
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 01 October 2019
Sec. Cell Death and Survival
Volume 7 - 2019 | https://doi.org/10.3389/fcell.2019.00212
Chemokine (C–C motif) ligand 19 (CCL19) is a critical regulator of the induction of T cell activation, immune tolerance, and inflammatory responses during continuous immune surveillance, homeostasis, and development. Migration of CC-chemokine receptor 7 (CCR7)-expressing cells to secondary lymphoid organs is a crucial step in the onset of adaptive immunity, which is initiated by a complex interaction between CCR7 and its cognate ligands. Recent advances in knowledge regarding the response of the CCL19-CCR7 axis to viral infections have elucidated the complex network of interplay among the invading virus, target cells and host immune responses. Viruses use various strategies to evade or delay the cytokine response, gaining additional time to replicate in the host. In this review, we summarize the impacts of CCL19 and CCR7 expression on the regulation of viral pathogenesis with an emphasis on the corresponding signaling pathways and adjuvant mechanisms. We present and discuss the expression, signaling adaptor proteins and effects of CCL19 and CCR7 as these molecules differentially impact different viral infections and viral life cycles in host homeostatic strategies. The underlying mechanisms discussed in this review may assist in the design of novel agents to modulate chemokine activity for viral prevention.
CCL19 and its receptor CCR7 control a diverse array of migratory events in adaptive immunity following antigen encounter by immunocytes. Recently, some rules governing the mechanisms of the CCL19-CCR7 axis in directing lymphocyte homing and encountering viruses have been clarified in vitro (Comerford et al., 2006; de Paz et al., 2007; Jafarnejad et al., 2017). In vivo, both the host’s antiviral and adjuvant-based immune responses are regulated by interactions among viral proteins, chemokine receptors and their downstream adaptor components. Changes in the bioavailability of CCR7/ligands (CCL19 and CCL21) may modulate the immunopathogenesis pathways of the host, thereby altering virus invasion (Comerford et al., 2013; Steen et al., 2014). Hence, we systematically evaluated the contributions of CCL19 and CCR7 expression polymorphisms, signal transduction and CCL19-based adjuvant mechanisms in viral infections.
CCL19 and CCL21 have a conserved tetra-cysteine motif but only share 32% amino acid identity. Structurally, CCL21 differs from CCL19 because it has a uniquely long C-terminal tail containing an extra 37 amino acids (6 cysteine residues) that are highly positively charged and capable of binding glycosaminoglycans (GAGs) (Nagira et al., 1997; Steen et al., 2014). CCL19 is secreted by mature dendritic cells (mDCs), while CCL21 is secreted from the endothelium of afferent lymphatic vessels (this has been shown in mice, but evidence in humans is lacking), and both are predominantly secreted by the lumen of high endothelial venules, the stromal cells of the draining lymph node and the spleen (Steen et al., 2014; Wang et al., 2018). Due to their differential structures and expression patterns, CCL19 and CCL21 display different binding affinities for specific heparin or heparan sulfate, and distinct signaling responses are required for in vivo functions (Rot and von Andrian, 2004; de Paz et al., 2007; Raju et al., 2015). CCR7 was the first identified lymphocyte-specific G-protein-coupled receptor (GPCR) with seven transmembrane spanning alpha helices (Birkenbach et al., 1993). CCR7 is expressed on double negative and single positive thymocytes, including naïve T cells, central memory T cells, regulatory T cells, naïve B cells, semi-mature/mature DCs and NK cells, and a minority of tumor cells, and it acts as a key regulator guiding homeostatic lymphocytes to secondary lymphoid organs (Ohl et al., 2004; Comerford et al., 2013; Hauser and Legler, 2016; Wang et al., 2018; Laufer et al., 2019). The CCR7-ligand axis carries out the following three fundamental “cellular reflexes”: message acquisition, semantic extraction and initiation of cell responses (Bardi et al., 2001; Rot and von Andrian, 2004; Griffith et al., 2014). Chemokine receptor internalization due to binding with a chemokine helps regulate chemokine activities (Rot and von Andrian, 2004). CCL19 is the only chemokine known to effectively stimulate β-arrestin-mediated CCR7 phosphorylation and internalization, leading to receptor desensitization and antigen-presenting dendritic cell (DC) migration (Bardi et al., 2001; Tian et al., 2014; Anderson C. et al., 2016). In particular, CCL19 displays obvious concentration- and time-dependent internalization in CD4+ and CD8+ T cells, which differs from CCL21 (Hjortø et al., 2016). Both ligands are able to activate G-protein signaling and elicit 3D chemotaxis and Ca2+ flux, but CCL19 has been shown to be relatively more potent (Bardi et al., 2001; Steen et al., 2014; Hjortø et al., 2016) (Figure 1).
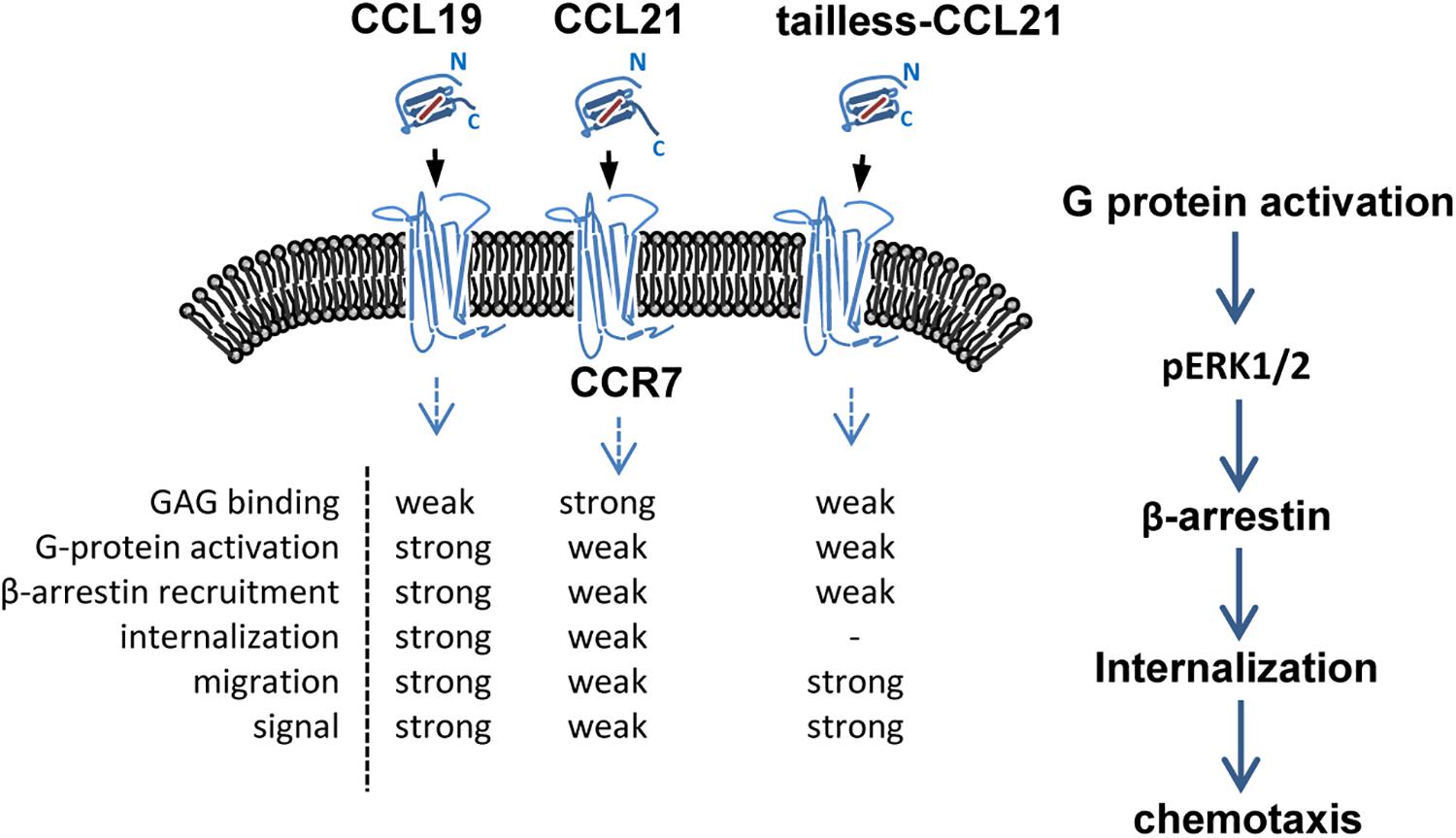
Figure 1. Schematic of CCR7 and its ligands. CCL19, CCL21 and tailless CCL21 bind CCR7, a 7-transmembrane receptor. Binding of receptor/ligands results in GPCR activation and consequent internalization, followed by a decrease in the surface-exposed receptor and activation of certain intracellular pathways. GAG, glycosaminoglycan.
Chemokines constitute a class of cytokines that control immunocyte migration to infection and inflammation sites in many biological processes. In different virus–host interactions, chemokine receptors may play a sensory function in the immune system, resulting in the production of the characteristic fingerprints of chemokines (Chensue, 2001; Alcami, 2003). The chemokine system can be mimicked by viruses, and viral proteins can act as antagonists or inappropriate agonists to use host chemokine receptors as modes of cellular invasion (Rot and von Andrian, 2004). For instance, human immunodeficiency virus type 1 (HIV-1) masquerades as a “chemokine” to promote its fusion with target cells (Murphy, 2001). Additionally, poxviruses and herpesviruses encode homologs of chemokine receptors that are expressed on their target cells, allowing the host chemokines to direct the infected cells to remote sites for viral dissemination (Alcami, 2003). Based on the essential roles of the CCL19-CCR7 axis in organizing immunological and inflammatory responses, we summarize in this review its pathogenic roles in some viral infection conditions, such as infections by HIV-1 (Wilflingseder et al., 2004; Cameron et al., 2010; Damås et al., 2012; Hong et al., 2012; Ramirez et al., 2014; Anderson J.L. et al., 2016), scrapie virus (Kim et al., 2018), respiratory syncytial virus (RSV) (Le Nouën et al., 2011; Inchley et al., 2013; Alturaiki et al., 2018), Epstein–Barr virus (EBV) (Ehlin-Henriksson et al., 2009; Dunham et al., 2017; Wu et al., 2017), influenza virus (Debes et al., 2004; Piqueras et al., 2006), dengue virus (DENV) (Wu et al., 2009, 2011; Hsu et al., 2015), hepatitis B virus (HBV) (Zhang et al., 2009; Cao et al., 2014), and West Nile virus (WNV) (Bardina et al., 2017). The CCL19-CCR7 axis also plays a role in vaccine-based protection against multiple viruses, such as HIV-1 (Hu et al., 2013), herpes simplex virus 1 (HSV-1) (Lee et al., 2003; Toka et al., 2003), HSV-2 (Yan et al., 2015), hepatitis C virus (HCV) (Hartoonian et al., 2014), and pseudorabies virus (Han et al., 2009). In addition, the CCL19-CCR7 interaction helps immune cells release antiviral-related cytokines (e.g., IFN-γ and IL-4), which promote T cell proliferation and antigen uptake by DC (Hu et al., 2013, 2017).
For many years, the focus on prophylactic vaccines aimed to elicit robust neutralizing antibody (Ab) responses. However, increasing evidence suggests that T cell-mediated immunity also plays a critical role in controlling persistent viral infections, such as HIV-1, cytomegalovirus (CMV), and HCV infections (Kallas et al., 2016). Recently, various promising prophylactic vaccines in conjunction with adjuvant cytokines or chemokines, such as CCL19, have been investigated to enhance virus-specific cellular immune responses through cytokine polyfunctionality. For future therapeutic initiatives, it is important to understand the roles of the CCL19/CCR7 axis in modulating immune cell migration and activation to potentially differentiate the good and bad effects. In this review, we evaluate the functional efficacies of CCL19 and CCR7 in viral infection and prevention, which may facilitate the development of more potent, durable and safe T cell-based anti-virus pharmaceuticals or vaccines.
CCR7+ immune effector cells become dysfunctional during some viral infections, followed by the decreased expression of CCR7 during adaptive immune responses (Förster et al., 2008). CCR7 presents as a defining factor for non-polarized central (CCR7+) and polarized effector memory (CCR7–) T cells (Unsoeld et al., 2002). CCR7 is expressed at high levels on naïve and central memory T cells and enables homeostasis T cell subsets to recirculate and home to T cell areas in lymphoid organs, such as the white pulp areas of the spleen and lymph nodes (Rot and von Andrian, 2004; Schaerli and Moser, 2005). During murine lymphocytic choriomeningitis virus (LCMV) infection, CCR7 is down-regulated on virus-specific CD8+ effector T cells in vivo (Potsch et al., 1999). The down-regulation of CCR7 expression on virus-specific CD8+ effector T cells renders the cells unresponsive to chemokines from secondary lymphoid tissues, which limits T cell homing. It has been speculated that the exclusion of CD8+ effector T cells from the T-zone may represent an important mechanism protecting professional antigen-presenting cells (APCs) against cytotoxic T cell attacks and, thus, preventing a premature decline in immune responses. During HIV-1 infection, the CCL19/CCR7 axis assists with establishing a latent infection. The CCR7 expression pattern is strongly correlated with increased HIV-1 viral reservoirs and is associated with chronic HIV-1 infection. Stimulation of HIV-1-infected primary CD4+ T cells with CCL19 results in the enhancement of both the motility of CCR7-dependent T cells and the permissiveness of resting memory T cells, leading to the efficient propagation of HIV-1 (Saleh et al., 2007; Hayasaka et al., 2015). In addition, the HIV-1 accessory protein Vpu induces the down-regulation of CCR7 expression on the surface of HIV-1-infected CD4+ T cells (Ramirez et al., 2014). Consistently, a clinical study showed that HIV-1-infected individuals lack CCR7 expression on natural killer (NK) cells (Hong et al., 2012). An HIV-1 and CMV coinfection study demonstrated that most (>70%) CD8+ effector T cells have a CCR7– phenotype in both the blood and lymph nodes (Ellefsen et al., 2002). Furthermore, mechanistic studies have shown that HIV-1 gp120 can mimic chemokine sequences and significantly promote chemokine receptor-dependent CD4+ target-cell migration to remote lymph nodes, which likely leads to enhanced viral dissemination (Murphy, 2001; Hayasaka et al., 2015).
Similarly, two previous studies shows that mice infected with scrapie virus (ME7 strain) have been shown to exhibit an impaired splenic white pulp structure and markedly diminished T-zone areas in the spleen due to the decreased splenic expression of CCL19 and CCL21 (Kim et al., 2016, 2018). Furthermore, decreased expression of T cell homing chemokines CCL19 and CCL21 resulted in a partial failure in CD4+ T cell recruitment to the spleen and expression of the memory marker CD44 on CCR7± CD4+ T cells was decreased in scrapie virus (ME7 strain)-infected mice compared to that in control mice (Table 1). Finally, high levels of the cellular prion protein [PrP(C)] and accumulated PrP(Sc) expressed by follicular DCs were detected in the ME7-infected spleens. During the infection of respiratory viruses, such as human respiratory syncytial virus (HRSV), human metapneumovirus (HMPV) and human parainfluenza virus type 3 (HPIV3), can induce incomplete or short-lived virus-specific immunity. These viruses can produce symptomatic reinfections throughout life without undergoing significant antigenic changes. As a class of professional presentation cells, DCs can provide antigen presentation in the context of viral infection. However, human monocyte-derived DCs (mo-DCs) stimulated with HRSV, HMPV, or HPIV3 show an inefficient increase in CCR7 expression unless a secondary stimulation with lipopolysaccharide (LPS) or a cocktail of proinflammatory cytokines is presented. In contrast, HRSV and HMPV infections induce less CCR7 expression and less efficient DC migration in response to CCL19 than HPIV3. The low expression of CCR7 mediates the inefficient migration of HRSV- and HMPV-stimulated DCs to lymphoid organs and causes impaired adaptive responses to these viruses, ultimately leading to abundant virus replication and tissue damage, thus resulting in more severe disease (Le Nouën et al., 2011; Inchley et al., 2013) (Table 1).
During EBV infection, CCR7 expression likely plays an important role in pathogenesis. Compared with uninfected tonsillar B cells, CCR7 expression, which is critical for the migration of B cells into lymphoid tissues, has been shown to decline from day 2 after EBV infection and be undetectable by day 14 (Ehlin-Henriksson et al., 2009) (Table 1). In addition, migration of EBV-infected cells toward CCL19 or CCL21 is impaired, although the expression of CCR7 in infected cells is similar to that in uninfected controls (Ehlin-Henriksson et al., 2009; Hauser and Legler, 2016; Dunham et al., 2017; Wu et al., 2017). CCR7 plays an essential role in B cell trafficking in lymphoid tissue, and its activation has been shown to be strictly dependent on a highly conserved cellular DNA binding factor (CBF1) as a blockade of or a deficiency in this factor results in the repression of immunoglobulin (Ig) expression in the context of EBV infection (Maier et al., 2005, 2006).
During Influenza A virus infection, the Th1-based immune response dominates the immune process (Doherty et al., 1997). CCL19/CCL21 and CCR7 are well-known to be essential for fulfilling the important role of recruiting T cells into the lung and other peripheral specialized microenvironments within tissues (important pathogen entry sites). CCR7 plays an important role in the migration of T cells from lymph nodes and Peyer’s patches through high endothelial venules during mucosal immune protection (Förster et al., 1999; Debes et al., 2004). However, compared to uninfected mice, CCR7 expression has been shown to be down-regulated on influenza virus-specific CD4+ T cells obtained from the spleen, mesenteric lymph nodes (MLNs) and the lungs at the peak of infection (Debes et al., 2004) (Figure 2), indicating that these cells lost the ability to recirculate after the response to viral antigens (Ags).
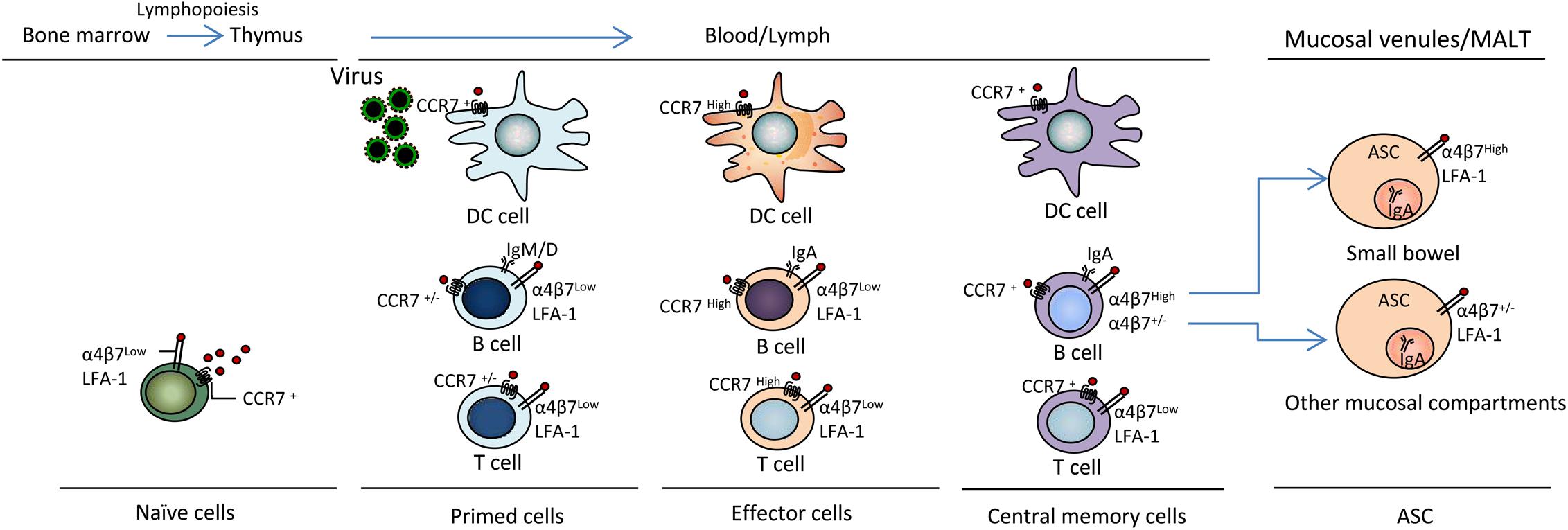
Figure 2. CCL19 and CCR7 mediate virus antigen uptake and immunocyte activation and differentiation. DC, B and T lymphopoiesis are characterized by sequential changes in migration properties. Surface receptor and Ig expression occurs during T lymphopoiesis in bone marrow and the thymus during immune response initiation and the generation of effector T cells for participation in active defense at virus entry sites in peripheral tissues, including blood, lymph, mucosal venules and MALT; thereby, inflammation and the establishment of memory cells for immune surveillance occur. α4β7, integrin α4β7; LFA-1, lymphocyte function-associated antigen-1; ASC, antibody-secreting cell; MALT, mucosal-associated lymphoid tissue. Other mucosal compartments: colon, alveolus, mammary and salivary glands, genital mucosa.
High expression of CCR7 on immunocytes has been observed in DENV infection. DCs are the most important target cells, and CCR7 expression has been shown to be increased on DENV-stimulated mo-DCs (Wu et al., 2011). However, mo-DCs with increased CCR7 expression have a greater opportunity to interact with abundant T cells and begin to secrete cytokines, metalloproteinases, and chemokines in the T-zone of draining lymph nodes. The clinical manifestations of DENV infection include dengue fever and potentially fatal phenomena, such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The interaction between DENV and target immune effectors, cytokines or chemokines may lead to the development of severe clinical manifestations, such as DHF (Wu et al., 2009). In clinical therapy, some medicines, such as leflunomide, inhibit the excessive production of cytokines and chemokines from DENV-infected mo-DCs by suppressing mo-DC maturation (Wu et al., 2011). Similarly, DENV infection has been shown to specifically increase the mRNA and protein levels of Gal-9. Additionally, Gal-9 small interfering RNA downregulates CCR7 expression and suppresses DENV-specific DC migration toward the chemoattractants CCL19 and CCL21. Thus, a Gal-9 inhibitor might be useful for preventing immunopathogenesis in DENV infection (Hsu et al., 2015). Similar functional molecules might be useful in drug development to prevent DENV immunopathogenesis by reducing the number of CCR7-expressing cells.
Via interacting with its receptor (CCR7), CCL19 has the potential to activate the signaling pathway enabling HIV-1 to enter the nucleus of resting (memory) T cells (Saleh et al., 2016; Cameron et al., 2010; Anderson J.L. et al., 2016). HIV-1 latent infection in resting memory CD4+ T cells is a major barrier to HIV-1 eradication. The reversal of proviral latency has attracted much attention as a curative strategy for HIV-1 infection (Cillo et al., 2014). CCL19-treated CXCR4-expressing CD4+ T cells have been shown to exhibit increased permissiveness for HIV-1 production, thereby facilitating provirus post-integration and latency (Anderson J.L. et al., 2016). Furthermore, inoculation of CD4+ T cells with HIV causes a modest down-regulation (as shown via flow cytometry) of CCR7 expression and a slight increase of CCR5 expression after stimulation with CCL19 (Anderson J.L. et al., 2016). CCR5 is a co-receptor essential for HIV-1 entry into susceptible cells and is an attractive target for controlling HIV-1 infection (Li et al., 2015). In addition, CCL19 and mDCs co-culture with CD4+ T cells has been shown to be beneficial for CCR5- and CXCR4-tropic virus latent infection in vitro (Anderson J.L. et al., 2016).
In the context of chronic HBV infection, mDCs are highly migratory, which is accompanied by the up-regulation of CCR7 expression on hepatic mDCs and an increase in the response to CCL19 (Abe et al., 2004). In two studies, immature hepatic DCs did not respond to any tested chemokines, despite the expression of mRNA transcripts encoding the appropriate receptors for these chemokines, and CCR7 expression was strongly enhanced in response to DC maturation (Abe et al., 2004; Thomson and Knolle, 2010). Hepatic mDCs play a critical role in promoting immune tolerance by producing IL-10 and TGFβ, activating regulatory T cells or regulatory B cells and suppressing effector T cell proliferation (Abe et al., 2004; Liu et al., 2018). Compared with the robust responses at clinical onset, HBV-specific CD8+ T cell responses are rather weak and limited, which may lead to viral persistence and disease progression (Nitschke et al., 2016; Fisicaro et al., 2017; Wieland et al., 2017). However, acute inflammation may convert hepatic mDCs from a tolerogenic phenotype, allowing these cells to activate T cells (Abe et al., 2004; Thomson and Knolle, 2010).
CCR7 expression can induce the migration of antigen-specific effector and central memory T cells to the lymph nodes via the chemotactic response to CCL19 (Abe et al., 2004). The major phenotype and functions of pathogen-specific CD8+ T cells may differ in different viral infections (Appay et al., 2002; Romero et al., 2007). The quantitative and qualitative compositions of the immune cells in the liver markedly differ from those in secondary lymphoid organs, including the spleen, lymph nodes and peripheral blood. The hepatic CD8+/CD4+ T cell ratio is 3.5:1; however, this ratio is the reverse of the 1:2 CD8+/CD4+ T cell ratio found in secondary lymphoid organs (Horst et al., 2016). CD8+ T cells play crucial roles in HBV control and liver inflammation. Several studies have investigated CCR7 expression by HBV-specific CD8+ T cells in the context of chronic HBV infection (Boettler et al., 2006; Zhang et al., 2009). Up-regulation of programed death-1 (PD-1) expression can impair HBV-specific memory CD8+ T cell responses, resulting in the functional suppression of IFN-γ production. Additionally, blockage of PD-1/PD-L1 interactions in vitro increases the frequency of HBV-specific CD8+ T cells, and enhances CCR7, CD45RA, and CD127 expression in these cells, resulting in increased cell proliferation and IFN-γ production (Zhang et al., 2009) (Table 1).
Chronic infections with blood-borne pathogens, such as HIV-1, HBV and HCV, tend to increase the development of memory homeostasis T cells (Carotenuto et al., 2011; Cao et al., 2014; Cillo et al., 2014; Baskic et al., 2017). During primary viral infections, virus-specific T cell responses are vigorous; however, once a persistent infection is established, both virus-specific CD4+ and CD8+ T cells become dysfunctional or difficult to detect ex vivo (Cao et al., 2014). Virus-specific CD8+ effector T cells play a critical role in eliminating HIV-1, EBV, HBV, CMV and HCV infections, and the expression patterns of CCR7, CD27, and CD28 exhibit similar characteristics during the primary infection phase and chronic phase of a persistent infection (Appay et al., 2002; Romero et al., 2007). The CCR7+ CD27+, CCR7– CD27+, and CCR7– CD27– phenotypes can represent the early, median, and late stages of memory CD8+ T cell differentiation, respectively, while a down-regulated expression of these molecules indicates an inability of T cells to differentiate toward the effector phenotype. Several studies report that the removal of effector T cells from circulation favors the recruitment of CCR7+ naïve cells, which may result in an impairment in the generation of functionally competent memory T cells and an inability to control viral replication (Appay et al., 2002).
Mature dendritic cells and T cells that express CCR7 enhance the velocity of T cell locomotion within the lymph nodes and, thus, increase the likelihood that T cells encounter DCs (Mora and von Andrian, 2008). In influenza virus infection, high levels of CCR7+ IFN-γ+ CD4+ T cells have been observed in lung draining lymph nodes (Debes et al., 2004). A study investigating the kinetics of chemokine expression showed that the levels of CCL19, CCL22, and CXCL13 are significantly up-regulated during the third wave (8 to 24 h) of influenza virus infection, which helps CCR7+ DCs reach the lymphoid organs and attract naïve T and B cells (Piqueras et al., 2006) (Table 1).
The major adaptive immune lymphocytes present in the lungs of human infants who have died from severe RSV infection are B cells (Welliver et al., 2007; Reed et al., 2009). The B cell differentiation factor BAFF is an indicator of pulmonary Ab responses after HRSV, HMPV, H1N1, bocavirus, rhinovirus and Mycoplasma pneumoniae infections (McNamara et al., 2013). CCL19 and other chemokines, particularly CXCL12, CXCL13 and CCL21, influence B cell and human blood DC (i.e., plasmacytoid and myeloid DCs) differentiation, migration and homeostasis (Freire-de-Lima et al., 2017; Alturaiki et al., 2018). The expression kinetics of these chemokines has been reported to be related to the airway epithelial innate immune response to respiratory virus infections. CCL19 has been shown to be expressed in lung tissues 1, 2, and 7 days after infection with RSV (Le Nouën et al., 2011; Inchley et al., 2013; Alturaiki et al., 2018). However, in the context of influenza virus infection, CCL19 and CCL21 levels have been reported to be increased during the late wave (Comerford et al., 2006). These variable results may be explained by differences in the models or sampling methods used (Alturaiki et al., 2018) (Table 1).
During homeostasis, CCR7 regulates the homing of T cells into lymphoid organs. During WNV infections, CCR7+ DCs regulate the homing of T cells expressing the cognate ligands CCL19 or CCL21 into the lymph nodes immediately following infection and restrict leukocyte migration into the brain. Leukocyte hypercellularity within the central nervous system (CNS) contributes to CNS viremia, neuroinflammation, and increased mortality. Thus, CCR7 acts as a host defense restriction factor limiting neuroinflammation during acute WNV infection (Bardina et al., 2017) (Table 1).
Taken together, these data show that host responses to viral infections involve distinct effectors of innate and adaptive immunity and that the lymphocyte mobilization mediated by these effectors needs to be coordinated to ensure protection. Both inflammatory and homeostatic chemokines are involved in the control of the trafficking of effector or memory cells. Inflammatory chemokines determine the cellular infiltrates at sites of pathogen entry, whereas inflammation and homeostatic chemokines regulate the inflammation-independent, continuous trafficking of memory cells through healthy peripheral tissues.
The CCL19-CCR7 axis is involved in the constitutive migration and homing of lymphocytes. Furthermore, the CCL19-CCR7 axis has been shown to perform other biological activities, such as regulation of DC morphologic change and thymic T cell development and suppression of DC apoptosis, which can lead to the regulation of adaptive immunity and tolerance (Yanagawa and Onoe, 2002; Müller and Lipp, 2003; Sánchez-Sánchez et al., 2004; Förster et al., 2008; Raju et al., 2015). This axis performs its biological functions by activation of the G protein-coupled receptor kinase (GPK)/β-arrestin that transduces the binding of extracellular stimuli to intracellular signaling (Zidar et al., 2009; Tian et al., 2014). CCL19 but not CCL21 induces robust β-arrestin 2 recruitment and results in serine/threonine-phosphorylated receptor CCR7 internalization to endosomal vesicles, thereby efficiently limits receptor susceptibility to extracellular ligands (Kohout et al., 2004) (Figure 1). In addition, CCL19 has been shown to be more efficient than CCL21 in activating ERK1/2 (part of the MAP kinase cascade) through Gαi subunit (Kohout et al., 2004; Raju et al., 2015) and increasing Ca2+ flux through the Gβγ subunit (Yoshida et al., 1998; Otero et al., 2008) (Figure 3). CCR7, as a homeostatic chemokine receptor, inhibits adenylate cyclase, and limits the level of intracellular cyclic adenosine monophosphate (cAMP) and the activation of protein kinase A (PKA) (Steen et al., 2014; Raju et al., 2015). Similar to other GPCRs, CCR7 plays a crucial role in activating or inhibiting downstream signaling adaptors in viral infections through G-protein-promoted secondary messengers, including cAMP, Ca2+, and phosphoinositides (Steen et al., 2014; Raju et al., 2015) (Figure 3). The following three principle modes are involved in the GPCR homeostatic regulation: desensitization (receptor becomes refractory to continued stimuli), internalization (receptors are physically removed from the cell surface by endocytosis) and down-regulation (cellular receptor levels are decreased) (Tian et al., 2014). In addition to inducing directional steering in cells, CCR7 provides costimulatory and survival cues (Bock et al., 2016). Moreover, CCR7-mediated human T cell polarization and migration have been shown to be linked to protein–protein interactions in cell signaling across multiple cellular compartments (Raju et al., 2015).
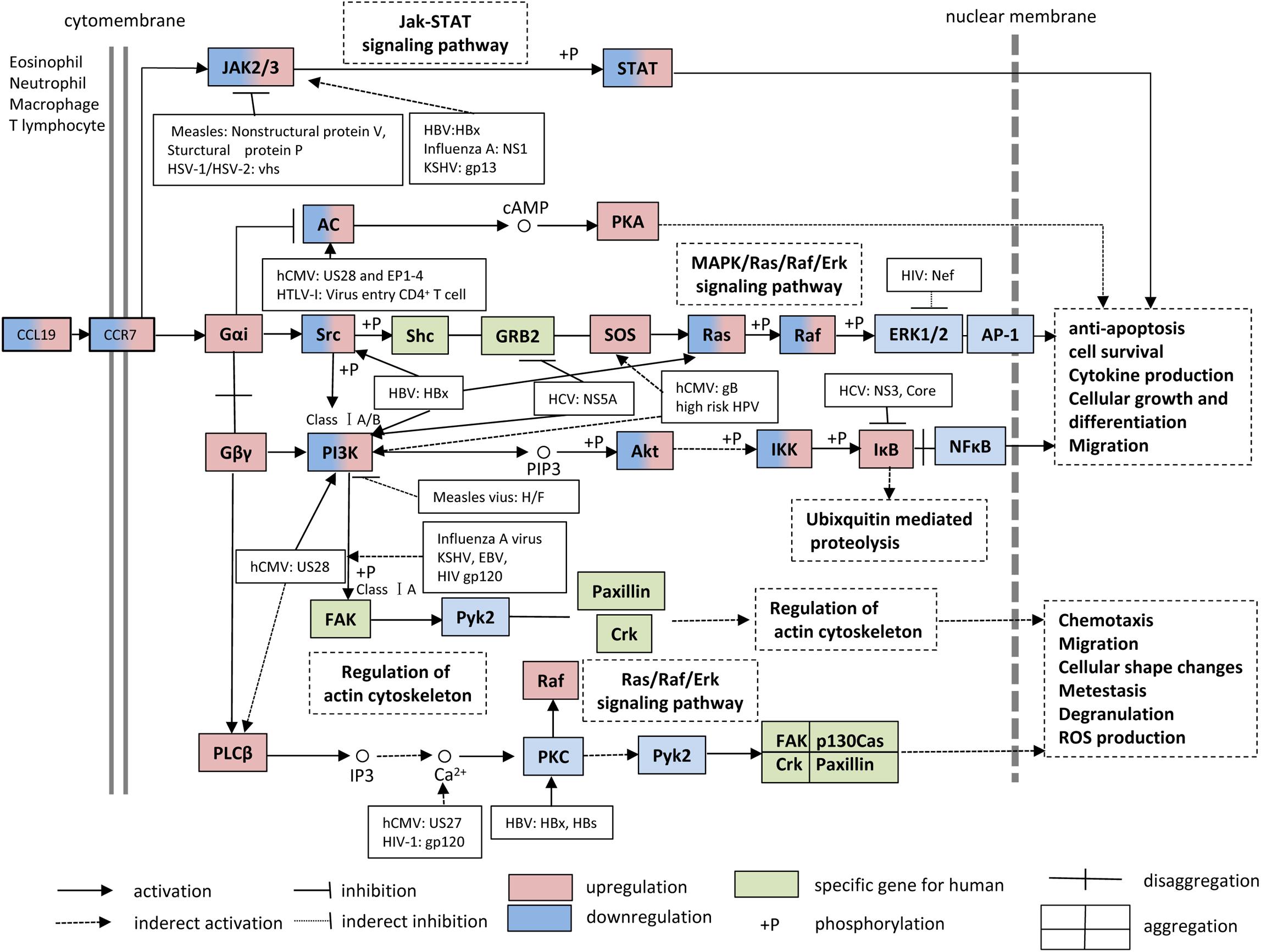
Figure 3. CCL19 and CCR7 signaling pathways. During viral infections, many studies have supported the notion that the interaction between CCL19 and CCR7 facilitates the up-regulation/down-regulation of downstream signaling adaptor molecules and results in anti-apoptosis, cell survival, cytokine production, cellular growth, differentiation, chemotaxis, and migration, etc. KSHV, Kaposi sarcoma-associated herpesvirus; HTLV, human T-cell leukemia virus 1; HPV, human papillomavirus.
Over the past few years, numerous CCL19-based signaling-related adaptor molecules have been reported in CCR7 signaling pathways, and their specific functions during viral infections are summarized in Figure 3. Viruses have been well-demonstrated to have highly efficient strategies to modulate and prevent the transduction of apoptotic signals to favor their infections through viral proteins, such as the HBx gene product of HBV (Shin et al., 2016). For this review, we searched CCR7-associated chemokine signaling pathways in the KEGG PATHWAY Database1 and discuss the multiple regulatory mechanisms of CCR7 signaling and the influences on CCR7 functions during viral infections.
The HIV-1 accessory protein Vpu reduces CCR7 expression on CD4+ T cells (Ramirez et al., 2014). Vpu specifically interacts and colocalizes with CCR7 in the trans-Golgi network in which CCR7 is retained. The stimulation of HIV-1-infected primary CD4+ T cells with CCL19 reduces mobilization of Ca2+, reduces phosphorylation of Erk1/2, and impairs migration toward CCL19 (Ramirez et al., 2014). Studies concerning CCL19-CCR7 signaling have shown that CCL19-induced signaling proteins mediate HIV-1 integration in CD4+ T cells at several integration sites and that this process is suppressed by inhibitors of the PI3K, NF-κB and MEK/Ras/Raf signaling pathways (Raju et al., 2015; Saleh et al., 2016) (Figure 3). Recently, the Food and Drug Administration (FDA) approved the Janus Kinase (JAK) inhibitor ruxolitinib to diminish the release of multiple cytokines and thereby prevent their effects on latency reversal in HIV-1-positive patients. A combination of ingenol compounds with the JAK inhibitor may represent a novel strategy for HIV-1 eradication (Spivak et al., 2016).
The infection of DCs with DENV causes cell maturation and probably enhances cell migration to lymphoid organs to promote interactions with T cells (Mathew and Rothman, 2008). At 48 h post-infection, the number of CCR7+ mo-DCs increases, and the increase in cell number is accompanied by the significant activation of the COX-2-PGE2 signaling pathway in migrating DCs (Wu et al., 2009). All MAPK inhibitors and the COX-2 inhibitor celecoxib suppress DENV-induced PGE2 production to basal levels. The mechanisms involved in the activation of COX-2 include the activation of the IKK-NF-κB and MAPK-activator protein-1 (AP-1) signaling pathways (Wu et al., 2009; Hsu et al., 2015). Several COX-2 upstream signaling molecules have been suggested to be useful for the treatment or control of viral infections. For example, the blockade of ERK, which is upstream of the MKK1/2 kinase, suppresses virus infectivity by inhibiting early gene expression in human CMV (hCMV) infections (Johnson et al., 2001). Additionally, leflunomide has been shown to be an effective therapeutic drug for DENV infection. Leflunomide inhibits DENV-induced mo-DC migration in response to the chemoattractant CCL19 by suppressing CCR7 expression and the NF-κB and AP-1 signaling pathways (Wu et al., 2011).
Cytokines have been successfully used as molecular adjuvants to promote virus-specific humoral and cellular immune responses by modifying the magnitude, intensity, nature, and duration of the responses (Sin et al., 1999; Kanagavelu et al., 2012; Gupta et al., 2015; Doosti et al., 2019). The positive effects of chemokines as immunomodulators of vaccines against viral infections remain to be further evaluated. CCL19 has been postulated as a promising adjuvant candidate for vaccines against both cancers and infectious diseases due to its paramount importance in immune response formation and its diverse effects on DC and lymphocyte migration and activation. CCL19 also controls lymphocyte localization during T cell development in response to immunization. Activated T cells lead to alterations in the expression of various molecules, including integrins (α4β3, LFA-1), selectins, and chemokine receptors, leading to the modulation of key intracellular signaling events that promote T cell proliferation, differentiation, and migration to inflamed tissues (Murdoch and Finn, 2000; Tahamtan et al., 2018; Justo-Junior et al., 2019) (Figure 2).
In the context of viral neutralizing antibodies (nAbs), studies have shown that both monomeric gp120 and trimeric gp140 HIV-1 vaccines induce neutralizing antibody responses but still lack sufficient breadth to effectively protect against diverse HIV-1 isolates (García-Arriaza et al., 2017; Shen et al., 2017). HSV-2 gB or gD subunit vaccines could induce high levels of neutralizing Abs but could not efficiently protect mice against a viral challenge (Zhu et al., 2014). Thus, effective vaccines may be needed to broadly induce neutralizing Abs. DNA vaccines are generally considered suboptimal for inducing humoral responses, particularly in humans. Strategies to overcome these limitations include the optimization of plasmid backbones, the use of molecular adjuvants and changing the delivery methods. We previously explored the possibility that the use of chemokine CCL19 or CCL28 could optimize systemic and mucosal humoral responses to a HIV-1 vaccine candidate gp140, which is the primary target of Ab-mediated antiviral functions (Hu et al., 2013). In the CCL19 adjuvant group, CCR7 showed a high expression on activated B cells, T cells and DCs in secondary lymphoid organs, which was beneficial for increasing the chance of interactions among B cells, T cells, and DCs in the local tissue (Table 2).
In the context of cellular immune responses, several potential roles of molecular adjuvants, such as cytokines and costimulatory molecules, in vaccination strategies have been investigated. Currently, various promising prophylactic vaccines focused on inducing substantial vaccine-specific T cell responses have been developed (Hartoonian et al., 2014; Rosendahl Huber et al., 2014). Increased breadth in the vaccine-induced T cell response has been found to be beneficial against many chronic pathogens (Kallas et al., 2016; Panagioti et al., 2016). The molecular adjuvant CCL19 plays an important role in augmenting the trafficking of T cell-based vaccines into regional T cell compartments through efficient CCR7 gene transduction and participates in the priming and preparation of antigen-specific T cells and the production of Abs (Müller and Lipp, 2003; Aritomi et al., 2010).
In chronic viral infections, memory B and T cells are more numerically and functionally superior to neutralizing antibodies than the corresponding naïve precursor cells that are present before infection as memory lymphocytes tend to induce rapid and powerful recall responses (Panagioti et al., 2016, 2018). Because neutralizing antibodies fail to provide control over persisting viral infections (e.g., herpesviruses, HIV-1, and HCV), therapeutic immunizations should focus on generating strong cellular T cell-based immunity and more virus-specific memory immunocytes. Live attenuated, synthetic and subunit vaccines are all able to elicit central memory T cells, effector memory T cells and resident memory T cells, and these responses are highly dependent on the addition of adjuvants and the route of administration (Schaerli and Moser, 2005; Mueller et al., 2013; Khan et al., 2017; Gebhardt et al., 2018).
Substantial evidence indicates that CCL19-adjuvanted vaccine recipients produce CD4+/CD8+ T cells more promptly and at higher levels than recipients administered a control vector lacking CCL19 (Table 2). In a study coadministering HSV-1 vaccine and plasmids encoding CCL19 (pCCL19) and HSV-1 gB (pgB), CCR7high DCs were activated and migrated, resulting in the enhancement of antigen uptake and presumably the augmentation of naïve T cell priming. Compared with pCCL19 delivered alone, the codelivery of pCCL19 and pgB induced a notable increase in lung CD11c+ DCs. In the pCCL19-codelivery group, the CD8+ T cells isolated from the lungs produced more IFN-γ. Consistently, it appears that CCL19 codelivery may lead to the induction of functional memory CD8+ T cells (Lee et al., 2003; Toka et al., 2003). The high level of IFN-γ observed in these CD8+ T cells may suggest that the enhanced potential to secrete IFN-γ plays a notable protective role in lethal mucosal HSV-1 challenges. In our HIV-1 and HSV-2 vaccine studies, adjuvant CCL19 codelivery produces a balanced enhancement of HIV-1 gp140- or HSV-2 gB-specific Th1/Th2 responses (Hu et al., 2013; Yan et al., 2015). Such enhancements appear to be associated with the mobilization of abundant CCR7+ immunocyte migration to secondary lymphoid organs and mucosal tissue. Similarly, a previous HCV vaccine study showed that CCL19 contributes to the activation of DCs and CD4+ T cells, enhances CD8+ T cell accumulation and helps attract rare virus-specific T cells in the context of HCV core DNA vaccination in mice (Hartoonian et al., 2014). CCL19 as molecular adjuvant is helpful in increasing the naïve T cell sensitivity to low density Ag presentation. Such functional studies might be helpful for human virus vaccine development.
Regarding virus challenges, whether enhanced immunity mediated by adjuvant CCL19 can protect animals against genital tract mucosal infections has been tested, and previous studies have demonstrated that CCL19 enhances the protective responses to an HSV-1 challenge (Lee et al., 2003; Toka et al., 2003). In these studies, a CCL19 protective vaccination regimen was shown to elicit increased serum and vaginal HSV-1 gB-specific IgG and IgA Ab levels through intranasal mucosal immunization. CCL19 appeared to play a pivotal role in increasing Ab responses (levels of total Abs, virus-specific Abs and neutralizing Abs). Furthermore, CCL19 enhanced gB-specific immune responses by improving the kinetics and distribution of the adaptive immune response to the codelivered antigen and protected animals against genital infection by HSV-2. Finally, the results showed that the fusion constructs induced strong HSV-2 gB-specific IgG and IgA Ab levels in mouse sera and vaginal fluids. In a different study, we revealed that a gB-CCL19 fusion construct exhibited a stronger ability to increase the numbers of CCR7+ immunocytes in secondary lymphoid tissue and IgA+ cells in mucosal sites (Figure 2). The enhanced systemic and vaginal mucosal Abs protected the tested mice against a lethal intravaginal HSV-2 challenge in vivo (Yan et al., 2015). These findings may provide an effective strategy for the design of vaccines against mucosal infection by sexually transmitted viruses.
This review seeks to address how we can apply the current understanding of CCL19 and CCR7 functions to better understand the pathogenesis of viral infections and better treat or prevent viral diseases. CCL19 and CCR7 are essential players in the balance between immunity and tolerance. CCL19 promotes activation-induced cell death (AICD) in antigen-specific CD4+ T cells, and this chemokine regulates not only T cell mobilization but also the post-activation fate of T cells (Yasuda et al., 2007; Förster et al., 2008). It is a key step to understanding the appropriate initial programing signals as the signaling pathways of CCR7 elicited by CCL19 during priming or boosting influences the development of lymphocytes. Information regarding the differences in anatomical location, activation, and differentiation between memory T cells in lymphoid organs and those in non-inducing lymphoid organs could also be very valuable (Thomson and Knolle, 2010). We believe that recent advances in the field of target cell signaling stimulation coupled with animal models that express viral antigens could provide an opportunity to directly address some of these questions in a living animal. Such work will not only improve our understanding of CCL19 and CCR7 expression and their functions during viral infections but may also provide tools for the design of new therapeutic strategies for the treatment of viral infections and the prevention of invading pathogens.
Several lymphocyte-targeted chemokines, such as CCL19, CCL21 and CCL28, are known to be important for regulating adaptive immune responses during viral infection, and their engagement with cognate receptors can result in enhanced T cell activation, expansion, and survival as well as the establishment of long-term memory. The most important feature of the immune system is its ability to produce protective immune responses against pathogens while maintaining tolerance to self-antigens and innocuous environmental Ags. Therefore, chemokines have the potential to serve as effective immunomodulatory components of prophylactic vaccines against chronic viruses (Hartoonian et al., 2014; Kallas et al., 2016).
YY, RC, XW, and KH analyzed the data. YY drafted the manuscript. LH, ML, and QH reviewed and finalized the manuscript. All authors read and approved the final manuscript.
This work was funded by the Wuxi Technology Bureau Scientific and Technology Project (CSE31N1607), Nanjing Medical University Project (2015NJMUZD078), Wuxi Key Medical Talents Program (ZDRC024), Wuxi Health and Family Planning Commission Appropriate Technology Extension Project (MS201702), National Natural Science Foundation of China (81701550 and 81572009), Open Research Fund Program of the State Key Laboratory of Virology of China (2019IOV005), and Deutsche Forschungsgemeinschaft (RTG1949 and Transregio TRR60).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abe, M., Zahorchak, A., Colvin, B., and Thomson, A. (2004). Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation 78, 762–765. doi: 10.1097/01.TP.0000130450.61215.3B
Alcami, A. (2003). Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3, 36–50. doi: 10.1038/nri980
Alturaiki, W., McFarlane, A. J., Rose, K., Corkhill, R., McNamara, P. S., Schwarze, J., et al. (2018). Expression of the B cell differentiation factor BAFF and chemokine CXCL13 in a murine model of Respiratory Syncytial Virus infection. Cytokine 110, 267–271. doi: 10.1016/j.cyto.2018.01.014
Anderson, C., Solari, R., and Pease, J. E. (2016). Biased agonism at chemokine receptors: obstacles or opportunities for drug discovery? J. Leukoc. Biol. 99, 901–909. doi: 10.1189/jlb.2MR0815-392R
Anderson, J. L., Mota, T. M., Evans, V. A., Kumar, N., Rezaei, S. D., Cheong, K., et al. (2016). Understanding factors that modulate the establishment of HIV latency in resting CD4+ T-cells in vitro. PLoS One 11:e0158778. doi: 10.1371/journal.pone.0158778
Appay, V., Dunbar, P. R., Callan, M., Klenerman, P., Gillespie, G. M. A., Papagno, L., et al. (2002). Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8, 379–385. doi: 10.1038/nm0402-379
Aritomi, K., Kuwabara, T., Tanaka, Y., Nakano, H., Yasuda, T., Ishikawa, F., et al. (2010). Altered antibody production and helper T cell function in mice lacking chemokines CCL19 and CCL21-Ser. Microbiol. Immunol. 54, 691–701. doi: 10.1111/j.1348-0421.2010.00266.x
Bardi, G., Lipp, M., Baggiolini, M., and Loetscher, P. (2001). The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur. J. Immunol. 31, 3291–3297.
Bardina, S. V., Brown, J. A., Michlmayr, D., Hoffman, K. W., Sum, J., Pletnev, A. G., et al. (2017). Chemokine receptor Ccr7 restricts fatal west nile virus encephalitis. J. Virol. 91, e02409–e02416. doi: 10.1128/jvi.02409-16
Baskic, D., Vukovic, V. R., Popovic, S., Djurdjevic, P., Zaric, M., Nikolic, I., et al. (2017). Cytokine profile in chronic hepatitis C: an observation. Cytokine 96, 185–188. doi: 10.1016/j.cyto.2017.04.008
Birkenbach, M., Josefsen, K., Yalamanchili, R., Lenoir, G., and Kieff, E. (1993). Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J. Virol. 67, 2209–2220.
Bock, A., Bermudez, M., Krebs, F., Matera, C., Chirinda, B., Sydow, D., et al. (2016). Ligand binding ensembles determine graded agonist efficacies at a G protein-coupled receptor. J. Biol. Chem. 291, 16375–16389. doi: 10.1074/jbc.M116.735431
Boettler, T., Panther, E., Bengsch, B., Nazarova, N., Spangenberg, H. C., Blum, H. E., et al. (2006). Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80, 3532–3540. doi: 10.1128/jvi.80.7.3532-3540.2006
Cameron, P. U., Saleh, S., Sallmann, G., Solomon, A., Wightman, F., Evans, V. A., et al. (2010). Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 107, 16934–16939. doi: 10.1073/pnas.1002894107
Cao, W., Qiu, Z., Zhu, T., Li, Y., Han, Y., and Li, T. (2014). CD8+ T cell responses specific for hepatitis B virus core protein in patients with chronic hepatitis B virus infection. J. Clin. Virol. 61, 40–46. doi: 10.1016/j.jcv.2014.06.022
Carotenuto, P., Artsen, A., Osterhaus, A. D., and Pontesilli, O. (2011). Reciprocal changes of naïve and effector/memory CD8+T lymphocytes in chronic hepatitis B virus infection. Viral Immunol. 24, 27–33. doi: 10.1089/vim.2010.0067
Chensue, S. W. (2001). Molecular machinations: chemokine signals in host-pathogen interactions. Clin. Microbiol. Rev. 14, 821–835. doi: 10.1128/CMR.14.4.821-835.2001
Cillo, A. R., Sobolewski, M. D., Bosch, R. J., Fyne, E., Piatak, M. Jr., Coffin, J. M., et al. (2014). Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. U.S.A. 111, 7078–7083. doi: 10.1073/pnas.1402873111
Comerford, I., Harata-Lee, Y., Bunting, M. D., Gregor, C., Kara, E. E., and McColl, S. R. (2013). A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 24, 269–283. doi: 10.1016/j.cytogfr.2013.03.001
Comerford, I., Milasta, S., Morrow, V., Milligan, G., and Nibbs, R. (2006). The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur. J. Immunol. 36, 1904–1916. doi: 10.1002/eji.200636327
Damås, J. K., Øktedalen, O., Ueland, T., Landrø, L., Barstad, J., Müller, F., et al. (2012). Enhanced levels of CCL19 in patients with advanced acquired immune deficiency syndrome (AIDS). Clin. Exp. Immunol. 167, 492–498. doi: 10.1111/j.1365-2249.2011.04524.x
de Paz, J. L., Moseman, E. A., Noti, C., Polito, L., von Andrian, U. H., and Seeberger, P. H. (2007). Profiling heparin-chemokine interactions using synthetic tools. ACS Chem. Biol. 2, 735–744. doi: 10.1021/cb700159m
Debes, G. F., Bonhagen, K., Wolff, T., Kretschmer, U., Krautwald, S., Kamradt, T., et al. (2004). CC chemokine receptor 7 expression by effector/memory CD4+ T cells depends on antigen specificity and tissue localization during influenza A virus infection. J. Virol. 78, 7528–7535. doi: 10.1128/jvi.78.14.7528-7535.2004
Doherty, P. C., Topham, D. J., Tripp, R. A., Cardin, R. D., Brooks, J. W., and Stevenson, P. G. (1997). Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159, 105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x
Doosti, M., Nassiri, M., Nasiri, K., Tahmoorespur, M., and Zibaee, S. (2019). Immunogenic evaluation of FMD virus immuno-dominant epitopes coupled with IL-2/FcIgG in BALB/c mice. Microb. Pathog. 132, 30–37. doi: 10.1016/j.micpath.2019.04.019
Dunham, J., van Driel, N., Eggen, B. J., Paul, C., ‘t Hart, BA., Laman, J. D., et al. (2017). Analysis of the cross-talk of Epstein-Barr virus-infected B cells with T cells in the marmoset. Clin. Transl. Immunol. 6:e127. doi: 10.1038/cti.2017.1
Ehlin-Henriksson, B., Liang, W., Cagigi, A., Mowafi, F., Klein, G., and Nilsson, A. (2009). Changes in chemokines and chemokine receptor expression on tonsillar B cells upon Epstein-Barr virus infection. Immunology 127, 549–557. doi: 10.1111/j.1365-2567.2008.03029.x
Ellefsen, K., Harari, A., Champagne, P., Bart, P. A., Sékaly, R. P., and Pantaleo, G. (2002). Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur. J. Immunol. 32, 3756–3764.
Fisicaro, P., Barili, V., Montanini, B., Acerbi, G., Ferracin, M., Guerrieri, F., et al. (2017). Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 23, 327–336. doi: 10.1038/nm.4275
Förster, R., Davalos-Misslitz, A. C., and Rot, A. (2008). CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8, 362–371. doi: 10.1038/nri2297
Förster, R., Schubel, A., Breitfeld, D., Kremmer, E., Renner-Müller, I., Wolf, E., et al. (1999). CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33. doi: 10.1016/s0092-8674(00)80059-8
Freire-de-Lima, L., Nardy, A. F. F. R., Ramos-Junior, E. S., Conde, L., Santos Lemos, J., Fonseca, L. M. D., et al. (2017). Multiple myeloma cells express key immunoregulatory cytokines and modulate the monocyte migratory response. Front. Med. 4:92. doi: 10.3389/fmed.2017.00092
García-Arriaza, J., Perdiguero, B., Heeney, J. L., Seaman, M. S., Montefiori, D. C., Yates, N. L., et al. (2017). HIV/AIDS vaccine candidates based on replication-competent recombinant poxvirus NYVAC-C-KC expressing trimeric gp140 and gag-derived virus-like particles or lacking the viral molecule B19 that inhibits type I Interferon activate relevant HIV-1-specific B and T cell immune functions in nonhuman primates. J. Virol. 91, e02182–e02198. doi: 10.1128/jvi.02182-16
Gebhardt, T., Palendira, U., Tscharke, D. C., and Bedoui, S. (2018). Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 283, 54–76. doi: 10.1111/imr.12650
Griffith, J. W., Sokol, C. L., and Luster, A. D. (2014). Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 32, 659–702. doi: 10.1146/annurev-immunol-032713-120145
Gupta, S., Clark, E. S., Termini, J. M., Boucher, J., Kanagavelu, S., LeBranche, C. C., et al. (2015). DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers. J. Virol. 89, 4158–4169. doi: 10.1128/jvi.02904-14
Han, Y. W., Aleyas, A. G., George, J. A., Kim, S. J., Kim, H. K., Yoo, D. J., et al. (2009). Genetic co-transfer of CCR7 ligands enhances immunity and prolongs survival against virulent challenge of pseudorabies virus. Immunol. Cell Biol. 87, 91–99. doi: 10.1038/icb.2008.69
Hartoonian, C., Sepehrizadeh, Z., Tabatabai Yazdi, M., Jang, Y. S., Langroudi, L., Amir Kalvanagh, P., et al. (2014). Enhancement of immune responses by co-delivery of CCL19/MIP-3beta chemokine plasmid with HCV core DNA/protein immunization. Hepat. Mon. 14:e14611. doi: 10.5812/hepatmon.14611
Hauser, M. A., and Legler, D. F. (2016). Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J. Leukoc. Biol. 99, 869–882. doi: 10.1189/jlb.2MR0815-380R
Hayasaka, H., Kobayashi, D., Yoshimura, H., Nakayama, E. E., Shioda, T., and Miyasaka, M. (2015). The HIV-1 Gp120/CXCR4 axis promotes CCR7 ligand-dependent CD4 T cell migration: CCR7 homo- and CCR7/CXCR4 hetero-oligomer formation as a possible mechanism for up-regulation of functional CCR7. PLoS One 10:e0117454. doi: 10.1371/journal.pone.0117454
Hjortø, G. M., Larsen, O., Steen, A., Daugvilaite, V., Berg, C., Fares, S., et al. (2016). Differential CCR7 targeting in dendritic cells by three naturally occurring CC-Chemokines. Front. Immunol. 7:568. doi: 10.3389/fimmu.2016.00568
Hong, H. S., Ahmad, F., Eberhard, J. M., Bhatnagar, N., Bollmann, B. A., Keudel, P., et al. (2012). Loss of CCR7 expression on CD56bright NK cells is associated with a CD56dimCD16+ NK cell-like phenotype and correlates with HIV viral load. PLoS One 7:e44820. doi: 10.1371/journal.pone.0044820.t001
Horst, A. K., Neumann, K., Diehl, L., and Tiegs, G. (2016). Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell. Mol. Immunol. 13, 277–292. doi: 10.1038/cmi.2015.112
Hsu, Y. L., Wang, M. Y., Ho, L. J., Huang, C. Y., and Lai, J. H. (2015). Up-regulation of galectin-9 induces cell migration in human dendritic cells infected with dengue virus. J. Cell. Mol. Med. 19, 1065–1076. doi: 10.1111/jcmm.12500
Hu, K., Luo, S., Tong, L., Huang, X., Jin, W., Huang, W., et al. (2013). CCL19 and CCL28 augment mucosal and systemic immune responses to HIV-1 gp140 by mobilizing responsive immunocytes into secondary lymph nodes and mucosal tissue. J. Immunol. 191, 1935–1947. doi: 10.4049/jimmunol.1300120
Hu, Z., Li, Y., Van Nieuwenhuijze, A., Selden, H. J., Jarrett, A. M., Sorace, A. G., et al. (2017). CCR7 modulates the generation of thymic regulatory T cells by altering the composition of the thymic dendritic cell compartment. Cell Rep. 21, 168–180. doi: 10.1016/j.celrep.2017.09.016
Inchley, C. S., Østerholt, H. C. D., Sonerud, T., Fjaerli, H. O., and Nakstad, B. (2013). Downregulation of IL7R, CCR7, and TLR4 in the cord blood of children with Respiratory Syncytial Virus disease. J. Infect. Dis. 208, 1431–1435. doi: 10.1093/infdis/jit336
Jafarnejad, M., Zawieja, D. C., Brook, B. S., Nibbs, R. J. B., and Moore, J. E. (2017). A novel computational model predicts key regulators of chemokine gradient formation in lymph nodes and site-specific roles for CCL19 and ACKR4. J. Immunol. 199, 2291–2304. doi: 10.4049/jimmunol.1700377
Johnson, R. A., Ma, X. L., Yurochko, A. D., and Huang, E. S. (2001). The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 82, 493–497. doi: 10.1099/0022-1317-82-3-493
Justo-Junior, A. S., Villarejos, L. M., Lima, X. T. V., Nadruz, W. Jr., Sposito, A. C., Mamoni, R. L., et al. (2019). Monocytes of patients with unstable angina express high levels of chemokine and pattern-recognition receptors. Cytokine 113, 61–67. doi: 10.1016/j.cyto.2018.06.008
Kallas, E., Huik, K., Türk, S., Pauskar, M., Jõgeda, E. L., Šunina, M., et al. (2016). T cell distribution in relation to HIV/HBV/HCV coinfections and intravenous drug use. Viral Immunol. 29, 464–470. doi: 10.1089/vim.2016.0057
Kanagavelu, S. K., Snarsky, V., Termini, J. M., Gupta, S., Barzee, S., Wright, J. A., et al. (2012). Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine. Vaccine 30, 691–702. doi: 10.1016/j.vaccine.2011.11.088
Khan, A. A., Srivastava, R., Chentoufi, A. A., Kritzer, E., Chilukuri, S., Garg, S., et al. (2017). Bolstering the number and function of HSV-1-specific CD8+ effector memory T cells and tissue-resident memory T cells in latently infected trigeminal ganglia reduces recurrent ocular herpes infection and disease. J. Immunol. 199, 186–203. doi: 10.4049/jimmunol.1700145
Kim, S., Han, S., Kim, T., Nam, J., Kim, Y. S., Choi, E. K., et al. (2018). Prolonged follicular helper T cell responses in ME7 scrapie-infected mice. Prion 12, 1–8. doi: 10.1080/19336896.2018.1458573
Kim, S., Han, S., Lee, H. S., Kim, Y. S., Choi, E. K., and Kim, M. Y. (2016). Impaired spleen structure and chemokine expression in ME7 scrapie-infected mice. Immunobiology 221, 871–878. doi: 10.1016/j.imbio.2016.03.008
Kohout, T. A., Nicholas, S. L., Perry, S. J., Reinhart, G., Junger, S., and Struthers, R. S. (2004). Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem. 279, 23214–23222. doi: 10.1074/jbc.M402125200
Laufer, J. M., Kindinger, I., Artinger, M., Pauli, A., and Legler, D. F. (2019). CCR7 is recruited to the immunological synapse, acts as co-stimulatory molecule and drives LFA-1 clustering for efficient T cell adhesion through ZAP70. Front. Immunol. 9:3115. doi: 10.3389/fimmu.2018.03115
Le Nouën, C, Hillyer, P., Winter, C. C., McCarty, T., Rabin, R. L., Collins, P. L., et al. (2011). Low CCR7-mediated migration of human monocyte derived dendritic cells in response to human Respiratory Syncytial Virus and human metapneumovirus. PLoS Pathog. 7:e1002105. doi: 10.1371/journal.ppat.1002105
Lee, Y., Eo, S. K., Rouse, R. J. D., and Rouse, B. T. (2003). Influence of CCR7 ligand DNA preexposure on the magnitude and duration of immunity. Virology 312, 169–180. doi: 10.1016/s0042-6822(03)00199-5
Li, C., Guan, X., Du, T., Jin, W., Wu, B., Liu, Y., et al. (2015). Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J. Gen. Virol. 96, 2381–2393. doi: 10.1099/vir.0.000139
Liu, J., Yu, Q., Wu, W., Huang, X., Broering, R., Werner, M., et al. (2018). TLR2 stimulation strengthens intrahepatic myeloid-derived cell-mediated T cell tolerance through inducing kupffer cell expansion and IL-10 production. J. Immunol. 200, 2341–2351. doi: 10.4049/jimmunol.1700540
Maier, S., Santak, M., Mantik, A., Grabusic, K., Kremmer, E., Hammerschmidt, W., et al. (2005). A somatic knockout of CBF1 in a human B-cell line reveals that induction of CD21 and CCR7 by EBNA-2 is strictly CBF1 dependent and that downregulation of immunoglobulin M is partially CBF1 independent. J. Virol. 79, 8784–8792. doi: 10.1128/jvi.79.14.8784-8792.2005
Maier, S., Staffler, G., Hartmann, A., Höck, J., Henning, K., Grabusic, K., et al. (2006). Cellular target genes of epstein-barr virus nuclear antigen 2. J. Virol. 80, 9761–9771. doi: 10.1128/jvi.00665-06
Mathew, A., and Rothman, A. L. (2008). Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol. Rev. 225, 300–313. doi: 10.1111/j.1600-065X.2008.00678.x
McNamara, P. S., Fonceca, A. M., Howarth, D., Correia, J. B., Slupsky, J. R., Trinick, R. E., et al. (2013). Respiratory Syncytial Virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax 68, 76–81. doi: 10.1136/thoraxjnl-2012-202288
Mora, J. R., and von Andrian, U. H. (2008). Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 1, 96–109. doi: 10.1038/mi.2007.14
Mueller, S. N., Gebhardt, T., Carbone, F. R., and Heath, W. R. (2013). Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161. doi: 10.1146/annurev-immunol-032712-095954
Müller, G., and Lipp, M. (2003). Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking. Microcirculation 10, 325–334. doi: 10.1038/sj.mn.7800197
Murdoch, C., and Finn, A. (2000). Chemokine receptors and their role in inflammation and infectious diseases. Blood 95, 3032–3043.
Murphy, P. M. (2001). Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2, 116–122. doi: 10.1038/84214
Nagira, M., Imai, T., Hieshima, K., Kusuda, J., Ridanpää, M., Takagi, S., et al. (1997). Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that Is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J. Biol. Chem. 272, 19518–19524. doi: 10.1074/jbc.272.31.19518
Nitschke, K., Luxenburger, H., Kiraithe, M. M., Thimme, R., and Neumann-Haefelin, C. (2016). CD8+ T-cell responses in hepatitis B and C: the (HLA-) A, B, and C of hepatitis B and C. Dig. Dis. 34, 396–409. doi: 10.1159/000444555
Ohl, L., Mohaupt, M., Czeloth, N., Hintzen, G., Kiafard, Z., Zwirner, J., et al. (2004). CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288. doi: 10.1016/j.immuni.2004.06.014
Otero, C., Eisele, P. S., Schaeuble, K., Groettrup, M., and Legler, D. F. (2008). Distinct motifs in the chemokine receptor CCR7 regulate signal transduction, receptor trafficking and chemotaxis. J. Cell. Sci. 121, 2759–2767. doi: 10.1242/jcs
Panagioti, E., Klenerman, P., Lee, L. N., van der Burg, S. H., and Arens, R. (2018). Features of effective T cell-inducing vaccines against chronic viral infections. Front. Immunol. 9:276. doi: 10.3389/fimmu.2018.00276
Panagioti, E., Redeker, A., van Duikeren, S., Franken, K. L., Drijfhout, J. W., van der Burg, SH., et al. (2016). The breadth of synthetic long peptide vaccine-induced CD8+ T cell responses determines the efficacy against mouse cytomegalovirus infection. PLoS Pathog. 12:e1005895. doi: 10.1371/journal.ppat.1005895
Piqueras, B., Connolly, J., Freitas, H., Palucka, A. K., and Banchereau, J. (2006). Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood 107, 2613–2618. doi: 10.1182/blood2005-07-2965
Potsch, C., Vöhringer, D., and Pircher, H. (1999). Distinct migration patterns of naive and effector CD8 T cells in the spleen_ correlation with CCR7 receptor expression and chemokine reactivity. Eur. J. Immunol. 29, 3562–3570.
Raju, R., Gadakh, S., Gopal, P., George, B., Advani, J., Soman, S., et al. (2015). Differential ligand-signaling network of CCL19/CCL21-CCR7 system. Database 2015:bav106. doi: 10.1093/database/bav106
Ramirez, P. W., Famiglietti, M., Sowrirajan, B., DePaula-Silva, A. B., Rodesch, C., Barker, E., et al. (2014). Downmodulation of CCR7 by HIV-1 vpu results in impaired migration and chemotactic signaling within CD4+ T cells. Cell Rep. 7, 2019–2030. doi: 10.1016/j.celrep.2014.05.015
Reed, J. L., Welliver, T. P., Sims, G. P., McKinney, L., Velozo, L., Avendano, L., et al. (2009). Innate immune signals modulate antiviral and polyreactive antibody responses during severe Respiratory Syncytial Virus infection. J. Infect. Dis. 199, 1128–1138. doi: 10.1086/597386
Romero, P., Zippelius, A., Kurth, I., Pittet, M. J., Touvrey, C., Iancu, E. M., et al. (2007). Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 178, 4112–4119. doi: 10.4049/jimmunol.178.7.4112
Rosendahl Huber, S., van Beek, J., de Jonge, J., Luytjes, W., and van Baarle, D. (2014). T cell responses to viral infections-opportunities for peptide vaccination. Front. Immunol. 5:171. doi: 10.3389/fimmu.2014.00171
Rot, A., and von Andrian, U. H. (2004). Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 22, 891–928. doi: 10.1146/annurev.immunol.22.012703.104543
Saleh, S., Lu, H. K., Evans, V., Harisson, D., Zhou, J., Jaworowski, A., et al. (2016). HIV integration and the establishment of latency in CCL19-treated resting CD4+ T cells require activation of NF-κB. Retrovirology 13:49. doi: 10.1186/s12977-016-0284-7
Saleh, S., Solomon, A., Wightman, F., Xhilaga, M., Cameron, P. U., and Lewin, S. R. (2007). CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection a novel model of HIV-1 latency. Blood 110, 4161–4164. doi: 10.1182/blood-2007-06-097907
Sánchez-Sánchez, N., Riol-Blanco, L., de la Rosa, G., Puig-Kröger, A., García-Bordas, J., Martín, D., et al. (2004). Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood 104, 619–625. doi: 10.1182/blood-2003-11-3943
Schaerli, P., and Moser, B. (2005). Chemokines: control of primary and memory T-cell traffic. Immunol. Res. 31, 57–74. doi: 10.1385/IR:31:1:57
Shen, X., Bogers, W. M., Yates, N. L., Ferrari, G., Dey, A. K., Williams, W. T., et al. (2017). Cross-linking of a CD4-mimetic miniprotein with HIV-1 env gp140 alters kinetics and specificities of antibody responses against HIV-1 env in macaques. J. Virol. 91, e00401–e00417. doi: 10.1128/jvi.00401-17
Shin, G. C., Kang, H. S., Lee, A. R., and Kim, K. H. (2016). Hepatitis B virus–triggered autophagy targets TNFRSF10B/death receptor 5 for degradation to limit TNFSF10/TRAIL response. Autophagy 12, 2451–2466. doi: 10.1080/15548627.2016.1239002
Sin, J. I., Kim, J. J., Arnold, R. L., Shroff, K. E., McCallus, D., Pachuk, C., et al. (1999). IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 162, 2912–2921.
Spivak, A. M., Larragoite, E. T., Coletti, M. L., Macedo, A. B., Martins, L. J., Bosque, A., et al. (2016). Janus kinase inhibition suppresses PKC-induced cytokine release without affecting HIV-1 latency reversal ex vivo. Retrovirology 13:88. doi: 10.1186/s12977-016-0319-0
Steen, A., Larsen, O., Thiele, S., and Rosenkilde, M. M. (2014). Biased and g protein-independent signaling of chemokine receptors. Front. Immunol. 5:277. doi: 10.3389/fimmu.2014.00277
Tahamtan, A., Tavakoli-Yaraki, M., Shadab, A., Rezaei, F., Marashi, S. M., Shokri, F., et al. (2018). The role of cannabinoid receptor 1 in the immunopathology of Respiratory Syncytial Virus. Viral Immunol. 31, 292–298. doi: 10.1089/vim.2017.0098
Thomson, A. W., and Knolle, P. A. (2010). Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 10, 753–766. doi: 10.1038/nri2858
Tian, X., Kang, D. S., and Benovic, J. L. (2014). Beta-arrestins and G protein-coupled receptor trafficking. Handb. Exp. Pharmacol. 219, 173–186. doi: 10.1007/978-3-642-41199-1_9
Toka, F. N., Gierynska, M., and Rouse, B. T. (2003). Codelivery of CCR7 ligands as molecular adjuvants enhances the protective immune response against herpes simplex virus type 1. J. Virol. 77, 12742–12752. doi: 10.1128/jvi.77.23.12742-12752.2003
Unsoeld, H., Krautwald, S., Voehringer, D., Kunzendorf, U., and Pircher, H. (2002). Cutting edge: CCR7+ and CCR7- memory T cells do not differ in immediate effector cell function. J. Immunol. 169, 638–641. doi: 10.4049/jimmunol.169.2.638
Wang, Z., Wang, L., Qian, T., and Chu, Y. (2018). Chemokines and receptors in intestinal B lymphocytes. J. Leukoc. Biol. 103, 807–819. doi: 10.1002/jlb.1ru0717-299rr
Welliver, T. P., Garofalo, R. P., Hosakote, Y., Hintz, K. H., Avendano, L., Sanchez, K., et al. (2007). Severe human lower respiratory tract illness caused by Respiratory Syncytial Virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195, 1126–1136. doi: 10.1086/512615
Wieland, D., Hofmann, M., and Thimme, R. (2017). Overcoming CD8+ T-cell exhaustion in viral hepatitis: lessons from the mouse model and clinical perspectives. Dig. Dis. 35, 334–338. doi: 10.1159/000456584
Wilflingseder, D., Müllauer, B., Schramek, H., Banki, Z., Pruenster, M., Dierich, M. P., et al. (2004). HIV-1-induced migration of monocyte-derived dendritic cells is associated with differential activation of MAPK pathways. J. Immunol. 173, 7497–7505. doi: 10.4049/jimmunol.173.12.7497
Wu, L., Ehlin-Henriksson, B., Zhou, X., Zhu, H., Ernberg, I., Kis, L. L., et al. (2017). Epstein-Barr virus (EBV) provides survival factors to EBV+ diffuse large B-cell lymphoma (DLBCL) lines and modulates cytokine induced specific chemotaxis in EBV+ DLBCL. Immunology 152, 562–573. doi: 10.1111/imm.12792
Wu, W. L., Ho, L. J., Chang, D. M., Chen, C. H., and Lai, J. H. (2009). Triggering of DC migration by dengue virus stimulation of COX-2-dependent signaling cascadesin vitrohighlights the significance of these cascades beyond inflammation. Eur. J. Immunol. 39, 3413–3422. doi: 10.1002/eji.200939306
Wu, W. L., Ho, L. J., Chen, P. C., Tsai, Y. T., Hsu, S. T., Chang, D. M., et al. (2011). Immunosuppressive effects and mechanisms of leflunomide in dengue virus infection of human dendritic cells. J. Clin. Immunol. 31, 1065–1078. doi: 10.1007/s10875-011-9578-7
Yan, Y., Hu, K., Deng, X., Guan, X., Luo, S., Tong, L., et al. (2015). Immunization with HSV-2 gB-CCL19 fusion constructs protects mice against lethal vaginal challenge. J. Immunol. 195, 329–338. doi: 10.4049/jimmunol.1500198
Yanagawa, Y., and Onoe, K. (2002). CCL19 induces rapid dendritic extension of murine dendritic cells. Blood 100, 1948–1956. doi: 10.1182/blood-2002-01-0260
Yasuda, T., Kuwabara, T., Nakano, H., Aritomi, K., Onodera, T., et al. (2007). Chemokines CCL19 and CCL21 promote activation-induced cell death of antigen-responding T cells. Blood 109, 449–456. doi: 10.1182/blood-2006-04-018101
Yoshida, R., Nagira, M., Kitaura, M., Imagawa, N., Imai, T., and Yoshie, O. (1998). Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol. Chem. 273, 7118–7122. doi: 10.1074/jbc.273.12.7118
Zhang, Z., Jin, B., Zhang, J. Y., Xu, B., Wang, H., Shi, M., et al. (2009). Dynamic decrease in PD-1 expression correlates with HBV-specific memory CD8 T-cell development in acute self-limited hepatitis B patients. J. Hepatol. 50, 1163–1173. doi: 10.1016/j.jhep.2009.01.026
Zhu, X. P., Muhammad, Z., Wang, J. G., Lin, W., Guo, S. K., and Zhang, W. (2014). HSV-2 vaccine: current status and insight into factors for developing an efficient vaccine. Viruses 6, 371–390. doi: 10.3390/v6020371
Keywords: CCL19, CCR7, chemotaxis, antivirus, signaling, adjuvant
Citation: Yan Y, Chen R, Wang X, Hu K, Huang L, Lu M and Hu Q (2019) CCL19 and CCR7 Expression, Signaling Pathways, and Adjuvant Functions in Viral Infection and Prevention. Front. Cell Dev. Biol. 7:212. doi: 10.3389/fcell.2019.00212
Received: 30 April 2019; Accepted: 18 September 2019;
Published: 01 October 2019.
Edited by:
Bin Li, Shanghai Jiao Tong University School of Medicine, ChinaReviewed by:
Shulin Qin, University of Pittsburgh, United StatesCopyright © 2019 Yan, Chen, Wang, Hu, Huang, Lu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihua Huang, aHVhbmdsaWh1YTE5NjRAc2luYS5jb20=; Mengji Lu, bWVuZ2ppLmx1QHVuaS1kdWUuZGU=; Qinxue Hu, cWh1QHdoLmlvdi5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.