fuiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Coppolino, Z2NvcHBvbGlub0B1bmljei5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.