
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 12 April 2016
Sec. Membrane Traffic and Organelle Dynamics
Volume 4 - 2016 | https://doi.org/10.3389/fcell.2016.00024
This article is part of the Research Topic Tethering of transport vesicles in secretory and endocytic pathways: mechanisms and players View all 18 articles
Exocytosis involves the fusion of intracellular secretory vesicles with the plasma membrane, thereby delivering integral membrane proteins to the cell surface and releasing material into the extracellular space. Importantly, exocytosis also provides a source of lipid moieties for membrane extension. The tethering of the secretory vesicle before docking and fusion with the plasma membrane is mediated by the exocyst complex, an evolutionary conserved octameric complex of proteins. Recent findings indicate that the exocyst complex also takes part in other intra-cellular processes besides secretion. These various functions seem to converge toward defining a direction of membrane growth in a range of systems from fungi to plants and from neurons to cilia. In this review we summarize the current knowledge of exocyst function in cell polarity, signaling and cell-cell communication and discuss implications for plant and animal health and disease.
Exocytosis involves the fusion of intracellular secretory vesicles with the plasma membrane (PM), thereby delivering integral membrane proteins at the cell surface and releasing material into the extracellular space, such as hormones or components of the extracellular matrix. Importantly, exocytosis also provides a source of lipid moieties for membrane extension. The addition of new membranes to specific areas of the cell periphery is a fundamental requirement for growth, polarity, or division and is therefore critical for cell function and tissue development.
A further understanding of the mechanisms regulating secretory vesicle sorting, transport, and targeting is beginning to deepen. Cargoes emanating from intracellular endomembrane compartments are transported by motor proteins along cytoskeletal tracks toward polarized areas of the PM. The initial contact between the vesicle and the PM (aka tethering) is mediated by the exocyst complex, which consists of eight proteins named Sec (for “secretion”) or Exo (for “exocyst related”): Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 (TerBush and Novick, 1995; TerBush et al., 1996). The exocyst complex, aided by Sec1/Munc-18, is thought to bridge the SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) on opposing membranes (2 target t-SNAREs and 1 vesicular v-SNARE) (Hong and Lev, 2014). This paired trans-SNARE complex docks the vesicle to the receiving membrane and finally induces lipid fusion.
The exocyst complex has been extensively studied using Saccharomyces cerevisiae as a model organism. But even within the budding yeast community, the mechanisms of its assembly, and mode of action are controversial. It was originally proposed that the assembly of the exocyst complex is sequential. In this model, Sec3 and Exo70 sit at the PM and this localization is independent of the secretion and transport machineries (Finger et al., 1998). At the PM, Sec3, and Exo70 interact with phosphatidylinositol (4,5)-bisphosphate (PIP2) (Boyd et al., 2004; He et al., 2007; Liu et al., 2007; Pleskot et al., 2015) and assemble the rest of the exocyst complex, which is trafficked to the PM on vesicles in an actin-dependent manner (Jin et al., 2011). For these reasons, Sec3 and Exo70 were described as landmarks for polarized secretion (Boyd et al., 2004). This model implies that at least two sub-complexes independently exist prior to the formation of the full complex, one at the PM and one on the secretory vesicle. However, convincing evidence later indicated that the endogenous untagged Sec3 protein behaves similarly to the other exocyst subunits, thus calling the landmark model into question (Roumanie et al., 2005). Furthermore, the fully assembled octameric complex, but not any sub-complex, could be isolated from S. cerevisiae, in the most advanced biochemical study of the exocyst complex to date (Heider et al., 2016).
The complex is highly conserved across evolution (Koumandou et al., 2007) and it was long assumed that the properties and mechanisms of action of the exocyst in budding yeast also applied to other organisms. In recent years, the interest that the exocyst has raised in fungi, plants and animal systems, has unraveled new functions, similarities and differences with the paradigmatic yeast model. This review aims to summarize some of them, and to further highlight the importance of the exocyst complex in normal cell function and pathological situations. The interplay between the exocyst and its regulators have been extensively reviewed elsewhere (e.g., Wu et al., 2008; Guichard et al., 2014; Mukherjee et al., 2014; Shirakawa and Horiuchi, 2015) and are not emphasized here.
Fungi have a single homolog encoding gene for each of the eight exocyst components (TerBush and Novick, 1995; TerBush et al., 1996; Guo et al., 1999, 2015; Wang et al., 2002, 2003; Li et al., 2007; Kohli et al., 2008; Taheri-Talesh et al., 2008; Panepinto et al., 2009; Jones and Sudbery, 2010; Bendezu et al., 2012; Jourdain et al., 2012; Giraldo et al., 2013; Irieda et al., 2014; Kwon et al., 2014; Riquelme et al., 2014; Chavez-Dozal et al., 2015a,b; Gupta et al., 2015), and most of these genes are essential for viability except exo70 in Schizosaccharomyces pombe, sec3 in S. cerevisiae, Candida albicans, and Aspergillus niger, sec5 in Neurospora crassa, and both exo70 and sec5 in Magnaporthe oryzae (Finger and Novick, 1997; Wang et al., 2002; Li et al., 2007; Kohli et al., 2008; Kim D. U. et al., 2010; Giraldo et al., 2013; Kwon et al., 2014; Riquelme et al., 2014). Thus, the same component of the exocyst may not share identical interactions within the complex in different fungi. However, co-purification and co-immunoprecipitation experiments conducted in N. crassa and in M. oryzae indicate that in these two fungi at least, the exocyst exists as an octameric complex (Riquelme et al., 2014; Gupta et al., 2015). All members of the exocyst have a similar fold made of long helical bundles, which are thought to pack against one another to form the exocyst complex (Munson and Novick, 2006; Heider et al., 2016). It is therefore possible that structural redundancies exist between fungal species.
Fungi exhibit different modes of growth with a wide variety of sizes and shapes (Figure 1). Filamentous fungi such as N. crassa, Ashbya gossypii, Aspergillus nidulans, Fusarium oxysporum, or M. oryzae form hyphae. Hyphae are long cylindrical structures that show sustained polar growth at their tips and that are able to form a branched network of hyphae (aka mycelium). In multicellular hyphae, individual cells are separated by a septum, but aseptated, unicellular hyphae also exist. Yeast are unicellular fungi that do not normally form hyphae. The fission yeast Schizosaccharomyces (e.g., S. pombe) are rod- shaped, and like filamentous fungi, grow by tip extension and form a septum perpendicular to the cell's long axis. By contrast, budding yeast (e.g., S. cerevisiae) are round or ovoid and divide by budding. Dimorphic fungi (e.g., C. albicans, Ustilago maydis, or Zymoseptoria tritici) are capable of switching from a yeast form to a hyphal or pseudohyphal form, where the pseudohyphae are branching chains of cells that fail to separate after mitosis. In yeast and most common model fungi, non-essential genes can be fully deleted and for genes that are essential for viability, conditional mutants can be generated (the fungus is only mutant under certain experimental conditions). Thus, a number of mutants of the exocyst complex have been created in various fungal species (TerBush and Novick, 1995; TerBush et al., 1996; Guo et al., 1999; Wang et al., 2002, 2003; Li et al., 2007; Bendezu et al., 2012; Jourdain et al., 2012; Giraldo et al., 2013; Kwon et al., 2014; Riquelme et al., 2014; Chavez-Dozal et al., 2015a,b; Gupta et al., 2015). This has allowed the identification of the different cellular functions of the exocyst complex in fungi.
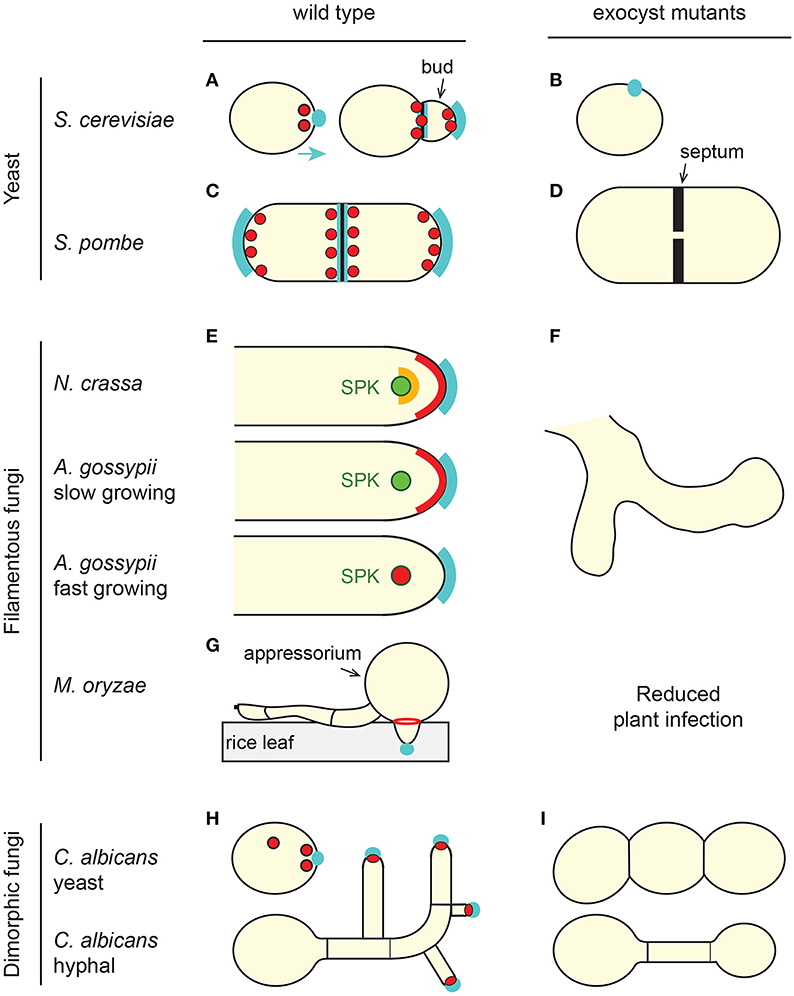
Figure 1. The exocyst complex in fungi. (A,C,E,G,H) Overall localization of the exocyst complex (red and orange) in wild-type cells of representative fungi. For simplicity, S. pombe cells (C,D) are represented as both elongating and dividing. In all fungi, the exocyst localizes at sites of polarized secretion and polarized growth (cyan), the bud tip in budding cells (A,H), cell tip area in tip-growing fungi (C,E) or septal area in dividing cells (A,C). In some cases individual sub-units or sub-complexes are present at different sub-cellular locations, which is only illustrated here for N. crassa (E, red and orange). The exocyst localizes as a ring at the base of the M. oryzae appressorium during rice leaf infection (G). SPK, Spitzenkörper (green). (B,D,F,I) Typical cellular phenotype of exocyst mutants. In the absence of a functional exocyst, most fungal species have a morphological or loss of polarity phenotype, failing to grow a bud (B), growing wider (D), branching out (F), or failing to branch (I). Note that in the case of the hyphal form of C. albicans, three different phenotypes were described. We arbitrarily present here a hypo-branching and globular tip phenotype reported for a sec3 defective mutant. Some fungi also have a cytokinetic defect (D,I). See text for details and references.
The filamentous growth is an extreme polarized process that requires the selection of one growth site and its maintenance by selective transport and targeting of secretory vesicles toward what will constitute the hyphal tip. At the apex, these secretory vesicles are incorporated to specific domains of the PM, where they allow its expansion. They ensure the delivery of material and enzymes involved in the synthesis of the fungal cell walls and the release of proteins to the extracellular space. The polarization site is marked by polarity cues that enable the assembly of the machinery responsible for polar growth. Once the site has been chosen, the multiprotein complex termed the polarisome is deposited at cell tips by microtubules (MTs) (Feierbach et al., 2004; Takeshita et al., 2008; Takeshita and Fischer, 2011) and orchestrates the nucleation of actin cables by formins, along which the secretory vesicles are transported (Pruyne and Bretscher, 2000a,b; Pruyne et al., 2002). At the hyphal tip, fungi have a Spitzenkörper (SPK, “tip body”), a sub-apical membrane rich region where secretory vesicles continuously accumulate prior to fusion with the PM (Riquelme et al., 2007; Verdin et al., 2009; Sanchez-Leon et al., 2011; Richthammer et al., 2012; Sanchez-Leon and Riquelme, 2015). The SPK is also composed of ribosomes, MTs, actin microfilaments, ER and Golgi equivalents (GEs) and an amorphous material of undefined nature (Girbardt, 1969; Grove and Bracker, 1970; Howard and Aist, 1979; Howard, 1981; Bourett and Howard, 1991; Roberson and Vargas, 1994; Steinberg, 2007; Pinar et al., 2013). Although it is not an organelle in strict sense, the SPK is a very dynamic apical cluster that behaves as a single unit to drive direction and rate of growth at the hyphal apex (Bartnicki-García, 2002; Araujo-Palomares et al., 2007; Kohli et al., 2008; Taheri-Talesh et al., 2008) and can be seen as a Vesicle Supply Center (VSC) (Bartnicki-Garcia et al., 1989, 2000; Bartnicki-García, 1990). After their accumulation in the SPK and before fusion with the PM, the SPK vesicles interact finally with components of the polarized growth machinery, such as the exocyst complex (Kohli et al., 2008; Riquelme et al., 2014).
It is commonly accepted that exocyst components are at the apex of growing fungal hyphae or yeast ends, forming a crescent that follows the curvature of the tip (Sudbery, 2011). Systematic and comparative observations suggest that the spatio-temporal localization of the exocyst complex subunits is probably more complex, and possibly fungus-dependent (Figure 1). In hyphae of C. albicans, A. nidulans, A. oryzae, M. oryzae, and Z. tritici, all the exocyst components studied localize to their hyphal tip surface but this localization is not associated to the position of the SPK (Taheri-Talesh et al., 2008; Jones and Sudbery, 2010; Hayakawa et al., 2011; Sudbery, 2011; Giraldo et al., 2013; Guo et al., 2015; Gupta et al., 2015). In A. gossypii, the sub-cellular localization of Exo70, Sec3, and Sec5 depends on the rate of hyphal growth. In slow-growing hyphae, they form a cap at the tip of the hyphae while in fast growing hyphae they accumulate at the SPK (Kohli et al., 2008). In interphase fission yeast cells, all subunits co-localize at cell poles but in the form of patches rather than an apical crescent (Wang et al., 2002; Bendezu et al., 2012; Jourdain et al., 2012). They are also seen on cytoplasmic trafficking vesicles (Bendezu et al., 2012). By contrast, and regardless of the growth rate, in mature hyphae of N. crassa four subunits localize in a crescent at the foremost apical region of the hyphae, two subunits accumulate at the SPK outer layer and SEC-3 is at the interface between these two structures (Riquelme et al., 2014). Moreover, the localization of SEC-6 at the PM, but not EXO-70, is independent of the secretory pathway. This is in marked contrast to the situation in S. cerevisiae, where SEC3 and EXO70 are associated with the PM and do not depend on the secretory pathway, whilst other subunits are carried on the secretory vesicles (Boyd et al., 2004). These findings support the hypothesis that sub-complexes exist in some, but possibly not all fungi. The results further indicate that different members of the exocyst may act as landmarks for polarized secretion in different fungi and that the mode of assembly of the exocyst complex may differ between fungi.
In yeast where travel distances are short (approx. 10 μm), the vesicular subunits of the exocyst complex are trafficked toward the cell periphery by an actin-based mechanism (Boyd et al., 2004; Jones and Sudbery, 2010; Bendezu et al., 2012). In filamentous fungi distances are much longer (approx. 100 μm in hyphae from U. maydis, Schuster et al., 2011) and MTs organize the long-range transport of exocyst-carrying vesicles whereas actin distributes vesicles locally at the cell tip (Kohli et al., 2008; Hayakawa et al., 2011; Riquelme et al., 2014). The SPK may constitute the transition zone where vesicles switch from MTs to actin (Harris et al., 2005; Riquelme et al., 2014). This is in a manner reminiscent of the situation in neurons where actin-based motors support the switch of endomembrane compartments from MTs to the actin-rich periphery (Woolner and Bement, 2009). Hence, the mode of transport of the exocyst subunits in filamentous fungi may simply depend on the travel distance.
The interdependence of the polarisome, the SPK and the exocyst to orchestrate hyphal tip expansion is evidenced by their relative localization at the hyphal tips. However, this relative localization exhibits differences within fungi and suggests variable modes of interplay between the three components. In N. crassa, EXO-70, and EXO-84 partially co-localize with the outer layer of the SPK (Verdin et al., 2009) and suggest that the exocyst is involved in the delivery of the vesicles from the SPK to the PM (Riquelme et al., 2014). In this organism, the polarisome component SPA-2 co-localizes and interacts with the SPK and spreads radially from there to the PM (Araujo-Palomares et al., 2009). A change in the position of the SPK displaces SPA-2 (Araujo-Palomares et al., 2009). In M. oryzae, the SPK marker Mlc1 co-localizes with FM4-64 in what it constitutes the SPK (Giraldo et al., 2013). In this fungus, Spa2 forms a bright spot at the hyphal tips in a structure similar to that formed by the SPK, while all the exocyst components are forming a cap at the PM (Gupta et al., 2015). In C. albicans, the normal localization of the SPK, the polarisome and the exocyst is disturbed by the lack of Sec15 however, the three components re-localized after Sec15 is expressed again (Jones and Sudbery, 2010; Chavez-Dozal et al., 2015b). By contrast, mutations in fission yeast Sec3 affect the localization of the formin For3 but the rest of the polarisome remains unaffected (Jourdain et al., 2012).
In fungal mutants of the exocyst, post-Golgi vesicles accumulate in the cytoplasm and secretion is affected (e.g., Panepinto et al., 2009; Chavez-Dozal et al., 2015a). As a result, cell wall components are abnormally deposited and confer sensitivity to cell wall stressors or degrading enzymes (Panepinto et al., 2009; Chavez-Dozal et al., 2015a,b). Furthermore, Caballero-Lima and co-workers proposed a model for C. albicans hyphal tip growth in which the density of exocyst components determine the rate of cell wall-synthesizing-containing vesicles that fuse with the hyphal tip (Caballero-Lima et al., 2013). In yeast, like in other higher eukaryotes, the exocyst is critical for cytokinesis and cells usually die because of a failure to build two new PM and cell walls (aka septum) between the two daughter cells (Wang et al., 2002, 2003; Fendrych et al., 2010; Neto et al., 2013b; Chavez-Dozal et al., 2015a; Perez et al., 2015). In fission yeast, the endo-β-glucanases required for cell separation during cytokinesis are located at the septum in a process mediated by the exocyst (Martin-Cuadrado et al., 2005). Accordingly, cells in which the exocyst complex is mutated fail to divide and often develop multiple thick septa (Wang et al., 2002; Jourdain et al., 2012) (Figure 1D). These cells are also wider than the wild-type and show an accumulation of secretory vesicles that do not fuse with the PM at cell tips (Jourdain et al., 2012). Surprisingly however, they do not seem to have any other cell wall defects besides a thickening of their septa (Jourdain et al., 2012). More specifically, it has been reported that the localization of the exocyst components in the cortex of S. pombe, and not cell shape or cell wall synthesis regulators, defines its growth pattern (Abenza et al., 2015). Similarly equatorial, but not tip endocytosis is affected in a mutant of sec8 (Gachet and Hyams, 2005). Thus, in fission yeast, the exocyst complex may play a different role during polarized cell growth and during cytokinesis. Mutant cells often show mating defects because they fail to secrete mating hormones, form a mating projection and develop a forespore membrane and cell wall (Wiederkehr et al., 2003; Sharifmoghadam et al., 2010). Fungal exocyst mutants are also characterized by the development of multiple new tips, which result in hyperbranching proposed to be due to the role that the exocyst plays in the organization of the SPK (Riquelme et al., 2014; Chavez-Dozal et al., 2015b) (Figure 1F). However, the involvement of the exocyst complex in cellular branching is likely to be more complex. Firstly, S. pombe cells can be “T-shaped” but the growth of a third pole does not depend on the exocyst. Secondly, in some cases loss of exocyst function in C. albicans—which normally has a SPK, and in neurons—which do not have a SPK, equally reduces cellular branching (Vega and Hsu, 2001; Chavez-Dozal et al., 2015a) (Figures 1I, 3B).
In contrast to exocytosis, endocytosis is responsible for the uptake of extracellular material and the recycling of lipids and surface proteins. A large number of conserved endocytic proteins participate in the steps of endocytosis and their ordered recruitment and function have been extensively characterized (Kaksonen et al., 2006). Nevertheless, what defines the sites of endocytosis remains unknown. The idea of a spatiotemporal coordination of exocytosis and endocytosis is intuitive as cell shape, size or symmetry can only be achieved if membrane addition is balanced by subtraction. At the hyphal tip of A. nidulans, exocytosis (at the front) and endocytosis (at the rim) have been proposed to be spatially coupled, while in C. albicans a new model for hyphal growth has been developed in which the form of the hyphae can be predicted if cell wall synthase enzymes are removed or inactivated from subapical regions of the hyphal membrane (Taheri-Talesh et al., 2008; Caballero-Lima et al., 2013). In yeast, endocytosis happens in actin patches which are normally localized at sites of active growth or division (e.g., Gachet and Hyams, 2005). In exocyst mutants however, endocytic patches assemble but are delocalized and fail to internalize, and uptake of endocytic dyes is perturbed (Riezman, 1985; Gachet and Hyams, 2005; Jourdain et al., 2012; Jose et al., 2015). Moreover, members of the yeast exocyst complex genetically and physically interact with the endocytic machinery (Jourdain et al., 2012; Jose et al., 2015). This was also observed in higher eukaryotes (Sommer et al., 2005; Zuo et al., 2006). In fission yeast, the exocyst does not appear to co-localize with actin patches in an heterogeneous population of cells in which polarity is permanently maintained (Jourdain et al., 2012). The reason may be that endocytosis and exocytosis are coupled during polarity establishment, but not maintenance (Jose et al., 2015). In conclusion, the exocyst complex is ideally placed to coordinate the balance between endo- and exocytosis (Jourdain et al., 2012; Gupta et al., 2015; Jose et al., 2015). Directly or indirectly, the exocyst complex may in fact regulate all cellular actin structures- cables, patches and actomyosin rings. Besides a defect in actin patches distribution and function, the constriction and disassembly of the cytokinetic actomyosin ring is compromised in S. pombe mutants of sec3 and these cells have no actin cables (Jourdain et al., 2012). In S. pombe, actin cables and the exocyst constitute two distinct but partially redundant morphogenetic pathways that contribute together to the robustness of the polarization machinery (Bendezu and Martin, 2011). The fact that Sec3 regulates both explains that sec3 mutant cells are mis-shapened (Figure 1D). How these findings extend to filamentous fungi remains to be determined but the answer may lie in the organization of the actin-rich SPK (Riquelme et al., 2014; Chavez-Dozal et al., 2015b).
Fungi are organisms with an enormous ecological and economic impact that in many ways are beneficial for humankind (Rokem, 2007). However, fungi can cause diseases in plants and animals having serious implications on human health and on the ecosystem (Fisher et al., 2012). The impact of fungal diseases is clearly manifested in crops. The rice blast fungus M. oryzae is one of the most destructive plant pathogens and in 2012 it was voted first of a Top 10 fungal plant pathogens list (Dean et al., 2012). This highlights the economic importance of this fungus that has become a model organism for the study of host-pathogen interactions.
M. oryzae infection of rice plants is mediated by an appressorium. This dome-shape specialized infection structure re-polarizes at its base where it generates the penetration hypha to rupture the leaf cuticle and invade the plant tissue. A report on the role of the exocyst complex during the appressorium-mediated infection by this plant pathogenic fungi has been recently published (Gupta et al., 2015). Through the generation of fluorescently tagged proteins, the different sub-units of the M. oryzae exocyst have been observed to assemble at the appressorium pore before emergence of the penetration peg (Figure 1G). This localization of the exocyst complex requires the prior formation of the septin ring (Dagdas et al., 2012) and indicates that polarized exocytosis is required for initiating polarity and protrusion of the penetration hypha. This result is consistent with the exocyst's role as a network hub, connecting endocytosis and exocytosis to optimize polarized growth (Jose et al., 2015). In fact, the targeted gene deletion of exo70 and sec5 results in a high reduction in the total protein secreted by the fungus, including the virulence-associated factor, spore tip mucilage (STM). In line with these results, Exo70 and Sec5 are required for full virulence of M. oryzae in plants and in a temperature-sensitive mutant of sec6, the disassembly of the exocyst complex causes a reduction in the virulence of the disease (Gupta et al., 2015).
During host invasion, pathogens secrete small molecules or effectors to suppress host immune responses and support pathogen growth. Based on their localization pattern, M. oryzae effector proteins can be classified in two groups. Apoplastic effectors accumulate extracellularly at the host-pathogen interface. By contrast, cytoplasmic effectors accumulate at a host derived structure termed the biotrophic interfacial complex (BIC) (Khang et al., 2010; Giraldo et al., 2013). In 2013, Giraldo et al. showed that apoplastic effectors are actively secreted via the conventional secretory pathway previously defined in filamentous fungi, and that cytoplasmic effectors are host-translocated by an unconventional mechanism involving the exocyst complex (Giraldo et al., 2013). The results obtained by Gupta et al. also suggest that disruption of the exocyst complex at the appressorium pore could inhibit secretion of virulence-associated proteins at later stages of appressorium development prior to penetration peg emergence (Gupta et al., 2015). Previously in Cryptococcus neoformans, it was also suggested that different virulence factors use different secretory pathways, such as through exosomes (see below) (Panepinto et al., 2009). Furthermore, the repression of Sec6 and Sec15 in the human pathogen C. albicans leads to a reduced secretion of aspartyl proteases and lipases involved in virulence (Chavez-Dozal et al., 2015a,b). However, the reduced macrophage death produced by the Sec6 mutant may also be a consequence of its defect in filamentation and hyphal branching (Chavez-Dozal et al., 2015a).
During infection of cucumber with Colletotrichum orbiculare, fluorescently labeled effectors are actively accumulated in a ring-like structure which resembles the M. oryzae BIC (Irieda et al., 2014). In the same study, Irieda et al. observed that the delivery of these effectors is Sec4- and Sec22-dependent and, as a consequence, the loss of either of these two proteins causes a defect in virulence (Irieda et al., 2014).
These findings could indicate that fungal effectors are delivered to the host through different secretion routes involving distinct exocyst subunits but that the secretory pathway may be conserved in pathogenic fungi with similar infection strategies.
The exocyst complex exists in higher plants. This is true for dicotyledonous (“broad leaf”) and monocotyledonous (“grass”) plants that include key cereal crops such as rice and wheat. All exocyst components identified in metazoans and fungi have been conserved in plants. However, most subunits exist as paralogs. In the model plant Arabidopsis thaliana there are commonly two isoforms for a single subunit (SEC3, SEC5, SEC10, and SEC15) increasing to three paralogs for Exo84 and to over 20 for Exo70 (Elias et al., 2003; Synek et al., 2006; Hala et al., 2008). Whole-genome duplications have occurred frequently during higher plant speciation and this has generated closely related sub-families of proteins (Maere et al., 2005; Synek et al., 2006). The functional redundancy between isoforms further complicates genetic analyses. Many of the most severe mutant alleles show reduced transmission in haploid gametes when alone or combined with mutant alleles of other complex members (Synek et al., 2006; Hala et al., 2008). Moreover, mutation of the two A. thaliana SEC3 loci (that contain two SEC3 isoforms) results in embryo lethality (Zhang et al., 2013). The vast majority of plant exocyst functional data come from heritable loss-of-function alleles and not from conditional or non-heritable phenotypes. Any allele that is lethal in the haploid state would not be transmitted in either germline. This is unlike the situation in fungi where conditional alleles have been used extensively for functional analyses. It is therefore possible that all components of the exocyst are critical for plant life even though there is only evidence from selected subunits. The use of CRISPR/Cas9 mediated gene targeting (Hyun et al., 2015), inducible RNA interference (RNAi) approaches effective against essential genes (e.g., Ketelaar et al., 2004) and the identification of the drug endosidin 2 (Zhang et al., 2016) are likely to further expand the range of plant exocyst phenotypes. Recently, tissue-specific expression of RNAi for each exocyst subunit was used to demonstrate that the exocyst is essential for Arabidopsis fertilization (Safavian et al., 2015), elegantly demonstrating the use of this technique to circumvent lethal phenotypes.
The body of evidence for these individual components functioning as a complex is convincing (Zarsky et al., 2013). Binary interactions and partial-complex purification are consistent with conservation of “canonical” core subunit interactions. Five interactions between complex members have been confirmed through co-immunoprecipitation from plant tissue and nine through yeast 2-hybrid (Hala et al., 2008; Fendrych et al., 2010; Zarsky et al., 2013). These interaction data are broadly in line with the recent work exploring the structure of the S. cerevisiae complex (Heider et al., 2016). Data describing the structural biology of the plant exocyst is extremely limited. Interpretations of plant exocyst architecture are built upon primary sequence conservation in key structural domains, and supported by secondary structure prediction (Zarsky et al., 2013). Cross-kingdom complementation experiments have not been reported for plant exocyst components. However, a plant Exo70 has been found to have activity in a mammalian cell type (Ding et al., 2014), although Exo70 is known to exert effects on membranes in isolation from other complex members (Zhao et al., 2013).
Visual co-localization of exocyst components in planta has generated a variety of endomembrane localization patterns that suggests a complex and dynamic interplay between the subunits. Much of this variety could however be due to isoform-specific, tissue-specific and label-induced effects. Each plant tissue appears to have a distinct combination of paralog expression (Hala et al., 2008; Li et al., 2010), thus creating unique opportunities for component distribution and interactions. Further complication arises from stimulus-responsive expression of some paralogs (Chong et al., 2010; Pecenkova et al., 2011). At least four A. thaliana fluorescent protein fusions used to localize the exocyst have been shown to complement their respective mutant phenotypes (SEC3a, Exo84b, SEC8, and Exo70B1; Fendrych et al., 2010; Zhang et al., 2013) and the majority of observations using these probes underpin the familiar dogma that the plant exocyst is recruited to the PM. An exception is Exo70B1. This fusion protein complements its cognate mutant phenotype but is recruited to autophagosomes where Exo70B1 contributes to efficient autophagy (Kulich et al., 2013). Immunofluorescence of fixed pollen tubes associates endogenous SEC6, SEC8, and Exo70A1 to a shared population of endomembrane compartments at the growing tip and to the PM of the tube (Hala et al., 2008). Pollen tubes are cells that grow extremely rapidly through tip-focused secretion and are exploited as a model tissue for understanding plant cell polarity and exocytosis. Taken together these experiments support the existence of a plant exocyst complex that functions in a broadly analogous manner to its fungal and metazoan counterparts.
Cytokinesis in plants requires the building of a new cell wall between two daughter cells. This is orchestrated by a cytoskeletal array unique to plants named the phragmoplast. The phragmoplast is derived from the disassembly of the anaphase spindle with the purpose of guiding a cloud of vesicular material to the site of cell plate construction (Figure 2C). The phenotypes of A. thaliana Exo84 isoform “b” and the SEC3 locus support the proposal that the exocyst is necessary for organized cytokinesis and cell plate maturation (Fendrych et al., 2010; Zhang et al., 2013) (Figure 2D). 3.5% of cells mutant for Exo84b showed evidence of failed cytokinesis (Fendrych et al., 2010) while SEC3 mutants showed “normal” cell plate maturation but no organization of cell plate orientation, causing early embryo arrest (Zhang et al., 2013). Close observation of Exo70 isoform “A1” mutants has also revealed a subtle defect in the process of cell plate assembly (Fendrych et al., 2010). The process of cell plate deposition is performed in close co-operation with the TRAPPII membrane-tethering complex. This is largely a sequential division of responsibilities during the maturation process but co-immunoprecipitation experiments suggest the possibility of physical bridging between the TRAPPII and exocyst complexes (Rybak et al., 2014).
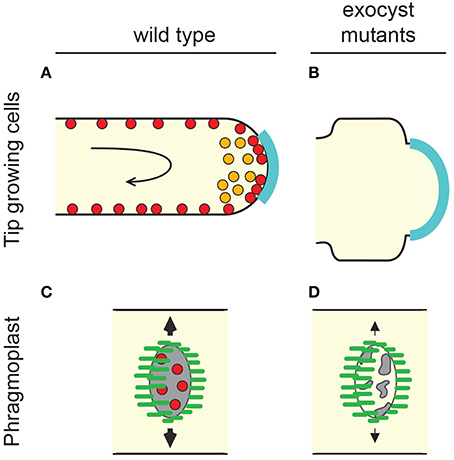
Figure 2. The plant exocyst supports tip-growth and cell division. (A) Tip-growing cells in plants (pollen tubes and root hairs) incorporate membrane and other cargoes delivered via vesicles to the apical growth zone of the PM (cyan). Rapid, rotational streaming of cytoplasm (black arrow) occurs behind the apex and is driven by myosin and actin cables. In pollen tubes SEC6, SEC8, and Exo70 label endomembrane compartments within the apex (orange). In both pollen tubes and root hairs exocyst components decorate the PM in a punctate pattern (red). (B) Loss of exocyst causes shortening and broadening of tip-growing cells. This is potentially consistent with a hypothetical widening of the growth zone (cyan) but could also be caused by alterations in cell wall properties that could result in turgor-driven expansion. (C) Plant cells produce new cell plates (gray) through the action of a cytoskeletal array known as the phragmoplast (microtubule organization within the phragmoplast is depicted in green). The phragmoplast expands from the former site of the spindle and advances toward the outer cell walls where the cell plate will eventually fuse. Exocyst components are enriched at the developing plate during two distinct plate growth phases (red) (Fendrych et al., 2010; Zhang et al., 2013; Rybak et al., 2014). (D) Exocyst mutants show a variety of cell plate phenotypes including reduction in the rate of cell plate expansion, increase in the probability of cell plate collapse, and alteration of the content of developing cell walls. See text for details and other references.
Work on cell plates of dividing cells and the tips of pollen tubes have demonstrated bulk recruitment of exocyst subunits but the crowding of secretory machinery at these structures prevents the observation of recruitment sequences. Two studies have utilized interphase cells to observe “real-time” exocyst dynamics (at rapid frame acquisition rates) across relatively large areas of PM and cell cortex (Fendrych et al., 2013; Zhang et al., 2013). Both studies showed that exocyst components are recruited transiently as punctate structures that can be resolved using diffraction-limited fluorescence microscopy. SEC3A-GFP punctae exist at densities of 0.4–0.6 /μm2 and appear immobile (Zhang et al., 2013). These patches exist for a median time of only 4 s before disappearing from the membrane. This is likely through a process of patch disassembly as no punctate structures decorated with SEC3-GFP can be identified using spinning disk confocal microscopy within the cytoplasm (Zhang et al., 2013). Fluorescent protein fusions to SEC6, SEC8, Exo70A1, and Exo84b are also distributed in punctae at the PM and have been recorded at higher densities of 1.2–1.6/ μm2 (Fendrych et al., 2013). Co-localization analysis confirmed that over a third of punctae shared binary combinations of SEC6, SEC8, and Exo84b. These binary combinations arrived and departed in synchrony with a residency time of over 10 s; significantly longer than the SEC3-GFP study. A vesicle-resident SNARE protein (VAMP721) was found to co-occupy a small but statistically significant number of Exo84b patches suggesting that exocyst punctae can coincide with vesicles pausing at the PM (Fendrych et al., 2013). Co-imaging of SEC3A and further exocyst components is urgently required to consolidate the observations but a model is emerging where the exocyst assembles, or arrives in a preassembled state, to exist as a transient, anchored nano-structure at the PM that can interface with stationary vesicles. Equally these exocyst patches/particles are capable of completing their residency at the cell cortex without the presence of a vesicle, possibly suggesting that the plant exocyst provides windows of opportunity for PM-vesicle interaction and fusion (Fendrych et al., 2013; Zhang et al., 2013).
Any discussion of the molecular function of the plant exocyst must include the diversity of the plant Exo70 subunit. This gene family has shown broad diversification linked to functional specification. There are 23 Exo70 genes within the A. thaliana genome and these consist of a series of fundamental subclasses that are conserved across higher plants and are named alphabetically from A to I (Synek et al., 2006). The functional specialization of individual Exo70 isoforms has been demonstrated experimentally. Exo70A1 of A. thaliana is widely expressed with mutant phenotypes that suggest this isoform has a basal secretory function for cell wall and cuticle development in multiple cell types (Fendrych et al., 2010; Kulich et al., 2010; Li et al., 2013). Moreover, the function of this isoform has an impact upon organ development through the plant hormone auxin. The trafficking of auxin efflux proteins is inefficient in exo70a1 mutants. This causes an abnormal distribution of the hormone and perturbs signaling processes that guide root growth (Drdova et al., 2013). The A. thaliana Exo70B1 isoform is required for autophagy and delivery of endomembrane compartments to the vacuole (Kulich et al., 2013). This transport pathway plays a critical role in receptor signaling during plant immune responses and the closely related Exo70B2 isoform has a pathogen defense phenotype (Pecenkova et al., 2011). Exo70C1 contributes to pollen tube growth (Li et al., 2010). Exo70E2 promotes the formation of double membrane secretory organelles (Ding et al., 2014) and Exo70H4 is required for callose deposition (a carbohydrate cell wall polymer) in specific cell types (Kulich et al., 2015).
The properties of specific Exo70 variants are conserved across kingdoms. Exo70 isoforms capable of forming exosomes destined for unconventional secretion can do so effectively in mammalian cells (Ding et al., 2014). This suggests exciting opportunities for endomembrane manipulation through heterologous expression of novel Exo70 isoforms. It also postulates a model where the general membrane tethering and fusion-regulating properties of the exocyst complex can be directed using specific Exo70 “warheads.” The cells of higher plants place unusual demands on secretion machinery, requiring cargoes to be sorted within a thin layer of cytoplasm to distinct destinations spread over a vast cell surface. The proliferation of Exo70 may have occurred in part in response to this demand for overlapping yet spatially and temporally distinct routes for secretory traffic.
A comparative analysis of exocyst function across eukaryote kingdoms is perhaps best performed in tip-growing plant cells. These are analogous in their growth style to fungal hyphae and some membrane protrusions of animals and protozoa. Most plant cells are considered to expand using “intercalary” growth where new cell wall material and new PM are integrated across relatively wide areas. Tip-growing cells are less common but include pollen tubes (the structures that emerge from pollen grains and deliver sperm nuclei to oocytes) and root hairs (long tubular extensions of root epidermal cells that anchor roots and provide surface area for water and nutrient uptake). In these cells exocytosis is focused, but not necessarily exclusive, to the very tip of the cellular extension (Rounds and Bezanilla, 2013). Thick actin bundles and myosin-mediated motor action dominate transport processes throughout most of the cytoplasm of tip-growing cells but toward the tip the actin cytoskeleton is frayed into finer, dynamic filamentous arrays that are essential for cargo integration at the cell cortex. The details of this arrangement differ between pollen tubes and root hairs but in both cases bulk endomembrane compartment transport does not extend fully to the tip where the cytoplasm is enriched with exocytic and endocytic endomembrane compartments. There is no defined analog of the SPK.
Components of the exocyst have been identified at the apex of tip-growing cells (Figure 2A). Immunofluorescence using antibodies raised to SEC6, SEC8, and Exo70A1 show these components located within the secretory vesicle-mass at the tip of pollen tubes (Hala et al., 2008). The same study identified genes encoding four subunits as being significant contributors to pollen tube growth (SEC5, SEC6, SEC8, and SEC15). Mutant tubes showed a tendency for increased width and reduced length (Figure 2B). These features are indicators of a loss of growth polarity that are also associated with aberrant small GTPase signaling and impaired actin dynamics. A forward genetics screen in maize identified roothairless1 (rth1), a mutant where root hairs initiate but do not elongate. Cloning of the gene revealed it to be an isoform of SEC3 (Wen et al., 2005). Surprisingly, despite the catastrophic impact on root hair tip-growth, pollen tube function appears normal (Wen et al., 2005). This may indicate genetic redundancy with other SEC3 paralogs. In A. thaliana loss-of-function alleles of SEC3a have proven to be embryo lethal (Zhang et al., 2013) but transmission through the male germline is again unaffected. Unlike exocyst mutants of neurons and some filamentous fungi, no pollen tube branching phenotypes have been reported from plant exocyst mutants. An allele of Exo70A1 does however cause an increase in root hair branching from wild-type levels of 22–56% (Synek et al., 2006).
Live-cell imaging of SEC3a-GFP in root hairs has shown no enrichment at the tip. Instead the protein decorates the PM in a punctate pattern but at an even intensity along the length of the growing root hair with only a 30% enrichment at the tip (Figure 2A) (Zhang et al., 2013). It is not known whether this behavior is specific to SEC3a or, alternatively, whether other exocyst components are localized in a pattern more analogous to that observed in pollen tubes. It is interesting to note that SEC6, SEC8, and Exo70A1 also decorate the PM of pollen tubes in a punctate pattern when observed beyond the tip-growth zone (Hala et al., 2008). In summary, exocyst components are essential for tip growth in plants but they are not necessarily precise markers for indicating sites of tip-polarized exocytosis.
Fungal tip growth is distinctly different. Long-distance transport in fungal hyphae, like in mammalian cells utilizes bi-directional movement on MTs rather than unidirectional transport on actin cables. The exchange between distribution systems occurs in the zone of the SPK, so an absence of “long range” to “short range” transference may also explain the lack of a plant SPK analog. Equivalent endomembrane compartments do accumulate at the tip of plant cells but the borders of these masses are less defined. The distribution of plant exocyst components in tip growing cells is, in general, less focused to the polar site of cargo delivery in comparison to fungal hyphae. “Foci” of exocyst components appear transiently at the plant PM for periods of 4–10 s throughout the tip-growing cell, yet paradoxically the complex is required for tip-focused secretion. One speculative interpretation is that the cross-kingdom comparison suggests that plant exocyst localization is not necessarily indicative of an active exocyst population. This is in contrast to the fungal exocyst where localization is tightly correlated with activity.
Very recently the plant exocyst has emerged as a potential key player in plant-pathogen interactions. The evidence for this can be grouped into three streams: 1/mutant phenotypes that reduce basal immune defenses; 2/sub-cellular localizations associated with microbe contact; 3/gene-for-gene interactions that suggest exocyst components are targets of pathogen effector proteins. The most direct evidence is the impact upon microbial interactions caused by the loss of function of the host exocyst. This has not been explored in a methodical fashion using all exocyst subunits but two Exo70 isoforms of A. thaliana were identified as being expressed in response to pathogen challenge (Pecenkova et al., 2011). Loss of function phenotypes of these two genes include increased susceptibility to the bacterial phytopathogen Pseudomonas syringae and aberrant defense structures in response to powdery mildew (Pecenkova et al., 2011). Pathogen perception appears to be a key role for Exo70B2. Disruption of Exo70B2 activity perturbs the function of receptors that respond to microbial molecular patterns such as flagellin and chitin (Pecenkova et al., 2011; Stegmann et al., 2012). Defense responses are not activated efficiently without Exo70B2 function. These relationships between microbes and the exocyst could be important in an agricultural context. Transient gene silencing assays revealed that Exo70F function supports defense against powdery mildews in barley (Ostertag et al., 2013). This relationship is likely to extend to other closely related cereal crops such as wheat, rice and maize.
Many plant-microbe interactions are beneficial for plant life. Arbuscular mycorrhizas are root-fungal interactions that are promoted by reciprocal signaling between host and microbe. The plant benefits through increased efficiency of nutrient absorption and this process requires the development of specialized interfaces between plant and fungal cells. In Medicago truncatula multiple markers of exocytosis are recruited to these interfaces including Exo84 and Exo70I (Genre et al., 2012; Zhang et al., 2015). Exo70I function is critical for the formation of the interface and in its absence fungal growth into host cells is restricted and markers indicative of interface membrane identity are depleted. Remodeling of membranes to accommodate microbes therefore appears to be a role for the host exocyst complex.
Evidence is accumulating that multiple classes of phytopathogens have developed adaptations that specifically suppress or manipulate the host exocyst complex. The rice pathogen M. oryzae has a suite of secreted proteins to suppress host defense that includes the protein AVR-Pii. Co-immunoprecipitation from rice cells using this protein as bait isolated the Exo70 isoforms Exo70F2 and Exo70F3 (Fujisaki et al., 2015). A rice gene present in some cultivars (called Pii) can confer resistance to M. oryzae by responding to the presence of AVR-Pii. The rice Pii gene encodes a cytosolic receptor for AVR-Pii. Rice Exo70-F3 is required for M. oryzae AVR-Pii to trigger a response from rice Pii (Fujisaki et al., 2015). One proposed explanation for this is that Pii is “guarding” Exo70 and responds when pathogen effectors disrupt this interaction. These relationships are proving to be common in gene-for-gene defense responses. An analogous scenario has been described in A. thaliana powdery mildew interactions (Zhao et al., 2015). Here the host Exo70 isoform is Exo70B1 and the receptor is TIR-NBS2. Loss of Exo70B1 disrupts the interaction between the two host proteins and leads to constitutive activation of immune responses. Phytopathogenic oomycetes also target exocyst components. The Phytophthora infestans (potato blight) effector AVR1 binds SEC5 to disrupt secretion and programmed cell death responses (Du et al., 2015). These relationships infer that the host exocyst is a significant battleground in plant disease progression. Excitingly many of these examples are derived directly from critical crop plants and their agriculturally damaging pathogens. This opens the potential for immune system reinforcement, based around engineering of the exocyst complex, that will have high-value in safeguarding world food supplies.
The mammalian exocyst complex also contains eight sub-units that are ubiquitously expressed. They are often called by their Sec or Exo names for simplicity, but are officially labeled EXOC1 (= Sec3), EXOC2 (= Sec5), EXOC3 (= Sec6), EXOC4 (= Sec8), EXOC5 (= Sec10), EXOC6 (= Sec15), EXOC7 (= Exo70), and EXOC8 (= Exo84). In mammals, each sub-unit has several isoforms produced by alternative splicing (UniProt Consortium, 2015). In the absence of systematic spatio-temporal analysis, the significance of this diversification is at present largely unknown.
Existing full knockout (KO) mice of members of the exocyst show early embryonic lethality, from the blastocyst stage. The blastocyst forms when 64 cells differentiate into 2 lineages: the inner cell mass (ICM), which will give rise to the embryo; and a peripheral layer of cells, the trophoblast which will give rise to the placenta and mediate the attachment of the embryo to the uterus wall. The blastocyst then implants in the uterus through its trophoblast. The later gastrula reorganizes as an ectoderm, mesoderm, and endoderm that will subsequently develop into tissues and organs. Sec3−/− embryos have a peri-implantation lethal phenotype, due to defects in ICM proliferation (Mizuno et al., 2015). Sec8 null homozygous mutant mice initiate gastrulation but show a defect in mesoderm formation (Friedrich et al., 1997). Other conditional, partial, or heterozygous mutant mice are characterized by a range of tissue-specific developmental defects. A conditional Sec10 KO mouse exhibits defects in the organogenesis of its genito-urinary track. It develops a type of chronic kidney disease characterized by the obstruction of the urinary tract. This leads to complete anuria in newborns, with death occurring 6–14 h after birth (Fogelgren et al., 2015). Sec15 is involved in late erythroid differentiation in the bone marrow (Bloom and Simon-Stoos, 1997). Mice in which Sec15 is truncated (aka hbd, hemoglobin-deficit mouse) fail to accumulate iron in their reticulocytes and have small reticulocytes (Lim et al., 2005; White et al., 2005; Garrick and Garrick, 2007). The phenotype can be rescued by transplantation of normal bone marrow and transplantation of a sick bone marrow into healthy mice gives rise to a hbd phenotype. Bizarrely, these Sec15 mutant mice have no other phenotypic defects despite the fact the protein is expressed in other, non-hematopoietic tissues (Lim et al., 2005).
The exocyst is involved in the development of the placenta from the blastocyst trophoblast. The syncitiotrophoblast is a non-proliferative layer of the trophobtast. It is directly in contact with the mother blood and facilitates nutrient and gas exchange between mother and fetus. It also secretes signaling hormones and lytic enzymes that cause apoptosis of the epithelium of the uterus, necessary for its implantation. Some subunits of the exocyst complex are present in the apical membrane of the human syncytiotrophoblast (Vandre et al., 2012; Gonzalez et al., 2014). ExoC1-6 show a punctuate signal throughout the cytosplasm, whereas Exo70 and Exo84 are also enriched at or near the apical PM. It was suggested that alterations in exocyst function may be associated with preclampsia, a trophoblastic condition that leads to poor placental vascularization.
It is probable that the ubiquitous expression and multiple functions of the exocyst complex that we develop below are the source of these dramatic and early embryonic phenotypes.
Neurons are highly polarized cells, typically made of one long axon and several shorter, arborized dendrites. These neurites branch out from a cell body and extend to form connections with neighboring cells and ultimately establish complex circuits. Dendrites typically function to receive signals while axons send signals to neighboring cells. At the tip of the axon, a specialized type of exocytosis, called synaptic transmission regulates neurotransmitter release. Surprisingly, the exocyst is not involved in the tethering of these specialized vesicles (Murthy et al., 2003). It was suggested that the exocyst marks the sites of future synapse formation but plays no role in the mature synapse (Hazuka et al., 1999). This hypothesis is challenged by the fact Sec6 was observed at the PM of mature synaptic terminals (Vik-Mo et al., 2003). It is now apparent that the exocyst is present at multiple sub-cellular locations, which raises the question of its role(s) in neuron biology.
In drosophila, the development of the optic circuits is well documented and it is possible to know if a photoreceptor neuron reaches its target partner neuron at the right ganglion. sec15−/− neurons extend normally and form normal synapses but fail to select their right synaptic partner (Mehta et al., 2005). At the distal tip of extending neurites, growth cones probe the environment and guide neurite outgrowth. Growth cones are very dynamic structures that continuously extend and retract actin-rich membrane protrusions such as filopodia and lamellipodia. Axons and dendrites outgrowth therefore relies on membrane expansion and cytoskeletal remodeling. Highlighting the prominent role played by the exocyst in the determination of neuronal cell polarity, neurite outgrowth is impaired in the absence of functional exocyst subunits in various systems such as primary neurons, cultured PC12 cells or multicellular model organisms (Figure 3B) (Vega and Hsu, 2001; Murthy et al., 2003; Lalli, 2009; Das et al., 2014; Peng et al., 2015). This phenotype further accounts for the role of the exocyst during neuroblast migration and brain development (Letinic et al., 2009; Das et al., 2014).
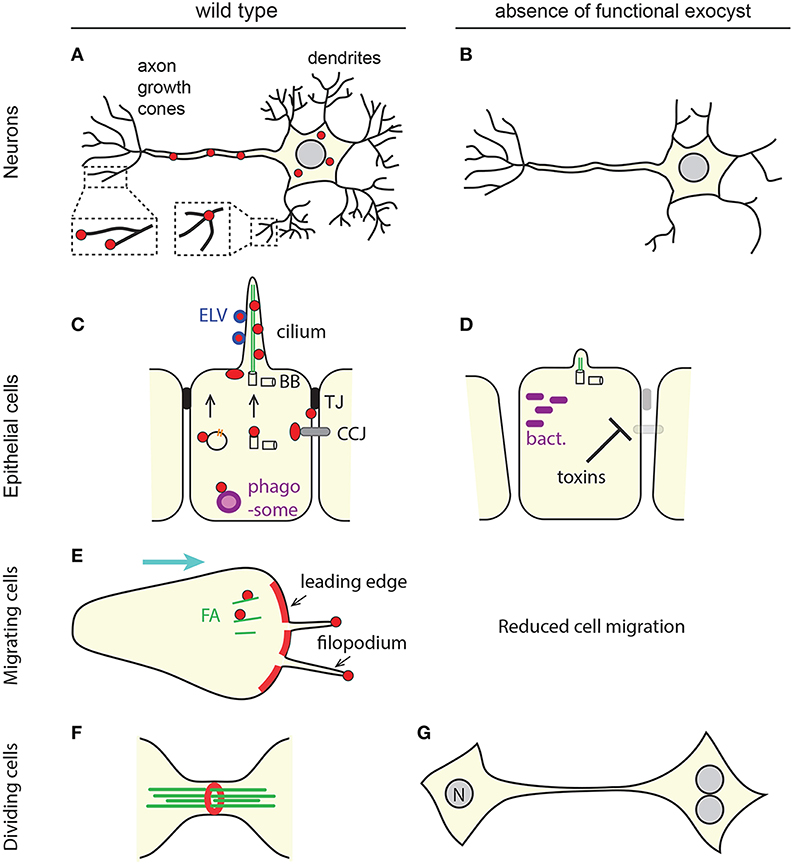
Figure 3. Some of the multiple roles of the animal exocyst complex. (A,C,E,F) Overall localization of the exocyst in physiological conditions (red). For simplicity, the differential localization of individual sub-units is not represented here. The exocyst is mostly present at zones of bulk (leading edge, E), or “finger-like” (neurites, A; cilia, C; filopodia, E) membrane extension. (A) In neurons, some subunits accumulate at dentritic branching points, but not at growing tips of dendrites, whereas others localize to the tip of axon filopodia. (C,E) The exocyst localizes at sites of cell-cell or cell-matrix junctions (tight junctions, TJ, black; cell-cell junctions, CCJ, gray; focal adhesions, FA, green). It was also reported to be associated with trafficking vesicles carrying cell surface receptors (orange), with the migrating basal body (BB), with phagosomes or phagosome-fusing endosomes (purple) and with extra-cellular exosome-like vesicles (ELV, blue). (F) The exocyst can organize as a ring. (B,D,G) Cellular phenotypes associates with a loss of exocyst function. Protrusions necessary for cell function or migration are not formed, cell polarity and tissue integrity are compromised, phagosome acidification is altered which constitutes a window of opportunity for pathogenic bacteria, cells fail to complete cytokinesis, and undergo extra mitoses (nucleus, N). (D) Some toxins secreted by bacteria impair the delivery of the exocyst at cell junctions. See text for details and references.
Axon and dendrite outgrowth may however rely on different mechanisms. In developing hippocampal neurons, the exocyst is highly enriched in axon growth cones and in filopodia, where it probably participates in membrane extension by mediating the fusion of plasmalemmal precursor vesicles with the PM, at the most distal end of the axon (Figure 3A) (Hazuka et al., 1999; Dupraz et al., 2009). The exocyst may also orchestrate actin organization within filopodia (Hertzog and Chavrier, 2011) (see below). In mice skin melanoma cells, Exo70 induces filopodia formation through its ability to deform membranes (Zhao et al., 2013) and it is possible that Exo70 plays a similar function in neurite outgrowth. At the membrane of the synapse, the exocyst may recruit and act as a scaffold for plasticity related proteins, such as post synaptic density 95 (PSD-95) (Riefler et al., 2003). A scaffolding role was also suggested during starvation- and pathogen-induced autophagy, where the exocyst mediates the assembly and activation of the autophagy machinery (Bodemann et al., 2011).
Unlike axons, terminal dendrites may not grow by direct tip extension or rely on distal, targeted exocytosis (Peng et al., 2015). Instead, the source of material for terminal dendrite outgrowth is thought to be provided by a unidirectional lateral diffusion from primary dendrites. Exocytosis and the exocyst are not at the distal tips of dendrites but concentrate at dendritic branches, from where they orchestrate dendritic arborization (Figure 3A) (Peng et al., 2015; Taylor et al., 2015; Zou et al., 2015). In C. elegans PVD neurons- a highly branched network of sensory neurons that envelope the worm and regulate its body posture and movement (Albeg et al., 2011), loss of function mutants of exoc-8 and sec-8 cause dendritic arborization defects, due to the fact pro-branching trans-membrane proteins are not delivered to the proximal dendritic membranes (Zou et al., 2015). A role of the exocyst in arborization rather than tip growth was also reported in drosophila tracheal terminal cells and may therefore not be neuron-specific (Jones et al., 2014).
Several exocyst subunits interact in a Ral-dependent manner with the Par3–aPKC-Par6 complex (Partitioning-defective and atypical protein kinase C), a master regulator of polarity (Lalli, 2009). The exocyst-Par axis exists in other cell types besides neurons, where it similarly regulates polarity (e.g., Rosse et al., 2009; Zuo et al., 2009; Bryant et al., 2010). It appears that the exocyst targets Par-aPKC to polarized zones and conversely aPKC actively regulates the polarized delivery of the exocyst (Rosse et al., 2009). As well as targeting polarity factors at sites of polarized growth, the exocyst also regulates the delivery of receptors. Exo70 is required to insert the IGF-1 receptor into the membrane of the growth cone, which stimulates axon elongation and membrane expansion through the Rho GTPase TC10 (Dupraz et al., 2009; Fujita et al., 2013).
The long distance transportation of exocyst subunits along the axon is mediated by MTs, which explains that Sec6 and Sec8 were immunolocalized to the cell body and axon and that the exocyst was co-immunoprecipitated with MTs in rat brain lysates (Vega and Hsu, 2001). However, there is to date no reported evidence that the exocyst complex directly regulates the dynamics or organization of MTs like it does with the actin cytoskeleton.
The role of the exocyst complex in polarization is further exemplified in epithelial apico-basal polarity (Blankenship et al., 2007). Epithelial cells are held together and to the matrix through specialized junctions that help orient the resulting tissue. Adherens junctions and desmosomes are type of cell-cell junctions, in which adhesion molecules on opposing cells interact and bridge cytoskeletal component of neighboring cells. Upon initiation of cadherin-mediated cell-cell adhesion, the exocyst relocates from the cytoplasm to sites of cell-cell contacts, where it interacts with junctional proteins (Grindstaff et al., 1998; Yeaman et al., 2004). In an invasive cell line that expresses no cadherin, the exocyst is however no longer localized to sites of cell-cell contacts, but instead relocates to protrusions that preclude cell migration (Spiczka and Yeaman, 2008) (Figures 3C,E). Conversely, the exocyst is involved in efficient targeting of these adhesion molecules. Interactions between type I phosphatidylinositol-4-phosphate 5-kinases and Exo70 governs the binding of the epithelial cadherin E-cadherin. Knockdown (KD) of Exo70 perturbs correct clustering of E-cadherin at adherens junctions and KD of Sec3 causes inefficient targeting of desmosomes (Andersen and Yeaman, 2010; Xiong et al., 2012). In certain growth conditions, epithelial Madin-Darby canine kidney cells (MDCK) organize as a hollow cyst and if stimulated, can extend tubules from the basolateral surface of individual cells. In non-polarized MDCK cells, the exocyst is cytosolic but in polarized cyst cells and mature tubule cells, it localizes to tight junctions (Lipschutz et al., 2000) (Figure 3C). Tight junctions seal epithelial cells and play two functions: they limit the passage of small molecules between cells and they block lateral diffusion within the lipid bilayers. They are therefore critical to maintain an apical and a basolateral domain and are a central platform for regulation by the Par complex with which the exocyst collaborates. Accordingly, over-expression of Sec10 in MDCK cells results in increased tubulogenesis due to an increased delivery of baso-lateral proteins (Lipschutz et al., 2000).
The role of the exocyst complex in epithelium polarity has consequences for embryonic development. In fly embryos, cellularization is the process by which a large syncytium containing thousands of nuclei is subdivided into separate cells. Sec5 mediates cellularization by directing the addition of new membranes from the apical end of the lateral membranes (Murthy et al., 2010).
A function of epithelia is to create a physical barrier between the external milieu and the body interior. Because it strengthens cell-cell junctions, the exocyst protects epithelium barrier integrity (Figure 3D). Cholera toxin weakens intercellular bonds at the basolateral surface of the intestinal epithelium, by inhibiting the Rab11/exocyst-mediating trafficking of E-cadherins to adherens junctions. This leads to a massive efflux of water across the intestinal epithelium, characteristic of the water diarrhea observed in patients infected with Vibrio cholera (Guichard et al., 2013). Similarly, two toxins secreted by Bacillus antracis target Rab11/Sec15, affect the formation and signaling at adherens junctions and lead to the disruption of the endothelial barrier and the lethal vascular leakage associated with systemic anthrax (Guichard et al., 2010).
Another protective function of the exocyst complex may come from its ability to somehow regulate endocytosis in metazoan like it does in yeast (Oztan et al., 2007; Jourdain et al., 2012; Jose et al., 2015). Sec10 protects kidney epithelium integrity against oxidative stress associated with kidney ischemia and reperfusion (I/R) injury, by increasing EGF receptor endocytosis and signaling, and downstream activation of the MAPK pathway (Park et al., 2010; Fogelgren et al., 2014). Sec10 may also be involved in kidney recovery after I/R injury, which may have implications for transplantation and vascular surgery (Park et al., 2010).
The exocyst participates in cell adhesion with the substratum. Silencing of Exo70 by siRNA inhibits cell spreading on fibronectin-coated substratum (Hertzog et al., 2012). On the one hand, the exocyst participates in the RalA-dependent delivery of focal adhesion proteins such as integrins at the leading edge of migrating cells (Balasubramanian et al., 2010; Thapa et al., 2012). Cell adhesion to the substratum is a prerequisite to cell migration and interfering with exocyst function leads to cell motility defects (Spiczka and Yeaman, 2008). Accordingly, some, but not all exocyst subunits colocalizes and co-fractionates with paxillin-containing focal complexes in pseudopods of migrating cancer cells (Spiczka and Yeaman, 2008) (Figure 3E). Neuron guidance defects observed in Sec15−/− drosophila photoreceptors are also associated with defects in the trafficking of adhesion molecules (Mehta et al., 2005). On the other hand, upon cell detachment from the substratum and re-adhesion, Exo70 regulates the recycling of the mechanosensing and endocytic protein caveolin-1 to the PM, away from focal adhesions (Hertzog et al., 2012). Opposite from its role as a tether or scaffold, the exocyst may therefore also mediate the exclusion of factors from specialized PM domains.
Disruption of the exocyst complex affects cytokinesis in animal cells and yeast in a similar manner (Wang et al., 2002; Echard et al., 2004; VerPlank and Li, 2005; Neto et al., 2013a; Giansanti et al., 2015). In both cases, cytokinesis involves the formation of a medial actin ring, which, upon constriction, drags the PM. Animal cytokinesis does not involve septum formation and further differs from yeast cytokinesis by the fact that the ingressed cleavage furrow leaves a narrow cytoplasmic bridge between daughter cells at the end of cytokinesis. This midbody is made of overlapping MTs and many other proteins whose function is to define the site of abscission. In drosophila the exocyst complex localizes at the cleavage furrow and is involved in the early stages of furrow ingression (Giansanti et al., 2015). Later, it localizes at the site of abscission (Skop et al., 2004). More specifically, the exocyst forms a midbody ring through its interaction with centriolin, a protein classically associated with centrosome maturation (Gromley et al., 2005) (Figure 3F). Similar to loss of centrosome function, loss of exocyst function leads to the accumulation of several multinucleated cells connected by long intracellular bridges that went through a second mitosis without having divided (Chen et al., 2006) (Figure 3G). These findings support the notion of a putative collaboration between the centrosome or centrosome-associated proteins and the exocyst complex. This is strengthened by the observation that centrosome and endosomes interact (Hehnly et al., 2012). The exocyst also tethers recycling endosomes but it is unknown whether this is in connection with its interaction with centrosome proteins at the midbody (Fielding et al., 2005; Chen et al., 2006; Hehnly et al., 2012; Neto et al., 2013a). The discovery that centriolin/exocyst connects the two organelles may shed light on the functional significance of this association. The implications go beyond the understanding of cell division, as both centrosomes and membrane-bound vesicles are involved in other cellular processes such as polarized migration and ciliogenesis.
Most mammalian cells build primary cilia, sensory organelles that act as chemo- or mechanosensors and transducers of signals that regulate key developmental signaling pathways. Primary cilia have a characteristic “9+0” architecture made up of nine doublet MTs arranged in a ring and surrounded by a ciliary membrane that is continuous with the PM. The primary cilium is extended from a basal body derived from one of the two centrioles of the cell's centrosome (Dawe et al., 2007). Ciliogenesis is a multi-stage process initiated by the translocation of the centrioles to the apical membrane. Centrioles then acquire two sets of distal and subdistal appendages (aka centriole maturation). During centrosome migration, a ciliary vesicle that is probably derived from the Golgi apparatus encapsulates the distal end of the mother centriole through association with the distal appendages (Sorokin, 1962; Schmidt et al., 2012; Joo et al., 2013). The exact mechanism behind centriole migration toward the apical cell surface is elusive but requires actin and the ciliary vesicle (Boisvieux-Ulrich et al., 1987, 1990; Lemullois et al., 1987, 1988; Pan et al., 2007; Park et al., 2008; Kim J. et al., 2010; Pitaval et al., 2010; Yan and Zhu, 2013; Kim et al., 2015). The ciliary vesicle fuses with the PM in a manner similar to exocytosis, thereby docking the centrioles into the cortical cytoskeleton (Ghossoub et al., 2011). After removal of a capping protein from its distal end, the mother centriole elongates an axoneme and the cilium is extended. Both rely on intraflagellar transport (IFT) (Scholey, 2003), a MT-directed trafficking mechanism that facilitates the bi-directional movement of cargoes between the cilium base and tip. Ciliary cargoes are necessary for cilia assembly, maintenance and signaling. IFT involves the recruitment and assembly of the IFT machinery with cargo at the cilium base and links the ciliary membrane to the basal body (Deane et al., 2001).
Besides a localization at tight junctions the exocyst complex also localizes at cilia in MDCK cells (Liu Q. et al., 2007). In fact, the exocyst complex displays several sub-ciliary locations (Figure 3C). Some exocyst sub-units were visualized at the base of the primary cilium (Rogers et al., 2004; Babbey et al., 2010; Seixas et al., 2016). There, the exocyst is believed to traffic and dock Golgi-derived vesicles carrying ciliary proteins. In support of this, Sec10 directly interacts with the ciliary proteins IFT20, IFT88, and polycystin-2, and the expression levels of some of these proteins depend on the level of expression of Sec10 (Zuo et al., 2009; Fogelgren et al., 2015). Moreover, the KD of Sec10 in MDCK cells and of Sec15 in a human retinal pigment epithelial cell line reduce cilium length, whereas the overexpression of Sec10 increases cilium length (Zuo et al., 2009; Feng et al., 2012) (Figure 3D). Antisense morpholinos of sec10 in zebrafish produce curly tail up, left-right asymmetry defects, small eyes and oedema, that are characteristic of defective cilia (Fogelgren et al., 2015).
Some sub-units of the exocyst complex are also (e.g., Sec10) or solely (e.g., Exo70 and Exo84) present within the cilium itself (Feng et al., 2012; Chacon-Heszele et al., 2014; Seixas et al., 2016). This uniform distribution along cilia, but not at the cilium tip, is surprising and raises the question of the ciliary role of the exocyst. One possibility is that like in other organisms, ciliary vesicles actually exist that could carry exocyst proteins (Chacon-Heszele et al., 2014).
All members of the exocyst complex and most of its regulators, are also present in exosome-like vesicles (ELV), attached to the outer surface of cilia (Chacon-Heszele et al., 2014). ELV are released into the extracellular medium by budding of the PM at the ciliary base. They are considered a form of cell-cell communication, because they can bind to the neighboring cilium and release cilia-specific membrane proteins (Hogan et al., 2009; Wood et al., 2013). The ciliary matrix is continuous with the cytoplasm but at the transition zone, a physical barrier controls ciliary entry, and exit (Garcia-Gonzalo and Reiter, 2012). One hypothesis is that ELV are too large to pass this barrier but must be delivered to the nascent cilium of the same or adjacent cell as they contain proteins and lipids necessary for ciliogenesis. In this scenario, the exocyst would be involved in the release or uptake of ELV from and to cilia, or may unload or traffic taken up ELV cargoes along the cilium (Chacon-Heszele et al., 2014). This would explain the uniform localization of the exocyst within the cilium. Exosomes are not cilia-specific but the role of the exocyst in their biology may be (Hyenne et al., 2015). Fungi also secrete exosomes. In C. neoformans, Sec6 RNAi mutants are less virulent to mice than the WT because they cannot secrete exosomes and deliver virulence factors (Panepinto et al., 2009). It is possible that, like in epithelial cells, the exocyst complex controls exosome release by the fungus, or uptake by the host.
Another possible role of the exocyst complex may be in basal body positioning. Centriolin and cenexin/ODF2 are centriole appendage proteins and they mediate the association of the exocyst and some of its regulators with the mother centriole (Hehnly et al., 2012). Centrin on the basal body may bind the exocyst on the ciliary vesicle. In other words, the centrin-exocyst interaction bridges the basal body and ciliary vesicle (Park et al., 2008). In some respect this ciliary vesicle/centrosome interaction is similar to the endosome/centrosome interaction mentioned above (Hehnly et al., 2012). By targeting the ciliary vesicle toward the PM, the exocyst may therefore participate in the migration, planar positioning or docking of the basal body (Park et al., 2008; Huang and Lipschutz, 2014).
A number of inherited diseases, collectively termed ciliopathies, have been linked with defects in genes that affect cilium biogenesis or function (Waters and Beales, 2011). Ciliopathies share many clinical features, with kidney cysts, retinal degeneration and skeletal malformations often seen in combination with central nervous system developmental defects. The most severe are lethal in early gestation or shortly after birth. Exo84 was identified as a Joubert syndrome gene in a family originally diagnosed with an early onset neurodevelopmental disorder (Dixon-Salazar et al., 2012). Exome sequencing conducted on Arab families with Meckel-Gruber syndrome identified a single nucleotide pathogenic mutation in Sec8 (Shaheen et al., 2013). No other exocyst members have so far been associated with ciliopathies, probably because alterations are lethal. However, Sec10 genetically and physically interacts with the ciliary protein polycystin-2 whose mutation leads to autosomal dominant polycystic kidney disease (ADPKD), and knock down of Sec10 causes the generation of multiple kidney cysts with short primary cilia, a hallmark of ADPKD (Fogelgren et al., 2011; Seixas et al., 2016). Thus, two, possibly three, exocyst genes happen to be ciliopathy genes.
Many ciliary proteins now appear to have both ciliary and non-ciliary functions that may equally account for the cellular defects that yield ciliopathies. This is very likely to be the case for the exocyst, but it is clear that the complex has a role to play in cilium assembly and function.
Tunneling nanotubes (TNTs) are a type of intercellular communication that bridge two or more cells over long distances with no contact with a substratum (Rustom et al., 2004). They are used to shuttle material from cell to cell and to transduce molecular and electrical signals. TNTs were visualized in vitro in various cell types but they also exist in vivo (Gerdes et al., 2013; Ady et al., 2014). In macrophages and HeLa cells, TNT outgrowth can be induced de novo by expression of M-Sec (aka TNFaip2), a protein that shows some structural homology with Sec6. M-Sec co-fractionates with Sec6 but does not substitute for Sec6 within the exocyst complex and does not integrate into the exocyst complex. However, M-Sec-induced TNT formation depends on the exocyst complex and on its activator the small GTPase RalA (Hase et al., 2009). The M-Sec-exocyst-Ral association initiates TNT formation, possibly by organizing the actin cytoskeleton but it is not clear at present whether they also participate in TNT fusion with the target cell. M-Sec expression is restricted to myeloid lineages, but the role of the RalA-exocyst axis in TNT induction is likely to be conserved in other cell types (Gousset et al., 2013). One proposed mechanism of TNTs formation is through filopodia conversion (Bukoreshtliev et al., 2009) and the RalA-exocyst interaction is essential for filopodia formation in neurons (Sugihara et al., 2002).
TNTs are also exploited by pathogens to spread and evade antibody neutralization (e.g., HIV, prion). The HIV-1 protein Nef is involved in AIDS pathogenesis notably by promoting nanotube formation. HIV-1 Nef was shown to interact with five members of the exocyst complex and depletion of SEC5 impairs Nef-mediated nanotube formation (Mukerji et al., 2012). Besides a role in TNT formation, the Nef-exocyst interaction regulates the chemotactic response of T lymphocytes to antigen recognition. Surprisingly given the role of both in membrane trafficking, the exocyst is not involved in the perturbation of membrane trafficking by Nef, but in the inhibition of actin remodeling and associated cell spreading (Imle et al., 2015).
The canonical role of the exocyst complex in secretion, membrane expansion and delivery of membrane proteins explains in part its involvement in cancer. The invadopodium is an actin-rich, “finger-like,” protrusion of the PM that breaches the extra cellular matrix prior to cancer invasion and metastasis. The exocyst participates in the secretion of degrading matrix metalloproteinases (MMPs) and promotes invadopodium formation by adding new membranes at the tip of the nascent invadopodia. It also controls the Arp2/3-mediated actin polymerization by directly interacting with the Arp2/3 complex and its activator WASH (Liu et al., 2009; Monteiro et al., 2013). In hepatocellular carcinoma cells, the exocyst positions receptors at the PM such as the G-coupled chemokine receptors CXCR4 (Cepeda et al., 2015), whose overexpression is known in a range of tumor types, where it plays a central role in tumor growth and metastasis (Domanska et al., 2013).
The epithelial-mesenchymal transition (EMT) is the process by which epithelial cells lose their interaction with the matrix and neighboring cells and acquire the motile and invasive characteristics of mesenchymal cells. It is involved in cell migration during embryonic development as well as in cancer metastasis. Splice variants of Exo70, generated through the activity of the pre-mRNA splicing factor ESRP1, are involved in EMT (Lu et al., 2013). The level of expression of the mesenchymal isoform of Exo70 correlates with cancer metastasis in the lung of a mouse model and expression of epithelial Exo70 prevents metastasis. Unlike the epithelial isoform, the mesenchymal splice variant of Exo70 interacts with the Arp2/3 complex and stimulates actin branching in lamelipodia and invadopodia, thus promoting motility and invasion. The effect on actin organization, but not secretion, is responsible for this phenotype. Thus, alternative splicing of members of the exocyst complex can change their function dramatically and have implications in disease.
Invasive cancer cells are characterized by an abundance of filopodia, “finger-like” protrusions similar in architecture to invadopodia, that extend from the cell edge and whose functions include cell adhesion and migration. In mice skin melanoma cells, Exo70 induces filopodia formation through its ability to generate negative curvature on the PM in a manner similar to BAR domain proteins (Zhao et al., 2013). This curvature may create a physical space necessary to accommodate the Arp2/3-mediated actin polymerization that drives filopodia outgrowth. On the other hand, over-expression of Exo70 can produce filopodia that are devoid of actin at their most distal tip, suggesting that the membrane deforming activity of Exo70 prevails over its function on actin organization (Zhao et al., 2013). No other exocyst subunit can similarly deform membranes, nor induce filopodia formation. Thus, this membrane-bending activity is probably not a property of the exocyst as a whole, but of a free pool of oligomerized Exo70 (Zhao et al., 2013).
Approximately a third of all human cancers contain oncogenic mutations in the gene encoding the GTPase Ral, which is then produced in its constitutive state (Issaq et al., 2010). Sec5 and Exo84 are effectors of Ral and KD of Sec5 and Exo84 in human embryonic kidney cells (HEK) leads to a reduced Ral-driven tumorigenicity (Issaq et al., 2010). Thus, the role of Ral in tumoriginicity is at least in part through the exocyst complex. In this instance, the Ral-exocyst complex may act as a scaffolding unit that recruits a ikappaB kinase that activates Akt and inhibits apoptosis in cancer cells (Chien and White, 2008). Similarly, KD of Sec8 in flies allows JIP4, a JNK related protein, to interact with proteins in growth signaling pathways, preventing apoptosis (Tanaka et al., 2014). This has important implications in cell immortality which is associated with cancer. The role of the Ral-exocyst axis in abscission may further account for the generation of aneuploidy, a hallmark of cancer (Chen et al., 2006). Finally, in human cell lines, Ral and Exo84 are required for stress-induced autophagy, whose alterations can lead to cancer (Bodemann et al., 2011).
In replicating cells, un-repaired DNA damage is propagated in progeny and commonly leads to cancer. Endogenous and exogenous DNA damage is very frequent and therefore necessitates the existence of a DNA damage response (DDR) machinery that faithfully repairs the DNA. Sec8 has been shown to interact with 33 DDR and chromatin modifying proteins and to modulate the response to DNA damage. Ablation of Sec8 leads to an increase in the frequency of DNA repair but generates chromosomal aberrations (Torres et al., 2015). Therefore, paradoxically, the absence of Sec8 induces genomic instability. Sec8 may participate in the cellular response to DNA damage by restraining the spacio-temporal recruitment of chromatin remodeling proteins in the absence of appropriate DDR signaling, thus promoting a faithful repair. Depletion of Sec8 also confers radioresistance, which has implications for the treatment by radiotherapy of colorectal cancers, in which Sec8 is deleted (Ashktorab et al., 2010; Torres et al., 2015). The G1/S checkpoint is another mechanism by which propagation of damaged DNA can be prevented. Sec8 inhibits cell growth and promotes cell-cycle arrest at the G1/S phase by controlling p21 expression, a protein that inhibits the activity of cyclins at the G1 checkpoint (Tanaka et al., 2014).
Phagocytosis is a form of endocytosis in which a large, solid particle is recognized, wrapped around an outgrowing host PM and internalized by engulfment. The resulting phagosome maturates into a phagolysosome upon fusion with the acidic lysosomal and endosomal compartments. In mammalian cells, phagocytosis removes invading pathogens and clears apoptotic cells during tissue homeostasis and remodeling. A systematic analysis of the phagosome proteome in drosophila identified members of the exocyst complex (Stuart et al., 2007). In mammalian HeLA cells, depletion of Exo70 by siRNA is associated with a reduced uptake of large, but not small particles. Whether the whole exocyst is present in nascent phagosomes is controversial and it was suggested that complex assembly and involvement may be transient (Mohammadi and Isberg, 2013).
The exocyst also has a role in phagosome maturation. KD of exocyst proteins in endothelial cells greatly reduces phagosome acidification and bacterial elimination (Figure 3D). Exocyst proteins were observed on Rab11-positive recycling endosomes that dynamically fuse with the phagosome and may mediate the interaction between these two organelles (Rauch et al., 2014) (Figure 3C). These findings further indicate that the exocyst complex associates with and mediates the fusion of intra-cellular compartments and does not solely play a role at the PM.
The exocyst is therefore involved in host defense, but it can on the contrary promote bacteria and parasite entry and invasion. Many Gram-negative intracellular bacterial pathogens use a secretion system to inject virulence factors into the host cell through the formation of a translocation pore. In Salmonella typhimurium, one of the members of the T3SS translocation complex, SipC, directly interacts with actin and with several members of the exocyst complex. The recruitment of the exocyst at the invadosomes, the sites of invasion, is thought to increase membrane expansion and create a membrane ruffling that will lead to the internalization of Salmonella by macropinocytosis. In support of this, KD of Sec5 reduces membrane ruffling and bacterial invasion (Nichols and Casanova, 2010).
Trypanosoma cruzi is an obligate intracellular parasite responsible for Chagas disease that causes damage to the heart and central nervous system. During invasion, the parasite makes a parasitophorous vacuole (PV) in which it will reside and develop, protected from the host's lysosome. In the process, T. cruzi injures the host's PM, opening large wounds through which extra cellular calcium can engulf. This sudden increase in calcium influx triggers a burst in calcium-mediated exocytosis of lysosomes, which, upon fusion with the PM and active removal of lesions by endocytosis, participate in wound healing and PM repair (Andrews et al., 2014). Conversely, PM repair is required for T. cruzi invasion. The PV is in fact derived from the host's endosomal or lysosomal compartment (Fernandes et al., 2011). Exo70 and Sec8 were shown to accumulate in nascent T. cruzi vacuoles and at the sites of wounding and to be required for both PM repair and T. cruzi invasion (Fernandes et al., 2015). This study raises the possibility that the exocyst, which is traditionally associated with constitutive exocytosis, is also involved in Ca2+-mediated, regulated exocytosis. This would have implications for wound repair in other tissues. In budding yeast, damage of the cell wall and PM triggers the reorientation of the actin cytoskeleton and exocyst complex from sites of polarized growth to the wounded site, thus avoiding competition between cell polarization and wound healing (Kono et al., 2012).
Pathogens use the recycling pathway and the exocyst complex to exit infected cells and spread to neighboring cells. Porphyromonas gingivalis is a bacterium pathogen that penetrates gingival epithelial cells by exploiting the endocytic pathway of its host. Expression of dominant negative versions and KD of exocyst components compromise the recycling of the pathogen back to the PM, its exit from the host, and therefore its spreading to neighboring cells (Takeuchi et al., 2011).
Interestingly, the genomes of a few parasitic protists and ciliates contain no or a reduced number of exocyst subunits (Koumandou et al., 2007; Klinger et al., 2013), indicating that the exocyst is not absolutely required for cellular life. However, it was suggested that this is not a consequence of evolution but an adaptation to the lifestyle and anatomy of these parasites in which directed exocytosis can occur without any specialized machinery (Klinger et al., 2013).
Membrane expansion, cell polarity and cell division are key fundamental cellular processed that are central in all kingdoms for tissue development and cell function. It is therefore not surprising that the mechanisms and factors that orchestrate these processes have been conserved across evolution. Besides, the mechanisms of pathogen infection or cell invasion are rather similar between kingdoms. The fact that the exocyst complex is essential for survival in fungi and animals, along with the fact it is linked to a number of diseases, confirms its pivotal and universal role.
Although very few complementation experiments have been carried out, the composition and function of individual sub-units would seem to be essentially conserved from plants to animals, despite little primary sequence similarities. Multi-cellular organisms have developed different ways to deal with tissue specificity and needs for differential regulation. Splice variants probably account for this specialization in plants and metazoan, but plants also have paralogs that are expressed from distinct loci (Cvrckova et al., 2012; Lu et al., 2013). Either way, the implications of this diversification are currently far from being clear.
The exocyst is made of eight proteins and the full octameric complex clearly assembles in all species. Whether sub-complexes exist is a matter of debate and may be species-dependent. The idea that sub-complexes may exist originally came from studies of S. cerevisiae (Boyd et al., 2004). However, no stable sub-complex has so far been purified biochemically from this yeast, but a stable holocomplex was (Heider et al., 2016). In filamentous fungi, plants and animals, the dilemma comes from observations that different exocyst members localize to different sub-cellular locations. In higher eukaryotes few studies have systematically reported the localization of all subunits in the same cell, under the same experimental conditions. Evidence that some exocyst members at least are localized to distinct cellular regions is however convincing. The existence of combinations of exocyst components could be a mean to provide functional diversity in these complex systems. This later hypothesis would explain why the individually KO of all sub-units in plants and animals does not give rise to the same exact phenotype.
Research in each kingdom has focussed on different aspects of exocyst biology but similarities and differences clearly exist that could be exploited for a better understanding of this complex in health and disease.
MMU wrote the chapter on fungi. MD wrote the chapter on plants. CH helped write the chapter on animals. HD helped write the chapter on cilia. IJ wrote most of the chapter on animals, made the figures and coordinated the preparation of the ms.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Jo Costello, Andrew Early, and Rhiannon White for their editing of the manuscript.
Abenza, J. F., Couturier, E., Dodgson, J., Dickmann, J., Chessel, A., Dumais, J., et al. (2015). Wall mechanics and exocytosis define the shape of growth domains in fission yeast. Nat. Commun. 6, 8400. doi: 10.1038/ncomms9400
Ady, J. W., Desir, S., Thayanithy, V., Vogel, R. I., Moreira, A. L., Downey, R. J., et al. (2014). Intercellular communication in malignant pleural mesothelioma: properties of tunneling nanotubes. Front. Physiol. 5:400. doi: 10.3389/fphys.2014.00400
Albeg, A., Smith, C. J., Chatzigeorgiou, M., Feitelson, D. G., Hall, D. H., Schafer, W. R., et al. (2011). C. elegans multi-dendritic sensory neurons: morphology and function. Mol. Cell. Neurosci. 46, 308–317. doi: 10.1016/j.mcn.2010.10.001
Andersen, N. J., and Yeaman, C. (2010). Sec3-containing exocyst complex is required for desmosome assembly in mammalian epithelial cells. Mol. Biol. Cell 21, 152–164. doi: 10.1091/mbc.E09-06-0459
Andrews, N. W., Almeida, P. E., and Corrotte, M. (2014). Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol. 24, 734–742. doi: 10.1016/j.tcb.2014.07.008
Araujo-Palomares, C. L., Castro-Longoria, E., and Riquelme, M. (2007). Ontogeny of the spitzenkorper in germlings of Neurospora crassa. Fungal Genet. Biol. 44, 492–503. doi: 10.1016/j.fgb.2006.10.004
Araujo-Palomares, C. L., Riquelme, M., and Castro-Longoria, E. (2009). The polarisome component SPA-2 localizes at the apex of Neurospora crassa and partially colocalizes with the Spitzenkorper. Fungal Genet. Biol. 46, 551–563. doi: 10.1016/j.fgb.2009.02.009
Ashktorab, H., Schaffer, A. A., Daremipouran, M., Smoot, D. T., Lee, E., and Brim, H. (2010). Distinct genetic alterations in colorectal cancer. PLoS ONE 5:e8879. doi: 10.1371/journal.pone.0008879
Babbey, C. M., Bacallao, R. L., and Dunn, K. W. (2010). Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am. J. Physiol. Renal Physiol. 299, F495–F506. doi: 10.1152/ajprenal.00198.2010
Balasubramanian, N., Meier, J. A., Scott, D. W., Norambuena, A., White, M. A., and Schwartz, M. A. (2010). RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling. Curr. Biol. 20, 75–79. doi: 10.1016/j.cub.2009.11.016
Bartnicki-García, S. (1990). “Role of vesicles in apical growth and a new mathematical model of hyphal morphogenesis,” in Tip Growth in Plants and Fungal Cells, ed I. Heath (San Diego, CA: Academic), 211–232.
Bartnicki-García, S. (2002). “Hyphal tip growth: outstanding questions,” in Molecular Biology of Fungal Development, ed H. Osiewacz (New York, NY: Marcel Dekker), 29–58.
Bartnicki-Garcia, S., Bracker, C. E., Gierz, G., Lopez-Franco, R., and Lu, H. (2000). Mapping the growth of fungal hyphae: orthogonal cell wall expansion during tip growth and the role of turgor. Biophys. J. 79, 2382–2390. doi: 10.1016/S0006-3495(00)76483-6
Bartnicki-Garcia, S., Hergert, F., and Gierz, G. (1989). Computer simulation of fungal morphogenesis and the mathematical basis for hyphal (tip) growth. Protoplasma 153, 46–57. doi: 10.1007/BF01322464
Bendezu, F. O., and Martin, S. G. (2011). Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol. Biol. Cell 22, 44–53. doi: 10.1091/mbc.E10-08-0720
Bendezu, F. O., Vincenzetti, V., and Martin, S. G. (2012). Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. PLoS ONE 7:e40248. doi: 10.1371/journal.pone.0040248
Blankenship, J. T., Fuller, M. T., and Zallen, J. A. (2007). The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J. Cell Sci. 120, 3099–3110. doi: 10.1242/jcs.004770
Bloom, M. L., and Simon-Stoos, K. L. (1997). The hemoglobin-deficit mouse: analysis of phenotype and hematopoiesis in the transplant model. Blood 90, 2062–2067.
Bodemann, B. O., Orvedahl, A., Cheng, T., Ram, R. R., Ou, Y. H., Formstecher, E., et al. (2011). RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144, 253–267. doi: 10.1016/j.cell.2010.12.018
Boisvieux-Ulrich, E., Laine, M. C., and Sandoz, D. (1987). In vitro effects of benzodiazepines on ciliogenesis in the quail oviduct. Cell Motil. Cytoskeleton 8, 333–344. doi: 10.1002/cm.970080406
Boisvieux-Ulrich, E., Laine, M. C., and Sandoz, D. (1990). Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell Tissue Res. 259, 443–454. doi: 10.1007/BF01740770
Bourett, T. M., and Howard, R. J. (1991). Ultrastructural immunolocalization of actin in a fungus. Protoplasma 163, 199–202. doi: 10.1007/BF01323344
Boyd, C., Hughes, T., Pypaert, M., and Novick, P. (2004). Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 167, 889–901. doi: 10.1083/jcb.200408124
Bryant, D. M., Datta, A., Rodriguez-Fraticelli, A. E., Peranen, J., Martin-Belmonte, F., and Mostov, K. E. (2010). A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 12, 1035–1045. doi: 10.1038/ncb2106
Bukoreshtliev, N. V., Wang, X., Hodneland, E., Gurke, S., Barroso, J. F., and Gerdes, H. H. (2009). Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 583, 1481–1488. doi: 10.1016/j.febslet.2009.03.065
Caballero-Lima, D., Kaneva, I. N., Watton, S. P., Sudbery, P. E., and Craven, C. J. (2013). The spatial distribution of the exocyst and actin cortical patches is sufficient to organize hyphal tip growth. Eukaryotic Cell 12, 998–1008. doi: 10.1128/EC.00085-13
Cepeda, E. B., Dediulia, T., Fernando, J., Bertran, E., Egea, G., Navarro, E., et al. (2015). Mechanisms regulating cell membrane localization of the chemokine receptor CXCR4 in human hepatocarcinoma cells. Biochim. Biophys. Acta 1853, 1205–1218. doi: 10.1016/j.bbamcr.2015.02.012
Chacon-Heszele, M. F., Choi, S. Y., Zuo, X., Baek, J. I., Ward, C., and Lipschutz, J. H. (2014). The exocyst and regulatory GTPases in urinary exosomes. Physiol. Rep. 2:e12116. doi: 10.14814/phy2.12116
Chavez-Dozal, A. A., Bernardo, S. M., Rane, H. S., Herrera, G., Kulkarny, V., Wagener, J., et al. (2015a). The Candida albicans Exocyst Subunit Sec6 Contributes to Cell Wall Integrity and Is a Determinant of Hyphal Branching. Eukaryotic Cell 14, 684–697. doi: 10.1128/EC.00028-15
Chavez-Dozal, A. A., Bernardo, S. M., Rane, H. S., and Lee, S. A. (2015b). Functional Analysis of the Exocyst Subunit Sec15 in Candida albicans. Eukaryotic Cell 14, 1228–1239. doi: 10.1128/EC.00147-15
Chen, X. W., Inoue, M., Hsu, S. C., and Saltiel, A. R. (2006). RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J. Biol. Chem. 281, 38609–38616. doi: 10.1074/jbc.M512847200
Chien, Y., and White, M. A. (2008). Characterization of RalB-Sec5-TBK1 function in human oncogenesis. Meth. Enzymol. 438, 321–329. doi: 10.1016/S0076-6879(07)38022-1
Chong, Y. T., Gidda, S. K., Sanford, C., Parkinson, J., Mullen, R. T., and Goring, D. R. (2010). Characterization of the Arabidopsis thaliana exocyst complex gene families by phylogenetic, expression profiling, and subcellular localization studies. New Phytol. 185, 401–419. doi: 10.1111/j.1469-8137.2009.03070.x
Cvrckova, F., Grunt, M., Bezvoda, R., Hala, M., Kulich, I., Rawat, A., et al. (2012). Evolution of the land plant exocyst complexes. Front. Plant Sci. 3:159. doi: 10.3389/fpls.2012.00159
Dagdas, Y. F., Yoshino, K., Dagdas, G., Ryder, L. S., Bielska, E., Steinberg, G., et al. (2012). Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 336, 1590–1595. doi: 10.1126/science.1222934
Das, A., Gajendra, S., Falenta, K., Oudin, M. J., Peschard, P., Feng, S., et al. (2014). RalA promotes a direct exocyst-Par6 interaction to regulate polarity in neuronal development. J. Cell Sci. 127, 686–699. doi: 10.1242/jcs.145037
Dawe, H. R., Farr, H., and Gull, K. (2007). Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci. 120, 7–15. doi: 10.1242/jcs.03305
Dean, R., Van Kan, J. A., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Spanu, P. D., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
Deane, J. A., Cole, D. G., Seeley, E. S., Diener, D. R., and Rosenbaum, J. L. (2001). Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11, 1586–1590. doi: 10.1016/S0960-9822(01)00484-5
Ding, Y., Wang, J., Chun Lai, J. H., Ling Chan, V. H., Wang, X., Cai, Y., et al. (2014). Exo70E2 is essential for exocyst subunit recruitment and EXPO formation in both plants and animals. Mol. Biol. Cell 25, 412–426. doi: 10.1091/mbc.E13-10-0586
Dixon-Salazar, T. J., Silhavy, J. L., Udpa, N., Schroth, J., Bielas, S., Schaffer, A. E., et al. (2012). Exome sequencing can improve diagnosis and alter patient management. Sci. Transl. Med. 4, 138ra178. doi: 10.1126/scitranslmed.3003544
Domanska, U. M., Kruizinga, R. C., Nagengast, W. B., Timmer-Bosscha, H., Huls, G., de Vries, E. G., et al. (2013). A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur. J. Cancer 49, 219–230. doi: 10.1016/j.ejca.2012.05.005
Drdova, E. J., Synek, L., Pecenkova, T., Hala, M., Kulich, I., Fowler, J. E., et al. (2013). The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J. 73, 709–719. doi: 10.1111/tpj.12074
Du, Y., Mpina, M. H., Birch, P. R., Bouwmeester, K., and Govers, F. (2015). Phytophthora infestans RXLR Effector AVR1 interacts with exocyst component Sec5 to manipulate plant immunity. Plant Physiol. 169, 1975–1990. doi: 10.1104/pp.15.01169
Dupraz, S., Grassi, D., Bernis, M. E., Sosa, L., Bisbal, M., Gastaldi, L., et al. (2009). The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J. Neurosci. 29, 13292–13301. doi: 10.1523/JNEUROSCI.3907-09.2009
Echard, A., Hickson, G. R., Foley, E., and O'Farrell, P. H. (2004). Terminal cytokinesis events uncovered after an RNAi screen. Curr. Biol. 14, 1685–1693. doi: 10.1016/j.cub.2004.08.063
Elias, M., Drdova, E., Ziak, D., Bavlnka, B., Hala, M., Cvrckova, F., et al. (2003). The exocyst complex in plants. Cell Biol. Int. 27, 199–201. doi: 10.1016/S1065-6995(02)00349-9
Feierbach, B., Verde, F., and Chang, F. (2004). Regulation of a formin complex by the microtubule plus end protein tea1p. J. Cell Biol. 165, 697–707. doi: 10.1083/jcb.200403090
Fendrych, M., Synek, L., Pecenkova, T., Drdova, E. J., Sekeres, J., de Rycke, R., et al. (2013). Visualization of the exocyst complex dynamics at the plasma membrane of Arabidopsis thaliana. Mol. Biol. Cell 24, 510–520. doi: 10.1091/mbc.E12-06-0492
Fendrych, M., Synek, L., Pecenkova, T., Toupalova, H., Cole, R., Drdova, E., et al. (2010). The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell 22, 3053–3065. doi: 10.1105/tpc.110.074351
Feng, S., Knodler, A., Ren, J., Zhang, J., Zhang, X., Hong, Y., et al. (2012). A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J. Biol. Chem. 287, 15602–15609. doi: 10.1074/jbc.M111.333245
Fernandes, M. C., Corrotte, M., Miguel, D. C., Tam, C., and Andrews, N. W. (2015). The exocyst is required for trypanosome invasion and the repair of mechanical plasma membrane wounds. J. Cell Sci. 128, 27–32. doi: 10.1242/jcs.150573
Fernandes, M. C., Cortez, M., Flannery, A. R., Tam, C., Mortara, R. A., and Andrews, N. W. (2011). Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion. J. Exp. Med. 208, 909–921. doi: 10.1084/jem.20102518
Fielding, A. B., Schonteich, E., Matheson, J., Wilson, G., Yu, X., Hickson, G. R., et al. (2005). Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 24, 3389–3399. doi: 10.1038/sj.emboj.7600803
Finger, F. P., Hughes, T. E., and Novick, P. (1998). Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell 92, 559–571. doi: 10.1016/S0092-8674(00)80948-4
Finger, F. P., and Novick, P. (1997). Sec3p is involved in secretion and morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell 8, 647–662. doi: 10.1091/mbc.8.4.647
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. doi: 10.1038/nature10947
Fogelgren, B., Lin, S. Y., Zuo, X., Jaffe, K. M., Park, K. M., Reichert, R. J., et al. (2011). The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet. 7:e1001361. doi: 10.1371/journal.pgen.1001361
Fogelgren, B., Polgar, N., Lui, V. H., Lee, A. J., Tamashiro, K. K., Napoli, J. A., et al. (2015). Urothelial defects from targeted inactivation of exocyst Sec10 in mice cause ureteropelvic junction obstructions. PLoS ONE 10:e0129346. doi: 10.1371/journal.pone.0129346
Fogelgren, B., Zuo, X., Buonato, J. M., Vasilyev, A., Baek, J. I., Choi, S. Y., et al. (2014). Exocyst Sec10 protects renal tubule cells from injury by EGFR/MAPK activation and effects on endocytosis. Am. J. Physiol. Renal Physiol. 307, F1334–F1341. doi: 10.1152/ajprenal.00032.2014
Friedrich, G. A., Hildebrand, J. D., and Soriano, P. (1997). The secretory protein Sec8 is required for paraxial mesoderm formation in the mouse. Dev. Biol. 192, 364–374. doi: 10.1006/dbio.1997.8727
Fujisaki, K., Abe, Y., Ito, A., Saitoh, H., Yoshida, K., Kanzaki, H., et al. (2015). Rice Exo70 interacts with a fungal effector, AVR-Pii, and is required for AVR-Pii-triggered immunity. Plant J. 83, 875–887. doi: 10.1111/tpj.12934
Fujita, A., Koinuma, S., Yasuda, S., Nagai, H., Kamiguchi, H., Wada, N., et al. (2013). GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PLoS ONE 8:e79689. doi: 10.1371/journal.pone.0079689
Gachet, Y., and Hyams, J. S. (2005). Endocytosis in fission yeast is spatially associated with the actin cytoskeleton during polarised cell growth and cytokinesis. J. Cell Sci. 118, 4231–4242. doi: 10.1242/jcs.02530
Garcia-Gonzalo, F. R., and Reiter, J. F. (2012). Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J. Cell Biol. 197, 697–709. doi: 10.1083/jcb.201111146
Garrick, M. D., and Garrick, L. M. (2007). Loss of rapid transferrin receptor recycling due to a mutation in Sec15l1 in hbd mice. Biochim. Biophys. Acta 1773, 105–108. doi: 10.1016/j.bbamcr.2006.09.032
Genre, A., Ivanov, S., Fendrych, M., Faccio, A., Zarsky, V., Bisseling, T., et al. (2012). Multiple exocytotic markers accumulate at the sites of perifungal membrane biogenesis in arbuscular mycorrhizas. Plant Cell Physiol. 53, 244–255. doi: 10.1093/pcp/pcr170
Gerdes, H. H., Rustom, A., and Wang, X. (2013). Tunneling nanotubes, an emerging intercellular communication route in development. Mech. Dev. 130, 381–387. doi: 10.1016/j.mod.2012.11.006
Ghossoub, R., Molla-Herman, A., Bastin, P., and Benmerah, A. (2011). The ciliary pocket: a once-forgotten membrane domain at the base of cilia. Biol. Cell 103, 131–144. doi: 10.1042/BC20100128
Giansanti, M. G., Vanderleest, T. E., Jewett, C. E., Sechi, S., Frappaolo, A., Fabian, L., et al. (2015). Exocyst-dependent membrane addition is required for anaphase cell elongation and cytokinesis in Drosophila. PLoS Genet. 11:e1005632. doi: 10.1371/journal.pgen.1005632
Giraldo, M. C., Dagdas, Y. F., Gupta, Y. K., Mentlak, T. A., Yi, M., Martinez-Rocha, A. L., et al. (2013). Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 4, 1996. doi: 10.1038/ncomms2996
Girbardt, M. (1969). Die ultrastruktur der apikalregion von pilzhyphen. Protoplasma 67, 413–441. doi: 10.1007/BF01254905
Gonzalez, I. M., Ackerman, W. E. IV, Vandre, D. D., and Robinson, J. M. (2014). Exocyst complex protein expression in the human placenta. Placenta 35, 442–449. doi: 10.1016/j.placenta.2014.04.015
Gousset, K., Marzo, L., Commere, P. H., and Zurzolo, C. (2013). Myo10 is a key regulator of TNT formation in neuronal cells. J. Cell Sci. 126, 4424–4435. doi: 10.1242/jcs.129239
Grindstaff, K. K., Yeaman, C., Anandasabapathy, N., Hsu, S. C., Rodriguez-Boulan, E., Scheller, R. H., et al. (1998). Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93, 731–740. doi: 10.1016/S0092-8674(00)81435-X
Gromley, A., Yeaman, C., Rosa, J., Redick, S., Chen, C. T., Mirabelle, S., et al. (2005). Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123, 75–87. doi: 10.1016/j.cell.2005.07.027
Grove, S. N., and Bracker, C. E. (1970). Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkorper. J. Bacteriol. 104, 989–1009.
Guichard, A., Cruz-Moreno, B., Aguilar, B., van Sorge, N. M., Kuang, J., Kurkciyan, A. A., et al. (2013). Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell Host Microbe 14, 294–305. doi: 10.1016/j.chom.2013.08.001
Guichard, A., McGillivray, S. M., Cruz-Moreno, B., van Sorge, N. M., Nizet, V., and Bier, E. (2010). Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature 467, 854–858. doi: 10.1038/nature09446
Guichard, A., Nizet, V., and Bier, E. (2014). RAB11-mediated trafficking in host-pathogen interactions. Nat. Rev. Microbiol. 12, 624–634. doi: 10.1038/nrmicro3325
Guo, M., Kilaru, S., Schuster, M., Latz, M., and Steinberg, G. (2015). Fluorescent markers for the Spitzenkorper and exocytosis in Zymoseptoria tritici. Fungal Genet. Biol. 79, 158–165. doi: 10.1016/j.fgb.2015.04.014
Guo, W., Grant, A., and Novick, P. (1999). Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 274, 23558–23564. doi: 10.1074/jbc.274.33.23558
Gupta, Y. K., Dagdas, Y. F., Martinez-Rocha, A. L., Kershaw, M. J., Littlejohn, G. R., Ryder, L. S., et al. (2015). Septin-dependent assembly of the exocyst is essential for plant infection by Magnaporthe oryzae. Plant Cell 27, 3277–3289. doi: 10.1105/tpc.15.00552
Hala, M., Cole, R., Synek, L., Drdova, E., Pecenkova, T., Nordheim, A., et al. (2008). An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20, 1330–1345. doi: 10.1105/tpc.108.059105
Harris, S. D., Read, N. D., Roberson, R. W., Shaw, B., Seiler, S., Plamann, M., et al. (2005). Polarisome meets spitzenkorper: microscopy, genetics, and genomics converge. Eukaryotic Cell 4, 225–229. doi: 10.1128/EC.4.2.225-229.2005
Hase, K., Kimura, S., Takatsu, H., Ohmae, M., Kawano, S., Kitamura, H., et al. (2009). M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427–1432. doi: 10.1038/ncb1990
Hayakawa, Y., Ishikawa, E., Shoji, J. Y., Nakano, H., and Kitamoto, K. (2011). Septum-directed secretion in the filamentous fungus Aspergillus oryzae. Mol. Microbiol. 81, 40–55. doi: 10.1111/j.1365-2958.2011.07700.x
Hazuka, C. D., Foletti, D. L., Hsu, S. C., Kee, Y., Hopf, F. W., and Scheller, R. H. (1999). The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. 19, 1324–1334.
He, B., Xi, F., Zhang, X., Zhang, J., and Guo, W. (2007). Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 26, 4053–4065. doi: 10.1038/sj.emboj.7601834
Hehnly, H., Chen, C. T., Powers, C. M., Liu, H. L., and Doxsey, S. (2012). The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr. Biol. 22, 1944–1950. doi: 10.1016/j.cub.2012.08.022
Heider, M. R., Gu, M., Duffy, C. M., Mirza, A. M., Marcotte, L. L., Walls, A. C., et al. (2016). Subunit connectivity, assembly determinants and architecture of the yeast exocyst complex. Nat. Struct. Mol. Biol. 23, 59–66. doi: 10.1038/nsmb.3146
Hertzog, M., and Chavrier, P. (2011). Cell polarity during motile processes: keeping on track with the exocyst complex. Biochem. J. 433, 403–409. doi: 10.1042/BJ20101214
Hertzog, M., Monteiro, P., Le Dez, G., and Chavrier, P. (2012). Exo70 subunit of the exocyst complex is involved in adhesion-dependent trafficking of caveolin-1. PLoS ONE 7:e52627. doi: 10.1371/journal.pone.0052627
Hogan, M. C., Manganelli, L., Woollard, J. R., Masyuk, A. I., Masyuk, T. V., Tammachote, R., et al. (2009). Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 20, 278–288. doi: 10.1681/ASN.2008060564
Hong, W., and Lev, S. (2014). Tethering the assembly of SNARE complexes. Trends Cell Biol. 24, 35–43. doi: 10.1016/j.tcb.2013.09.006
Howard, R. J. (1981). Ultrastructural analysis of hyphal tip cell growth in fungi: Spitzenkorper, cytoskeleton and endomembranes after freeze-substitution. J. Cell Sci. 48, 89–103.
Howard, R. J., and Aist, J. R. (1979). Hyphal tip cell ultrastructure of the fungus Fusarium: improved preservation by freeze-substitution. J. Ultrastruct. Res. 66, 224–234. doi: 10.1016/S0022-5320(79)90120-5
Huang, L., and Lipschutz, J. H. (2014). Cilia and polycystic kidney disease, kith and kin. Birth Defects Res. C Embryo Today 102, 174–185. doi: 10.1002/bdrc.21066
Hyenne, V., Apaydin, A., Rodriguez, D., Spiegelhalter, C., Hoff-Yoessle, S., Diem, M., et al. (2015). RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 211, 27–37. doi: 10.1083/jcb.201504136
Hyun, Y., Kim, J., Cho, S. W., Choi, Y., Kim, J. S., and Coupland, G. (2015). Site-directed mutagenesis in Arabidopsis thaliana using dividing tissue-targeted RGEN of the CRISPR/Cas system to generate heritable null alleles. Planta 241, 271–284. doi: 10.1007/s00425-014-2180-5
Imle, A., Abraham, L., Tsopoulidis, N., Hoflack, B., Saksela, K., and Fackler, O. T. (2015). Association with PAK2 enables functional interactions of lentiviral nef proteins with the exocyst complex. MBio 6, e01309–e01315. doi: 10.1128/mBio.01309-15
Irieda, H., Maeda, H., Akiyama, K., Hagiwara, A., Saitoh, H., Uemura, A., et al. (2014). Colletotrichum orbiculare secretes virulence effectors to a biotrophic interface at the primary hyphal neck via exocytosis coupled with SEC22-mediated traffic. Plant Cell 26, 2265–2281. doi: 10.1105/tpc.113.120600
Issaq, S. H., Lim, K. H., and Counter, C. M. (2010). Sec5 and Exo84 foster oncogenic ras-mediated tumorigenesis. Mol. Cancer Res. 8, 223–231. doi: 10.1158/1541-7786.MCR-09-0189
Jin, Y., Sultana, A., Gandhi, P., Franklin, E., Hamamoto, S., Khan, A. R., et al. (2011). Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev. Cell 21, 1156–1170. doi: 10.1016/j.devcel.2011.10.009
Jones, L. A., and Sudbery, P. E. (2010). Spitzenkorper, exocyst, and polarisome components in Candida albicans hyphae show different patterns of localization and have distinct dynamic properties. Eukaryotic Cell 9, 1455–1465. doi: 10.1128/EC.00109-10
Jones, T. A., Nikolova, L. S., Schjelderup, A., and Metzstein, M. M. (2014). Exocyst-mediated membrane trafficking is required for branch outgrowth in drosophila tracheal terminal cells. Dev. Biol. 390, 41–50. doi: 10.1016/j.ydbio.2014.02.021
Joo, K., Kim, C. G., Lee, M. S., Moon, H. Y., Lee, S. H., Kim, M. J., et al. (2013). CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc. Natl. Acad. Sci. U.S.A. 110, 5987–5992. doi: 10.1073/pnas.1220927110
Jose, M., Tollis, S., Nair, D., Mitteau, R., Velours, C., Massoni-Laporte, A., et al. (2015). A quantitative imaging-based screen reveals the exocyst as a network hub connecting endocytosis and exocytosis. Mol. Biol. Cell 26, 2519–2534. doi: 10.1091/mbc.E14-11-1527
Jourdain, I., Dooley, H. C., and Toda, T. (2012). Fission yeast sec3 bridges the exocyst complex to the actin cytoskeleton. Traffic 13, 1481–1495. doi: 10.1111/j.1600-0854.2012.01408.x
Kaksonen, M., Toret, C. P., and Drubin, D. G. (2006). Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 7, 404–414. doi: 10.1038/nrm1940
Ketelaar, T., Allwood, E. G., Anthony, R., Voigt, B., Menzel, D., and Hussey, P. J. (2004). The actin-interacting protein AIP1 is essential for actin organization and plant development. Curr. Biol. 14, 145–149. doi: 10.1016/j.cub.2004.01.004
Khang, C. H., Berruyer, R., Giraldo, M. C., Kankanala, P., Park, S. Y., Czymmek, K., et al. (2010). Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22, 1388–1403. doi: 10.1105/tpc.109.069666
Kim, D. U., Hayles, J., Kim, D., Wood, V., Park, H. O., Won, M., et al. (2010). Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 28, 617–623. doi: 10.1038/nbt.1628
Kim, J., Jo, H., Hong, H., Kim, M. H., Kim, J. M., Lee, J. K., et al. (2015). Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat. Commun. 6, 6781. doi: 10.1038/ncomms7781
Kim, J., Lee, J. E., Heynen-Genel, S., Suyama, E., Ono, K., Lee, K., et al. (2010). Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464, 1048–1051. doi: 10.1038/nature08895
Klinger, C. M., Klute, M. J., and Dacks, J. B. (2013). Comparative genomic analysis of multi-subunit tethering complexes demonstrates an ancient pan-eukaryotic complement and sculpting in Apicomplexa. PLoS ONE 8:e76278. doi: 10.1371/journal.pone.0076278
Kohli, M., Galati, V., Boudier, K., Roberson, R. W., and Philippsen, P. (2008). Growth-speed-correlated localization of exocyst and polarisome components in growth zones of Ashbya gossypii hyphal tips. J. Cell Sci. 121, 3878–3889. doi: 10.1242/jcs.033852
Kono, K., Saeki, Y., Yoshida, S., Tanaka, K., and Pellman, D. (2012). Proteasomal degradation resolves competition between cell polarization and cellular wound healing. Cell 150, 151–164. doi: 10.1016/j.cell.2012.05.030
Koumandou, V. L., Dacks, J. B., Coulson, R. M., and Field, M. C. (2007). Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol. Biol. 7:29. doi: 10.1186/1471-2148-7-29
Kulich, I., Cole, R., Drdová, E., Cvrcková, F., Soukup, A., Fowler, J., et al. (2010). Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytol. 188, 615–625. doi: 10.1111/j.1469-8137.2010.03372.x
Kulich, I., Pecenkova, T., Sekeres, J., Smetana, O., Fendrych, M., Foissner, I., et al. (2013). Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14, 1155–1165. doi: 10.1111/tra.12101
Kulich, I., Vojtikova, Z., Glanc, M., Ortmannova, J., Rasmann, S., and Zarsky, V. (2015). Cell wall maturation of Arabidopsis trichomes is dependent on exocyst subunit EXO70H4 and involves callose deposition. Plant Physiol. 168, 120–131. doi: 10.1104/pp.15.00112
Kwon, M. J., Arentshorst, M., Fiedler, M., de Groen, F. L., Punt, P. J., Meyer, V., et al. (2014). Molecular genetic analysis of vesicular transport in Aspergillus niger reveals partial conservation of the molecular mechanism of exocytosis in fungi. Microbiology 160, 316–329. doi: 10.1099/mic.0.074252-0
Lalli, G. (2009). RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J. Cell Sci. 122, 1499–1506. doi: 10.1242/jcs.044339
Lemullois, M., Boisvieux-Ulrich, E., Laine, M. C., Chailley, B., and Sandoz, D. (1988). Development and functions of the cytoskeleton during ciliogenesis in metazoa. Biol. Cell 63, 195–208. doi: 10.1016/0248-4900(88)90058-5
Lemullois, M., Klotz, C., and Sandoz, D. (1987). Immunocytochemical localization of myosin during ciliogenesis of quail oviduct. Eur. J. Cell Biol. 43, 429–437.
Letinic, K., Sebastian, R., Toomre, D., and Rakic, P. (2009). Exocyst is involved in polarized cell migration and cerebral cortical development. Proc. Natl. Acad. Sci. U.S.A. 106, 11342–11347. doi: 10.1073/pnas.0904244106
Li, C. R., Lee, R. T., Wang, Y. M., Zheng, X. D., and Wang, Y. (2007). Candida albicans hyphal morphogenesis occurs in Sec3p-independent and Sec3p-dependent phases separated by septin ring formation. J. Cell Sci. 120, 1898–1907. doi: 10.1242/jcs.002931
Li, S., Chen, M., Yu, D., Ren, S., Sun, S., Liu, L., et al. (2013). EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 25, 1774–1786. doi: 10.1105/tpc.113.112144
Li, S., van Os, G. M., Ren, S., Yu, D., Ketelaar, T., Emons, A. M., et al. (2010). Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol. 154, 1819–1830. doi: 10.1104/pp.110.164178
Lim, J. E., Jin, O., Bennett, C., Morgan, K., Wang, F., Trenor, C. C. III, et al. (2005). A mutation in Sec15l1 causes anemia in hemoglobin deficit (hbd) mice. Nat. Genet. 37, 1270–1273. doi: 10.1038/ng1659
Lipschutz, J. H., Guo, W., O'Brien, L. E., Nguyen, Y. H., Novick, P., and Mostov, K. E. (2000). Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol. Biol. Cell 11, 4259–4275. doi: 10.1091/mbc.11.12.4259
Liu, J., Yue, P., Artym, V. V., Mueller, S. C., and Guo, W. (2009). The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol. Biol. Cell 20, 3763–3771. doi: 10.1091/mbc.E08-09-0967
Liu, J., Zuo, X., Yue, P., and Guo, W. (2007). Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell 18, 4483–4492. doi: 10.1091/mbc.E07-05-0461
Liu, Q., Tan, G., Levenkova, N., Li, T., Pugh, E. N. Jr., Rux, J. J., et al. (2007). The proteome of the mouse photoreceptor sensory cilium complex. Mol. Cell. Proteomics 6, 1299–1317. doi: 10.1074/mcp.M700054-MCP200
Lu, H., Liu, J., Liu, S., Zeng, J., Ding, D., Carstens, R. P., et al. (2013). Exo70 isoform switching upon epithelial-mesenchymal transition mediates cancer cell invasion. Dev. Cell 27, 560–573. doi: 10.1016/j.devcel.2013.10.020
Maere, S., De Bodt, S., Raes, J., Casneuf, T., Van Montagu, M., Kuiper, M., et al. (2005). Modeling gene and genome duplications in eukaryotes. Proc. Natl. Acad. Sci. U.S.A. 102, 5454–5459. doi: 10.1073/pnas.0501102102
Martin-Cuadrado, A. B., Morrell, J. L., Konomi, M., An, H., Petit, C., Osumi, M., et al. (2005). Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell 16, 4867–4881. doi: 10.1091/mbc.E04-12-1114
Mehta, S. Q., Hiesinger, P. R., Beronja, S., Zhai, R. G., Schulze, K. L., Verstreken, P., et al. (2005). Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 46, 219–232. doi: 10.1016/j.neuron.2005.02.029
Mizuno, S., Takami, K., Daitoku, Y., Tanimoto, Y., Dinh, T. T., Mizuno-Iijima, S., et al. (2015). Peri-implantation lethality in mice carrying megabase-scale deletion on 5qc3.3 is caused by Exoc1 null mutation. Sci. Rep. 5:13632. doi: 10.1038/srep13632
Mohammadi, S., and Isberg, R. R. (2013). Cdc42 interacts with the exocyst complex to promote phagocytosis. J. Cell Biol. 200, 81–93. doi: 10.1083/jcb.201204090
Monteiro, P., Rosse, C., Castro-Castro, A., Irondelle, M., Lagoutte, E., Paul-Gilloteaux, P., et al. (2013). Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J. Cell Biol. 203, 1063–1079. doi: 10.1083/jcb.201306162
Mukerji, J., Olivieri, K. C., Misra, V., Agopian, K. A., and Gabuzda, D. (2012). Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology 9, 33. doi: 10.1186/1742-4690-9-33
Mukherjee, D., Sen, A., and Aguilar, R. C. (2014). RhoGTPase-binding proteins, the exocyst complex and polarized vesicle trafficking. Small GTPases 5:e28453. doi: 10.4161/sgtp.28453
Munson, M., and Novick, P. (2006). The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 13, 577–581. doi: 10.1038/nsmb1097
Murthy, M., Garza, D., Scheller, R. H., and Schwarz, T. L. (2003). Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37, 433–447. doi: 10.1016/S0896-6273(03)00031-X
Murthy, M., Teodoro, R. O., Miller, T. P., and Schwarz, T. L. (2010). Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development 137, 2773–2783. doi: 10.1242/dev.048330
Neto, H., Balmer, G., and Gould, G. (2013a). Exocyst proteins in cytokinesis: regulation by Rab11. Commun. Integr. Biol. 6:e27635. doi: 10.4161/cib.27635
Neto, H., Kaupisch, A., Collins, L. L., and Gould, G. W. (2013b). Syntaxin 16 is a master recruitment factor for cytokinesis. Mol. Biol. Cell 24, 3663–3674. doi: 10.1091/mbc.E13-06-0302
Nichols, C. D., and Casanova, J. E. (2010). Salmonella-directed recruitment of new membrane to invasion foci via the host exocyst complex. Curr. Biol. 20, 1316–1320. doi: 10.1016/j.cub.2010.05.065
Ostertag, M., Stammler, J., Douchkov, D., Eichmann, R., and Huckelhoven, R. (2013). The conserved oligomeric Golgi complex is involved in penetration resistance of barley to the barley powdery mildew fungus. Mol. Plant Pathol. 14, 230–240. doi: 10.1111/j.1364-3703.2012.00846.x
Oztan, A., Silvis, M., Weisz, O. A., Bradbury, N. A., Hsu, S. C., Goldenring, J. R., et al. (2007). Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol. Biol. Cell 18, 3978–3992. doi: 10.1091/mbc.E07-02-0097
Pan, J., You, Y., Huang, T., and Brody, S. L. (2007). RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J. Cell Sci. 120, 1868–1876. doi: 10.1242/jcs.005306
Panepinto, J., Komperda, K., Frases, S., Park, Y. D., Djordjevic, J. T., Casadevall, A., et al. (2009). Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 71, 1165–1176. doi: 10.1111/j.1365-2958.2008.06588.x
Park, K. M., Fogelgren, B., Zuo, X., Kim, J., Chung, D. C., and Lipschutz, J. H. (2010). Exocyst Sec10 protects epithelial barrier integrity and enhances recovery following oxidative stress, by activation of the MAPK pathway. Am. J. Physiol. Renal Physiol. 298, F818–F826. doi: 10.1152/ajprenal.00596.2009
Park, T. J., Mitchell, B. J., Abitua, P. B., Kintner, C., and Wallingford, J. B. (2008). Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871–879. doi: 10.1038/ng.104
Pecenkova, T., Hala, M., Kulich, I., Kocourkova, D., Drdova, E., Fendrych, M., et al. (2011). The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J. Exp. Bot. 62, 2107–2116. doi: 10.1093/jxb/erq402
Peng, Y., Lee, J., Rowland, K., Wen, Y., Hua, H., Carlson, N., et al. (2015). Regulation of dendrite growth and maintenance by exocytosis. J. Cell Sci. 128, 4279–4292. doi: 10.1242/jcs.174771
Perez, P., Portales, E., and Santos, B. (2015). Rho4 interaction with exocyst and septins regulates cell separation in fission yeast. Microbiology 161, 948–959. doi: 10.1099/mic.0.000062
Pinar, M., Pantazopoulou, A., Arst, H. N. Jr., and Penalva, M. A. (2013). Acute inactivation of the Aspergillus nidulans Golgi membrane fusion machinery: correlation of apical extension arrest and tip swelling with cisternal disorganization. Mol. Microbiol. 89, 228–248. doi: 10.1111/mmi.12280
Pitaval, A., Tseng, Q., Bornens, M., and Thery, M. (2010). Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J. Cell Biol. 191, 303–312. doi: 10.1083/jcb.201004003
Pleskot, R., Cwiklik, L., Jungwirth, P., Zarsky, V., and Potocky, M. (2015). Membrane targeting of the yeast exocyst complex. Biochim. Biophys. Acta 1848, 1481–1489. doi: 10.1016/j.bbamem.2015.03.026
Pruyne, D., and Bretscher, A. (2000a). Polarization of cell growth in yeast. J. Cell Sci. 113(Pt 4), 571–585.
Pruyne, D., and Bretscher, A. (2000b). Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113(Pt 3), 365–375.
Pruyne, D., Evangelista, M., Yang, C., Bi, E., Zigmond, S., Bretscher, A., et al. (2002). Role of formins in actin assembly: nucleation and barbed-end association. Science 297, 612–615. doi: 10.1126/science.1072309
Rauch, L., Hennings, K., and Aepfelbacher, M. (2014). A role for exocyst in maturation and bactericidal function of staphylococci-containing endothelial cell phagosomes. Traffic 15, 1083–1098. doi: 10.1111/tra.12189
Richthammer, C., Enseleit, M., Sanchez-Leon, E., Marz, S., Heilig, Y., Riquelme, M., et al. (2012). RHO1 and RHO2 share partially overlapping functions in the regulation of cell wall integrity and hyphal polarity in Neurospora crassa. Mol. Microbiol. 85, 716–733. doi: 10.1111/j.1365-2958.2012.08133.x
Riefler, G. M., Balasingam, G., Lucas, K. G., Wang, S., Hsu, S. C., and Firestein, B. L. (2003). Exocyst complex subunit sec8 binds to postsynaptic density protein-95 (PSD-95): a novel interaction regulated by cypin (cytosolic PSD-95 interactor). Biochem. J. 373, 49–55. doi: 10.1042/bj20021838
Riezman, H. (1985). Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell 40, 1001–1009. doi: 10.1016/0092-8674(85)90360-5
Riquelme, M., Bartnicki-Garcia, S., Gonzalez-Prieto, J. M., Sanchez-Leon, E., Verdin-Ramos, J. A., Beltran-Aguilar, A., et al. (2007). Spitzenkorper localization and intracellular traffic of green fluorescent protein-labeled CHS-3 and CHS-6 chitin synthases in living hyphae of Neurospora crassa. Eukaryotic Cell 6, 1853–1864. doi: 10.1128/EC.00088-07
Riquelme, M., Bredeweg, E. L., Callejas-Negrete, O., Roberson, R. W., Ludwig, S., Beltran-Aguilar, A., et al. (2014). The Neurospora crassa exocyst complex tethers Spitzenkorper vesicles to the apical plasma membrane during polarized growth. Mol. Biol. Cell 25, 1312–1326. doi: 10.1091/mbc.E13-06-0299
Roberson, R. W., and Vargas, M. M. (1994). The tubulin cytoskeleton and its sites of nucleation in hyphal tips of Allomyces macrogynus. Protoplasma 182, 19–31. doi: 10.1007/BF01403685
Rogers, K. K., Wilson, P. D., Snyder, R. W., Zhang, X., Guo, W., Burrow, C. R., et al. (2004). The exocyst localizes to the primary cilium in MDCK cells. Biochem. Biophys. Res. Commun. 319, 138–143. doi: 10.1016/j.bbrc.2004.04.165
Rokem, J. S. (2007). “Industrial mycology,” in Biotechnology, eds H. Doelle and E. DaSilva (Oxford: Encyclopedia of Life Support Systems (EOLSS)). Avaiable online at: http://www.eolss.net/
Rosse, C., Formstecher, E., Boeckeler, K., Zhao, Y., Kremerskothen, J., White, M. D., et al. (2009). An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol. 7:e1000235. doi: 10.1371/journal.pbio.1000235
Roumanie, O., Wu, H., Molk, J. N., Rossi, G., Bloom, K., and Brennwald, P. (2005). Rho GTPase regulation of exocytosis in yeast is independent of GTP hydrolysis and polarization of the exocyst complex. J. Cell Biol. 170, 583–594. doi: 10.1083/jcb.200504108
Rounds, C. M., and Bezanilla, M. (2013). Growth mechanisms in tip-growing plant cells. Annu. Rev. Plant Biol. 64, 243–265. doi: 10.1146/annurev-arplant-050312-120150
Rustom, A., Saffrich, R., Markovic, I., Walther, P., and Gerdes, H. H. (2004). Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. doi: 10.1126/science.1093133
Rybak, K., Steiner, A., Synek, L., Klaeger, S., Kulich, I., Facher, E., et al. (2014). Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev. Cell 29, 607–620. doi: 10.1016/j.devcel.2014.04.029
Safavian, D., Zayed, Y., Indriolo, E., Chapman, L., Ahmed, A., and Goring, D. R. (2015). RNA silencing of exocyst genes in the stigma impairs the acceptance of compatible pollen in Arabidopsis. Plant Physiol. 169, 2526–2538. doi: 10.1104/pp.15.00635
Sanchez-Leon, E., and Riquelme, M. (2015). Live imaging of beta-1,3-glucan synthase FKS-1 in Neurospora crassa hyphae. Fungal Genet. Biol. 82, 104–107. doi: 10.1016/j.fgb.2015.07.001
Sanchez-Leon, E., Verdin, J., Freitag, M., Roberson, R. W., Bartnicki-Garcia, S., and Riquelme, M. (2011). Traffic of chitin synthase 1 (CHS-1) to the Spitzenkorper and developing septa in hyphae of Neurospora crassa: actin dependence and evidence of distinct microvesicle populations. Eukaryotic Cell 10, 683–695. doi: 10.1128/EC.00280-10
Schmidt, K. N., Kuhns, S., Neuner, A., Hub, B., Zentgraf, H., and Pereira, G. (2012). Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J. Cell Biol. 199, 1083–1101. doi: 10.1083/jcb.201202126
Scholey, J. M. (2003). Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19, 423–443. doi: 10.1146/annurev.cellbio.19.111401.091318
Schuster, M., Kilaru, S., Fink, G., Collemare, J., Roger, Y., and Steinberg, G. (2011). Kinesin-3 and dynein cooperate in long-range retrograde endosome motility along a nonuniform microtubule array. Mol. Biol. Cell 22, 3645–3657. doi: 10.1091/mbc.E11-03-0217
Seixas, C., Choi, S. Y., Polgar, N., Umberger, N. L., East, M. P., Zuo, X., et al. (2016). Arl13b and the exocyst interact synergistically in ciliogenesis. Mol. Biol. Cell 27, 308–320. doi: 10.1091/mbc.E15-02-0061
Shaheen, R., Faqeih, E., Alshammari, M. J., Swaid, A., Al-Gazali, L., Mardawi, E., et al. (2013). Genomic analysis of Meckel-Gruber syndrome in Arabs reveals marked genetic heterogeneity and novel candidate genes. Eur. J. Hum. Genet. 21, 762–768. doi: 10.1038/ejhg.2012.254
Sharifmoghadam, M. R., de Leon, N., Hoya, M., Curto, M. A., and Valdivieso, M. H. (2010). Different steps of sexual development are differentially regulated by the Sec8p and Exo70p exocyst subunits. FEMS Microbiol. Lett. 305, 71–80. doi: 10.1111/j.1574-6968.2010.01915.x
Shirakawa, R., and Horiuchi, H. (2015). Ral GTPases: crucial mediators of exocytosis and tumourigenesis. J. Biochem. 157, 285–299. doi: 10.1093/jb/mvv029
Skop, A. R., Liu, H., Yates, J. III, Meyer, B. J., and Heald, R. (2004). Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305, 61–66. doi: 10.1126/science.1097931
Sommer, B., Oprins, A., Rabouille, C., and Munro, S. (2005). The exocyst component Sec5 is present on endocytic vesicles in the oocyte of Drosophila melanogaster. J. Cell Biol. 169, 953–963. doi: 10.1083/jcb.200411053
Sorokin, S. (1962). Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363–377. doi: 10.1083/jcb.15.2.363
Spiczka, K. S., and Yeaman, C. (2008). Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J. Cell Sci. 121, 2880–2891. doi: 10.1242/jcs.031641
Stegmann, M., Anderson, R. G., Ichimura, K., Pecenkova, T., Reuter, P., Zarsky, V., et al. (2012). The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 24, 4703–4716. doi: 10.1105/tpc.112.104463
Steinberg, G. (2007). Hyphal growth: a tale of motors, lipids, and the Spitzenkorper. Eukaryotic Cell 6, 351–360. doi: 10.1128/EC.00381-06
Stuart, L. M., Boulais, J., Charriere, G. M., Hennessy, E. J., Brunet, S., Jutras, I., et al. (2007). A systems biology analysis of the Drosophila phagosome. Nature 445, 95–101. doi: 10.1038/nature05380
Sudbery, P. (2011). Fluorescent proteins illuminate the structure and function of the hyphal tip apparatus. Fungal Genet. Biol. 48, 849–857. doi: 10.1016/j.fgb.2011.02.004
Sugihara, K., Asano, S., Tanaka, K., Iwamatsu, A., Okawa, K., and Ohta, Y. (2002). The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat. Cell Biol. 4, 73–78. doi: 10.1038/ncb720
Synek, L., Schlager, N., Elias, M., Quentin, M., Hauser, M. T., and Zarsky, V. (2006). AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J. 48, 54–72. doi: 10.1111/j.1365-313X.2006.02854.x
Taheri-Talesh, N., Horio, T., Araujo-Bazan, L., Dou, X., Espeso, E. A., Penalva, M. A., et al. (2008). The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell 19, 1439–1449. doi: 10.1091/mbc.E07-05-0464
Takeshita, N., and Fischer, R. (2011). On the role of microtubules, cell end markers, and septal microtubule organizing centres on site selection for polar growth in Aspergillus nidulans. Fungal Biol. 115, 506–517. doi: 10.1016/j.funbio.2011.02.009
Takeshita, N., Higashitsuji, Y., Konzack, S., and Fischer, R. (2008). Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 19, 339–351. doi: 10.1091/mbc.E07-06-0523
Takeuchi, H., Furuta, N., Morisaki, I., and Amano, A. (2011). Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cell. Microbiol. 13, 677–691. doi: 10.1111/j.1462-5822.2010.01564.x
Tanaka, T., Iino, M., and Goto, K. (2014). Knockdown of Sec8 enhances the binding affinity of c-Jun N-terminal kinase (JNK)-interacting protein 4 for mitogen-activated protein kinase kinase 4 (MKK4) and suppresses the phosphorylation of MKK4, p38, and JNK, thereby inhibiting apoptosis. FEBS J. 281, 5237–5250. doi: 10.1111/febs.13063
Taylor, C. A., Yan, J., Howell, A. S., Dong, X., and Shen, K. (2015). RAB-10 regulates dendritic branching by balancing dendritic transport. PLoS Genet. 11:e1005695. doi: 10.1371/journal.pgen.1005695
TerBush, D. R., Maurice, T., Roth, D., and Novick, P. (1996). The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483–6494.
TerBush, D. R., and Novick, P. (1995). Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 130, 299–312. doi: 10.1083/jcb.130.2.299
Thapa, N., Sun, Y., Schramp, M., Choi, S., Ling, K., and Anderson, R. A. (2012). Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev. Cell 22, 116–130. doi: 10.1016/j.devcel.2011.10.030
Torres, M. J., Pandita, R. K., Kulak, O., Kumar, R., Formstecher, E., Horikoshi, N., et al. (2015). Role of the exocyst complex component Sec6/8 in genomic stability. Mol. Cell. Biol. 35, 3633–3645. doi: 10.1128/MCB.00768-15
UniProt Consortium (2015). UniProt: a hub for protein information. Nucleic Acids Res. 43, D204–D212. doi: 10.1093/nar/gku989
Vandre, D. D., Ackerman, W. E. IV, Tewari, A., Kniss, D. A., and Robinson, J. M. (2012). A placental sub-proteome: the apical plasma membrane of the syncytiotrophoblast. Placenta 33, 207–213. doi: 10.1016/j.placenta.2011.12.010
Vega, I. E., and Hsu, S.-C. (2001). The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. 21, 3839–3848.
Verdin, J., Bartnicki-Garcia, S., and Riquelme, M. (2009). Functional stratification of the Spitzenkorper of Neurospora crassa. Mol. Microbiol. 74, 1044–1053. doi: 10.1111/j.1365-2958.2009.06917.x
VerPlank, L., and Li, R. (2005). Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol. Biol. Cell 16, 2529–2543. doi: 10.1091/mbc.E04-12-1090
Vik-Mo, E. O., Oltedal, L., Hoivik, E. A., Kleivdal, H., Eidet, J., and Davanger, S. (2003). Sec6 is localized to the plasma membrane of mature synaptic terminals and is transported with secretogranin II-containing vesicles. Neuroscience 119, 73–85. doi: 10.1016/S0306-4522(03)00065-4
Wang, H., Tang, X., and Balasubramanian, M. K. (2003). Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe. Genetics 164, 1323–1331.
Wang, H., Tang, X., Liu, J., Trautmann, S., Balasundaram, D., McCollum, D., et al. (2002). The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 13, 515–529. doi: 10.1091/mbc.01-11-0542
Waters, A. M., and Beales, P. L. (2011). Ciliopathies: an expanding disease spectrum. Pediatr. Nephrol. 26, 1039–1056. doi: 10.1007/s00467-010-1731-7
Wen, T. J., Hochholdinger, F., Sauer, M., Bruce, W., and Schnable, P. S. (2005). The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol. 138, 1637–1643. doi: 10.1104/pp.105.062174
White, R. A., Boydston, L. A., Brookshier, T. R., McNulty, S. G., Nsumu, N. N., Brewer, B. P., et al. (2005). Iron metabolism mutant hbd mice have a deletion in Sec15l1, which has homology to a yeast gene for vesicle docking. Genomics 86, 668–673. doi: 10.1016/j.ygeno.2005.09.015
Wiederkehr, A., Du, Y., Pypaert, M., Ferro-Novick, S., and Novick, P. (2003). Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell 14, 4770–4782. doi: 10.1091/mbc.E03-04-0229
Wood, C. R., Huang, K., Diener, D. R., and Rosenbaum, J. L. (2013). The cilium secretes bioactive ectosomes. Curr. Biol. 23, 906–911. doi: 10.1016/j.cub.2013.04.019
Woolner, S., and Bement, W. M. (2009). Unconventional myosins acting unconventionally. Trends Cell Biol. 19, 245–252. doi: 10.1016/j.tcb.2009.03.003
Wu, H., Rossi, G., and Brennwald, P. (2008). The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 18, 397–404. doi: 10.1016/j.tcb.2008.06.007
Xiong, X., Xu, Q., Huang, Y., Singh, R. D., Anderson, R., Leof, E., et al. (2012). An association between type Igamma PI4P 5-kinase and Exo70 directs E-cadherin clustering and epithelial polarization. Mol. Biol. Cell 23, 87–98. doi: 10.1091/mbc.E11-05-0449
Yan, X., and Zhu, X. (2013). Branched F-actin as a negative regulator of cilia formation. Exp. Cell Res. 319, 147–151. doi: 10.1016/j.yexcr.2012.08.009
Yeaman, C., Grindstaff, K. K., and Nelson, W. J. (2004). Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J. Cell Sci. 117, 559–570. doi: 10.1242/jcs.00893
Zarsky, V., Kulich, I., Fendrych, M., and Pecenkova, T. (2013). Exocyst complexes multiple functions in plant cells secretory pathways. Curr. Opin. Plant Biol. 16, 726–733. doi: 10.1016/j.pbi.2013.10.013
Zhang, C., Brown, M. Q., van de Ven, W., Zhang, Z. M., Wu, B., Young, M. C., et al. (2016). Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc. Natl. Acad. Sci. U.S.A. 113, E41–E50. doi: 10.1073/pnas.1521248112
Zhang, X., Pumplin, N., Ivanov, S., and Harrison, M. J. (2015). EXO70I is required for development of a sub-domain of the periarbuscular membrane during arbuscular mycorrhizal symbiosis. Curr. Biol. 25, 2189–2195. doi: 10.1016/j.cub.2015.06.075
Zhang, Y., Immink, R., Liu, C. M., Emons, A. M., and Ketelaar, T. (2013). The Arabidopsis exocyst subunit SEC3A is essential for embryo development and accumulates in transient puncta at the plasma membrane. New Phytol. 199, 74–88. doi: 10.1111/nph.12236
Zhao, T., Rui, L., Li, J., Nishimura, M. T., Vogel, J. P., Liu, N., et al. (2015). A truncated NLR protein, TIR-NBS2, is required for activated defense responses in the exo70B1 mutant. PLoS Genet. 11:e1004945. doi: 10.1371/journal.pgen.1004945
Zhao, Y., Liu, J., Yang, C., Capraro, B. R., Baumgart, T., Bradley, R. P., et al. (2013). Exo70 generates membrane curvature for morphogenesis and cell migration. Dev. Cell 26, 266–278. doi: 10.1016/j.devcel.2013.07.007
Zou, W., Yadav, S., DeVault, L., Nung Jan, Y., and Sherwood, D. R. (2015). RAB-10-dependent membrane transport is required for dendrite arborization. PLoS Genet. 11:e1005484. doi: 10.1371/journal.pgen.1005484
Zuo, X., Guo, W., and Lipschutz, J. H. (2009). The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell 20, 2522–2529. doi: 10.1091/mbc.E08-07-0772
Keywords: exocyst complex, fungi, plants, mammals, pathogens
Citation: Martin-Urdiroz M, Deeks MJ, Horton CG, Dawe HR and Jourdain I (2016) The Exocyst Complex in Health and Disease. Front. Cell Dev. Biol. 4:24. doi: 10.3389/fcell.2016.00024
Received: 31 December 2015; Accepted: 11 March 2016;
Published: 12 April 2016.
Edited by:
Mary Munson, University of Massachusetts Medical School, USAReviewed by:
Viktor Zarsky, Charles University, Czech RepublicCopyright © 2016 Martin-Urdiroz, Deeks, Horton, Dawe and Jourdain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabelle Jourdain, aS5qb3VyZGFpbkBleGV0ZXIuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.