- Department of Biotechnology, Delft University of Technology, Delft, Netherlands
Isothermal titration calorimetry (ITC) is a popular chemical analysis technique that can be used to measure macromolecular interactions and chemical and physical processes. ITC involves the measurement of heat flow to and from a measurement cell after each injection during a titration experiment. ITC has been useful to measure the thermodynamics of macromolecular interactions such as protein-ligand or protein-protein binding affinity and also chemical processes such as enzyme catalyzed reactions. The use of ITC in biocatalysis has a number of advantages as ITC enables the measurement of enzyme kinetic parameters in a direct manner and, in principle, can be used for most enzymes and substrates. ITC approaches have been developed to measure reversible and irreversible enzyme inhibition, the effects of molecular crowding on enzyme activity, the activity of immobilized enzymes and the conversion of complex polymeric substrates. A disadvantage is that in order to obtain accurate kinetic parameters special care has to be taken in proper experimental design and data interpretation, which unfortunately is not always the case in reported studies. Furthermore, special caution is necessary when ITC experiments are performed that include solvents, reducing agents and may have side reactions. An important bottleneck in the use of calorimetry to measure enzyme activity is the relatively low throughput, which may be solved in the future by sensitive chip based microfluidic enzyme calorimetric devices.
Introduction
Calorimetry is the quantitative measurement of heat, heat flow and/or heat capacity, as part of a chemical or physical process. A calorimeter is a device to perform calorimetry. This can be done in different ways, such as a microcalorimeter or a differential scanning calorimeter. A very popular calorimeter that has found its way in many (bio)chemical labs since the early 2000s is the isothermal titration calorimeter (ITC). The popularity of the ITC is easily explained. The machine is easy to operate and provides information rich experimental data. But the simplicity can be somewhat misleading as experiments that are not well designed may lead to data that is wrongly interpreted.
The field of biocalorimetry has been dominated by the measurement of biological activity on complex substrates, e.g. microbial growth on wood materials (Wadsö et al., 2017). Such experiments allow the measurement of respiratory heat, and can be used to quantitatively monitor growth on such complex substrates. With the advent of sensitive calorimeters with smaller measurement cells more biological applications became feasible, such as the thermodynamic characterization of protein-DNA, protein-protein and protein-ligand interactions (Wiseman et al., 1989). Already in the early 1990s a number of examples of enzyme calorimetry were reported, such as the measurement of cytochrome c oxidase, acylase and nitrogenase (Watt, 1990; Morin and Freire, 1991; Williams and Toone, 1993). The measurement of enzyme kinetics using ITC became more popular after ITC instruments with small measurement cells became widely adopted. ITC can be used, in principle, as a generic technique to measure the enzyme kinetic parameters of most enzymes with most substrates.
The wide field involving ITC to study protein-protein or protein-ligand (metal ion, inhibitor, cofactor) interactions, thermodynamics of membrane proteins, as well as the study of protein stability are excluded from this review, but excellent reviews can be found elsewhere (Freire et al., 1990; Jelesarov and Bosshard, 1999; Ward and Holdgate, 2001; Holdgate, 2001; O'Brien and Haq, 2004; Grossoehme et al., 2010; Draczkowski, et al., 2014; Rajarathnam and Rösgen, 2014; Quinn, et al., 2016). A number of (tutorial) reviews on the study of enzyme kinetics using ITC are already available (Todd and Gomez, 2001; O'Brien and Haq, 2004; Bianconi, 2007; Demarse et al., 2013; Quinn and Hansen, 2019; Wang, et al., 2020; Ott, et al., 2021). The focus of this minireview is to cover developments in enzyme calorimetry that are relevant for the biocatalysis field.
Enzyme Calorimetry
Multi Injection Method
The most common way to measure enzyme kinetics using ITC is the multi injection method. The method is based on the addition of substrate aliquots to an enzyme solution in the ITC cell and to follow the heat flow to or from the measurement cell over time (Todd and Gomez, 2001; Bianconi, 2007). Since the heat transfer in the form of the ITC power is proportional to the rate of the reaction, the average of the power of the (shifted) baseline after each injection can be plotted versus the substrate concentration to result in a kinetic curve that in many cases will look like a Michaelis-Menten curve. In order to measure enzyme kinetics two different measurements have to be conducted. First the apparent molar enthalpy of the reaction has to be measured. This can be done by the conversion of a known amount of substrate using a relatively high enzyme concentration in order to allow the reaction to progress until completion or until the chemical equilibrium has been reached. The total heat change, which is equal to the area under the curve of an ITC thermogram can be used to obtain the apparent molar enthalpy using Eq. 1:
QITC is the total heat [Pfinal ] is the final product concentration. Vcell is the volume of the ITC measurement cell, and ΔHITC is the apparent molar enthalpy. It is important to realize that the determined apparent molar enthalpy is dependent on the experimental conditions, most notably the buffer and pH. The ionization enthalpy of the buffer will contribute to the total heat if the reaction involves protonation or deprotonation. It is therefore good practice to perform the measurement of the enthalpy and the enzyme activity measurement under the same conditions.
Secondly, the enzyme activity is recorded as the power of the shifted baseline after each injection of substrate using a relatively low enzyme concentration. By using the apparent molar enthalpy this observed power can be translated to the enzyme activity using Eqs 2, 3:
There are, however, several conditions that have to be met in order to obtain reliable kinetic parameters using this method. Unfortunately these conditions have not always been properly considered in literature, and the method is far too generally applied and too carelessly interpreted. For an example of a badly conducted ITC experiment (i.e., time between injections too short) one can look at in the article of Philpott and co-workers published in Science (Shi et al., 2008). Unfortunately, there are many more examples of published bad ITC data, and as a consequence thermodynamic and kinetic parameters in literature that are incorrect.
The first important factor that needs to be accounted for is the amount of consumed substrate during the reaction. Since a new aliquot of substrate is added with each injection and continuously substrate is consumed, the precise substrate concentration is always changing over time. This effect can be minimized by keeping the overall conversion during the reaction very low, e.g. below 5%. In that way the substrate concentration is always nearly equal to the added substrate and the rate can be assumed to reflect the initial rate. At the same time the product level has to be kept to a minimum, at least in cases where product inhibition might occur. The second problem is more fundamental, and it is due to the inherent sluggishness of the change of heat flow in a calorimeter. This effect, caused by the thermal inertia of the measurement solution and instrument components, is reflected by the instrumental time constant of the device. There are methods to correct the observed data using physical equations such as the Tian equation (Hofelich, et al., 2001; Wadsö, 2010; Jesús, et al., 2012). Alternatively one can choose to use long time lags between each injection (e.g., >10 times the time constant of the ITC), to ensure a stable heat flow is reached, and the time constant is not relevant anymore. The downside of this is that the measurement time will become very long.
Single Injection Method
The single injection method involves the slow long injection of substrate to an enzyme solution in the cell (Transtrum et al., 2015; Quinn and Hansen, 2019). The single injection replaces the multiple separate injections. Unfortunately this means that the substrate concentration is continuously changing, and proper correction of the time constant is essential to obtain reliable results. Depending on the response time of the instrument the measurement will be slow, e.g., circa 30 min or longer. An interesting possibility is to combine the enthalpy and the activity measurement in a single experiment by allowing the reaction to run until completion. For reliable kinetic parameters special care has to be taken to choose a substrate concentration sufficiently high above KM and a suitable enzyme concentration for a reaction that is sufficiently slow to minimize the effects of the response time and sufficiently fast to complete the conversion of all substrate in a reasonable time (up to several hours). Even so, the measurement will be very sensitive to product inhibition and to problems with enzymes stability. For enzyme activity measurements in general this method is not recommended.
Initial Rate Calorimetry Using the IrCal Method
An alternative to the methods in Multi Injection Method and Single Injection Method that circumvents the problems associated with the time constant of the instrument is the IrCal method. This method involves the apparent initial rate measurements where the inherent sluggishness is bypassed by observing the change of the power signal in the first moments after injection. This has to be done for each substrate concentration, so a downside is that multiple different measurements are necessary to obtain a kinetic curve, on the plus side each measurement is relatively short (circa 10 min, excluding equilibration time). The obtained kinetic data of a range of different enzymes have been found to match very well to data obtained using independent methods (Honarmand Ebrahimi, et al., 2015). The IrCal method is therefore the method of choice to obtain accurate kinetic parameters using ITC, although the multi-injection approach can be suitable if the response time of the instrument is properly considered.
The calorimetric assessment of lignin oxidation by different bacterial and fungal laccases was performed using the IrCal method (Figure 1) (A. Islam et al., 2022). This showed that the extent of lignin conversion, as measured from the apparent molar enthalpy, and the rate of that conversion, obtained from the power, showed large differences among the different enzymes. Furthermore, it showed that ITC can be used as global measurement tool of enzymatic lignin conversion, which allows the direct comparison of different enzyme variants and sources.
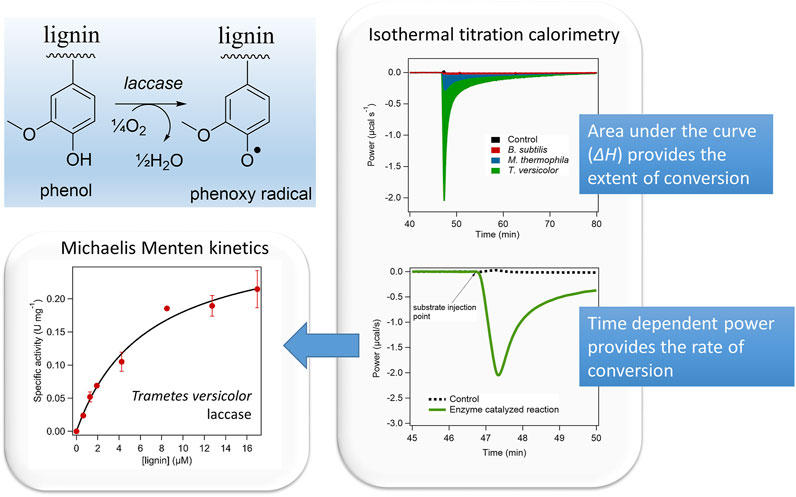
FIGURE 1. Isothermal titration calorimetry was used to assess the conversion of lignin by three different laccases. Isothermal Titration Calorimetry provides the molar enthalpy of the reaction, which reflects the extent of conversion and the time dependent power trace, which reflects the rate of the reaction. Large differences in the extent and kinetics of lignin conversion among the different bacterial and fungal laccases were found. Reprinted with permission from (Islam et al., 2022).
Applications of Enzyme Calorimetry
Enzyme Kinetics and Inhibition
Using the methods described in Enzyme Calorimetry it is possible to measure steady state kinetic parameters, such as KM and kcat. Specific applications of ITC to measure substrate or product inhibition have been explored. For example the product inhibition of RNase A by CMP has been determined using the change of the shape of the peak, characterized by the peak height, of sequential injections of the substrate cyclic CMP which is completely hydrolyzed after every injection (Cai, et al., 2001). The buildup of product slows down the reaction, which results in more shallow peaks that still have the same area under the curve. ITC has also been used to measure the action of reversible enzyme inhibitors by measuring enzyme kinetics in the presence of different inhibitor concentrations (Todd and Gomez, 2001). More conventionally ITC has been extensively used to measure binding thermodynamics of enzyme inhibitors (in the absence of substrates) (Weber and Salemme, 2003). A special application of ITC is to measure the effect of irreversible enzyme inhibitors by monitoring the time dependent change of the enzyme activity, which has been done for covalent inhibitors of prolyl oligopeptidase (Di Trani et al., 2018). In principle ITC can be used to measure the kinetics of the covalent modification of enzymes by the action of such inhibitors or by the change of residual enzyme activity during the reaction with an irreversible inhibitor.
Molecular Crowding
Enzyme kinetics is usually measured in vitro using dilute aqueous solutions, which is a very different environment as compared to the living cell (Lonhienne and Winzor, 2004; Maximova, et al., 2019). The total protein concentration in Escherichia coli is 230 mg ml−1 cell volume, and more generally the total protein is circa 20–30% of w/v for different prokaryotic or eukaryotic cell types (Brown, 1991). It is therefore interesting to measure enzyme properties in the presence of macromolecular crowders to better reflect the conditions of the cell. As such conditions may interfere with the UV-vis or fluorescence readout, ITC offers an alternative as it does not require optical transparency. Bovine serum albumin (BSA) or polyethylene glycol (PEG) have frequently been used as molecular crowders. The observed kcat and KM values of hexokinase in the presence of 250 mg ml−1 BSA were circa 20–30% lower and the ΔHr was 14% more negative for the crowded solution as compared to the dilute solution without BSA (Olsen, 2006).
Activity of Immobilized Enzymes
For ITC it is not necessary that the enzyme is in solution, although it has to be in a suspension that can be stirred in the calorimeter. The main boundary condition is that the enzyme and substrate need to be injectable into and removable from the ITC measurement cell. In practice this means that the particle size has to be below a few millimeter. Enzyme kinetic parameters were determined using ITC (multi injection approach) on beta-glucosidase immobilized on spherical polyelectrolyte brushes (Henzler, et al., 2008). Using this approach it was possible to measure apparent kinetic parameters for the immobilized enzyme with a 2-nitrophenol labelled glucose or the disaccharide cellobiose as substrates.
Difficult Substrates
Poorly soluble or complex polymeric substrates are generally very difficult to measure using conventional (e.g., UV-vis based) enzyme assays. Direct chemical analysis can be difficult in the case of polymeric substrates and products, e.g., in the case of biomass derived lignin. Here ITC can offer a pragmatic solution by measuring the overall conversion of lignin by an enzyme. This has been done for the lignin conversion by different laccase variants and showed that the extent of conversion and the rate of that conversion can be determined (Figure 1), although the precise molecular nature of the products needs to be determined independently (Islam et al., 2022).
Coupled Reactions
The power signal observed with ITC is dependent on the rate and on the reaction enthalpy. If the enzyme reaction is slow and/or has a modest reaction enthalpy it can be attractive to add an enzyme to convert the product quickly and with a high reaction enthalpy. Such coupled enzyme reactions can be used to enhance calorimetric response and measure calorimetry silent reactions. For example the ITC response for the xylanase kinetics was enhanced by using a coupled assay with carbohydrate oxidase and catalase (Baumann et al., 2011). The polymeric xylan was used as a substrate, which results in a polymer with reducing sugar groups which were oxidized using carbohydrate oxidase and O2 resulting in hydrogen peroxide that was disproportionated using catalase. As for any coupled assay it will be important to optimize the amounts of the coupling enzymes to ensure the rate is only limited by the first enzyme.
Limitations of Enzyme Calorimetry
Can all Reactions be Measured?
Many enzymes and substrates can be measured using ITC (Table 1), however, not all enzyme reactions can be measured. Reactions in which there is no net change of bond order, e.g., for many isomerases, the reaction enthalpy change will be small. As a consequence such reactions cannot be directly measured, which is also exemplified by the almost complete absence of reports on enzyme calorimetry of isomerases. However, using an enzymatic or chemical coupled reaction that is product specific, this limitation can be circumvented. Previously Todd and Gomez reported activity of chaperonin subunit GroEL as a representative of the isomerase enzyme classification, which is misleading as the measured activity was ATP hydrolysis, and not the protein refolding which is chaperonin catalyzed (Todd and Gomez, 2001). An example of a true isomerase reaction measured with ITC is the activity of l-rhamnose isomerase, which catalyzes the conversion of l-rhamnose to l-rhamnulose (Bai et al., 2015). The activity with different substrates was measured using the multi injection and the single injection method. Unfortunately these experiments have not been well conducted so the obtained kinetic parameters are not reliable. However, there is a clear enzyme reaction observed and interestingly large differences in the apparent ΔHr with different substrates of the enzyme were observed. The “calorimetry silent” reactions can still be interesting, as in such cases binding of equilibrium substrate/product mixtures is possible. This can provide interesting complimentary information to kinetic data that was independently determined.
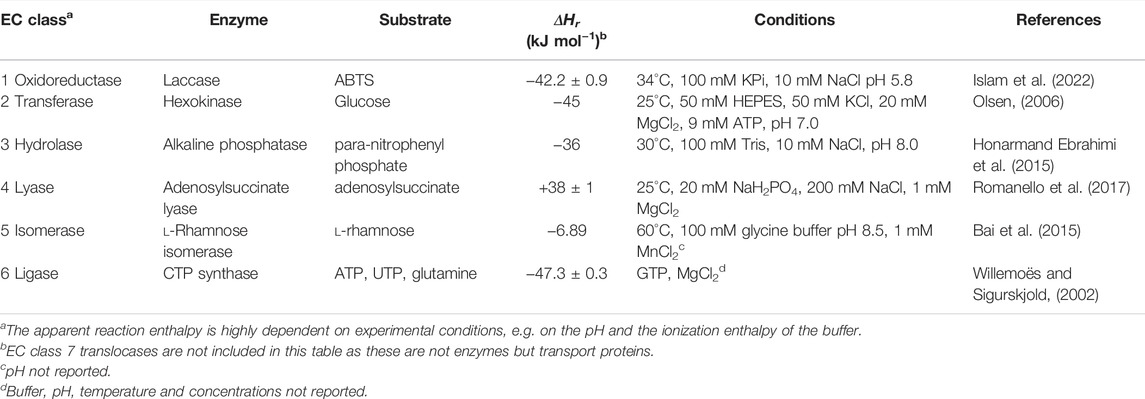
TABLE 1. The universality of enzyme calorimetry characterized by the apparent reaction enthalpies for enzyme reactions observed with ITC.
Solvents
Dilution of solvents in aqueous solutions will give very large calorimetric responses, which means that for ITC experiments both substrate and enzyme solutions should have identical solvent concentrations. For example the heat of dilution of ethanol in water is ΔHsoln = −10.1 kJ mol−1 at 25°C (Alexander and Hill, 1969). The dilution of an aqueous solution of methanol in water is a common positive control measurement to check if the ITC is performing well. If a substrate requires a co-solvent, the same co-solvent has to be present in the same concentration in the cell with the enzyme as well. This can give problems if the enzyme is not stable under such conditions. In order to perform ITC measurements using an organic solvent instead of water it is important to have the same solvent in the reference cell as well. Otherwise large differences in heat capacity of the solutions in the measurement and reference cell will lead to unreliable results.
Conditions
Although, this is not only true for calorimetry, experimental conditions under which the enzyme properties are measured have to be precisely reported and unless these conditions (such as temperature and pH) are included in the rate equations the reported kinetic parameters are highly conditional. ITC offers the advantage that the temperature control during the measurements is superior to most other used techniques. Reducing agents such as DTT are not chemically stable, and therefore produce significant background signals when used in ITC experiment. TCEP can be used instead of DTT or β-mercaptoethanol. In general it is recommended to avoid compounds that are not chemically stable in the measurement time of the experiment, and if their use cannot be prevented the concentration has to be kept to a minimum. Enzyme (usually in the cell) and substrate (usually in the syringe) has to be in solutions with preferably identical composition such as buffer concentration, pH and salt concentration. This is a downside as the enzyme assay conditions are not always the best conditions for enzyme stability.
Side-Reactions
Although calorimetry is an attractive technique as it can be used to measure many different chemical reactions, this also means that side reactions that are sufficiently fast and have a sufficient enthalpy change are observed together with the target reaction. An example of this is the measurement of different apparent reaction enthalpy values (ΔHr = −5.1 ± 0.2 and −3.3 ± 0.3) for the conversion of glucose and ATP to glucose-6-phosphate and ADP by two different yeast hexokinase isoenzymes (Bianconi, 2003). The reaction enthalpy should not be dependent on the type of enzyme, but only on the chemical reaction and physical conditions. In this case apparently the reaction is not exactly the same for both enzymes, which has been attributed to difference in the side reactions that may be catalyzed by each enzyme, such as ATP hydrolysis or protein phosphorylation.
Outlook
Because calorimetry is inherently slow it has been difficult to increase the throughput for biological calorimetric measurements. The key to increase the throughput is through miniaturization and using microfluidics. The development of miniaturized sensitive calorimeters has produced the field chip calorimetry (Lerchner et al., 2006; Lerchner, et al., 2008; Hartmann et al., 2014; van Schie, et al., 2018). Microfluidic chips in which calorimetric devices are integrated can be used to measure chemical reactions with a very short response time. The sensitivity of calorimetric devices has so far limited the applications of such chip based calorimetry devices to processes that will generate a large heat flow. The real breakthrough has to come from more sensitive devices that can record temperature changes in the sub-millikelvin range to allow measurement of enzyme reaction in microliter scale volumes. Besides the problem with sensitivity, mixing is also a bottleneck that awaits adequate technical solutions. In general turbulent mixers are incompatible with sensitive calorimetric devices as the friction heat will disturb the measurements. Diffusive mixing may therefore be preferred, but this requires very short diffusion distances in the nanometer range. Unfortunately to our knowledge no reported chip calorimeter designs has tackled all these technical problems. Enthalpy arrays have been successful to increase the throughput by performing many measurements in parallel in small volumes, although rigorous analysis of the kinetic data, high sensitivity and fast mixing are still challenging (Recht et al., 2008). Therefore sensitive and fast high throughput enzyme calorimetry is still a dream to be fulfilled. I envision that hyphenation of a chip based calorimetry device to powerful chemical analytical techniques such as mass spectrometry will produce a superior enzyme functional analysis method by combining direct measurement of the rate and the identification of the reaction products. This will enable breakthroughs in biocatalysis that are necessary to develop a more sustainable chemical industry.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alexander, D., and Hill, D. (1969). The Heats of Solution of Alcohols in Water. Aust. J. Chem. 22 (2), 347–356. doi:10.1071/CH9690347
Bai, W., Shen, J., Zhu, Y., Men, Y., Sun, Y., and Ma, Y. (2015). Characteristics and Kinetic Properties of L-Rhamnose Isomerase from Bacillus Subtilis by Isothermal Titration Calorimetry for the Production of D-Allose. Fstr 21 (1), 13–22. doi:10.3136/fstr.21.13
Baumann, M. J., Murphy, L., Lei, N., Krogh, K. B. R. M., Borch, K., and Westh, P. (2011). Advantages of Isothermal Titration Calorimetry for Xylanase Kinetics in Comparison to Chemical-Reducing-End Assays. Anal. Biochem. 410 (1), 19–26. doi:10.1016/j.ab.2010.11.001
Bianconi, M. L. (2003). Calorimetric Determination of Thermodynamic Parameters of Reaction Reveals Different Enthalpic Compensations of the Yeast Hexokinase Isozymes. J. Biol. Chem. 278 (21), 18709–18713. doi:10.1074/jbc.M211103200
Bianconi, M. L. (2007). Calorimetry of Enzyme-Catalyzed Reactions. Biophys. Chem. 126, 59–64. doi:10.1016/j.bpc.2006.05.017
Brown, G. C. (1991). Total Cell Protein Concentration as an Evolutionary Constraint on the Metabolic Control Distribution in Cells. J. Theor. Biol. 153 (2), 195–203. doi:10.1016/S0022-5193(05)80422-9
Cai, L., Cao, A., and Lai, L. (2001). An Isothermal Titration Calorimetric Method to Determine the Kinetic Parameters of Enzyme Catalytic Reaction by Employing the Product Inhibition as Probe. Anal. Biochem. 299 (1), 19–23. doi:10.1006/abio.2001.5397
Demarse, N. A., Killian, M. C., Hansen, L. D., and Quinn, C. F. (2013). “Determining Enzyme Kinetics via Isothermal Titration Calorimetry,” in Enzyme Engineering: Methods and Protocols. Editor J. C. Samuelson (Totowa, NJ: Humana Press), 21–30. doi:10.1007/978-1-62703-293-3_2
Di Trani, J. M., De Cesco, S., O’Leary, R., Plescia, J., do Nascimento, C. J., Moitessier, N., et al. (2018). Rapid Measurement of Inhibitor Binding Kinetics by Isothermal Titration Calorimetry. Nat. Commun. 9 (1), 893. doi:10.1038/s41467-018-03263-3
Draczkowski, P., Matosiuk, D., and Jozwiak, K. (2014). Isothermal Titration Calorimetry in Membrane Protein Research. J. Pharm. Biomed. Analysis 87, 313–325. doi:10.1016/j.jpba.2013.09.003
Freire, E., Mayorga, O. L., and Straume, M. (1990). Isothermal Titration Calorimetry. Anal. Chem. 62 (18), 950A–959A. doi:10.1021/ac00217a002
Grossoehme, N. E., Spuches, A. M., and Wilcox, D. E. (2010). Application of Isothermal Titration Calorimetry in Bioinorganic Chemistry. J. Biol. Inorg. Chem. 15 (8), 1183–1191. doi:10.1007/s00775-010-0693-3
Hartmann, T., Barros, N., Wolf, A., Siewert, C., Volpe, P. L. O., Schemberg, J., et al. (2014). Thermopile Chip Based Calorimeter for the Study of Aggregated Biological Samples in Segmented Flow. Sensors Actuators B Chem. 201, 460–468. doi:10.1016/j.snb.2014.05.024
Henzler, K., Haupt, B., and Ballauff, M. (2008). Enzymatic Activity of Immobilized Enzyme Determined by Isothermal Titration Calorimetry. Anal. Biochem. 378 (2), 184–189. doi:10.1016/j.ab.2008.04.011
Hofelich, T., Wadsö, L., Smith, A. L., Shirazi, H., and Mulligan, S. R. (2001). The Isothermal Heat Conduction Calorimeter: A Versatile Instrument for Studying Processes in Physics, Chemistry, and Biology. J. Chem. Educ. 78 (8), 1080. doi:10.1021/ed078p1080
Holdgate, G. A. (2001). Making Cool Drugs Hot: Isothermal Titration Calorimetry as a Tool to Study Binding Energetics. Biotechniques 31 (1), 164-passim.
Honarmand Ebrahimi, K., Hagedoorn, P.-L., Jacobs, D., and Hagen, W. R. (2015). Accurate Label-free Reaction Kinetics Determination Using Initial Rate Heat Measurements. Sci. Rep. 5 (1), 16380. doi:10.1038/srep16380
Islam, S. T. A., Zhang, J., Tonin, F., Hinderks, R., Deurloo, Y. N., Urlacher, V. B., et al. (2022). Isothermal Titration Calorimetric Assessment of Lignin Conversion by Laccases. Biotech Bioeng. 119 (2), 493–503. doi:10.1002/bit.27991
Jelesarov, I., and Bosshard, H. R. (1999). Isothermal Titration Calorimetry and Differential Scanning Calorimetry as Complementary Tools to Investigate the Energetics of Biomolecular Recognition. J. Mol. Recognit. 12 (1), 3–18. doi:10.1002/(sici)1099-1352(199901/02)12:1<3::aid-jmr441>3.0.co;2-6
Jesús, C., Socorro, F., and Rodríguez de Rivera, M. (2012). New Approach to Tian's Equation Applied to Heat Conduction and Liquid Injection Calorimeters. J. Therm. Anal. Calorim. 110 (3), 1523–1532. doi:10.1007/s10973-011-2117-1
Lerchner, J., Maskow, T., and Wolf, G. (2008). Chip Calorimetry and its Use for Biochemical and Cell Biological Investigations. Chem. Eng. Process. Process Intensif. 47, 991–999. doi:10.1016/j.cep.2007.02.014
Lerchner, J., Wolf, A., Wolf, G., Baier, V., Kessler, E., Nietzsch, M., et al. (2006). A New Micro-fluid Chip Calorimeter for Biochemical Applications. Thermochim. Acta 445, 144–150. doi:10.1016/j.tca.2005.07.011
Lonhienne, T. G. A., and Winzor, D. J. (2004). A Potential Role for Isothermal Calorimetry in Studies of the Effects of Thermodynamic Non-ideality in Enzyme-Catalyzed Reactions. J. Mol. Recognit. 17, 351–361. doi:10.1002/jmr.706
Maximova, K., Wojtczak, J., and Trylska, J. (2019). Enzyme Kinetics in Crowded Solutions from Isothermal Titration Calorimetry. Anal. Biochem. 567, 96–105. doi:10.1016/j.ab.2018.11.006
Morin, P. E., and Freire, E. (1991). Direct Calorimetric Analysis of the Enzymic Activity of Yeast Cytochrome C Oxidase. Biochemistry 30 (34), 8494–8500. doi:10.1021/bi00098a030
O'Brien, R., and Haq, I. (2004). “Applications of Biocalorimetry: Binding, Stability and Enzyme Kinetics,” in Biocalorimetry 2: Applications of Calorimetry in the Biological Sciences. Editors J. E. Ladbury, and M. L. Doyle (Hoboken, NJ: John Wiley & Sons), 1–34. doi:10.1002/0470011122.ch1
Olsen, S. N. (2006). Applications of Isothermal Titration Calorimetry to Measure Enzyme Kinetics and Activity in Complex Solutions. Thermochim. Acta 448 (1), 12–18. doi:10.1016/j.tca.2006.06.019
Ott, F., Rabe, K. S., Niemeyer, C. M., and Gygli, G. (2021). Toward Reproducible Enzyme Modeling with Isothermal Titration Calorimetry. ACS Catal. 11 (17), 10695–10704. doi:10.1021/acscatal.1c02076
Quinn, C. F., Carpenter, M. C., Croteau, M. L., and Wilcox, D. E. (2016). Isothermal Titration Calorimetry Measurements of Metal Ions Binding to Proteins. Meth. Enzymol. 567, 3–21. doi:10.1016/bs.mie.2015.08.021
Quinn, C. F., and Hansen, L. D. (2019). “Enzyme Kinetics Determined by Single-Injection Isothermal Titration Calorimetry,” in Microcalorimetry of Biological Molecules: Methods and Protocols. Editor E. Ennifar (New York, NY: Springer New York), 241–249. doi:10.1007/978-1-4939-9179-2_17
Rajarathnam, K., and Rösgen, J. (2014). Isothermal Titration Calorimetry of Membrane Proteins - Progress and Challenges. Biochimica Biophysica Acta (BBA) - Biomembr. 1838 (1), 69–77. doi:10.1016/j.bbamem.2013.05.023
Recht, M. I., De Bruyker, D., Bell, A. G., Wolkin, M. V., Peeters, E., Anderson, G. B., et al. (2008). Enthalpy Array Analysis of Enzymatic and Binding Reactions. Anal. Biochem. 377, 33–39. doi:10.1016/j.ab.2008.03.007
Romanello, L., Serrão, V. H. B., Torini, J. R., Bird, L. E., Nettleship, J. E., Rada, H., et al. (2017). Structural and Kinetic Analysis of Schistosoma Mansoni Adenylosuccinate Lyase ( Sm ADSL). Mol. Biochem. Parasitol. 214, 27–35. doi:10.1016/j.molbiopara.2017.03.006
Shi, H., Bencze, K. Z., Stemmler, T. L., and Philpott, C. C. (2008). A Cytosolic Iron Chaperone that Delivers Iron to Ferritin. Science 320 (5880), 1207–1210. doi:10.1126/science.1157643
Todd, M. J., and Gomez, J. (2001). Enzyme Kinetics Determined Using Calorimetry: A General Assay for Enzyme Activity? Anal. Biochem. 296, 179–187. doi:10.1006/abio.2001.5218
Transtrum, M. K., Hansen, L. D., and Quinn, C. (2015). Enzyme Kinetics Determined by Single-Injection Isothermal Titration Calorimetry. Methods 76, 194–200. doi:10.1016/j.ymeth.2014.12.003
van Schie, M. M. C. H., Ebrahimi, K. H., Hagen, W. R., and Hagedoorn, P.-L. (2018). Fast and Accurate Enzyme Activity Measurements Using a Chip-Based Microfluidic Calorimeter. Anal. Biochem. 544, 57–63. doi:10.1016/j.ab.2017.12.028
Wadsö, L., Johansson, S., and Bardage, S. (2017). Monitoring of Fungal Colonization of Wood Materials Using Isothermal Calorimetry. Int. Biodeterior. Biodegrad. 120, 43–51. doi:10.1016/j.ibiod.2017.02.003
Wadsö, L. (2010). Operational Issues in Isothermal Calorimetry. Cem. Concr. Res. 40 (7), 1129–1137. doi:10.1016/j.cemconres.2010.03.017
Wang, Y., Wang, G., Moitessier, N., and Mittermaier, A. K. (2020). Enzyme Kinetics by Isothermal Titration Calorimetry: Allostery, Inhibition, and Dynamics. Front. Mol. Biosci. 7, 583826. doi:10.3389/fmolb.2020.583826
Ward, W. H. J., and Holdgate, G. A. (2001). Isothermal Titration Calorimetry in Drug Discovery. Prog. Med. Chem. 38, 309–376. doi:10.1016/s0079-6468(08)70097-3
Watt, G. D. (1990). A Microcalorimetric Procedure for Evaluating the Kinetic Parameters of Enzyme-Catalyzed Reactions: Kinetic Measurements of the Nitrogenase System. Anal. Biochem. 187 (1), 141–146. doi:10.1016/0003-2697(90)90432-9
Weber, P. C., and Salemme, F. R. (2003). Applications of Calorimetric Methods to Drug Discovery and the Study of Protein Interactions. Curr. Opin. Struct. Biol. 13 (1), 115–121. doi:10.1016/S0959-440X(03)00003-4
Willemoës, M., and Sigurskjold, B. W. (2002). Steady-state Kinetics of the Glutaminase Reaction of CTP Synthase from Lactococcus Lactis. Eur. J. Biochem. 269 (19), 4772–4779. doi:10.1046/j.1432-1033.2002.03175.x
Williams, B. A., and Toone, E. J. (1993). Calorimetric Evaluation of Enzyme Kinetic Parameters. J. Org. Chem. 58 (13), 3507–3510. doi:10.1021/jo00065a010
Keywords: isothermal titration calorimetry, biocatalysis, enzyme kinetics, enzyme inhibition, reaction enthalpy
Citation: Hagedoorn P-L (2022) Isothermal Titration Calorimetry in Biocatalysis. Front. Catal. 2:906668. doi: 10.3389/fctls.2022.906668
Received: 28 March 2022; Accepted: 21 April 2022;
Published: 10 May 2022.
Edited by:
Felix Studt, Karlsruhe Institute of Technology (KIT), GermanyReviewed by:
José Cleiton Sousa dos Santos, University of International Integration of Afro-Brazilian Lusophony, BrazilCopyright © 2022 Hagedoorn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter-Leon Hagedoorn, cC5sLmhhZ2Vkb29ybkB0dWRlbGZ0Lm5s
 Peter-Leon Hagedoorn
Peter-Leon Hagedoorn