
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol., 12 March 2019
Sec. Signaling
Volume 7 - 2019 | https://doi.org/10.3389/fcell.2019.00033
This article is part of the Research TopicYAP1/Hippo Pathway in Development and DiseaseView all 6 articles
The first description of Hippo signaling in mammals a little more than 10 years ago showed a striking phenotype in the liver, linking the role of this signaling pathway to organ size control and carcinogenesis. Even though Hippo signaling has been extensively studied in the liver and other organs over the recent years, many open questions remain in our understanding of its role in hepatic physiology and disease. The functions of Hippo signaling extend well beyond cancer and organ size determination: components of upstream Hippo signaling and the downstream effectors YAP and TAZ are involved in a multitude of cell and non-cell autonomous functions including cell proliferation, survival, development, differentiation, metabolism, and cross-talk with the immune system. Moreover, regulation and biological functions of Hippo signaling are often organ or even cell type specific – making its role even more complex. Here, we give a concise overview of the role of Hippo signaling in the liver with a focus on cell-type specific functions. We outline open questions and future research directions that will help to improve our understanding of this important pathway in liver disease.
Several years after the identification and characterization of individual Hippo pathway members in Drosophila and mammals, the importance of Hippo signaling in the liver became evident with a striking phenotype: overexpression of YAP or expression of activated YAP resulted in dramatic overgrowth of the liver, identifying Hippo signaling as an important determinant in organ size control (Camargo et al., 2007; Dong et al., 2007). Rapid development of hepatocellular carcinoma (HCC) upon YAP overexpression further confirmed a potent oncogenic role of this protein (Dong et al., 2007). More recently, the investigation of Hippo signaling in non-parenchymal liver cells, including hepatic stellate cells (HSC) and liver sinusoidal endothelial cells (LSEC) has brought insight into the complex interplay between different hepatic cell types with profound impact on the pathophysiology of liver disease. Here, we provide an overview of Hippo signaling in the liver including recent advances and open questions along with future directions in the field.
Soon after the discovery of YAP function in murine liver, MST1 and MST2 protein kinases were confirmed as upstream Hippo pathway regulators that restrict YAP activation, tissue overgrowth, and carcinogenesis (Figure 1; Zhou et al., 2009; Lu et al., 2010; Song et al., 2010). In the same line, hepatic inactivation of the MST1/2-adaptor protein SAV1/WW45 resulted in YAP-associated cell proliferation and mutant mice ultimately developed tumors with characteristics of HCC and intrahepatic cholangiocarcinomas (ICC) (Lee et al., 2010; Lu et al., 2010). The conditional knock-out of Nf2, the mammalian homolog of the upstream Hippo regulator Merlin, led to a reduction in Lats1/2 phosphorylation and thereby activation of YAP, resulting in hepatic overgrowth and liver tumor development (Benhamouche et al., 2010; Zhang et al., 2010). Importantly, these findings established NF2 as a negative regulator of YAP and confirmed that a large part of the Drosophila Hippo pathway is conserved in mammals (Figure 1).
In all of these models, conditional inactivation of Hippo pathway genes was achieved by using either CAGGCre-ER transgenic mice (Zhou et al., 2009; Song et al., 2010) or an Albumin-driven Cre (Benhamouche et al., 2010; Lu et al., 2010; Zhang et al., 2010), which is also active in fetal hepatoblasts that give rise to bile duct cells. Mutant mice showed varying degrees of hepatocyte proliferation but also exhibited proliferation and expansion of a hepatic cell population with small nuclei around the portal triad, so-called “oval cells.” These cells were long considered to function as bipotent liver progenitor cells that can differentiate into hepatocytes and bile duct cells under certain conditions such as severe hepatocyte damage – a hypothesis that has been challenged by recent research (Tanimizu and Mitaka, 2014). The expansion of oval cells and the development of both HCC and ICC initially led to the speculation that tumors in Hippo pathway-inactivated models arise from these potential bipotent progenitor cells. However, recent studies suggest that these phenotypes arise from trans-differentiation of mutant hepatocytes and deregulated biliary morphogenesis (Yimlamai et al., 2014; Benhamouche-Trouillet et al., 2018). Several hepatocyte-specific transfection models can trigger the development of tumors with mixed differentiation: overexpression of YAP as well as inactivation of the upstream Hippo regulator Nf2 mediated by AAV-Cre induces de-differentiation of hepatocytes toward a progenitor-like phenotype (Yimlamai et al., 2014). Additionally, hydrodynamic tail vein injection of transposon-based expression constructs for constitutively active YAP and PIK3CA – the catalytic subunit of PI3K – resulted in formation of liver tumors with hepatocellular, cholangiocellular, or mixed HCC/ICC differentiation. In this model, tumors were characterized by activation of mTORC1/2, ERK/MAPK, and Notch pathways (Li et al., 2015). To date, the molecular basis for the cooperation between PI3K and YAP signaling in liver cancer is not well understood, but could be mediated be PI3K-induced upregulation of CD166, a cell surface protein that has been shown to positively regulate YAP activity (Ma et al., 2014). On the other hand, data from breast epithelial cells and colon cancer cells indicate that PI3K/PDK1/AKT signaling promotes YAP activity via LATS-dependent and -independent mechanisms (Zhao et al., 2018). However, if this mechanism is conserved in liver cancer and how it relates to cellular differentiation remains to be investigated. From what is known to date, YAP – and possibly other oncogenic pathways such as PI3K signaling – not only seem to promote proliferation and tumorigenesis in general, but also oncogenic plasticity of hepatocytes with trans-differentiation toward a progenitor-like or even biliary phenotype (Yimlamai et al., 2014; Fitamant et al., 2015; Font-Burgada et al., 2015; Patel et al., 2017). Notch signaling – a critical pathway in bile duct development – is a key candidate for triggering this differentiation switch. Of note, Notch signaling can be activated though upregulation of the YAP target genes such as Notch2 and Jag1 (Jeliazkova et al., 2013; Tschaharganeh et al., 2013; Yimlamai et al., 2014; Wu et al., 2017; Zhang S. et al., 2018). However, a study using liver-specific knock-out of Hippo pathway kinases LATS1 and LATS2 did not confirm a role of Notch signaling but indicated that active TGFβ signaling downstream of YAP induces trans-differentiation into bile duct cells (Lee et al., 2016). Additionally, YAP promotes the binding of transcriptional regulators HNF4A and FOXA2 to embryonic enhancer sites during hepatocyte differentiation to increase transcription of embryo-specific genes – a mechanism that could be hijacked in transformed cells to promote dedifferentiation and oncogenesis (Alder et al., 2014). Taken together, a complex pattern of oncogenic pathway interactions determines hepatocyte plasticity and tumor differentiation upon Hippo pathway inactivation that we are only beginning to understand.
In addition to promoting biliary differentiation in hepatocytes, YAP signaling is of considerable relevance bile ducts cells. High cytoplasmic and nuclear YAP expression in cholangiocytes in human and murine livers suggest that YAP activity is of relevance in bile duct cells, but functional studies dissecting the role of Hippo signaling in cholangiocytes are limited. (Bai et al., 2012; Patel et al., 2017), Nevertheless, several interesting observations in Albumin-Cre-based conditional knock-out models that activate Cre in precursors of both hepatocytes and cholangiocytes were made (Geisler et al., 2008): conditional knock-out of YAP alone or of both YAP and its ortholog TAZ – also known as WWTR1 – results in defects in bile duct morphogenesis with irregularly shaped and deformed intrahepatic bile ducts (Zhang et al., 2010; Lu et al., 2018), while knock-out of Nf2 resulted in the expansion of biliary structures (Benhamouche-Trouillet et al., 2018).
In humans, YAP is activated in ductular reactions in cholestatic liver disease and in non-alcoholic steatohepatitis (Bai et al., 2012; Anakk et al., 2013; Machado et al., 2015). In cholestatic liver damage after bile duct ligation in mice, this reactive proliferation and expansion of bile duct cells is markedly reduced in YAP-deficient livers – indicating that YAP activity is required for the formation of ductular reactions. In adult mice, genetic inactivation of YAP using the inducible Mx-Cre system results in profound hepatocyte damage and reduced compensatory proliferation of hepatocytes and bile duct cells, which is associated with increased mortality in comparison to wild type mice (Bai et al., 2012). Mechanistically, accumulation of bile acids in cholestatic liver disease seems to be an important activator of YAP downstream of the scaffolding protein IQGAP1, which is induced by bile acids – a mechanism that might also play a role in carcinogenesis (Anakk et al., 2013). Mechanistically, there is considerable evidence that IQGAP1 can directly bind to YAP to alter its transcriptional activity (Sayedyahossein et al., 2016). However, this interaction seems to be mostly inhibitory, indicating that a different, so far unknown mechanism might be responsible for IQGAP1-mediated YAP activation in the liver. In human cholangiocarcinoma, activated YAP is associated with poor prognosis, chemoresistance, angiogenesis, and chromosomal instability (Marti et al., 2015; Wu et al., 2016; Rizvi et al., 2018), and high TAZ expression correlates with a decreased survival after tumor resection (Xiao et al., 2016). In mice, expression of activated YAP and AKT in hepatocytes is sufficient to induce cholangiocarcinoma dependent on AKT/mTOR signaling, which likely cooperates with YAP in tumorigenesis and possibly promotes YAP activity (Zhang et al., 2017; Zhao et al., 2018). Furthermore, YAP is important for the maintenance of a cholangiocyte phenotype, as inhibition of YAP by overexpression of either LATS2 or of a dominant-negative variant of TEAD2 resulted in decreased expression of cholangiocyte markers (Zhang S. et al., 2018).
It has long been a mystery how the restoration of liver size almost exactly to its original volume is reliably achieved after liver resection. Hippo signaling seemed to be the ideal candidate for a pathway that governs controlled activation – and cessation – of hepatocyte proliferation during regeneration. Indeed, there is a robust activation of downstream YAP/TAZ about 24 h after partial hepatectomy followed by an activation of inhibitory Hippo kinases possibly to restrict excessive YAP/TAZ activation and halt proliferation once the liver approaches its original volume (Grijalva et al., 2014; Loforese et al., 2017; Lu et al., 2018). Liver-specific knock-out of YAP and TAZ results in impaired liver regeneration and delays restoration of liver mass (Lu et al., 2018), confirming the importance of Hippo signaling in liver regeneration. Contrarily, YAP and TAZ are not mandatory for completion of the regeneration process as alternative pathways can substitute for their activity to ensure liver regeneration – albeit with substantial delay (Michalopoulos, 2017). In fetal development, the Alb-Cre mediated conditional knockout of YAP in hepatocytes and cholangiocytes starting from embryonic day 13.5 resulted mainly in defective intrahepatic bile duct development – but interestingly in no overt phenotype in hepatocytes up to 8 weeks after birth (Zhang et al., 2010). In part, this finding has been attributed to rescue of YAP function by increased activity of its ortholog TAZ, similar to phenotypes overserved in zebrafish (Yi et al., 2018). But strikingly, deletion of both YAP and TAZ by Albumin-Cre leads to development of an almost-normal liver – and paradoxically results in an increase in liver size. This unexpected phenotype can be explained by an increase in hepatocyte damage with subsequent activation of other pro-proliferation pathways that are unable to control hepatic mass as tightly as Hippo signaling (Zhang et al., 2010; Lu et al., 2018). These findings highlight that pathway activation and interaction in embryonic development and during liver regeneration is characterized by a high plasticity to adopt to functional deficiency of major pro-proliferative pathways such as YAP/TAZ signaling. However, this functional redundancy does not seem to work as efficiently once the mice progress beyond a certain age: Older or diseased livers often fail to sufficiently regenerate as they are not able maintain adequate proliferative signaling. To some extent, this regeneration defect is due to hyperactive Hippo signaling – which is not sufficiently rescued by compensatory activation of other pro-proliferative pathways for reasons that are no completely understood to date. Silencing MST kinases by liposome-encapsulated siRNA restored expression of YAP target genes and improved liver regeneration in older animals (Loforese et al., 2017). In another study, a novel MST2 inhibitor reduced apoptosis and liver damage in acute aminacetophen-induced liver failure. Strikingly, a similar effect including subsiding fibrosis was observed in chronic liver injury after bile duct ligation or repeated CCl4-administration, respectively (Fan et al., 2016). Targeting upstream hippo kinases in human acute or chronic liver failure to restore regeneration is a promising approach with high clinical relevance – but must be weighed against the risk for oncogenic transformation especially in cirrhotic livers that inherently have a high risk for HCC development.
Hepatic stellate cells are the major cell type involved in liver fibrosis, but also play a critical role in acute repair after liver injury or partial hepatectomy (Kordes et al., 2014). Chronic liver damage triggers persistent activation of stellate cells with secretion and accumulation of excessive extracellular matrix (ECM) proteins, trans-differentiation of quiescent HSCs into myofibroblasts, cumulating in the formation of fibrotic scar tissue. The outcome of this dysregulated repair process is dictated by the molecular drivers that control HSC activation, which are therefore considered as candidates for targeted therapies. Recently, Hippo signaling has been identified as an important pathway in stellate cell activation. YAP is activated in HSCs during acute liver regeneration after partial hepatectomy or ischemia-reperfusion injury, and correspondingly in chronic injury in human fibrotic livers as well as in CCl4-induced fibrosis in mice (Mannaerts et al., 2015; Swiderska-Syn et al., 2016; Konishi et al., 2018). Importantly, sustained YAP activation in liver fibrosis is associated with an increase in ECM proteins and tissue stiffness (Dechene et al., 2010). The influence of matrix stiffness on YAP signaling is well established and mechanical cues are increasingly recognized as a mediator of pathological YAP activation in vivo: Increased matrix stiffness is associated with activation of cell surface receptors, that regulate F-actin polymerization in a RhoA-dependent manner to promote YAP/TAZ nuclear translocation and activation – probably providing an the molecular basis for YAP activation in HSCs and hepatocytes in fibrosis and possibly increasing carcinogenesis in cirrhotic livers (Dupont et al., 2011; Zhubanchaliyev et al., 2016). In acute liver injury, other pathways such as Hedgehog signaling likely contribute to YAP activation. After partial hepatectomy, mice with HSCs deficient for functional Hedgehog signaling show impaired activation of YAP in stellate cells and also in hepatocytes (Swiderska-Syn et al., 2016) – indicating that Hedgehog/YAP signaling mediates an important cross-talk between these cell types to ensure sufficient regeneration. While HSC activity is important for hepatic integrity following acute injury, sustained activation is deleterious in chronic liver damage – making YAP a potential target to prevent HSC activation and progression of fibrosis. Importantly, pharmacological inhibition of YAP/TEAD by verteporfin remarkably reduced HSC activation and impeded fibrogenesis that normally occurs after CCl4-treatment in mice (Mannaerts et al., 2015; Martin et al., 2016).
Other non-parenchymal cells with an essential role in liver injury are liver sinusoidal endothelial cells (LSECs), that have been shown to influence regeneration and fibrosis through angiocrine signaling to stellate cells in acute and chronic liver damage (Ding et al., 2014; Poisson et al., 2017). Co-culture experiments revealed that differentiated LSECs maintain HSC quiescence and that a dysregulated crosstalk between hepatocytes, LSECs, and HSCs in chronic liver injury contributes to fibrosis (Poisson et al., 2017). Interestingly, LSECs are also important in maintaining cell integrity after YAP activation in single hepatocytes. Combined hepatocyte and LSEC damage induced by ethanol – but not damage of hepatocytes or LSECs alone – changes the fate of YAP-activated hepatocytes from proliferation to apoptosis (Miyamura et al., 2017). YAP activation in LSECs has been shown to govern protein expression of HIF-1α and VEGF-A to promote angiogenesis in fibrosis – a process blocking nutrient transport from sinusoids and exacerbating the liver injury (Elpek, 2015; Zhang C. et al., 2018). Aside from triggering neo-angiogenesis in the diseased liver, it is highly likely that YAP/TAZ also play a major role in hepatic blood vessel formation during liver development, including in endothelial cell (EC) sprouting and junction maturation (Park and Kwon, 2018). However, a relevant expression of YAP/TAZ could not be detected in hepatic ECs at P5 and P12, which is in contrast to endothelial cells in developing retina and brain at the same time points – tissues where YAP and TAZ are functionally required for proper angiogenesis (Kim et al., 2017a). While this could indicate that YAP/TAZ signaling might not be relevant in liver angiogenesis, it is more likely that activation of YAP and TAZ does occur at earlier time points during liver development, where EC formation starts as early as E9.5 (Zhao and Duncan, 2005). If activation of YAP/TAZ signaling in endothelial cells plays a significant role in fibrosis and other liver diseases, however, remains to be investigated.
With the emerging importance of Hippo signaling in HSC, LSECs, and possibly endothelial cells in fibrosis and injury, any considerations to therapeutically target this pathway should take into account that liver regeneration will likely be impaired – possibly exacerbating liver injury in the long run.
In any tissue, cell proliferation and growth are dependent on nutrient availability. In this context, regulation of pro-proliferative YAP and TAZ by cellular metabolism is likely. Indeed, low energy levels trigger inhibition of YAP and TAZ, while high levels of glucose and fatty acids activate downstream YAP/TAZ transcription. On the other hand, YAP/TAZ themselves have been shown to promote key metabolic processes such as glycolysis to ensure an adequate energy supply in proliferating cells (Koo and Guan, 2018). In the liver, excess availability of nutrients can lead to steatosis and steatohepatitis (non-alcoholic fatty liver disease or NAFLD), in which fat accumulation results in inflammation and destruction hepatocytes, promoting the development of cirrhosis and liver cancer.
In murine models of NAFLD and in samples of human steatohepatitis an increase of YAP/TAZ levels can be observed – mainly in regenerative ductular reactions. In mice, viral expression of activated TAZ promotes inflammation in the liver, NAFLD, and tumor formation, linking TAZ – but not YAP – to an inflammatory signature in tumor development (Wang et al., 2016; Hagenbeek et al., 2018). Mechanistically, TAZ/TEAD directly activate Hedgehog signaling with increased expression of pro-fibrinogenic factors in HSC, including osteopontin, Timp1, and Col1a1 (Machado et al., 2015; Wang et al., 2016). Interestingly, a transposon screen in mice identified mutations in the Hippo adaptor protein Sav1 in NAFLD-associated tumors, but not those that developed in viral hepatitis (Kodama et al., 2018). One mechanism how steatosis could promote YAP/TAZ activation is through the obesity-associated protein JCAD that inhibits upstream LATS2 (Ye et al., 2017). To date, the differential role of YAP and TAZ is far from understood and most models of liver disease focus on the role of YAP. However, the abovementioned studies show that TAZ might have a unique role in steatohepatitis and the development of NAFLD-associated fibrosis.
Cirrhosis and chronic inflammation in the liver are leading risk factors for liver cancer (Massarweh and El-Serag, 2017). While YAP activation is an early event in HCC development (Perra et al., 2014), mutations within the Hippo pathway are rare – indicating for alternative regulatory pathways to promote YAP signaling. The search for upstream Hippo signaling regulators led to the identification of a variety of regulatory mechanisms ranging from mechanotransduction over GPCR signaling to interacting pathways, most prominently the Wnt signaling pathway (Meng et al., 2016; Dobrokhotov et al., 2018; Liu et al., 2018).
Given the increase in liver stiffness that occurs in cirrhosis, mechanical inputs are key candidates for oncogenic YAP activation that contributes to fibrosis progression and liver cancer. Mechanistically, several membrane-associated proteins such as Nf2/Merlin, scaffolding proteins of the angiomotin family, and WWC proteins have been shown to positively and – for certain members of the angiomotin family – negatively regulate Hippo signaling (Zhang et al., 2010; Li et al., 2012; Yi et al., 2013; Moleirinho et al., 2017; Hermann et al., 2018) – however, the relevance of these proteins in cirrhosis is unknown to date. Several interacting pathways, including Wnt and Notch signaling, play a role in the control of oncogenic YAP in liver cancer. In hepatocellular carcinoma, Notch signaling cannot only be activated by YAP, but also maintains a positive feedback loop in Mst1/2-deficient livers to enhance YAP signaling and tumorigenesis. In contrast, Wnt signaling has been shown to inhibit hepatic YAP/TAZ signaling – in part by interfering with the Notch-YAP feedback loop (Tschaharganeh et al., 2013; Kim et al., 2017b,c). Interestingly, inactivating mutations in the negative Wnt regulator AXIN do not lead to increased Wnt/β-catenin signaling in hepatocellular carcinoma as would be expected, but are instead associated with a proliferative phenotype and gene signatures enriched for Notch and YAP signaling indicating for a Wnt-independent direct or indirect inhibition of YAP by AXIN (Abitbol et al., 2018).
Downstream of activated YAP/TAZ, unchecked proliferation and deregulated cell cycle control are one of the key mechanisms driving tumor development. Several studies showed that YAP/TEAD co-operate with E2F transcription factors downstream of retinoblastoma signaling to promote proliferation in cancer, including in liver cancer (Ehmer et al., 2014; Kapoor et al., 2014; Ehmer and Sage, 2015; Fitamant et al., 2015; Hiemer et al., 2015; Shi et al., 2016). While low levels of active YAP are not sufficient to induce proliferation in quiescent livers, concomitant activation of other pro-proliferative signals in hepatic injury or inflammation give YAP-activated hepatocytes a proliferative advantage leading to their expansion (Ehmer et al., 2014; Su et al., 2015). Aside from proliferation, YAP signaling in HCC is involved in a multitude of cancer-associated pathways, including suppression of apoptosis, deregulated ER/unfolded protein response, and chromosomal instability (CNI) (Rosenbluh et al., 2012; Wu et al., 2015; Weiler et al., 2017). Last, but not least, Hippo signaling is a relevant player in the regulation of a protumoral inflammatory microenvironment. In Mst1/2-deficient livers, the YAP target Mcp1 triggers accumulation of tumor-infiltrating macrophages that impair immune clearance of transformed hepatocytes and promote HCC development (Guo et al., 2017; Kim et al., 2018). If the expanding role of Hippo signaling in cancer immunity is of any relevance in liver disease remains to be investigated, but could be important in optimizing strategies for HCC-targeted immunotherapies (Taha et al., 2018).
Effective targeting of Hippo signaling in vivo – either to promote liver regeneration or to inhibit fibrosis and cancer progression – presents an unmet need in liver disease. Hepatic inactivation of MST kinases or YAP by microencapsulated siRNA as well as YAP silencing by AAV-delivered shRNA has yielded promising efficiency in murine models, but a transition into clinics is not foreseeable yet (Fitamant et al., 2015; Yin et al., 2016; Loforese et al., 2017; Jiang et al., 2018). Recently, transient activation of YAP/TAZ signaling with improved murine liver regeneration has been achieved by inhibition of MST1/2 kinases using the novel compound XMU-MP-1 (Fan et al., 2016).
In contrast to regeneration, inhibition of YAP/TAZ is a promising therapeutic approach in cirrhosis, steatohepatitis, or hepatocellular carcinoma. Outcomes of YAP activation in the liver are mostly dependent on its interaction with TEAD transcription factors, making YAP/TEAD complexes a promising target. Verteporfin, a substance identified in a compound screen for YAP/TEAD inhibitors, impeded HCC development in Nf2-deficient livers (Liu-Chittenden et al., 2012). However, cellular toxicity by off-target effects as well as the production of reactive oxygen radicals upon light activation have hindered the transition of verteporfin into cancer therapy (Konstantinou et al., 2017). Recently, antiparasitic ivermectin was identified as an inhibitor of YAP/TEAD-dependent transcription – and was successfully used to inhibit YAP activation and hepatic overgrowth in Mob1a/1b-deficient livers. While the mechanism of YAP inhibition by ivermectin is not fully understood to date, the compound seems to have a good safety profile, making it a promising drug for in vivo YAP inhibition (Nishio et al., 2016). In esophageal adenocarcinoma cells, CA3 – a novel inhibitor of YAP/TEAD-dependent transcription – successfully reduced tumor cell growth together with established chemotherapy (Song et al., 2018). However, this compound has not been tested in the liver so far. In addition to direct YAP/TAZ inhibitors, compounds that target upstream regulators of Hippo signaling have been in the focus of several studies (Bae et al., 2017). In HCC cells, tankyrase inhibitors that upregulate members of the angiomotion family as well as aurokinase inhibitors restricted tumor cell growth by modification of YAP activity (Jia et al., 2017; Liu et al., 2017). While compounds that interfere with mechanotransduction by targeting F-actin or Rho have been successfully used to modify Hippo signaling and YAP activity in vitro, their efficiency in the liver cells is not known and the toxicity associated with interference of the cytoskeleton will likely limit their in vivo use (Bae et al., 2017). In Wnt-activated colon cancer cells, targeting of YES1 with the approved compound dasatinib inhibits tyrosine phosphorylation of YAP and activation of a β-catenin-YAP1-TBX5 transcriptional complex (Rosenbluh et al., 2012), while in mammary cells inhibition of PI3K/PDK1 was able to restrain the binding of PDK1 to SAV1, resulting disinhibition of upstream Hippo signaling, and reduction of YAP activity (Fan et al., 2013). Again, the efficiency of these compounds in liver cancer has not been investigated and the tissue specificity of YAP-interacting pathways will make the identification of targetable upstream regulators even more complicated. With several promising candidate drugs to inhibit YAP/TEAD being under development, their application is currently limited to preclinical studies and the first clinical trials that are expected in the near future will tell if the immense expectations in targeting YAP activity will hold true in the treatment of human disease.
Our knowledge about Hippo signaling in different hepatic cell types has expanded over the recent years (summarized in Figure 2), but important questions remain. In chronic and acute liver injury, we still do not fully comprehend the role of Hippo signaling in the interaction between HSC, LSEC, bile duct cells, and hepatocytes. Apart from hedgehog signaling, are there other pathways such as TGFbeta signaling involved and can these pathways be targeted? With therapeutic modification of Hippo signaling still in its infancy, it remains unclear if inhibition of YAP or TAZ in steatohepatitis, cirrhosis, and cancer might be deleterious in underlying liver disease as it could inhibit regeneration upon liver cell damage. It would therefore be more reasonable to target inflammation or tumor specific upstream regulators of Hippo signaling. However, the mechanisms that govern hepatic Hippo pathway regulation during liver development, in liver homeostasis in the adult liver, or in liver disease are not well understood. Additionally, the cell-specific differences in the outcome of YAP and TAZ activation remain to be investigated. It is of special relevance in this context that the role of YAP and TAZ is not completely redundant and deciphering the individual functions of both orthologs will certainly help to understand the role of Hippo signaling in the liver. To approach all these questions in the complex microenvironment of the liver, further studies will be needed.
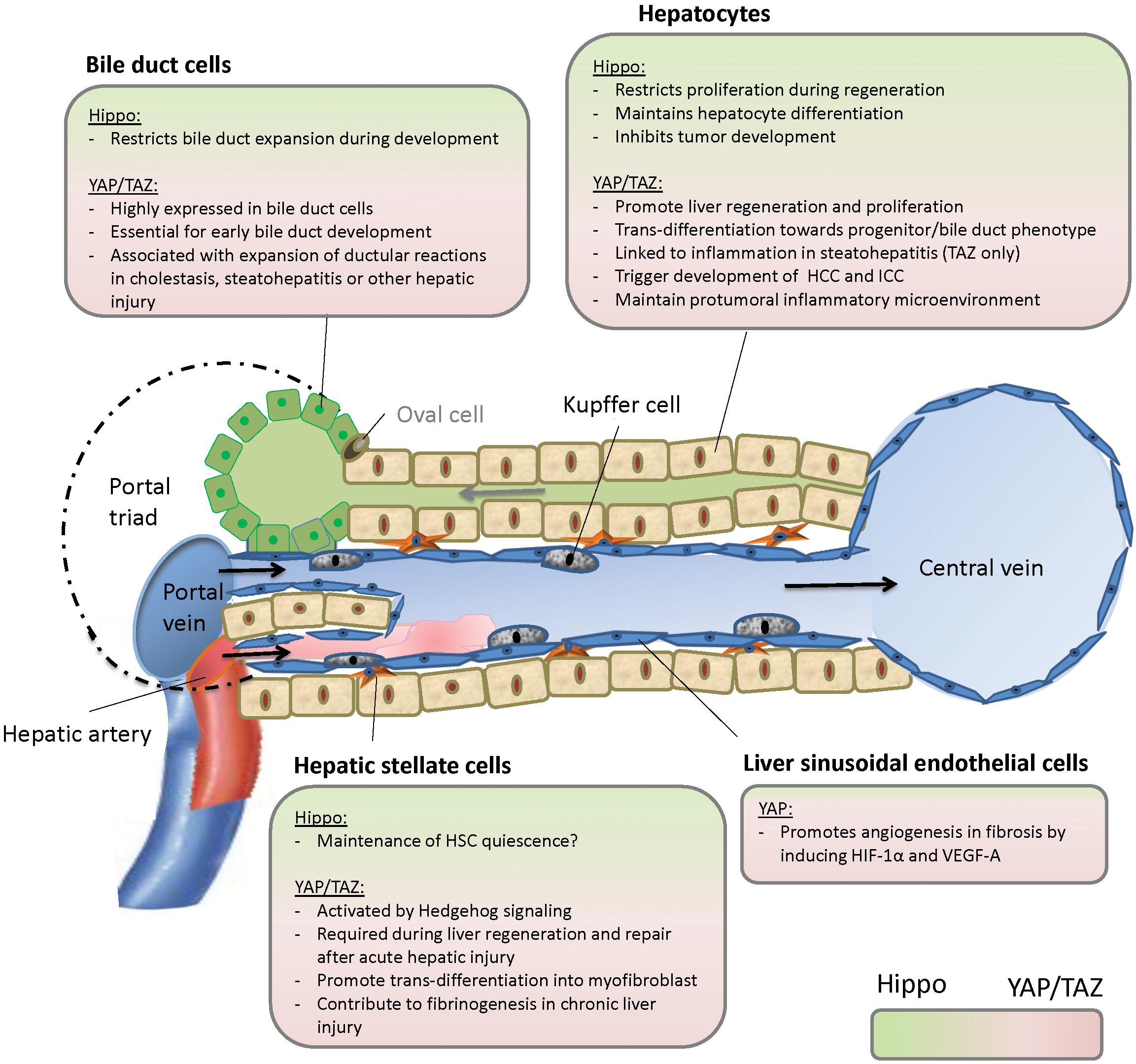
Figure 2. Hippo signaling in the liver. HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
SM and UE drafted and wrote the manuscript.
The work was funded by Wilhelm Sander-Stiftung, Germany (to UE, Grant No: 2017.115.1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abitbol, S., Dahmani, R., Coulouarn, C., Ragazzon, B., Mlecnik, B., Senni, N., et al. (2018). AXIN deficiency in human and mouse hepatocytes induces hepatocellular carcinoma in the absence of beta-catenin activation. J. Hepatol. 68, 1203–1213. doi: 10.1016/j.jhep.2017.12.018
Alder, O., Cullum, R., Lee, S., Kan, A. C., Wei, W., Yi, Y., et al. (2014). Hippo signaling influences HNF4A and FOXA2 enhancer switching during hepatocyte differentiation. Cell Rep. 9, 261–271. doi: 10.1016/j.celrep.2014.08.046
Anakk, S., Bhosale, M., Schmidt, V. A., Johnson, R. L., Finegold, M. J., and Moore, D. D. (2013). Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 5, 1060–1069. doi: 10.1016/j.celrep.2013.10.030
Bae, J. S., Kim, S. M., and Lee, H. (2017). The Hippo signaling pathway provides novel anti-cancer drug targets. Oncotarget 8, 16084–16098. doi: 10.18632/oncotarget.14306
Bai, H., Zhang, N., Xu, Y., Chen, Q., Khan, M., Potter, J. J., et al. (2012). Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 56, 1097–1107. doi: 10.1002/hep.25769
Benhamouche, S., Curto, M., Saotome, I., Gladden, A. B., Liu, C. H., Giovannini, M., et al. (2010). Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 24, 1718–1730. doi: 10.1101/gad.1938710
Benhamouche-Trouillet, S., O’Loughlin, E., Liu, C. H., Polacheck, W., Fitamant, J., McKee, M., et al. (2018). Proliferation-independent role of NF2 (merlin) in limiting biliary morphogenesis. Development 145:dev162123. doi: 10.1242/dev.162123
Camargo, F. D., Gokhale, S., Johnnidis, J. B., Fu, D., Bell, G. W., Jaenisch, R., et al. (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060. doi: 10.1016/j.cub.2007.10.039
Dechene, A., Sowa, J. P., Gieseler, R. K., Jochum, C., Bechmann, L. P., El Fouly, A., et al. (2010). Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology 52, 1008–1016. doi: 10.1002/hep.23754
Ding, B. S., Cao, Z., Lis, R., Nolan, D. J., Guo, P., Simons, M., et al. (2014). Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505, 97–102. doi: 10.1038/nature12681
Dobrokhotov, O., Samsonov, M., Sokabe, M., and Hirata, H. (2018). Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin. Transl. Med. 7:23. doi: 10.1186/s40169-018-0202-9
Dong, J., Feldmann, G., Huang, J., Wu, S., Zhang, N., Comerford, S. A., et al. (2007). Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133. doi: 10.1016/j.cell.2007.07.019
Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183. doi: 10.1038/nature10137
Ehmer, U., and Sage, J. (2015). Control of proliferation and cancer growth by the Hippo signaling pathway. Mol. Cancer Res. 14, 127–140. doi: 10.1158/1541-7786.MCR-15-0305
Ehmer, U., Zmoos, A. F., Auerbach, R. K., Vaka, D., Butte, A. J., Kay, M. A., et al. (2014). Organ size control is dominant over Rb family inactivation to restrict proliferation in vivo. Cell Rep. 8, 371–381. doi: 10.1016/j.celrep.2014.06.025
Elpek, G. O. (2015). Angiogenesis and liver fibrosis. World J. Hepatol. 7, 377–391. doi: 10.4254/wjh.v7.i3.377
Fan, F., He, Z., Kong, L. L., Chen, Q., Yuan, Q., Zhang, S., et al. (2016). Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci. Transl. Med. 8:352ra108. doi: 10.1126/scitranslmed.aaf2304
Fan, R., Kim, N. G., and Gumbiner, B. M. (2013). Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. U.S.A. 110, 2569–2574. doi: 10.1073/pnas.1216462110
Fitamant, J., Kottakis, F., Benhamouche, S., Tian, H. S., Chuvin, N., Parachoniak, C. A., et al. (2015). YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression. Cell Rep. doi: 10.1016/j.celrep.2015.02.027 [Epub ahead of print].
Font-Burgada, J., Shalapour, S., Ramaswamy, S., Hsueh, B., Rossell, D., Umemura, A., et al. (2015). Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766–779. doi: 10.1016/j.cell.2015.07.026
Geisler, F., Nagl, F., Mazur, P. K., Lee, M., Zimber-Strobl, U., Strobl, L. J., et al. (2008). Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology 48, 607–616. doi: 10.1002/hep.22381
Grijalva, J. L., Huizenga, M., Mueller, K., Rodriguez, S., Brazzo, J., Camargo, F., et al. (2014). Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G196–G204. doi: 10.1152/ajpgi.00077.2014
Guo, X., Zhao, Y., Yan, H., Yang, Y., Shen, S., Dai, X., et al. (2017). Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 31, 247–259. doi: 10.1101/gad.294348.116
Hagenbeek, T. J., Webster, J. D., Kljavin, N. M., Chang, M. T., Pham, T., Lee, H. J., et al. (2018). The Hippo pathway effector TAZ induces TEAD-dependent liver inflammation and tumors. Sci. Signal. 11:eaaj1757. doi: 10.1126/scisignal.aaj1757
Hermann, A., Wennmann, D. O., Gromnitza, S., Edeling, M., Van Marck, V., Sudol, M., et al. (2018). WW and C2 domain-containing proteins regulate hepatic cell differentiation and tumorigenesis through the hippo signaling pathway. Hepatology 67, 1546–1559. doi: 10.1002/hep.29647
Hiemer, S. E., Zhang, L., Kartha, V. K., Packer, T. S., Almershed, M., Noonan, V., et al. (2015). A YAP/TAZ-regulated molecular signature is associated with oral squamous cell carcinoma. Mol. Cancer Res. 13, 957–968. doi: 10.1158/1541-7786.MCR-14-0580
Jeliazkova, P., Jors, S., Lee, M., Zimber-Strobl, U., Ferrer, J., Schmid, R. M., et al. (2013). Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology 57, 2469–2479. doi: 10.1002/hep.26254
Jia, J., Qiao, Y., Pilo, M. G., Cigliano, A., Liu, X., Shao, Z., et al. (2017). Tankyrase inhibitors suppress hepatocellular carcinoma cell growth via modulating the Hippo cascade. PLoS One 12:e0184068. doi: 10.1371/journal.pone.0184068
Jiang, Y., Feng, D., Ma, X., Fan, S., Gao, Y., Fu, K., et al. (2018). Pregnane X receptor regulates liver size and liver cell fate via yes-associated protein activation. Hepatology 69, 343–358. doi: 10.1002/hep.30131
Kapoor, A., Yao, W., Ying, H., Hua, S., Liewen, A., Wang, Q., et al. (2014). Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 158, 185–197. doi: 10.1016/j.cell.2014.06.003
Kim, J., Kim, Y. H., Kim, J., Park, D. Y., Bae, H., Lee, D. H., et al. (2017a). YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest. 127, 3441–3461. doi: 10.1172/JCI93825
Kim, W., Khan, S. K., Gvozdenovic-Jeremic, J., Kim, Y., Dahlman, J., Kim, H., et al. (2017b). Hippo signaling interactions with Wnt/beta-catenin and Notch signaling repress liver tumorigenesis. J. Clin. Invest. 127, 137–152. doi: 10.1172/JCI88486
Kim, W., Khan, S. K., and Yang, Y. (2017c). Interacting network of Hippo, Wnt/beta-catenin and Notch signaling represses liver tumor formation. BMB Rep. 50, 1–2. doi: 10.5483/BMBRep.2017.50.1.196
Kim, W., Khan, S. K., Liu, Y., Xu, R., Park, O., He, Y., et al. (2018). Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut 67, 1692–1703. doi: 10.1136/gutjnl-2017-314061
Kodama, T., Yi, J., Newberg, J. Y., Tien, J. C., Wu, H., Finegold, M. J., et al. (2018). Molecular profiling of nonalcoholic fatty liver disease-associated hepatocellular carcinoma using SB transposon mutagenesis. Proc. Natl. Acad. Sci. U.S.A. 115, E10417–E10426. doi: 10.1073/pnas.1808968115
Konishi, T., Schuster, R. M., and Lentsch, A. B. (2018). Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 314, G471–G482. doi: 10.1152/ajpgi.00153.2017
Konstantinou, E. K., Notomi, S., Kosmidou, C., Brodowska, K., Al-Moujahed, A., Nicolaou, F., et al. (2017). Verteporfin-induced formation of protein cross-linked oligomers and high molecular weight complexes is mediated by light and leads to cell toxicity. Sci Rep. 7:46581. doi: 10.1038/srep46581
Koo, J. H., and Guan, K. L. (2018). Interplay between YAP/TAZ and metabolism. Cell Metab. 28, 196–206. doi: 10.1016/j.cmet.2018.07.010
Kordes, C., Sawitza, I., Gotze, S., Herebian, D., and Haussinger, D. (2014). Hepatic stellate cells contribute to progenitor cells and liver regeneration. J. Clin. Invest. 124, 5503–5515. doi: 10.1172/JCI74119
Lee, D. H., Park, J. O., Kim, T. S., Kim, S. K., Kim, T. H., Kim, M. C., et al. (2016). LATS-YAP/TAZ controls lineage specification by regulating TGFbeta signaling and Hnf4alpha expression during liver development. Nat. Commun. 7:11961. doi: 10.1038/ncomms11961
Lee, K. P., Lee, J. H., Kim, T. S., Kim, T. H., Park, H. D., Byun, J. S., et al. (2010). The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 8248–8253. doi: 10.1073/pnas.0912203107
Li, W., Cooper, J., Karajannis, M. A., and Giancotti, F. G. (2012). Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 13, 204–215. doi: 10.1038/embor.2012.11
Li, X., Tao, J., Cigliano, A., Sini, M., Calderaro, J., Azoulay, D., et al. (2015). Co-activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget 6, 10102–10115. doi: 10.18632/oncotarget.3546
Liu, F., Wang, G., Wang, X., Che, Z., Dong, W., Guo, X., et al. (2017). Targeting high Aurora kinases expression as an innovative therapy for hepatocellular carcinoma. Oncotarget 8, 27953–27965. doi: 10.18632/oncotarget.15853
Liu, H., Du, S., Lei, T., Wang, H., He, X., Tong, R., et al. (2018). Multifaceted regulation and functions of YAP/TAZ in tumors (Review). Oncol. Rep. 40, 16–28. doi: 10.3892/or.2018.6423
Liu-Chittenden, Y., Huang, B., Shim, J. S., Chen, Q., Lee, S. J., Anders, R. A., et al. (2012). Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305. doi: 10.1101/gad.192856.112
Loforese, G., Malinka, T., Keogh, A., Baier, F., Simillion, C., Montani, M., et al. (2017). Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2. EMBO Mol. Med. 9, 46–60. doi: 10.15252/emmm.201506089
Lu, L., Finegold, M. J., and Johnson, R. L. (2018). Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Exp. Mol. Med. 50:e423. doi: 10.1038/emm.2017.205
Lu, L., Li, Y., Kim, S. M., Bossuyt, W., Liu, P., Qiu, Q., et al. (2010). Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl. Acad. Sci. U.S.A. 107, 1437–1442. doi: 10.1073/pnas.0911427107
Ma, L., Wang, J., Lin, J., Pan, Q., Yu, Y., and Sun, F. (2014). Cluster of differentiation 166 (CD166) regulated by phosphatidylinositide 3-Kinase (PI3K)/AKT signaling to exert its anti-apoptotic role via yes-associated protein (YAP) in liver cancer. J. Biol. Chem. 289, 6921–6933. doi: 10.1074/jbc.M113.524819
Machado, M. V., Michelotti, G. A., Pereira, T. A., Xie, G., Premont, R., Cortez-Pinto, H., et al. (2015). Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J. Hepatol. 63, 962–970. doi: 10.1016/j.jhep.2015.05.031
Mannaerts, I., Leite, S. B., Verhulst, S., Claerhout, S., Eysackers, N., Thoen, L. F., et al. (2015). The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 63, 679–688. doi: 10.1016/j.jhep.2015.04.011
Marti, P., Stein, C., Blumer, T., Abraham, Y., Dill, M. T., Pikiolek, M., et al. (2015). YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology 62, 1497–1510. doi: 10.1002/hep.27992
Martin, K., Pritchett, J., Llewellyn, J., Mullan, A. F., Athwal, V. S., Dobie, R., et al. (2016). PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat. Commun. 7:12502. doi: 10.1038/ncomms12502
Massarweh, N. N., and El-Serag, H. B. (2017). epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control 24:1073274817729245. doi: 10.1177/1073274817729245
Meng, Z., Moroishi, T., and Guan, K. L. (2016). Mechanisms of Hippo pathway regulation. Genes Dev. 30, 1–17. doi: 10.1101/gad.274027.115
Michalopoulos, G. K. (2017). Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology 65, 1384–1392. doi: 10.1002/hep.28988
Miyamura, N., Hata, S., Itoh, T., Tanaka, M., Nishio, M., Itoh, M., et al. (2017). YAP determines the cell fate of injured mouse hepatocytes in vivo. Nat. Commun. 8:16017. doi: 10.1038/ncomms16017
Moleirinho, S., Hoxha, S., Mandati, V., Curtale, G., Troutman, S., Ehmer, U., et al. (2017). Regulation of localization and function of the transcriptional co-activator YAP by angiomotin. eLife 6:e23966. doi: 10.7554/eLife.23966
Nishio, M., Sugimachi, K., Goto, H., Wang, J., Morikawa, T., Miyachi, Y., et al. (2016). Dysregulated YAP1/TAZ and TGF-beta signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 113, E71–E80. doi: 10.1073/pnas.1517188113
Park, J. A., and Kwon, Y. G. (2018). Hippo-YAP/TAZ signaling in angiogenesis. BMB Rep. 51, 157–162. doi: 10.5483/BMBRep.2018.51.3.016
Patel, S. H., Camargo, F. D., and Yimlamai, D. (2017). Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology 152, 533–545. doi: 10.1053/j.gastro.2016.10.047
Perra, A., Kowalik, M. A., Ghiso, E., Ledda-Columbano, G. M., Di Tommaso, L., Angioni, M. M., et al. (2014). YAP activation is an early event and a potential therapeutic target in liver cancer development. J. Hepatol. 61, 1088–1096. doi: 10.1016/j.jhep.2014.06.033
Poisson, J., Lemoinne, S., Boulanger, C., Durand, F., Moreau, R., Valla, D., et al. (2017). Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 66, 212–227. doi: 10.1016/j.jhep.2016.07.009
Rizvi, S., Fischbach, S. R., Bronk, S. F., Hirsova, P., Krishnan, A., Dhanasekaran, R., et al. (2018). YAP-associated chromosomal instability and cholangiocarcinoma in mice. Oncotarget 9, 5892–5905. doi: 10.18632/oncotarget.23638
Rosenbluh, J., Nijhawan, D., Cox, A. G., Li, X., Neal, J. T., Schafer, E. J., et al. (2012). beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151, 1457–1473. doi: 10.1016/j.cell.2012.11.026
Sayedyahossein, S., Li, Z., Hedman, A. C., Morgan, C. J., and Sacks, D. B. (2016). IQGAP1 binds to yes-associated protein (YAP) and modulates its transcriptional activity. J. Biol. Chem. 291, 19261–19273. doi: 10.1074/jbc.M116.732529
Shi, Y., Bollam, S. R., White, S. M., Laughlin, S. Z., Graham, G. T., Wadhwa, M., et al. (2016). Rac1-mediated DNA damage and inflammation promote Nf2 tumorigenesis but also limit cell-cycle progression. Dev. Cell 39, 452–465. doi: 10.1016/j.devcel.2016.09.027
Song, H., Mak, K. K., Topol, L., Yun, K., Hu, J., Garrett, L., et al. (2010). Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. U.S.A. 107, 1431–1436. doi: 10.1073/pnas.0911409107
Song, S., Xie, M., Scott, A. W., Jin, J., Ma, L., Dong, X., et al. (2018). A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol. Cancer Ther. 17, 443–454. doi: 10.1158/1535-7163.MCT-17-0560
Su, T., Bondar, T., Zhou, X., Zhang, C., He, H., and Medzhitov, R. (2015). Two-signal requirement for growth-promoting function of Yap in hepatocytes. Elife 4:e02948. doi: 10.7554/eLife.02948
Swiderska-Syn, M., Xie, G., Michelotti, G. A., Jewell, M. L., Premont, R. T., Syn, W. K., et al. (2016). Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology 64, 232–244. doi: 10.1002/hep.28542
Taha, Z., Janse van Rensburg, H. J., and Yang, X. (2018). The hippo pathway: immunity and cancer. Cancers 10:E94. doi: 10.3390/cancers10040094
Tanimizu, N., and Mitaka, T. (2014). Re-evaluation of liver stem/progenitor cells. Organogenesis 10, 208–215. doi: 10.4161/org.27591
Tschaharganeh, D. F., Chen, X., Latzko, P., Malz, M., Gaida, M. M., Felix, K., et al. (2013). Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 144, 1530–1542.e12. doi: 10.1053/j.gastro.2013.02.009
Wang, X., Zheng, Z., Caviglia, J. M., Corey, K. E., Herfel, T. M., Cai, B., et al. (2016). Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 24, 848–862. doi: 10.1016/j.cmet.2016.09.016
Weiler, S. M. E., Pinna, F., Wolf, T., Lutz, T., Geldiyev, A., Sticht, C., et al. (2017). Induction of chromosome instability by activation of yes-associated protein and forkhead box M1 in liver cancer. Gastroenterology 152, 2037–2051.e22. doi: 10.1053/j.gastro.2017.02.018
Wu, H., Liu, Y., Jiang, X. W., Li, W. F., Guo, G., Gong, J. P., et al. (2016). Clinicopathological and prognostic significance of Yes-associated protein expression in hepatocellular carcinoma and hepatic cholangiocarcinoma. Tumour Biol. 37, 13499–13508. doi: 10.1007/s13277-016-5211-y
Wu, H., Wei, L., Fan, F., Ji, S., Zhang, S., Geng, J., et al. (2015). Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat. Commun. 6:6239. doi: 10.1038/ncomms7239
Wu, N., Nguyen, Q., Wan, Y., Zhou, T., Venter, J., Frampton, G. A., et al. (2017). The Hippo signaling functions through the Notch signaling to regulate intrahepatic bile duct development in mammals. Lab. Invest. 97, 843–853. doi: 10.1038/labinvest.2017.29
Xiao, H., Tong, R., Yang, B., Lv, Z., Du, C., Peng, C., et al. (2016). TAZ regulates cell proliferation and sensitivity to vitamin D3 in intrahepatic cholangiocarcinoma. Cancer Lett. 381, 370–379. doi: 10.1016/j.canlet.2016.08.013
Ye, J., Li, T. S., Xu, G., Zhao, Y. M., Zhang, N. P., Fan, J., et al. (2017). JCAD promotes progression of nonalcoholic steatohepatitis to liver cancer by inhibiting LATS2 kinase activity. Cancer Res. 77, 5287–5300. doi: 10.1158/0008-5472.CAN-17-0229
Yi, C., Shen, Z., Stemmer-Rachamimov, A., Dawany, N., Troutman, S., Showe, L. C., et al. (2013). The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci. Signal. 6:ra77. doi: 10.1126/scisignal.2004060
Yi, X., Yu, J., Ma, C., Li, L., Luo, L., Li, H., et al. (2018). Yap1/Taz are essential for the liver development in zebrafish. Biochem. Biophys. Res. Commun. 503, 131–137. doi: 10.1016/j.bbrc.2018.05.196
Yimlamai, D., Christodoulou, C., Galli, G. G., Yanger, K., Pepe-Mooney, B., Gurung, B., et al. (2014). Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338. doi: 10.1016/j.cell.2014.03.060
Yin, H., Bogorad, R. L., Barnes, C., Walsh, S., Zhuang, I., Nonaka, H., et al. (2016). RNAi-nanoparticulate manipulation of gene expression as a new functional genomics tool in the liver. J. Hepatol. 64, 899–907. doi: 10.1016/j.jhep.2015.11.028
Zhang, C., Bian, M., Chen, X., Jin, H., Zhao, S., Yang, X., et al. (2018). Oroxylin A prevents angiogenesis of LSECs in liver fibrosis via inhibition of YAP/HIF-1alpha signaling. J. Cell Biochem. 119, 2258–2268. doi: 10.1002/jcb.26388
Zhang, S., Wang, J., Wang, H., Fan, L., Fan, B., Zeng, B., et al. (2018). Hippo cascade controls lineage commitment of liver tumors in mice and humans. Am. J. Pathol. 188, 995–1006. doi: 10.1016/j.ajpath.2017.12.017
Zhang, N., Bai, H., David, K. K., Dong, J., Zheng, Y., Cai, J., et al. (2010). The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38. doi: 10.1016/j.devcel.2010.06.015
Zhang, S., Song, X., Cao, D., Xu, Z., Fan, B., Che, L., et al. (2017). Pan-mTOR inhibitor MLN0128 is effective against intrahepatic cholangiocarcinoma in mice. J. Hepatol. 67, 1194–1203. doi: 10.1016/j.jhep.2017.07.006
Zhao, R., and Duncan, S. A. (2005). Embryonic development of the liver. Hepatology 41, 956–967. doi: 10.1002/hep.20691
Zhao, Y., Montminy, T., Azad, T., Lightbody, E., Hao, Y., SenGupta, S., et al. (2018). PI3K positively regulates YAP and TAZ in mammary tumorigenesis through multiple signaling pathways. Mol. Cancer Res. 16, 1046–1058. doi: 10.1158/1541-7786.MCR-17-0593
Zhou, D., Conrad, C., Xia, F., Park, J. S., Payer, B., Yin, Y., et al. (2009). Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438. doi: 10.1016/j.ccr.2009.09.026
Keywords: hippo, yap, liver, hepatocellular carcinoma, HCC, cholangiocarcinoma, fibrosis, steatohepatitis
Citation: Manmadhan S and Ehmer U (2019) Hippo Signaling in the Liver – A Long and Ever-Expanding Story. Front. Cell Dev. Biol. 7:33. doi: 10.3389/fcell.2019.00033
Received: 04 December 2018; Accepted: 25 February 2019;
Published: 12 March 2019.
Edited by:
Stephano Spano Mello, University of Rochester, United StatesReviewed by:
Richard T. Premont, Harrington Discovery Institute, United StatesCopyright © 2019 Manmadhan and Ehmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ursula Ehmer, dXJzdWxhLmVobWVyQHR1bS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.