- 1Bioinformatics Research Center, North Carolina State University, Raleigh, NC, United States
- 2Center for Human Health and the Environment, North Carolina State University, Raleigh, NC, United States
- 3Department of Environmental and Occupational Health, University of Pittsburgh, Pittsburgh, PA, United States
- 4Department of Biological Sciences, North Carolina State University, Raleigh, NC, United States
- 5Department of Health, Behavior and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 6Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC, United States
- 7Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 8Department of Health Behavior and Policy, Virginia Commonwealth University, Richmond, VA, United States
- 9Department of Statistics, North Carolina State University, Raleigh, NC, United States
Background: Maternal diet during pregnancy has been shown to influence the child neuro-developmental outcomes. Studies examining effects of dietary patterns on offspring behavior are sparse.
Objective: Determine if maternal adherence to a Mediterranean diet is associated with child behavioral outcomes assessed early in life, and to evaluate the role of differentially methylated regions (DMRs) regulating genomically imprinted genes in these associations.
Methods: Among 325 mother/infant pairs, we used regression models to evaluate the association between tertiles of maternal periconceptional Mediterranean diet adherence (MDA) scores derived from a Food Frequency Questionnaire, and social and emotional scores derived from the Infant Toddler Social and Emotional Assessment (ITSEA) questionnaire in the second year of life. Methylation of nine genomically imprinted genes was measured to determine if MDA was associated with CpG methylation.
Results: Child depression was inversely associated with maternal MDA (Bonferroni-corrected p = 0.041). While controlling for false-discovery, compared to offspring of women with the lowest MDA tertile, those with MDA scores in middle and high MDA tertiles had decreased odds for atypical behaviors [OR (95% CI) = 0.40 (0.20, 0.78) for middle and 0.40 (0.17, 0.92) for highest tertile], for maladaptive behaviors [0.37 (0.18, 0.72) for middle tertile and 0.42 (0.18, 0.95) for highest tertile] and for an index of autism spectrum disorder behaviors [0.46 (0.23, 0.90) for middle and 0.35 (0.15, 0.80) for highest tertile]. Offspring of women with the highest MDA tertile were less likely to exhibit depressive [OR = 0.28 (0.12, 0.64)] and anxiety [0.42 (0.18, 0.97)] behaviors and increased odds of social relatedness [2.31 (1.04, 5.19)] behaviors when compared to low MDA mothers. Some associations varied by sex. Perinatal MDA score was associated with methylation differences for imprinted control regions of PEG10/SGCE [females: Beta (95% CI) = 1.66 (0.52, 2.80) – Bonferroni-corrected p = 0.048; males: -0.56 (-1.13, -0.00)], as well as both MEG3 and IGF2 in males [0.97 (0.00, 1.94)] and -0.92 (-1.65, -0.19) respectively.
Conclusion: In this ethnically diverse cohort, maternal adherence to a Mediterranean diet in early pregnancy was associated with favorable neurobehavioral outcomes in early childhood and with sex-dependent methylation differences of MEG3, IGF2, and SGCE/PEG10 DMRs.
Introduction
Numerous prenatal environmental exposures including smoking, stress, maternal obesity, and dietary factors have been associated with health and neurodevelopmental outcomes in children (Huizink et al., 2002; Wiebe et al., 2009; Steenweg-de Graaff et al., 2012; Gartstein and Skinner, 2017), with some exposures conferring life-long associations (House et al., 2016; Wyss et al., 2017). In a recent meta-analysis, pre-pregnancy obesity was estimated to be associated with a 51% increased risk of any neurodevelopmental impairment and a 62% increased risk of attention deficit hyperactivity disorder (ADHD) symptoms or ADHD related impairments (Sanchez et al., 2017). Prenatal nutrition may also have lasting effects on child neurodevelopment (Anjos et al., 2013; Lyall et al., 2014). While extensive research concerning maternal obesity, gestational weight gain, and child behavior has been conducted [for reviews see (Rivera et al., 2015; Contu and Hawkes, 2017; Sanchez et al., 2017)], less is known about particular types of maternal diets in humans and their effects on early childhood behavior.
Numerous studies suggest that there may be beneficial effects of maternal diet in pregnancy on child neurodevelopment, albeit, again with mixed results (Borge et al., 2017). Intakes of antioxidants, n-3 fatty acids, and foods rich in these nutrients have been associated with child developmental outcomes with inconsistent findings (Guxens et al., 2012; Brew et al., 2015; Delgado-Noguera et al., 2015; Emmett et al., 2015; Bolduc et al., 2016; Catena et al., 2016; Ishikawa et al., 2016; Julvez et al., 2016; Bruce-Keller et al., 2017; Lipton et al., 2017).
Far fewer studies have examined overall diet patterns (Borge et al., 2017). Among 23,020 children in the Norwegian Mother and Child Cohort Study, Jacka et al. (2013) reported that “unhealthy” maternal diet patterns during pregnancy were associated with increased externalizing child behaviors. A recent study in the United Kingdom suggested that maternal diets characterized by higher intakes of fruits and vegetables and lower intakes of meat and potatoes are associated with higher child IQ at age 8 years (Freitas-Vilela et al., 2017). In the Generation R study involving 3,104 children, investigators reported decreased levels of externalizing behaviors from children born to mothers who adhered to a Mediterranean diet during pregnancy, and increased child externalizing behaviors in children born to mothers who consumed a “Traditionally Dutch” diet consisting of high meat intake (processed and unprocessed), margarines and potatoes (Steenweg-de Graaff et al., 2014).
Precise definitions for the “Mediterranean diet” vary, but all share the following similarities: rich in fresh fruits, vegetables, legumes; higher amounts of mono-unsaturated fatty acids (MUFAs) such as olive oil, whole grains, and fish, while sparse in meat (Trichopoulou et al., 2003; Bach et al., 2006; Sofi, 2009; Korre et al., 2014). The Mediterranean diet has long been associated with a myriad of positive outcomes, both observationally and in randomized controlled trials. These include improved cognition in adults (Hardman et al., 2016), as well as reductions in cancer mortality (Trichopoulou et al., 2003; Schwingshackl et al., 2011; Dilis et al., 2012), all-cause mortality (Trichopoulou et al., 2003; Knoops et al., 2004; Panagiotakos et al., 2006), cardiovascular/chronic heart disease (CVD/CHD) mortality and morbidity (Renaud et al., 1995; Estruch et al., 2006; Martínez-González et al., 2008; Fung et al., 2009; Sofi, 2009; Kastorini et al., 2011), decreased incidence of diabetes mellitus (Estruch et al., 2006; Martínez-González et al., 2008; Esposito et al., 2009), as well as decreases in obesity related metrics (Iris Shai et al., 2008; Esposito et al., 2009; Leighton et al., 2009; Sofi, 2009; Verweij et al., 2011; Korre et al., 2014), and improvement of components of metabolic syndrome (Esposito et al., 2009; Leighton et al., 2009; Kastorini et al., 2011; Korre et al., 2014). In children, Mediterranean diet adherence (MDA) has been associated with reduced odds of ADHD and improved school performance (Esteban-Cornejo et al., 2016; Rios-Hernandez et al., 2017).
Although mechanisms linking Mediterranean diet and outcomes are still unclear, epigenetic modifications are hypothesized as one mechanism to explain associations between environmental cues such as maternal diet, and child temperament/behavior (Harper, 2005; Lee, 2015; Arpon et al., 2016; Lorite Mingot et al., 2017); for review see (Lillycrop and Burdge, 2015). Animal studies have demonstrated changes in DNA methylation in the offspring of mice from dams fed different diets during pregnancy (Vucetic et al., 2010). In addition, dietary choline deficiency in dams has been shown to alter methylation patterns in mouse fetal brains (Niculescu et al., 2006). In humans, low maternal MDA has recently been associated with altered CpG methylation of the imprinted MEG3-IG control region in female offspring (Gonzalez-Nahm et al., 2017). In other studies, imprinted IGF2 was altered at birth in offspring with periconceptional exposure to famine during world war II (1944–1945) (Tobi et al., 2009). Additional support for epigenetics as a mechanism for modulating behavior in humans is recent work identifying altered methylation in brain associated with schizophrenia and bipolar disorders (Xiao et al., 2014), altered cord-blood methylation patterns in DRD4 and 5-HTT associated with ADHD symptoms in children (van Mil et al., 2014), and work by Fuemmeler et al. (2016) relating methylation of the regulatory regions of imprinted genes with infant temperament.
Sex-specific methylation differences are common in the genome (Singmann et al., 2015). As shown in Gonzalez-Nahm’s work (Gonzalez-Nahm et al., 2017), differential methylation of imprinted genes from maternal dietary exposures in offspring can also be sex-specific. Additional evidence for sex-specific differences in the methylation of imprinted control regions (ICRs) in response to prenatal exposures comes from work showing that female infants, but not males, exhibited differential methylation of ICRs of IGF2 and H19 in response to maternal anxiety (Mansell et al., 2016). Likewise, lead exposures have been associated with sex-specific changes to the methylation patterns in multiple ICRs (Goodrich et al., 2015; Li Y. et al., 2016), and while not statistically significant, sex-specific differential methylation of the H19 and IFG2 DMRs were associated with prenatal exposure to cigarette smoke (Murphy et al., 2012a).
In the current study, we sought to test the hypothesis that maternal adherence to a dietary pattern favorably associated with multiple health outcomes (Mediterranean) would be associated with offspring behavior and changes in methylation of ICRs, many of which have been associated with other behavioral offspring outcomes from prenatal exposures.
Materials and Methods
NEST Cohort and Study Population
Study participants included mother and infant dyads enrolled in the Newborn Epigenetics STudy (NEST), a prospective cohort for which accrual protocols have been previously described in detail (Liu et al., 2012). The target population was first trimester pregnant women visiting prenatal clinics of Duke Hospital and Duke affiliated clinics. To be included, women had to be aged 18 years and older and speak English or Spanish. From these, women with an established HIV infection or who intended to relinquish custody of offspring were excluded. To facilitate specimen collection at birth, women who receive obstetrics care at hospitals other than Duke Obstetrics or Durham Regional Hospitals were also excluded. Between 2009 and 2011, 1700 pregnant women were enrolled, and 396 were lost to the cohort due to fetal wastage (n = 113), refusal of further participation (n = 146), received obstetric care at an outside hospital (n = 114), or other reasons (n = 23), such that 1,304 (76.7%) women remained enrolled in the study up to the time of analysis. The recruitment protocol was approved by the Duke University Institutional Review Board.
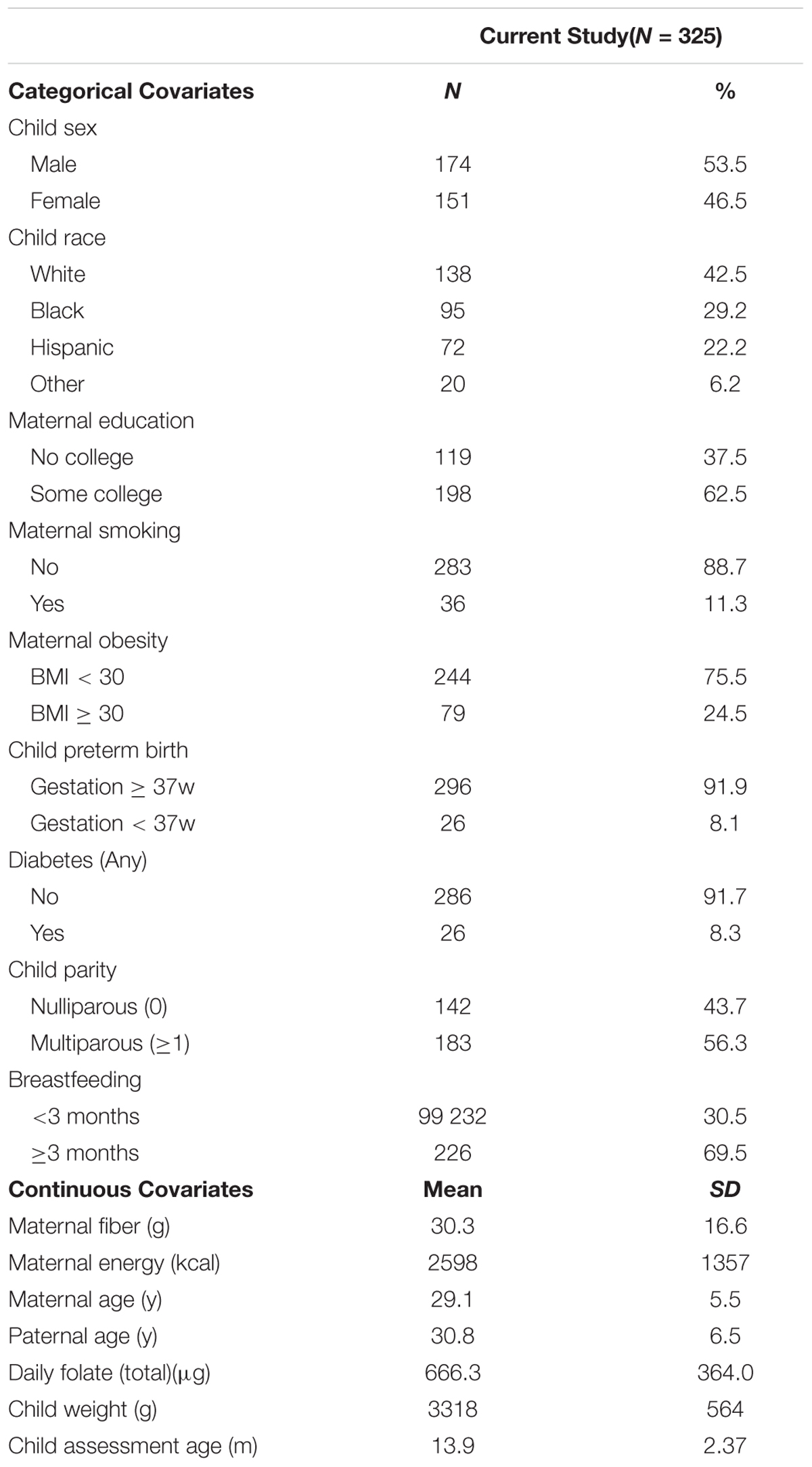
These analyses are limited to 325 mother child pairs (excluding multiple births) from which mothers completed both a periconceptional-food frequency questionnaire (FFQ) (University of Texas, MD Anderson Cancer Center Nutrition and Lifestyle Core Questionnaire 2008v.2) either upon enrollment or in their first trimester, and an Infant Toddler Social and Emotional Assessment (ITSEA) during the child’s second year of life. Because we were interested in maternal diet and because of the potential epigenetic vulnerability due to the requirement to maintain methylation at imprinted DMRs during the reprogramming that occurs immediately post-fertilization, mothers were specifically asked to recall foods consumed at or near their last menstrual cycle. FFQ responses were converted to intakes of foods, food groups, energy, and nutrients by Nutrition Quest1.
Child Behavioral Outcomes
The ITSEA (Carter et al., 2003) was administered by a parent, caregiver or staff to children aged 12–24 months (mean = 13.9 months) during a NEST follow-up visit. The ITSEA tool has been validated and used extensively to examine social-emotional behavioral outcomes and temperament in early-childhood (Carter et al., 2003, 2010). Scales of the ITSEA have demonstrated acceptable test–retest reliability and inter-rater reliability; Cronbach’s alpha coefficients for the internalizing and externalizing domains were 0.80 and 0.86, respectively (Carter et al., 2010). The ITSEA consists of questions regarding Externalizing behaviors (with domains for aggression-defiance, peer-aggression, and impulsive-activity), Internalizing behaviors (anxiety, separation-distress, depression-withdrawal, and inhibition-to-novelty), Dysregulation related behaviors (sleep, negative-emotionality, eating, and sensory-sensitivity), and Competency (attention, compliance, imitation/play, mastery-motivation, and empathy). In addition, the ITSEA contains 13 questions to assess Maladaptive behavior, 10 questions to assess Social-Relatedness behavior and 8 questions to assess Atypical behavior. We also examined a composite autism spectrum disorder index calculated from the problem and competency portions of the ITSEA as described in Kruizinga et al. (2014). With the exception of the Social-Relatedness category and all Competency domains, increased scores indicate adverse behavioral outcomes.
Infant Toddler Social and Emotional Assessment questions are scored from 0 to 2 (0 = Not True/Rarely, 1 = Somewhat, 2 = Very True/Often) and final domains are scaled by the number of questions in the domain. To examine associations of diet on ITSEA assessed child behaviors (Figure 1), and as many items were not normally distributed, even after log-transformation (Supplementary Figure 1), ITSEA behavior scores were divided into tertiles and associations with dietary exposures were assessed using ordinal logistic regression. We did not assess the peer aggression subscale of externalizing behavior due to lack of non-zero scoring (>2/3 of data were zero; data not shown).
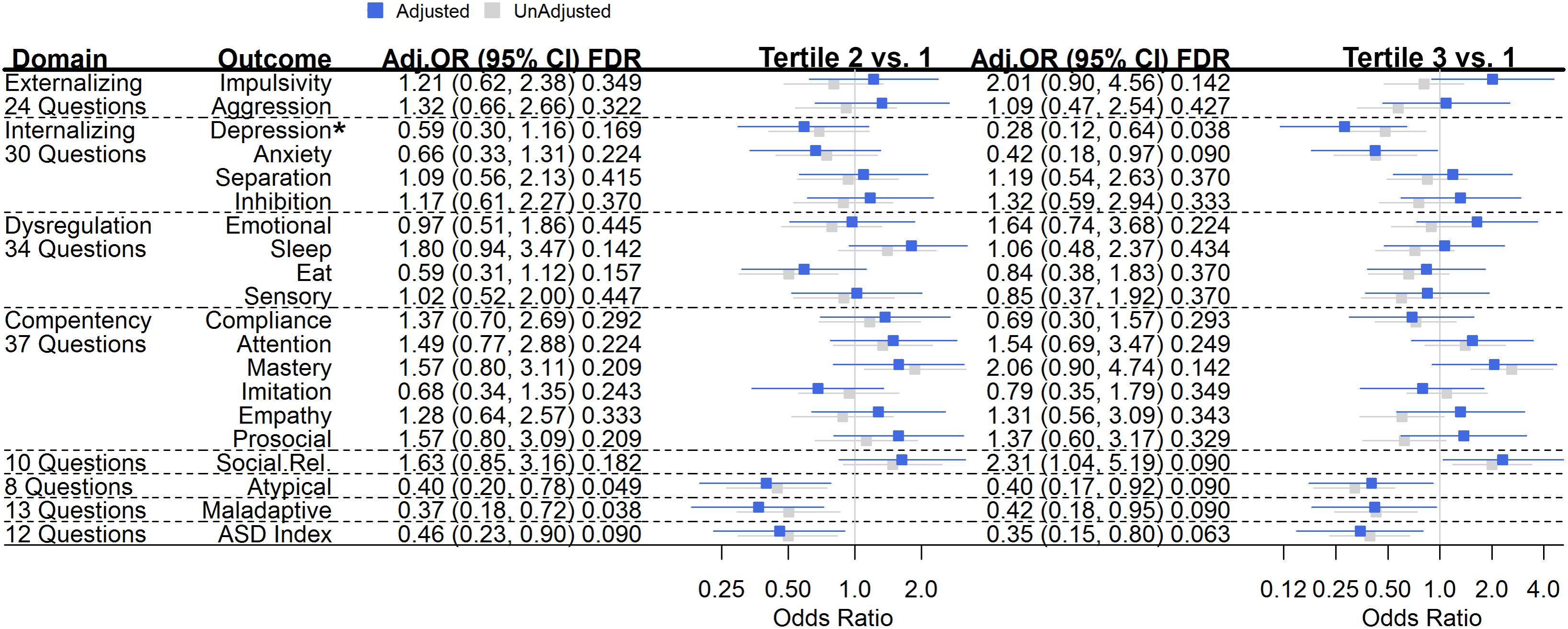
FIGURE 1. Maternal Mediterranean Diet Adherence (MDA) and Child Behavior Outcomes. For a given tertile of maternal MDA compared to tertile 1 (referent), the odds ratio (95% confidence interval) represents the risk of being in a higher tertile of behavioral outcome. Unadjusted (gray) and adjusted (blue) odds ratios (95% confidence intervals) are plotted. Estimates were adjusted for breastfeeding at least 3 months, age of child at behavioral assessment, maternal fiber intake, total calories, folate, education, diabetes, obesity, smoking, and age, as well as paternal age and child parity, premature birth, weight, race, and child sex. Q-values are shown and represent the false discovery rate (FDR) for each finding, which is automatically corrected for multiple testing. ∗Bonferroni-corrected p-trend = 0.041.
Mediterranean Diet Scoring
As described in detail by Gonzalez-Nahm et al. (2017), pregnant women’s MDA was calculated from the food frequency questionnaire (FFQ) upon enrollment soon after conception, concerning dietary consumption at or near their last menstrual period. In brief, intakes of food categories were adjusted by total energy to grams/1000 kcals and a modified version of Trichopoulou’s Mediterranean diet scoring was used (Trichopoulou et al., 2003). Participants were scored a 0 for below-median consumption of meats (including red meat, pork, poultry, game, but excluding processed meats) and a 1 for above-median consumption of the following: fruit (including fresh, dried and frozen, but excluding juice), vegetables (excluding vegetable juice and white potatoes), fish, dairy (including full-fat dairy but excluding dairy desserts), whole grains, nuts and seeds (including nut-butters), beans and legumes (including soy), and the ratio of mono-unsaturated fat to saturated fat. Alcohol consumption was not scored as it is not recommended during pregnancy and was extremely low in this cohort. The final Mediterranean diet score ranged from 0 to 9 with 9 as most adherent (Supplementary Figure 2). This composite MDA score was divided into tertiles (referent = first tertile) and subsequently used to assess associations of MDA with child behaviors. The normal distribution of these scores (Supplementary Figure 2) was maintained by offspring sex and by the sub-cohort used to assess associations of maternal MDA on CpG methylation.
DNA Methylation Assessment
For n = 142 children of the 325 included, 800 ng of genomic DNA from umbilical cord-blood was treated with sodium bisulfite. Treated DNA (40 ng) was used for bisulfite pyrosequencing (Fuemmeler et al., 2016; Gonzalez-Nahm et al., 2017) which included 48 CpG sites across 8 differentially methylated regions (DMRs) that regulate one more of nine imprinted genes [IGF2, H19, MEG3, MEG3-IG (regulates MEG3 and DKL1), NNAT, MEST, SGCE/PEG10, and PLAG1]. These regions are described in detail elsewhere (Fuemmeler et al., 2016; Cowley et al., 2018), as are complete assay conditions including primers and post-assay quality control (Murphy et al., 2012b; Nye et al., 2013; Gonzalez-Nahm et al., 2017). For the assessment of MDA on methylation, the number of subjects with maternal dietary information, all covariates, and cord blood methylation varied.
Statistical Analysis
For analysis of child behavioral outcomes, the following maternal covariates were included: age at delivery, education level (any college vs. none) pre-pregnancy obesity (BMI > 30 vs. BMI < 30), race (white, black, Hispanic, other), smoking during pregnancy, any of gestational, type I or type II diabetes, daily intake of folate (diet plus supplements: <400 μg, 400–800 μg, >800 μg), daily fiber intake, daily energy intake, and any breastfeeding of at least 3 months. We also adjusted for paternal age as well as child sex, child birthweight, full term status (≥37 weeks gestation or not), parity (nulliparous vs. not), and age of ITSEA assessment (Table 1). As neither post-birth-breastfeeding-status nor child age at ITSEA would be expected to impact cord-blood methylation patterns, these covariates were removed for assessment of associations of diet with differentially methylated imprinted gene DMR CpGs in cord-blood.
All analyses were done using R version 3.4.2 (R Development Core Team. R Foundation for Statistical Computing, Vienna, Austria). For associations of maternal diet (tertiles) with child behavior (tertiles), ordinal logistic regression was conducted using polr() from the MASS package (Venables and Ripley, 2002). Trend tests were conducted with ordinal logistic regression on tertiles of behavioral outcome while treating tertiles of maternal MDA exposure as a linear predictor (1 = least adherent, 3 = most adherent). In results, unless otherwise specified, trend p-values are not adjusted. For assessing the relationship between maternal dietary adherence and the percent methylation of CpGs (continuous) as well as the relationship between percent methylation of CpGs and child behavior (continuous), linear regression was conducted using lm() from the base R stats package. Correction for multiple comparisons was performed by (i) control of the family-wise error rate (FWER) at α= 0.05, and (ii) control of the false-discovery rate (FDR) at level 0.15. The use of FWER control is intended to highlight findings that pass the most rigorous false-positive standards, while the FDR procedure allows a proportion of false positives while maintaining a high proportion (0.85) of true discoveries. FWER control was achieved by applying the Bonferroni correction, augmented by permutation analysis for each set of tests. The permutation analyses used covariate-residualized of responses, permuted relative to the covariate-residualized predictors of interest. An alternate analysis used 10,000 permutations of DMR β values vs. maternal MDA, covariate-residualized and using the partial correlations as the test statistic. Control of the FWER over all DMRs was performed by using the minimum p-values over DMR as a statistic. In all instances, the permutation-adjusted p-values were only slightly more significant than the Bonferroni-corrected values, and did not result in additional significant findings, and so the Bonferroni-corrected values are reported for simplicity. FDR q-values were computed using the Bioconductor qvalue package (Storey et al., 2015), version 2.10.0 using default settings and are, by construction, already multiple comparison corrected.
Results
Cohort Demographics Outcome Assessment
Approximately half of the children assessed were male (53.5%) (Table 1). The majority of mothers identified as white (42.5%), with 29.2% as black, 22.2% as Hispanic, and the remainder (6.2%) classified as other. Nearly all births were full-term (91.9%) and 43.7% of children were born to nulliparous women. Amongst mothers, 62.5% had some college, 11.3% smoked while pregnant, and 24.5% were classified as obese (BMI ≥ 30) at or near conception. The prevalence of any of gestational, type I or type II diabetes was 8.3% (Table 1). Nearly 70% of mothers reported breastfeeding for at least 3 months. Children were between the age of 12 and 24 months when assessed for behavior (average age, 13.9 months). The distributions and medians for assessed behavioral outcomes are summarized in Supplementary Figure 1. With the exception of social relatedness index and the Competency subscales (compliance, attention, mastery, imitation, empathy, and prosocial), lower scores indicate favorable behavior outcomes.
Maternal Mediterranean Diet and Child Behavior Outcomes
We examined associations between maternal MDA at or near conception with ITSEA assessed childhood behaviors in offspring 12 and 24 months of age. The distribution of maternal MDA is shown in Supplementary Figure 2. Maternal MDA scores were divided into tertiles with tertile 1 (referent) as the least adherent.
Unadjusted and adjusted odds ratios for associations of maternal MDA and offspring behaviors assessed in the second year of age are displayed in Figure 1. Amongst Internalizing behaviors, when comparing offspring born to women with the lowest tertile of MDA, offspring of mothers in the highest tertile of MDA were less likely to score in a higher tertile of depression [OR (95% CI) = 0.28 (0.12, 0.64); p-trend = 0.002, Bonferroni-corrected p-trend = 0.041] and anxiety [0.42 (0.18, 0.97); p-trend = 0.041]. While not statistically significant, these associations persisted when comparing offspring of mothers in the middle tertile of MDA compared to those with mothers in the lowest level of MDA [depression: 0.59 (0.30, 1.16); anxiety: 0.66 (0.33, 1.31)]. The highest tertile of maternal MDA exposure was also associated with improved social relatedness behaviors [2.31 (1.04, 51.9); p-trend = 0.041] in offspring. Maternal MDA was not associated with Externalizing, Dysregulation or Competency behaviors (Figure 1).
When compared to offspring born to women with MDA scores in the lowest tertile, offspring of women with MDA scores in the middle tertile were less likely to report atypical behaviors [OR (95% CI) = (0.40, (0.20, 0.78)); associations of similar magnitude persisted when the highest tertile was compared to the lowest tertile (0.40 (0.17, 0.92); p-trend = 0.049]. Further, maternal MDA was inversely associated with maladaptive behaviors [middle tertile MDA vs. low – 0.37 (0.18, 0.72); high tertile MDA vs. low – 0.42, (0.18, 0.95); p-trend = 0.077]. Lastly, when compared with offspring of women with low MDA, offspring of women with middle and high scores of MDA were less likely to score in a higher tertile of autism spectrum index behaviors [middle tertile vs. low – 0.46, (0.23, 0.90); high tertile vs. low – 0.35 (0.15, 0.80); p-trend = 0.017] (Figure 1).
Child behavior can vary by sex; we examined if associations between maternal MDA and offspring behaviors did so (Supplementary Figure 3). In males, offspring born to mothers with the highest MDA scores were more likely to score in the next highest tertile of social relatedness behaviors [OR (95% CI) 3.20 (1.04, 10.25; p-trend = 0.032)]. Inverse associations of maternal MDA on depression, maladaptive, and ASD index behaviors were pronounced in females. Compared with daughters born to mothers with the lowest MDA scores, daughters born to mothers with MDA scores in the middle and high tertiles were much less likely to exhibit depression behaviors [middle tertile vs. low: 0.22 (0.06, 0.69); highest tertile vs. low: 0.05 (0.01, 0.22); Bonferroni-corrected p-trend = 0.002], or maladaptive behaviors [middle tertile vs. low: 0.23 (0.08, 0.63); highest tertile vs. low: 0.20 (0.05, 0.77); p-trend = 0.031] or ASD-related behaviors [middle tertile vs. low: 0.24 (0.08, 0.72); highest tertile vs. low: 0.08 (0.02, 0.38); Bonferroni-corrected p-trend = 0.017]. Females born to women in the highest tertile of MDA scores were more likely to exhibit increased mastery [5.53 (1.44, 22.2); p-trend = 0.012] and empathy [5.74 (1.36, 26.2); p-trend = 0.015] behaviors when compared with mothers in the lowest tertile of MDA scores.
Maternal MDA and CpG Methylation in Offspring
To test the hypothesis that associations of maternal MDA on offspring behavior are mediated through epigenetic mechanisms and taking into consideration work linking differential methylation control regions of imprinted genes with both offspring behavioral outcomes (Fuemmeler et al., 2016), and maternal diet (Rijlaarsdam et al., 2017), we examined the relationship of diet on CpG methylation of DMRs regulating nine imprinted genes. Building on prior research in our group on imprinted genes, we assessed the methylation status of 48 CpGs in the regulatory DMRs of nine imprinted genes for a subset of the cohort (n = 142 mother/child pairs). These include the DMRs of PLAGL1, H19, SGCE/PEG10, PEG3, NNAT, MEST, and two DMRs each for the DLK1/MEG3 and IGF2 domains. As mentioned in the introduction, since methylation of ICRs often vary by sex, we stratified on sex for these analyses and assessed whether maternal MDA was associated with the mean methylation percentage of CpGs at these loci with covariate-adjusted multiple linear regression (Figure 2). In females, maternal MDA was associated with an increase in mean methylation of the ICR of SGCD/PEG10 [β (95% CI) = 1.65 (0.52, 2.80); Bonferroni-corrected p-value = 0.048; Figure 2]. This translates to a 1.65% increase in mean methylation of the control region of IGF2 in offspring cord-blood for each unit increase in maternal MDA from zero to nine (Supplementary Figure 2). In males, after adjusting for a false discovery rate (FDR) of 0.15, maternal MDA was associated with a decrease in the mean methylation of CpGs in the IGF2 DMR [β (95% CI) = -0.92 (-1.65, -0.19); Figure 2] as well as with decreased methylation of the SGCE/PEG10 [-0.56 (-1.13, -0.00)], and PLAGL1 [-1.10 (-2.25, 0.04)] DMRs, and an increase in the mean methylation at the MEG3 DMR [β (95% CI) = 0.97 (0.00–1.97)]. For each of these findings, the effect estimates across the interrogated DMR were consistent in magnitude and direction (Supplementary Figure 4). Further, while not statistically significant in females, increased maternal MDA was consistently associated across the interrogated DMRs with increased methylation of CpGs in the MEG3 DMR, the intergenic MEG3-IG DMR and the PLAGL1 DMR (Supplementary Table 1 and Supplementary Figure 4).
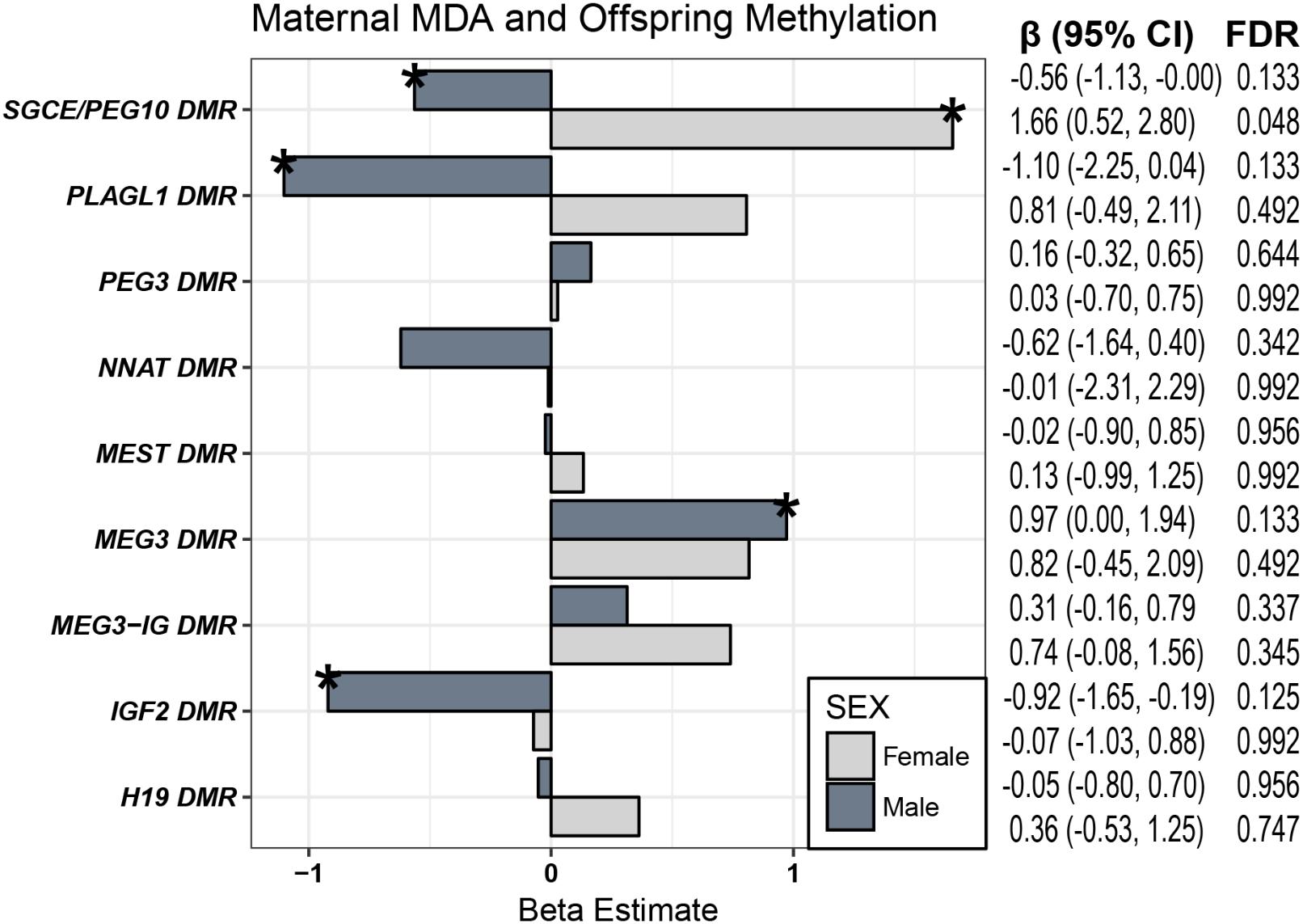
FIGURE 2. Maternal MDA and CpG Methylation. Females (light gray) and males (dark gray) were evaluated separately with linear regression for associations of maternal MDA on the average methylation status of the control region of 9 imprinted gene loci. Effect estimates for mean CpG methylation were adjusted for breastfeeding at least 3 months, age of child at behavioral assessment, maternal fiber intake, total calories, folate, education, diabetes, obesity, smoking, and age, as well as paternal age and child parity, premature birth, weight, race (∗FDR q < 0.15; n ranged from 51 to 75).
CpG Methylation and Child Behavior
For the significant associations of maternal MDA on offspring behavior (Figure 1) and maternal MDA on methylation of imprinted gene control regions (Figure 2), we examined associations of mean methylation on child behaviors (Supplementary Figure 5). In males, increased methylation of IGF2 was associated with increased ASD Composite Index behaviors and increased Atypical behavior, increased methylation of MEG3 was associated with increased Social Relatedness and decreased Maladaptive behaviors, increased methylation of SGCE/PEG10 was associated with increased Atypical behavior, while increased methylation of the PLAGL1 DMR was associated with increased Atypical and ASD Composite Index behaviors (Supplementary Figure 5). In females, increased methylation of the control region of SGCE/PEG10 was associated with decreased risk of depression, anxiety, and atypical behaviors. These estimates were unstable as evidenced by large confidence bounds and we were underpowered to conduct mediation analyses. In spite of this, we were able to identify multiple consistent associations of maternal MDA on child behavior, of maternal MDA on differential methylation of imprinted gene control regions, and of these DMRs on child behavior (Figure 3).
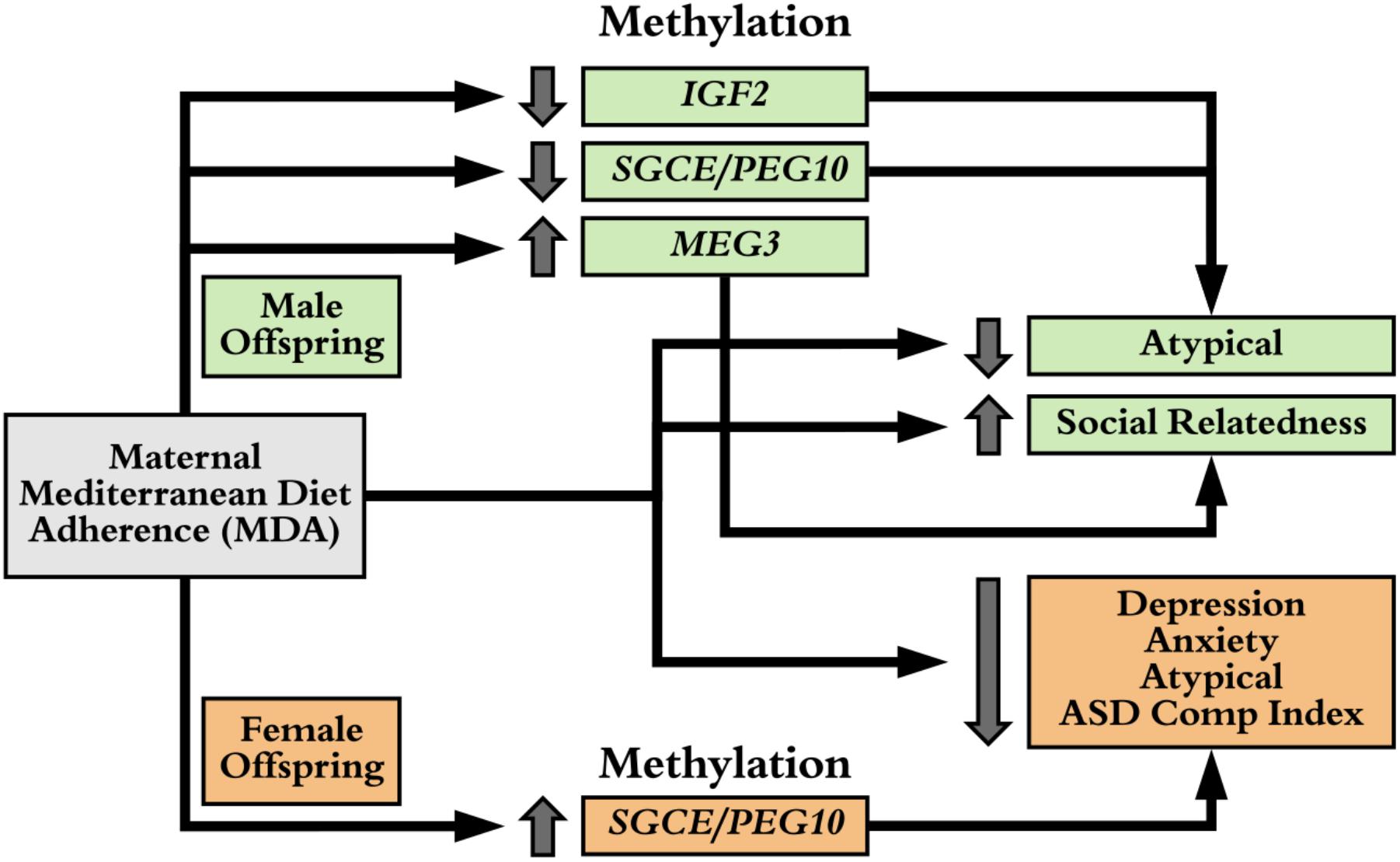
FIGURE 3. Sex Specific Maternal MDA and CpG Associations on Child Behavior. In females (orange), maternal MDA is associated with increased methylation in the control region of SGCE/PEG10 locus. In turn, this is associated with decreased odds of depression, anxiety, atypical, and Autism Spectrum Disorder (ASD) composite index behaviors, as is maternal MDA. In males (green), maternal MDA is associated with hypo-methylation of the control region of IGF2 and SGCE/PEG10 loci and hyper-methylation of the MEG3 control region which are, in turn, are consistently associated with maternal MDA associations on atypical and social relatedness behaviors.
Discussion
While much research has been conducted on the role of individual nutrients or foods on child outcomes, few studies have examined the potential role of the overall quality of maternal diet during pregnancy on child behavioral and other neurodevelopmental outcomes (Borge et al., 2017); the results so far are limited. In this study of a subset of the ethnically diverse NEST cohort, we observed a reduced risk of adverse child behaviors in relation to maternal diet patterns around the time of conception. Maternal MDA was associated with child social relatedness behaviors, and inversely associated with the internalization subscales of depression and anxiety. Further, maternal MDA was inversely associated with atypical, maladaptive, and ASD behavioral patterns. To minimize maternal depression as a potential confounder of the association between maternal MDA and offspring depression (Figure 1), we included maternal post-natal depression in our adjusted model and estimates were materially unchanged (data not shown). In addition, although covariate proportions differed (except sex and child birthweight), between our cohort and the parent cohort (data not shown), estimates for our significant findings were materially unchanged between unadjusted and adjusted models (Figure 1).
Our results suggest that during pregnancy, maternal adherence to the Mediterranean Diet, rich in legumes, vegetables, fish, and healthy fatty acids, and lower in red meat, may promote behavioral and emotional well-being in children. These findings are consistent with earlier studies reporting that maternal MDA, or to a dietary principal components pattern characterized as “healthy,” is beneficially associated with child behavioral and emotional problems (Jacka et al., 2013; Steenweg-de Graaff et al., 2014). In adults, adherence to this diet pattern is associated with better cognitive outcomes and decreased depression, suggesting a role of diet on cognition and emotional functioning (Crichton et al., 2013; Rienks et al., 2013; Trovato et al., 2014; Hardman et al., 2016; Aridi et al., 2017; Loughrey et al., 2017; Molendijk et al., 2018). Maternal adherence to this diet pattern during pregnancy has also been associated with protection from allergic disease in human offspring (Netting et al., 2014; Castro-Rodriguez and Garcia-Marcos, 2017). Given the rapid increase in the incidence of neurodevelopmental disorders in children (CDC, 2010; Visser et al., 2013, 2014), if replicated, our results relating maternal MDA during gestation with child neurodevelopmental outcomes may present new avenues for prevention.
Consistent with our findings, laboratory studies suggest that maternal diet during pregnancy may affect offspring behavioral health. Numerous studies in rodents have found associations between maternal diet and offspring neurodevelopment and behavior, such as recent work associating high maternal caloric intake with increased anxiety in male rats (Balsevich et al., 2016). Offspring from female rats fed a high fat diet pre-gestational through lactation suffered cognitive deficits that were ameliorated by dams who also had access to exercise (Moser et al., 2017). Anxiety, cognition, and compulsive behaviors were altered in male mice weaned from mothers who had received gut microbiota from high fat diet mice (Bruce-Keller et al., 2017). Rather than reductions in fat or increases in energy intake, the Mediterranean diet is thought to beneficially influence health outcomes as a result of increases in intakes of micronutrients, including MUFAs, antioxidant vitamins, and phytochemicals (Ros et al., 2014). Most rodent research to date has focused on the high-fat paradigm when examining outcomes. Further, nearly all studies examine high-fat in the context of excess energy, further reducing the ability to elucidate whether observed changes in offspring are due to excess body weight, excess energy or fat intake, or some combination.
Imprinted genes have parental allele specific silencing via epigenetics. These patterns are laid down during gametogenesis in imprinting control regions (Bartolomei and Ferguson-Smith, 2011). Germline ICRs are typically more epigenetically stable throughout life and aberrant epigenetic marks for imprinted gene ICRs are associated with effects on metabolism, neurodevelopment, and growth. Because of our findings that maternal MDA is associated with changes in methylation patterns of imprinted genes, changes which can vary by sex, we did re-examine associations of maternal MDA on child behavior stratified by sex despite being underpowered to gain real insight. For a subset of our cohort, we were able to conduct targeted assessment of the CpGs in the control regions of imprinted genes (Supplementary Figure 4), providing evidence for novel associations of maternal diet with changes in methylation in imprinted ICRs of offspring. Our data suggest that high maternal MDA is associated with altered methylation of the MEG3 ICR independent of sex, and of the SGCE/PEG10, PLAGL1 and IGF2 ICRs in a sex-dependent manner. These changes in methylation, are in turn, consistently associated with offspring behaviors that are also associated with maternal MDA (Figure 3).
We report here that maternal MDA is associated with increased methylation of the maternally expressed 3 (MEG3) ICR in males. Several lines of evidence suggest that increased methylation of the MEG3 ICR, regulating a known tumor suppressor (Zhou et al., 2012), is associated with decreased expression of MEG3, albeit with increased expression of the reciprocally imprinted DLK1 gene (Murphy et al., 2006). In genome-wide methylation arrays (Markunas et al., 2014), MEG3 DMR methylation was associated with maternal smoking and in human adults, DLK1 dysregulation has been associated with schizophrenia (Gardiner et al., 2012). In both mice and humans, hypo-methylation of the MEG3 control region has been associated with adverse neurobehavioral phenotypes (Kagami et al., 2015; Fuemmeler et al., 2016; Drobna et al., 2018). Microarray data from the mouse forebrain have shown differential spatial expression of the imprinted gene Gtl2 (aka Meg3) between the ventral and dorsal telencephalon of the mouse at a critical time point in the generation and migration of cortical neuronal populations (McLaughlin et al., 2006). In an effort to boost power, and given the sex independent associations of maternal MDA on the two control regions associated with MEG3, we examined if methylation at these loci mediated our findings of associations of maternal MDA on behavior, but were underpowered to draw conclusions (data not shown).
We also found sex-dependent associations of maternal MDA and the methylation of the epsilon sarcoglycan and paternally expressed gene 10 (SGCE/PEG10) ICR. PEG10 is essential for proper placental development, and over-expression of PEG10 protein has been associated with multiple tumor phenotypes (Okabe et al., 2003; Li X. et al., 2016; Peng et al., 2016), while differential methylation of the SGCE/PEG10 ICR has also been associated with cancer (Sepulveda et al., 2016). Hypomethylation of the SGCE/PEG10 ICR has been associated with higher expression of PEG10 as well as maternal stress and depression, although mechanisms are still unclear (Liu et al., 2012). In males at age 1 and 3, increased methylation at PEG10 has been associated with greater weight for length ratios (Gonzalez-Nahm et al., 2018), and paternal obesity has been associated with decreased methylation of PEG10 in sperm (Soubry et al., 2016).
Less is known about Pleiomorphic adenoma-like protein 1 (PLAGL1 or ZAC1). PLAGL1 is a zinc finger protein associated with cell growth suppression and with transient neonatal diabetes mellitus (Kamiya et al., 2000; Varrault et al., 2001). We found maternal MDA associated with hypomethylation of the ICR in males. Interestingly, in the EDEN cohort, maternal alcohol and dietary vitamin B2 were associated with ZAC1 methylation while increased ZAC1 methylation was associated with estimated fetal weight, weight at birth and at 1 year of age (Azzi et al., 2014).
Lastly, we report a strong association of maternal MDA with hypomethylation of insulin-like growth factor 2 (IGF2) in male offspring as well as an association of IGF2 hypomethylation with decreased atypical behavior (Figure 3). Hypomethylation of IGF2 and increased IGF2 protein have been associated with paternal obesity, as well as increased offspring obesity risk (Lawlor et al., 2012; Tobi et al., 2014; Dunford and Sangster, 2017). Decreased methylation of the IGF2 DMR has been associated with increased IGF2 transcripts (Murphy et al., 2012b) and protein (Hoyo et al., 2012). Differential methylation of IGF2 has been associated with both periconceptual exposure to extreme caloric restriction during the Dutch Famine (Tobi et al., 2009), and lower circulating folate concentrations (Hoyo et al., 2014). In the ALSPAC cohort, prenatal dietary patterns comprising high fat and high sugar were associated with lower IGF2 methylation and an earlier onset of ADHD behaviors (Rijlaarsdam et al., 2017). Dunford and Sangster provide a more thorough review of parental nutrition and offspring epigenetics in the context of metabolic syndrome risk (Dunford and Sangster, 2017).
Additional studies are needed to replicate these findings, but together, these data support mechanistic relationships between high adherence to Mediterranean diet and shifts in methylation of regulatory sequences of imprinted genes in offspring.
We adjusted for a wide array of covariates, including factors such as maternal education, BMI, maternal energy intake, and breastfeeding. Nonetheless, an important limitation of this research is uncertainty of the extent to which associations between child neurodevelopment and maternal diet quality during pregnancy may reflect differences in caregiving which are difficult to measure, or be influenced by confounding by factors such as maternal IQ (Der et al., 2006; Horta et al., 2015). Our study was limited to child outcomes in infancy; the persistence of effects in later life, and whether lasting effects may depend on childhood diet, remains uncertain. Though results are promising, more research is needed to elucidate whether this relationship is causal, and to identify the pathways through which maternal adherence to Mediterranean Diet may affect child behavior.
Our study also had some limitations including small sample sizes for stratified analyses. Despite this, all of the overall findings of associations of maternal MDA on offspring behavior and maternal MDA on offspring mean DMR methylation have an FDR < 0.15, suggesting these associations warrant further studies to replicate these findings. A lack of dietary data in infants to evaluate the extent to which neurodevelopmental outcomes may have been driven by postnatal dietary exposures other than breastfeeding is a limiting factor of this study, although dietary variability should be somewhat homogeneous at the age of behavioral assessment [12–24 months (mean = 13.9 months)]. Ideally, one would have whole-genome methylation data to identify the strongest associations of diet with CpG methylation. Our study has only focused on the effects at imprinted DMRs; there are likely effects at many other regions throughout the genome that more comprehensive epigenetic analyses would reveal. Although maternal MDA was associated with half of the imprinted gene regions evaluated, many of these imprinted gene control regions are related to major cell proliferation pathways such as TGF-β and TP53 which would impact vast downstream signaling pathways, so this may be expected. Further, we have not examined any potential effects on other epigenetic regulatory elements, including histone modifications or the expression and actions of non-coding RNAs. Additional epigenome-wide studies are needed to both replicate and clarify these findings and to characterize an epigenomic signature associated with maternal diet. Recall bias in dietary recall questionnaires is often an issue. To minimize recall bias, mothers in our study were asked to fill out a FFQ about the foods they ate near conception inside of a dozen weeks.
Conclusion
We report novel significant associations of maternal periconceptional MDA both with positive neurodevelopmental phenotypes in offspring as well as associations of maternal MDA on differential methylation of CpGs in the control regions of imprinted genes. If confirmed in other studies, these findings may pave the way for early identification of adverse behavior risk in offspring and for tailored interventions.
Data Availability
The datasets for this manuscript are not publicly available because: Human subjects were used with consent in this study, but public release of data is not approved under the IRB. Requests to access the datasets should be directed to CH for approval.
Ethics Statement
This study was carried outin accordance with the recommendations of the Duke University Health System Institutional Review Board. It conforms with special protections for pregnant women described in 45 CFR 46, Subpart B. The protocol was approved by the Duke University Health System Institutional Review Board. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
JH conceived and conducted the analyses, generated the figures and wrote the manuscript. MM, BF, FW, and CH supported the research and assisted with subject matter expertise, analysis plans, and manuscript writing and editing. RM, SG-N, ZH, JD, and SM each assisted with manuscript preparation, analysis support, and editing.
Funding
The research was supported by National Institute of Environmental Health Sciences of the National Institutes of Health (P01ES022831, R21ES014947, R01ES016772, R01HD084487, and P30ES025128) and by the U.S. Environmental Protection Agency (RD-83543701). Additional support was provided by the National Center for Advancing Translational Sciences (UL1TR001117).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Carole Grenier and her excellent skills and work with receipt and processing of the cord-blood specimens and pyrosequencing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2018.00107/full#supplementary-material
Footnotes
References
Anjos, T., Altmae, S., Emmett, P., Tiemeier, H., Closa-Monasterolo, R., Luque, V., et al. (2013). Nutrition and neurodevelopment in children: focus on NUTRIMENTHE project. Eur. J. Nutr. 52, 1825–1842. doi: 10.1007/s00394-013-0560-4
Aridi, Y. S., Walker, J. L., and Wright, O. R. L. (2017). The association between the mediterranean dietary pattern and cognitive health: a systematic review. Nutrients 9:E674. doi: 10.3390/nu9070674
Arpon, A., Riezu-Boj, J. I., Milagro, F. I., Marti, A., Razquin, C., Martinez-Gonzalez, M. A., et al. (2016). Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J. Physiol. Biochem. 73, 445–455. doi: 10.1007/s13105-017-0552-6
Azzi, S., Sas, T. C., Koudou, Y., Le Bouc, Y., Souberbielle, J. C., Dargent-Molina, P., et al. (2014). Degree of methylation of ZAC1 (PLAGL1) is associated with prenatal and post-natal growth in healthy infants of the EDEN mother child cohort. Epigenetics 9, 338–345. doi: 10.4161/epi.27387
Bach, A., Serra-Majem, L., Carrasco, J. L., Roman, B., Ngo, J., Bertomeu, I., et al. (2006). The use of indexes evaluating the adherence to the Mediterranean diet in epidemiological studies: a review. Public Health Nutr. 9, 132–146. doi: 10.1079/PHN2005936
Balsevich, G., Baumann, V., Uribe, A., Chen, A., and Schmidt, M. V. (2016). Prenatal exposure to maternal obesity alters anxiety and stress coping behaviors in aged mice. Neuroendocrinology 103, 354–368. doi: 10.1159/000439087
Bartolomei, M. S., and Ferguson-Smith, A. C. (2011). Mammalian genomic imprinting. Cold Spring Harb. Perspect. Biol. 3:a002592. doi: 10.1101/cshperspect.a002592
Bolduc, F. V., Lau, A., Rosenfelt, C. S., Langer, S., Wang, N., Smithson, L., et al. (2016). Cognitive enhancement in infants associated with increased maternal fruit intake during pregnancy: results from a birth cohort study with validation in an animal model. EBioMedicine 8, 331–340. doi: 10.1016/j.ebiom.2016.04.025
Borge, T. C., Aase, H., Brantsaeter, A. L., and Biele, G. (2017). The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: a systematic review and meta-analysis. BMJ Open 7:e016777. doi: 10.1136/bmjopen-2017-016777
Brew, B. K., Toelle, B. G., Webb, K. L., Almqvist, C., Marks, G. B., and CAPS investigators (2015). Omega-3 supplementation during the first 5 years of life and later academic performance: a randomised controlled trial. Eur. J. Clin. Nutr. 69, 419–424. doi: 10.1038/ejcn.2014.155
Bruce-Keller, A. J., Fernandez-Kim, S. O., Townsend, R. L., Kruger, C., Carmouche, R., Newman, S., et al. (2017). Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One 12:e0175577. doi: 10.1371/journal.pone.0175577
Carter, A. S., Briggs-Gowan, M. J., Jones, S. M., and Little, T. D. (2003). The infant-toddler social and emotional assessment (ITSEA): factor structure, reliability, and validity. J. Abnorm. Child Psychol. 31, 495–514. doi: 10.1023/A:1025449031360
Carter, A. S., Godoy, L., Wagmiller, R. L., Veliz, P., Marakovitz, S., and Briggs-Gowan, M. J. (2010). Internalizing trajectories in young boys and girls: the whole is not a simple sum of its parts. J. Abnorm. Child Psychol. 38, 19–31. doi: 10.1007/s10802-009-9342-0
Castro-Rodriguez, J. A., and Garcia-Marcos, L. (2017). What are the effects of a mediterranean diet on allergies and asthma in children? Front. Pediatr. 5:72. doi: 10.3389/fped.2017.00072
Catena, A., Munoz-Machicao, J. A., Torres-Espinola, F. J., Martinez-Zaldivar, C., Diaz-Piedra, C., Gil, A., et al. (2016). Folate and long-chain polyunsaturated fatty acid supplementation during pregnancy has long-term effects on the attention system of 8.5-y-old offspring: a randomized controlled trial. Am. J. Clin. Nutr. 103, 115–127. doi: 10.3945/ajcn.115.109108
CDC (2010). Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children — united states, 2003 and 2007. MMWR Morb. Mortal. Wkly. Rep. 59, 1439–1443.
Contu, L., and Hawkes, C. A. (2017). A review of the impact of maternal obesity on the cognitive function and mental health of the offspring. Int. J. Mol. Sci. 18:E1093. doi: 10.3390/ijms18051093
Cowley, M., Skaar, D. A., Jima, D. D., Maguire, R. L., Hudson, K. M., Park, S. S., et al. (2018). Effects of cadmium exposure on DNA methylation at imprinting control regions and genome-wide in mothers and newborn children. Environ. Health Perspect. 126:037003. doi: 10.1289/EHP2085
Crichton, G. E., Bryan, J., Hodgson, J. M., and Murphy, K. J. (2013). Mediterranean diet adherence and self-reported psychological functioning in an Australian sample. Appetite 70, 53–59. doi: 10.1016/j.appet.2013.06.088
Delgado-Noguera, M. F., Calvache, J. A., Bonfill Cosp, X., Kotanidou, E. P., and Galli-Tsinopoulou, A. (2015). Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 14:CD007901. doi: 10.1002/14651858.CD007901.pub3
Der, G., Batty, G. D., and Deary, I. J. (2006). Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ 333:945. doi: 10.1136/bmj.38978.699583.55
Dilis, V., Katsoulis, M., Lagiou, P., Trichopoulos, D., Naska, A., and Trichopoulou, A. (2012). Mediterranean diet and CHD: the Greek European prospective investigation into cancer and nutrition cohort. Br. J. Nutr. 108, 699–709. doi: 10.1017/S0007114512001821
Drobna, Z., Henriksen, A. D., Wolstenholme, J. T., Montiel, C., Lambeth, P. S., Shang, S., et al. (2018). Transgenerational effects of bisphenol a on gene expression and DNA methylation of imprinted genes in brain. Endocrinology 159, 132–144. doi: 10.1210/en.2017-00730
Dunford, A. R., and Sangster, J. M. (2017). Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: a systematic review. Diabetes Metab. Syndr. 11(Suppl. 2), S655–S662. doi: 10.1016/j.dsx.2017.04.021
Emmett, P. M., Jones, L. R., and Golding, J. (2015). Pregnancy diet and associated outcomes in the Avon longitudinal study of parents and children. Nutr. Rev. 73(Suppl. 3), 154–174. doi: 10.1093/nutrit/nuv053
Esposito, K., Maiorino, M. I., Ciotola, M., Di Palo, C., Scognamiglio, P., Gicchino, M., et al. (2009). Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann. Intern. Med. 151, 306–314. doi: 10.7326/0003-4819-151-5-200909010-00004
Esteban-Cornejo, I., Izquierdo-Gomez, R., Gomez-Martinez, S., Padilla-Moledo, C., Castro-Pinero, J., Marcos, A., et al. (2016). Adherence to the Mediterranean diet and academic performance in youth: the UP&DOWN study. Eur. J. Nutr. 55, 1133–1140. doi: 10.1007/s00394-015-0927-9
Estruch, R., Martiìnez-Gonzaìlez, M. A.ì, Corella, D., Salas-Salvadoì, J., Ruiz-Gutieìrrez, V., Covas, M. I., et al. (2006). Effects of a mediterranean-style diet on cardiovascular risk Factors: a randomized trial. Ann. Intern. Med. 145, 1–11. doi: 10.7326/0003-4819-145-1-200607040-00004
Freitas-Vilela, A. A., Pearson, R. M., Emmett, P., Heron, J., Smith, A. D., Emond, A., et al. (2017). Maternal dietary patterns during pregnancy and intelligence quotients in the offspring at 8 years of age: findings from the ALSPAC cohort. Matern. Child. Nutr. 14:e12431. doi: 10.1111/mcn.12431
Fuemmeler, B. F., Lee, C. T., Soubry, A., Iversen, E. S., Huang, Z., Murtha, A. P., et al. (2016). DNA methylation of regulatory regions of imprinted genes at birth and its relation to infant temperament. Genet. Epigenet. 8, 59–67. doi: 10.4137/GEG.S40538
Fung, T. T., Rexrode, K. M., Mantzoros, C. S., Manson, J. E., Willett, W. C., and Hu, F. B. (2009). Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 119, 1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736
Gardiner, E., Beveridge, N. J., Wu, J. Q., Carr, V., Scott, R. J., Tooney, P. A., et al. (2012). Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol. Psychiatry 17, 827–840. doi: 10.1038/mp.2011.78
Gartstein, M. A., and Skinner, M. K. (2017). Prenatal influences on temperament development: the role of environmental epigenetics. Dev. Psychopathol. doi: 10.1017/S0954579417001730 [Epub ahead of print].
Gonzalez-Nahm, S., Mendez, M., Robinson, W., Murphy, S. K., Hoyo, C., Hogan, V., et al. (2017). Low maternal adherence to a Mediterranean diet is associated with increase in methylation at the MEG3-IG differentially methylated region in female infants. Environ. Epigenet. 3:dvx007. doi: 10.1093/eep/dvx007
Gonzalez-Nahm, S., Mendez, M. A., Benjamin-Neelon, S. E., Murphy, S. K., Hogan, V. K., Rowley, D. L., et al. (2018). DNA methylation of imprinted genes at birth is associated with child weight status at birth, 1 year, and 3 years. Clin. Epigenet. 10:90. doi: 10.1186/s13148-018-0521-0
Goodrich, J. M., Sanchez, B. N., Dolinoy, D. C., Zhang, Z., Hernandez-Avila, M., Hu, H., et al. (2015). Quality control and statistical modeling for environmental epigenetics: a study on in utero lead exposure and DNA methylation at birth. Epigenetics 10, 19–30. doi: 10.4161/15592294.2014.989077
Guxens, M., Aguilera, I., Ballester, F., Estarlich, M., Fernandez-Somoano, A., Lertxundi, A., et al. (2012). Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ. Health Perspect. 120, 144–149. doi: 10.1289/ehp.1103469
Hardman, R. J., Kennedy, G., Macpherson, H., Scholey, A. B., and Pipingas, A. (2016). Adherence to a mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Front. Nutr. 3:22. doi: 10.3389/fnut.2016.00022
Harper, L. V. (2005). Epigenetic inheritance and the intergenerational transfer of experience. Psychol. Bull. 131, 340–360. doi: 10.1037/0033-2909.131.3.340
Horta, B. L., Loret de Mola, C., and Victora, C. G. (2015). Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 104, 14–19. doi: 10.1111/apa.13139
House, J. S., Wyss, A. B., Hoppin, J. A., Richards, M., Long, S., Umbach, D. M., et al. (2016). Early-life farm exposures and adult asthma and atopy in the Agricultural Lung Health Study. J. Allergy Clin. Immunol. 140, 249–256.e14. doi: 10.1016/j.jaci.2016.09.036
Hoyo, C., Daltveit, A. K., Iversen, E., Benjamin-Neelon, S. E., Fuemmeler, B., Schildkraut, J., et al. (2014). Erythrocyte folate concentrations, CpG methylation at genomically imprinted domains, and birth weight in a multiethnic newborn cohort. Epigenetics 9, 1120–1130. doi: 10.4161/epi.29332
Hoyo, C., Fortner, K., Murtha, A. P., Schildkraut, J. M., Soubry, A., Demark-Wahnefried, W., et al. (2012). Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control 23, 635–645. doi: 10.1007/s10552-012-9932-y
Huizink, A. C., de Medina, P. G., Mulder, E. J., Visser, G. H., and Buitelaar, J. K. (2002). Psychological measures of prenatal stress as predictors of infant temperament. J. Am. Acad. Child Adolesc. Psychiatry 41, 1078–1085. doi: 10.1097/00004583-200209000-00008
Iris Shai, R. D., Dan Schwarzfuchs, M. D., Yaakov Henkin, M. D., Danit, R., Shahar, R. D., Shula Witkow, R. D., et al. (2008). Weight loss with a low-carbohydrate, mediterranean, or low-fat diet. N. Engl. J. Med. 359, 229–241. doi: 10.1056/NEJMoa0708681
Ishikawa, Y., Tanaka, H., Akutsu, T., Koide, K., Sakuma, M., Okazaki, M., et al. (2016). Prenatal vitamin A supplementation associated with adverse child behavior at 3 years in a prospective birth cohort in Japan. Pediatr. Int. 58, 855–861. doi: 10.1111/ped.12925
Jacka, F. N., Ystrom, E., Brantsaeter, A. L., Karevold, E., Roth, C., Haugen, M., et al. (2013). Maternal and early postnatal nutrition and mental health of offspring by age 5 years: a prospective cohort study. J. Am. Acad. Child Adolesc. Psychiatry 52, 1038–1047. doi: 10.1016/j.jaac.2013.07.002
Julvez, J., Mendez, M., Fernandez-Barres, S., Romaguera, D., Vioque, J., Llop, S., et al. (2016). Maternal consumption of seafood in pregnancy and child neuropsychological development: a longitudinal study based on a population with high consumption levels. Am. J. Epidemiol. 183, 169–182. doi: 10.1093/aje/kwv195
Kagami, M., Mizuno, S., Matsubara, K., Nakabayashi, K., Sano, S., Fuke, T., et al. (2015). Epimutations of the IG-DMR and the MEG3-DMR at the 14q32.2 imprinted region in two patients with Silver-Russell Syndrome-compatible phenotype. Eur. J. Hum. Genet. 23, 1062–1067. doi: 10.1038/ejhg.2014.234
Kamiya, M., Judson, H., Okazaki, Y., Kusakabe, M., Muramatsu, M., Takada, S., et al. (2000). The cell cycle control gene ZAC/PLAGL1 is imprinted–a strong candidate gene for transient neonatal diabetes. Hum. Mol. Genet. 9, 453–460. doi: 10.1093/hmg/9.3.453
Kastorini, C. M., Milionis, H. J., Esposito, K., Giugliano, D., Goudevenos, J. A., and Panagiotakos, D. B. (2011). The effect of mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 57, 1299–1313. doi: 10.1016/j.jacc.2010.09.073
Knoops, K. T. B., de Groot, L. C. P. G. M., Kromhout, D., Perrin, A. E., Moreiras-Varela, O., Menotti, A., et al. (2004). Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA 292, 1433–1439. doi: 10.1001/jama.292.12.1433
Korre, M., Tsoukas, M. A., Frantzeskou, E., Yang, J., and Kales, S. N. (2014). Mediterranean diet and workplace health promotion. Curr. Cardiovasc. Risk Rep. 8:416. doi: 10.1007/s12170-014-0416-3
Kruizinga, I., Visser, J. C., van Batenburg-Eddes, T., Carter, A. S., Jansen, W., and Raat, H. (2014). Screening for autism spectrum disorders with the brief infant-toddler social and emotional assessment. PLoS One 9:e97630. doi: 10.1371/journal.pone.0097630
Lawlor, D. A., Relton, C., Sattar, N., and Nelson, S. M. (2012). Maternal adiposity–a determinant of perinatal and offspring outcomes? Nat. Rev. Endocrinol. 8, 679–688. doi: 10.1038/nrendo.2012.176
Lee, H. S. (2015). Impact of maternal diet on the epigenome during in utero life and the developmental programming of diseases in childhood and adulthood. Nutrients 7, 9492–9507. doi: 10.3390/nu7115467
Leighton, F., Polic, G., Strobel, P., Pérez, D., Martínez, C., Vásquez, L., et al. (2009). Health impact of Mediterranean diets in food at work. Public Health Nutr. 12, 1635–1643. doi: 10.1017/S1368980009990486
Li, X., Xiao, R., Tembo, K., Hao, L., Xiong, M., Pan, S., et al. (2016). PEG10 promotes human breast cancer cell proliferation, migration and invasion. Int. J. Oncol. 48, 1933–1942. doi: 10.3892/ijo.2016.3406
Li, Y., Xie, C., Murphy, S. K., Skaar, D., Nye, M., Vidal, A. C., et al. (2016). Lead exposure during early human development and DNA methylation of imprinted gene regulatory elements in adulthood. Environ. Health Perspect. 124, 666–673. doi: 10.1289/ehp.1408577
Lillycrop, K. A., and Burdge, G. C. (2015). Maternal diet as a modifier of offspring epigenetics. J. Dev. Orig. Health Dis. 6, 88–95. doi: 10.1017/S2040174415000124
Lipton, L. R., Brunst, K. J., Kannan, S., Ni, Y. M., Ganguri, H. B., Wright, R. J., et al. (2017). Associations among prenatal stress, maternal antioxidant intakes in pregnancy, and child temperament at age 30 months. J. Dev. Orig. Health Dis. 8, 638–648. doi: 10.1017/S2040174417000411
Liu, Y., Murphy, S. K., Murtha, A. P., Fuemmeler, B. F., Schildkraut, J., Huang, Z., et al. (2012). Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics 7, 735–746. doi: 10.4161/epi.20734
Lorite Mingot, D., Gesteiro, E., Bastida, S., and Sanchez-Muniz, F. J. (2017). Epigenetic effects of the pregnancy Mediterranean diet adherence on the offspring metabolic syndrome markers. J. Physiol. Biochem. 73, 495–510. doi: 10.1007/s13105-017-0592-y
Loughrey, D. G., Lavecchia, S., Brennan, S., Lawlor, B. A., and Kelly, M. E. (2017). The impact of the mediterranean diet on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Adv. Nutr. 8, 571–586. doi: 10.3945/an.117.015495
Lyall, K., Schmidt, R. J., and Hertz-Picciotto, I. (2014). Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epidemiol. 43, 443–464. doi: 10.1093/ije/dyt282
Mansell, T., Novakovic, B., Meyer, B., Rzehak, P., Vuillermin, P., Ponsonby, A. L., et al. (2016). The effects of maternal anxiety during pregnancy on IGF2/H19 methylation in cord blood. Transl. Psychiatry 6:e765. doi: 10.1038/tp.2016.32
Markunas, C. A., Xu, Z., Harlid, S., Wade, P. A., Lie, R. T., Taylor, J. A., et al. (2014). Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ. Health Perspect. 122, 1147–1153. doi: 10.1289/ehp.1307892
Martínez-González, M. A., de la Fuente-Arrillaga, C., Nunez-Cordoba, J. M., Basterra-Gortari, F. J., Beunza, J. J., Vazquez, Z., et al. (2008). Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ 336, 1348–1351. doi: 10.1136/bmj.39561.501007.BE
McLaughlin, D., Vidaki, M., Renieri, E., and Karagogeos, D. (2006). Expression pattern of the maternally imprinted gene Gtl2 in the forebrain during embryonic development and adulthood. Gene Expr. Patterns 6, 394–399. doi: 10.1016/j.modgep.2005.09.007
Molendijk, M., Molero, P., Ortuno Sanchez-Pedreno, F., Van der Does, W., and Angel Martinez-Gonzalez, M. (2018). Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 226, 346–354. doi: 10.1016/j.jad.2017.09.022
Moser, V. C., McDaniel, K. L., Woolard, E. A., Phillips, P. M., Franklin, J. N., and Gordon, C. J. (2017). Impacts of maternal diet and exercise on offspring behavior and body weights. Neurotoxicol. Teratol. 63, 46–50. doi: 10.1016/j.ntt.2017.07.002
Murphy, S. K., Adigun, A., Huang, Z., Overcash, F., Wang, F., Jirtle, R. L., et al. (2012a). Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene 494, 36–43. doi: 10.1016/j.gene.2011.11.062
Murphy, S. K., Huang, Z., and Hoyo, C. (2012b). Differentially methylated regions of imprinted genes in prenatal, perinatal and postnatal human tissues. PLoS One 7:e40924. doi: 10.1371/journal.pone.0040924
Murphy, S. K., Nolan, C. M., Huang, Z., Kucera, K. S., Freking, B. A., Smith, T. P., et al. (2006). Callipyge mutation affects gene expression in cis: a potential role for chromatin structure. Genome Res. 16, 340–346. doi: 10.1101/gr.4389306
Netting, M. J., Middleton, P. F., and Makrides, M. (2014). Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition 30, 1225–1241. doi: 10.1016/j.nut.2014.02.015
Niculescu, M. D., Craciunescu, C. N., and Zeisel, S. H. (2006). Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 20, 43–49. doi: 10.1096/fj.05-4707com
Nye, M. D., Hoyo, C., Huang, Z., Vidal, A. C., Wang, F., Overcash, F., et al. (2013). Associations between methylation of paternally expressed gene 3 (PEG3), cervical intraepithelial neoplasia and invasive cervical cancer. PLoS One 8:e56325. doi: 10.1371/journal.pone.0056325
Okabe, H., Satoh, S., Furukawa, Y., Kato, T., Hasegawa, S., Nakajima, Y., et al. (2003). Involvement of PEG10 in human hepatocellular carcinogenesis through interaction with SIAH1. Cancer Res. 63, 3043–3048.
Panagiotakos, D. B., Pitsavos, C., and Stefanadis, C. (2006). Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 16, 559–568. doi: 10.1016/j.numecd.2005.08.006
Peng, W., Fan, H., Wu, G., Wu, J., and Feng, J. (2016). Upregulation of long noncoding RNA PEG10 associates with poor prognosis in diffuse large B cell lymphoma with facilitating tumorigenicity. Clin. Exp. Med. 16, 177–182. doi: 10.1007/s10238-015-0350-9
Renaud, S., de Lorgeril, M., Delaye, J., Guidollet, J., Jacquard, F., Mamelle, N., et al. (1995). Cretan Mediterranean diet for prevention of coronary heart disease. Am. J. Clin. Nutr. 61, 1360S–1367S. doi: 10.1093/ajcn/61.6.1360S
Rienks, J., Dobson, A. J., and Mishra, G. D. (2013). Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur. J. Clin. Nutr. 67, 75–82. doi: 10.1038/ejcn.2012.193
Rijlaarsdam, J., Cecil, C. A., Walton, E., Mesirow, M. S., Relton, C. L., Gaunt, T. R., et al. (2017). Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J. Child Psychol. Psychiatry 58, 19–27. doi: 10.1111/jcpp.12589
Rios-Hernandez, A., Alda, J. A., Farran-Codina, A., Ferreira-Garcia, E., and Izquierdo-Pulido, M. (2017). The mediterranean diet and ADHD in children and adolescents. Pediatrics 139:e20162027. doi: 10.1542/peds.2016-2027
Rivera, H. M., Christiansen, K. J., and Sullivan, E. L. (2015). The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 9:194. doi: 10.3389/fnins.2015.00194
Ros, E., Martinez-Gonzalez, M. A., Estruch, R., Salas-Salvado, J., Fito, M., Martinez, J. A., et al. (2014). Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv. Nutr. 5, 330S–336S. doi: 10.3945/an.113.005389
Sanchez, C. E., Barry, C., Sabhlok, A., Russell, K., Majors, A., Kollins, S. H., et al. (2017). Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes. Rev. 19, 464–484. doi: 10.1111/obr.12643
Schwingshackl, L., Strasser, B., and Hoffmann, G. (2011). Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann. Nutr. Metab. 59, 176–186. doi: 10.1159/000334071
Sepulveda, J. L., Gutierrez-Pajares, J. L., Luna, A., Yao, Y., Tobias, J. W., Thomas, S., et al. (2016). High-definition CpG methylation of novel genes in gastric carcinogenesis identified by next-generation sequencing. Mod. Pathol. 29, 182–193. doi: 10.1038/modpathol.2015.144
Singmann, P., Shem-Tov, D., Wahl, S., Grallert, H., Fiorito, G., Shin, S. Y., et al. (2015). Characterization of whole-genome autosomal differences of DNA methylation between men and women. Epigenetics Chromatin 8:43. doi: 10.1186/s13072-015-0035-3
Sofi, F. (2009). The Mediterranean diet revisited: evidence of its effectiveness grows. Curr. Opin. Cardiol. 24, 442–446. doi: 10.1097/HCO.0b013e32832f056e
Soubry, A., Guo, L., Huang, Z., Hoyo, C., Romanus, S., Price, T., et al. (2016). Obesity-related DNA methylation at imprinted genes in human sperm: results from the TIEGER study. Clin. Epigenet. 8:51. doi: 10.1186/s13148-016-0217-2
Steenweg-de Graaff, J., Roza, S. J., Steegers, E. A., Hofman, A., Verhulst, F. C., Jaddoe, V. W., et al. (2012). Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am. J. Clin. Nutr. 95, 1413–1421. doi: 10.3945/ajcn.111.030791
Steenweg-de Graaff, J., Tiemeier, H., Steegers-Theunissen, R. P., Hofman, A., Jaddoe, V. W., Verhulst, F. C., et al. (2014). Maternal dietary patterns during pregnancy and child internalising and externalising problems. The Generation R Study. Clin. Nutr. 33, 115–121. doi: 10.1016/j.clnu.2013.03.002
Storey, J. D., Bass, A. J., Dabney, A., and Robinson, D. (2015). qvalue: Q-Value Estimation for False Discovery Rate Control. Available at: http://github.com/jdstorey/qvalue
Tobi, E. W., Goeman, J. J., Monajemi, R., Gu, H., Putter, H., Zhang, Y., et al. (2014). DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 5:5592. doi: 10.1038/ncomms6592
Tobi, E. W., Lumey, L. H., Talens, R. P., Kremer, D., Putter, H., Stein, A. D., et al. (2009). DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 18, 4046–4053. doi: 10.1093/hmg/ddp353
Trichopoulou, A., Costacou, T., Bamia, C., and Trichopoulos, D. (2003). Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 348, 2599–2608. doi: 10.1056/NEJMoa025039
Trovato, G. M., Catalano, D., Martines, G. F., Pace, P., and Trovato, F. M. (2014). Mediterranean diet: relationship with anxiety and depression. Ann. Neurol. 75:613. doi: 10.1002/ana.23991
van Mil, N. H., Steegers-Theunissen, R. P., Bouwland-Both, M. I., Verbiest, M. M., Rijlaarsdam, J., Hofman, A., et al. (2014). DNA methylation profiles at birth and child ADHD symptoms. J. Psychiatr. Res. 49, 51–59. doi: 10.1016/j.jpsychires.2013.10.017
Varrault, A., Bilanges, B., Mackay, D. J., Basyuk, E., Ahr, B., Fernandez, C., et al. (2001). Characterization of the methylation-sensitive promoter of the imprinted ZAC gene supports its role in transient neonatal diabetes mellitus. J. Biol. Chem. 276, 18653–18656. doi: 10.1074/jbc.C100095200
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S. 4th Edn. New York, NY: Springer.
Verweij, L. M., Coffeng, J., van Mechelen, W., and Proper, K. I. (2011). Meta-analyses of workplace physical activity and dietary behaviour interventions on weight outcomes. Obes. Rev. 12, 406–429. doi: 10.1111/j.1467-789X.2010.00765.x
Visser, S. N., Blumberg, S. J., Danielson, M. L., Bitsko, R. H., and Kogan, M. D. (2013). State-based and demographic variation in parent-reported medication rates for attention-deficit/hyperactivity disorder, 2007-2008. Prev. Chronic Dis. 10:E09. doi: 10.5888/pcd9.120073
Visser, S. N., Danielson, M. L., Bitsko, R. H., Holbrook, J. R., Kogan, M. D., Ghandour, R. M., et al. (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: united states, 2003-2011. J. Am. Acad. Child Adolesc. Psychiatry 53, 34–46e2. doi: 10.1016/j.jaac.2013.09.001
Vucetic, Z., Kimmel, J., Totoki, K., Hollenbeck, E., and Reyes, T. M. (2010). Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151, 4756–4764. doi: 10.1210/en.2010-0505
Wiebe, S. A., Espy, K. A., Stopp, C., Respass, J., Stewart, P., Jameson, T. R., et al. (2009). Gene-environment interactions across development: exploring DRD2 genotype and prenatal smoking effects on self-regulation. Dev. Psychol. 45, 31–44. doi: 10.1037/a0014550
Wyss, A. B., House, J. S., Hoppin, J. A., Richards, M., Hankinson, J. L., Long, S., et al. (2017). Raw milk consumption and other early-life farm exposures and adult pulmonary function in the Agricultural Lung Health Study. Thorax 73, 279–282. doi: 10.1136/thoraxjnl-2017-210031
Xiao, Y., Camarillo, C., Ping, Y., Arana, T. B., Zhao, H., Thompson, P. M., et al. (2014). The DNA methylome and transcriptome of different brain regions in schizophrenia and bipolar disorder. PLoS One 9:e95875. doi: 10.1371/journal.pone.0095875
Keywords: maternal diet, neuro-development, cord-blood methylation, child behavior disorders, ADHD-attention deficit disorder, autism spectrum disorder, epigenetics, imprinted genes
Citation: House JS, Mendez M, Maguire RL, Gonzalez-Nahm S, Huang Z, Daniels J, Murphy SK, Fuemmeler BF, Wright FA and Hoyo C (2018) Periconceptional Maternal Mediterranean Diet Is Associated With Favorable Offspring Behaviors and Altered CpG Methylation of Imprinted Genes. Front. Cell Dev. Biol. 6:107. doi: 10.3389/fcell.2018.00107
Received: 22 February 2018; Accepted: 20 August 2018;
Published: 07 September 2018.
Edited by:
Patrick McGowan, University of Toronto, CanadaReviewed by:
Craig A. Cooney, Central Arkansas Veterans Healthcare System Eugene J. Towbin Healthcare Center, United StatesAndrea Masotti, Ospedale Pediatrico Bambino Gesù (IRCCS), Italy
Copyright © 2018 House, Mendez, Maguire, Gonzalez-Nahm, Huang, Daniels, Murphy, Fuemmeler, Wright and Hoyo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John S. House, anNob3VzZUBuY3N1LmVkdQ==
 John S. House
John S. House Michelle Mendez3
Michelle Mendez3 Sarah Gonzalez-Nahm
Sarah Gonzalez-Nahm Susan K. Murphy
Susan K. Murphy Bernard F. Fuemmeler
Bernard F. Fuemmeler Fred A. Wright
Fred A. Wright