- 1Department of Cardio-thoracic Surgery, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
- 2Department of Cardio-Thoracic Surgery, Nanjing Drum Tower Hospital, Clinical College of Nanjing University of Chinese Medicine, Nanjing, China
Observational studies and randomised controlled trials (RCTs) have yielded conflicting results regarding the outcomes of multiple arterial grafts (MAG) vs. single arterial grafts (SAG) in coronary artery bypass graft (CABG) surgery. We conducted a comprehensive search across multiple databases for RCTs that directly compared MAG and SAG. The clinical outcomes assessed included all-cause mortality, cardiac-specific mortality, myocardial infarction (MI), repeat revascularization, stroke, sternal wound complications, and major bleeding. Outcomes were measured using hazard ratios (HR), relative risks (RR), and the corresponding 95% confidence intervals (CI). Eighteen RCTs involving 10,143 patients were included in the analysis. The follow-up period ranged from 6 months to 12.6 years, and the average age of the patients across the studies ranged between 56.3 and 77.3 years. MAG and SAG did not differ significantly in terms of the incidence of sternal wound complications, major bleeding, or stroke following CABG. However, the MAG group demonstrated a lower risk of all-cause mortality, cardiac mortality, MI, and repeat revascularization compared with the SAG group. MAG was associated with higher survival, lower risk of MI, and fewer repeat revascularization. Nonetheless, there were no significant differences in the incidence of sternal wound infections, major bleeding, and stroke between MAG and SAG.
Introduction
Coronary artery bypass grafting (CABG) is the surgical treatment of choice for severe multivessel coronary artery disease (CAD). Single arterial grafting (SAG) is the standard of care for patients undergoing CABG. Nonetheless, multiple arterial graft (MAG) is gaining popularity (1, 2), and there is debate regarding what patients benefit from MAG.
Revascularization with a left internal thoracic artery (LITA) graft to the left anterior descending artery is well established (1, 2). The radial artery (RA), right internal thoracic artery (RITA), and saphenous vein (SV) are grafts routinely used as the second conduit. Although a post hoc analysis of the SYNTAXES trial demonstrated the superiority of MAG over SAG for patients undergoing CABG (3), several surgeons favour the use of the SV because RCTs have failed to demonstrate a survival benefit of MAG over SAG, despite differences in vessel patency (4, 5). Possible reasons for this finding include underpowered studies (6) or inconclusive results due to discrepancies between the treatment allocated and the treatment received in the Arterial Revascularization Trial (ART) (5). To overcome the limitations of the ART, the ROMA trial (7), which is underway, will compare all MAG approaches and SAG without imposing on the surgeon which graft configuration should be adopted.
Although previous meta-analyses demonstrated the survival benefit of MAG, most included studies analysed observational data. Further, other studies evaluated single MAG configurations (8), or failed to provide as-treated data (9), or did not perform sensitivity analyses (10), or did not evaluate clinical outcomes such as wound infections, stroke, and myocardial infarction (MI) (9).
This study conducted a meta-analysis of RCTs, including all MAG approaches—bilateral internal thoracic artery (BITA), left internal thoracic artery (LITA)+RA, and BITA/LITA+RA)—to comprehensively and systematically compare the clinical outcomes of MAG and SAG. The primary outcomes were long-term survival and cardiac mortality. The secondary endpoints were repeat revascularization and MI. Postoperative complications included sternal wound infections (SWIs), stroke, and bleeding.
Results
Study selection and baseline characteristics
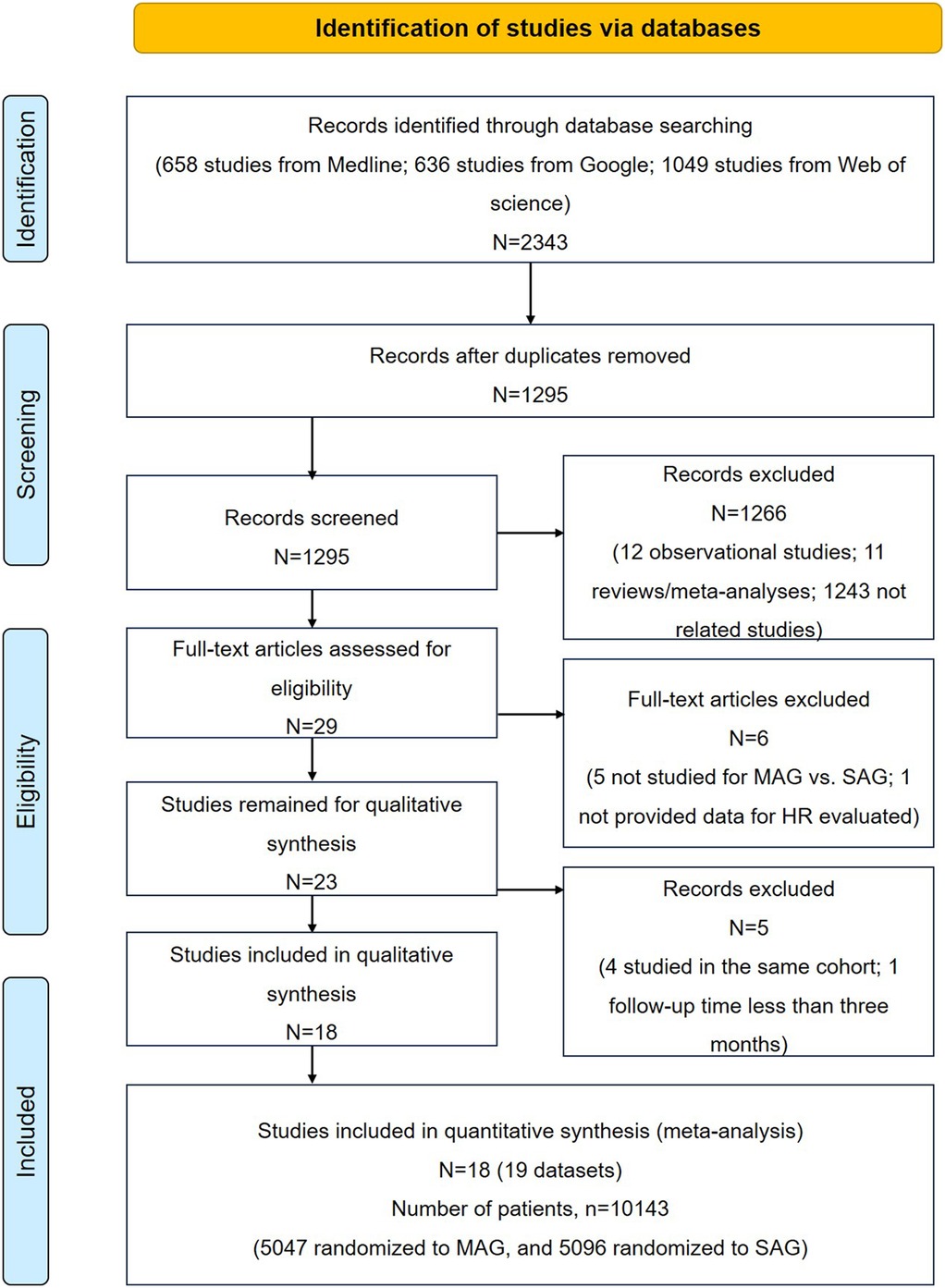
The study selection process is illustrated in Figure. 1. A total of 2,343 articles were initially included after searching PubMed, Google, and Web of Science databases. After removing 1,048 repetitive articles, we further excluded 1,266 records based on the title and abstract, including 12 observational reports, 11 reviews/meta-analyses, and 1,237 unrelated articles. Among the remaining 29 records, five articles did not compare MAG and SAG; one articles did not provide the estimated HR; four studies researched the same cohort; one study had a follow-up period of less than 3 months. Thus, 18 studies were included in this meta-analysis. Of which, sixteen was RCT studies (4, 6, 11–24), and two studies were post hoc analyses of observational data from randomised trials that evaluated alternative treatments, including the subsets from SYNTAX trail cited by Thuijs (3) and cited by EXCEL trials Thuijs (25). Given that both subsets were relevant to our research aim, and two previously comprehensive meta-analyses of RCTs, conducted by Changal et al. (26) and Magouliotis et al. (9), also included these two subsets as RCT group. Thus, we decided to incorporate these subsets into our meta-analysis.
The included studies were published from 1990 to 2022 and were conducted in the UK (19), Italy (21), Australia (4, 22), USA (3, 6, 17, 20, 23–25), Canada (15), Russia (12), Korea (13, 16), Denmark (18), Serbia (14), and multinational (11). The total sample size was 10,143 patients (MAG: 5,047; SAG: 5,096). Among the 5,047 patients assigned to the MAG group, 1,440 underwent RITA and LITA grafting, 850 underwent RA and LITA grafting, and 2,757 underwent RA/RITA and LITA grafting. The LITA to the LAD approach was used in all patients. The average age ranged from 56.3 to 77.3 years. Among these studies, some of them provided data on gender and comorbidities (Table 1; Supplementary Table S5). Among these studies, the sex distribution and the proportion of preoperative complications such as hypertension, diabetes mellitus, previous MI and COPD was similar between the two groups. The MAG group has a lower prevalence of obesity and peripheral arterial disease, but a higher incidence of hyper lipidaemia (Supplementary Table S6). The number of grafted vessels was similar across the groups. A total of 2,111 patients (20.8%) underwent off-pump CABG, with a similar distribution between the MAG and SAG groups. The mean follow-up period ranged from 0.5 to 12.6 years. Nine studies (2,234 MAG, 2,081 SAG) had follow-up periods of less than 5 years, six studies (836 MAG, 818 SAG) had follow-up periods of 5–10 years, and three studies (1,978 MAG, 2,197 SAG) had follow-up periods longer than 10 years. There may be some biases owing to patient selection, interventions, and outcomes reporting in our study. The assessment of the risk of bias is shown in Supplementary Figure S1.
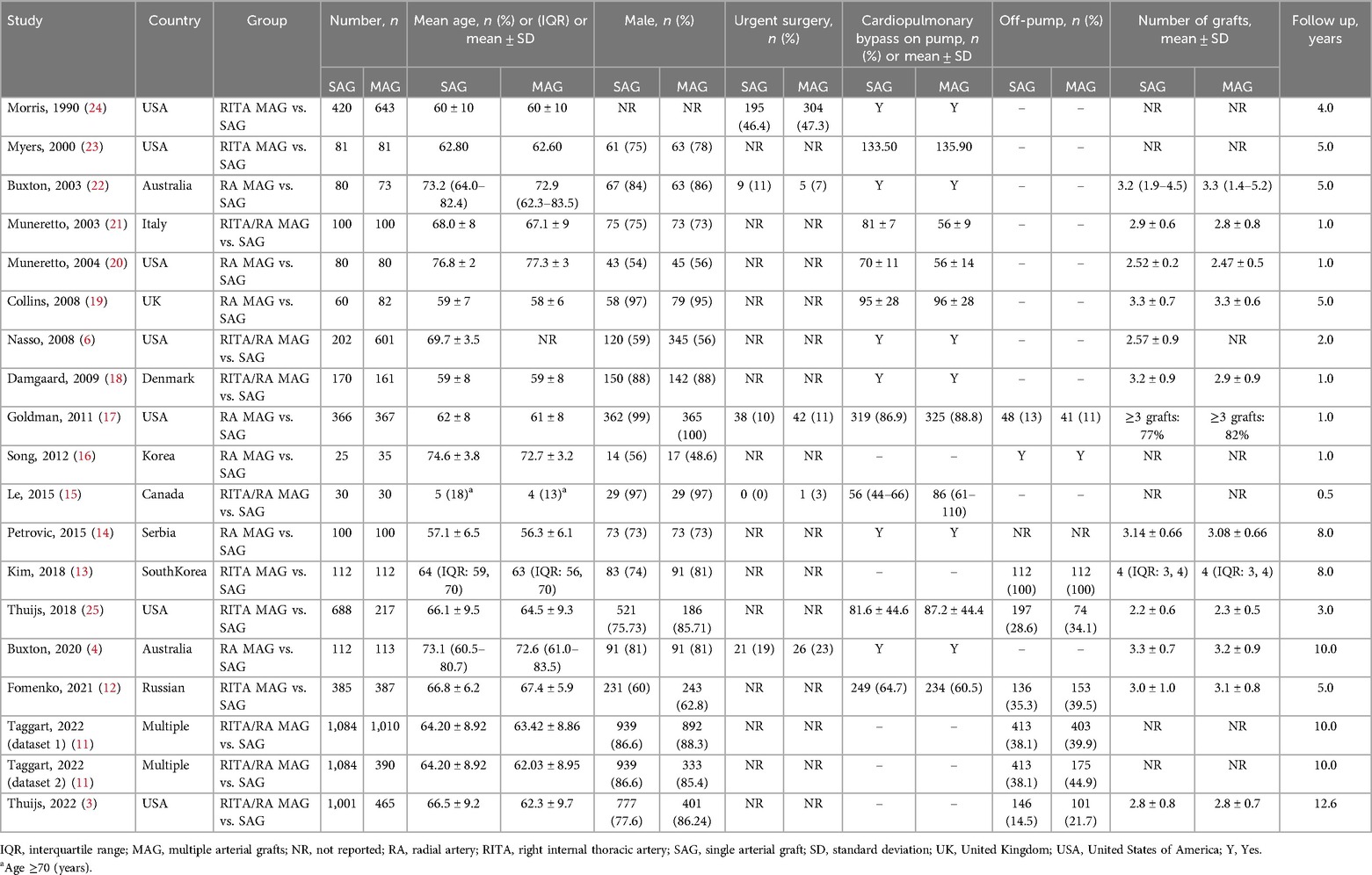
Table 1. Baseline characteristics of the studies included in the systematic review and meta-analysis.
Primary endpoints
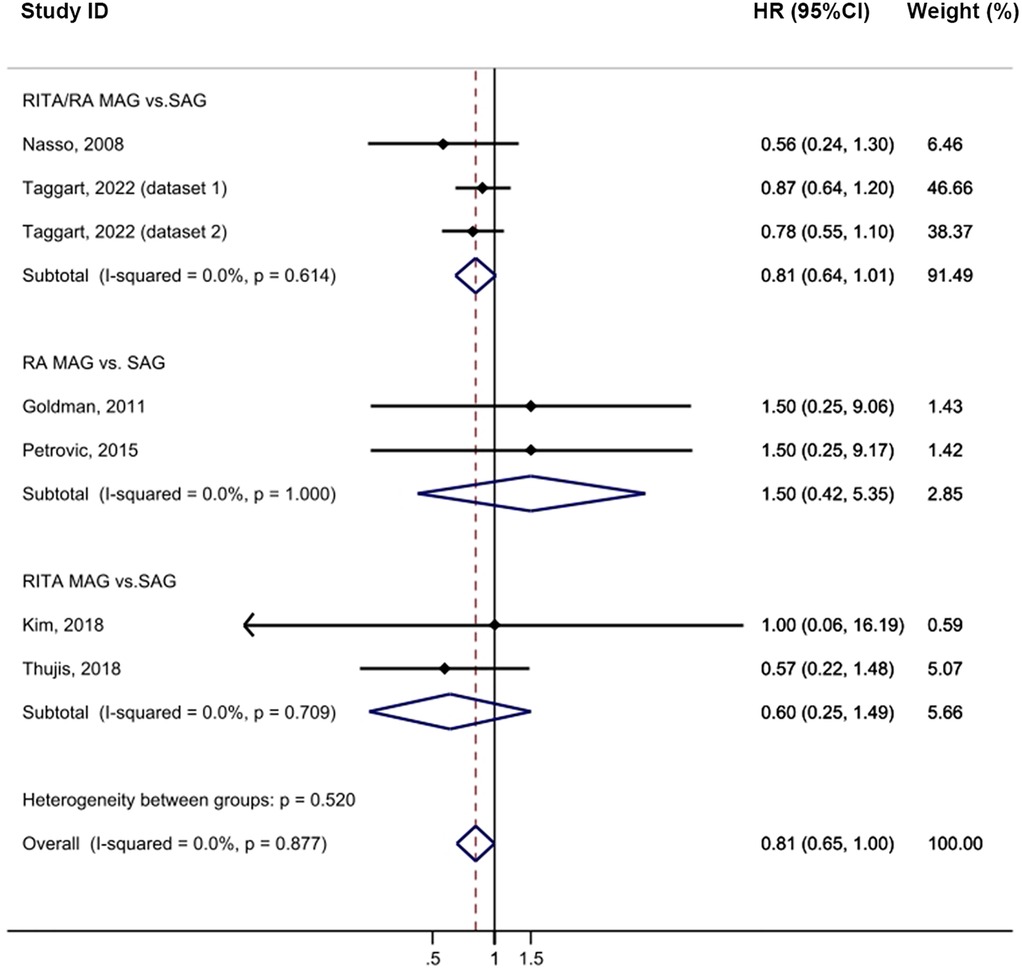
In the intention-to-treat analysis, MAG demonstrated a significant improvement in long-term survival compared to SAG [HR = 0.83, 95% confidence interval (CI) = 0.73–0.94, p = 0.004; Figure 2] with low heterogeneity observed (I2 = 0%). The funnel plot did not reveal any publication bias among the studies (Supplementary Figure S2A). Prespecified subgroup analyses identified a notable difference only in the RITA/RA vs. SAG group (HR = 0.77, 95% CI = 0.67–0.89, p = 0.001). Furthermore, among the follow-up subgroups, studies with a longer follow-up period, > 10 years, exhibited a larger effect size (HR = 0.78, 95% CI = 0.68–0.90, p = 0.001), which may be partly attributed to the larger sample size. The meta-analysis results showed that MAG effectively reduced all-cause mortality (p = 0.004). In the post hoc subgroup, MAG significantly decreased all-cause mortality (p = 0.012). Nevertheless, in the group of pure RCTs, MAG showed a trend towards reducing all-cause mortality, but the results did not reach the statistical significance (p = 0.053; Supplementary Table S12). Sensitivity analysis revealed that, after excluding the study by Morris (24), the result became more statistically significant (HR = 0.79, 95% CI = 0.70–0.91, p = 0.001; Supplementary Table S10).
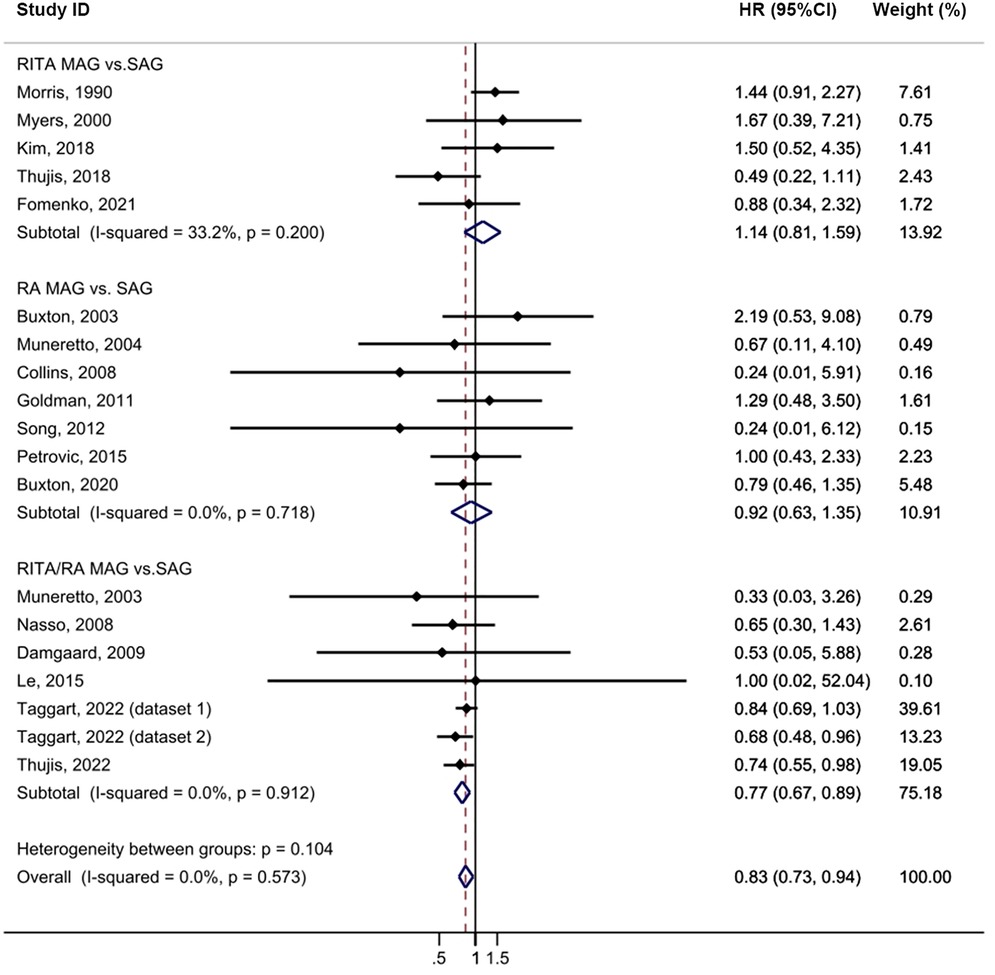
Figure 2. Forrest plot for all-cause mortality using intention-to-treat data. Horizontal lines represent 95% confidence intervals (CI). The rectangles represent the point estimate. The diamond represents the summary estimate (size of the diamond = 95% CI). The vertical line represents the reference of no increased risk.
Cardiac mortality was comparable between the MAG and SAG groups (HR = 0.81, 95% CI = 0.65–1.00, p = 0.05; Figure 3). No publication bias was evident in funnel plot analysis of these studies (Supplementary Figure S2B). Subgroup analyses revealed no significant differences between the established groups or across the different follow-up periods. Sensitivity analysis further showed that, upon excluding either the study by Goldman (17) or the study by Petrovic (14), the result became statistically significant (HR = 0.80, 95% CI = 0.64–0.99, p = 0.043; Supplementary Table S10).
Secondary endpoints
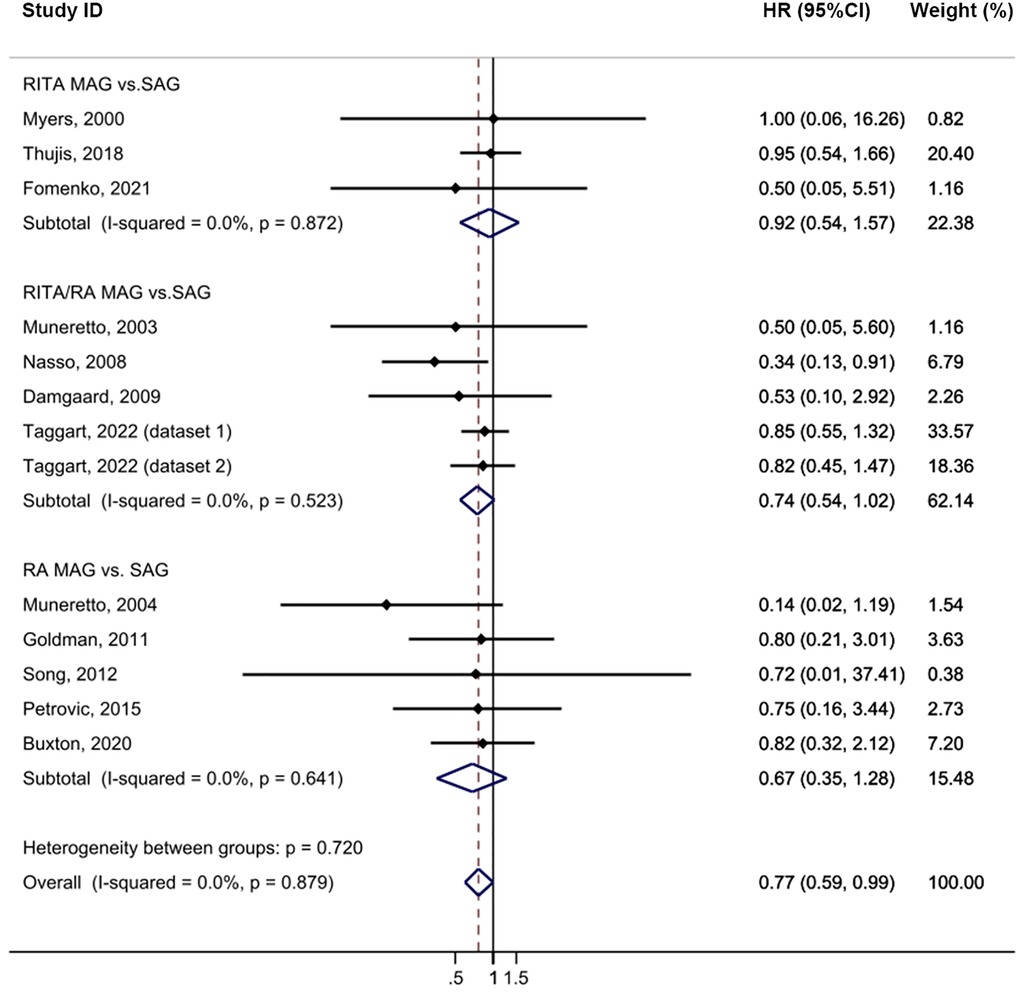
Compared to the SAG groups, the MAG group demonstrated a lower incidence of MI after CABG, with no heterogeneity (HR = 0.77, 95% CI = 0.59–0.99, p = 0.039, I2 = 0%; Figure 4). No publication bias was evident in funnel plot analysis of these studies (Supplementary Figure S2C). Subgroup analysis revealed that this significant effect was primarily driven by the RITA/RA vs. SAG subgroups (HR = 0.74, 95% CI = 0.54–1.02, p = 0.067) and subgroups with a follow-up time of less than 5 years (HR = 0.67, 95% CI = 0.44–1.02, p = 0.061). Sensitivity analysis further showed that, upon excluding the study by Thuijs (25), the result became more statistically significant (HR = 0.72, 95% CI = 0.55–0.96, p = 0.026; Supplementary Table S10).
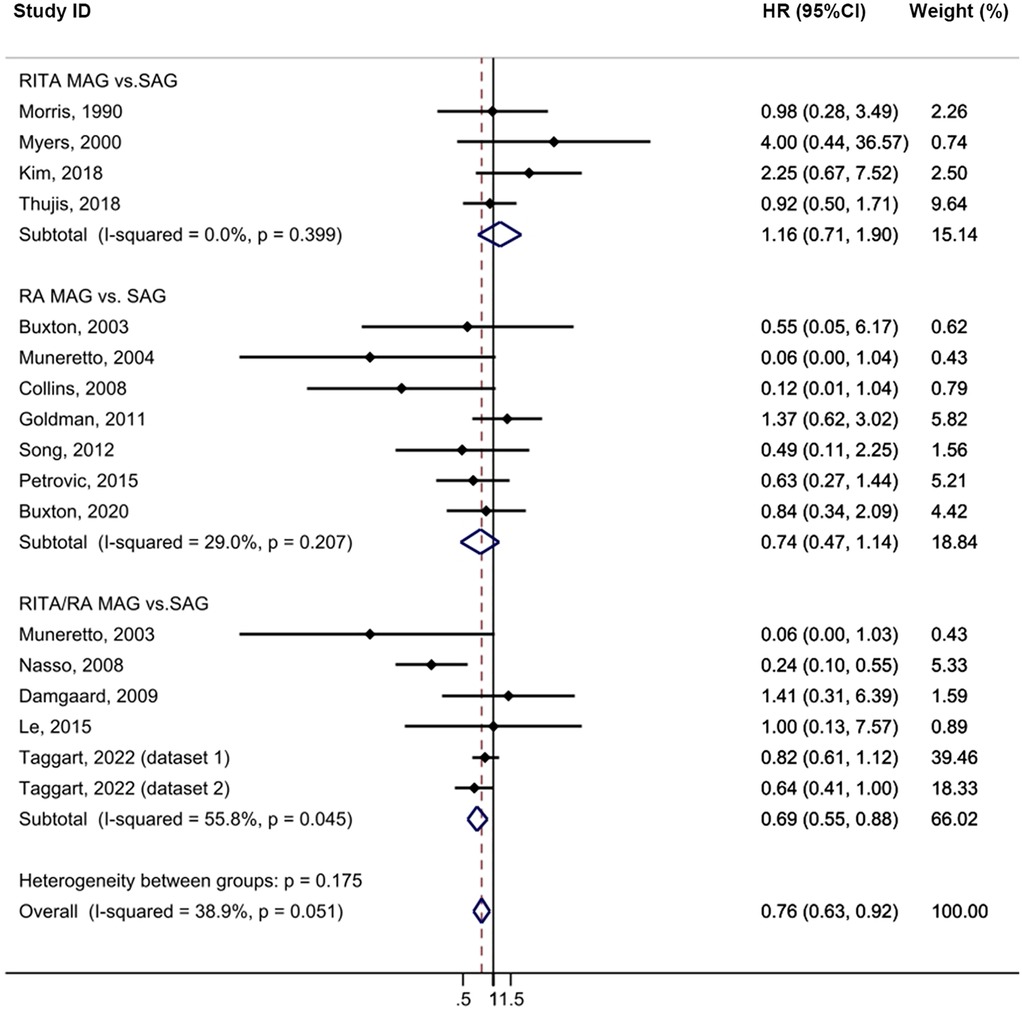
The MAG group exhibited a statistically significant lower incidence of repeat revascularization compared to the SAG group with minimal heterogeneity (HR = 0.76, 95% CI = 0.63–0.92, p = 0.004, I2 = 38.9%; Figure 5). No publication bias was evident in funnel plot analysis (Supplementary Figure S2D). Subgroup analysis revealed that the RITA/RA MAG subgroup had a statistically significant lower incidence of this complication compared to the SAG group (HR = 0.69, 95% CI = 0.55–0.88, p = 0.002). Furthermore, this effect was pronounced in the subgroup with a follow-up time exceeding 10 years (HR = 0.76, 95% CI = 0.60–0.97, p = 0.029). In the sensitivity analysis, the exclusion of a single study did not cause the outcome to lose its statistical significance (Supplementary Table S10).
Postoperative complications
There was no significant difference in the incidence of stroke between the MAG and SAG groups (HR = 0.83, 95% CI = 0.62–1.11, p = 0.214; Supplementary Figure S3). Similarly, the result of the subgroup analyses was not statistically significant. No publication bias was evident in funnel plot analysis (Supplementary Figure S2E). In the sensitivity analysis, the exclusion of any single study did not cause the outcome to be statistically significant (Supplementary Table S10).
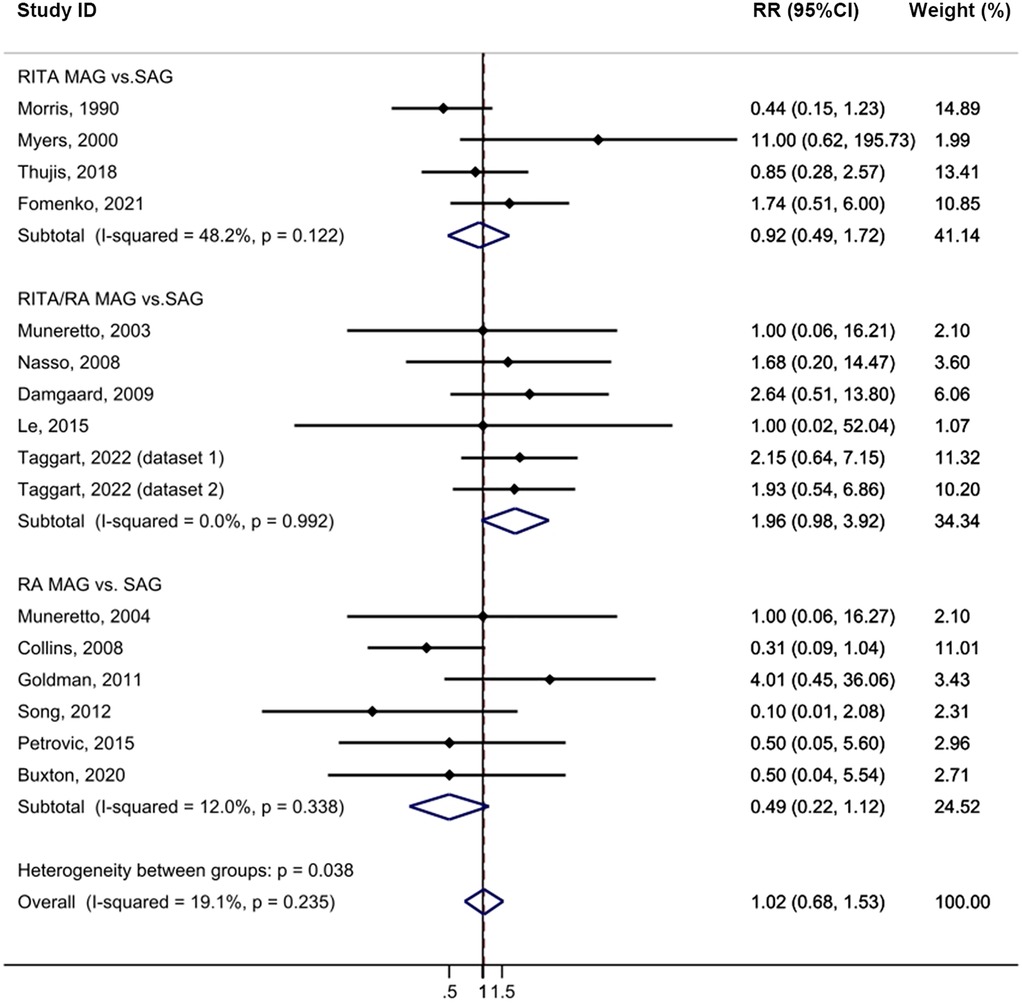
The MAG and SAG groups did not demonstrate any statistically significant difference in the incidence of sternal wound complications following CABG with minimal heterogeneity (HR = 1.02, 95% CI = 0.68–1.53, p = 0.919, I2 = 19.1%; Figure 6). No publication bias was evident in the funnel plot analysis of the studies (Supplementary Figure S2F), and the results of the subgroup analysis were not statistically significant. Sensitivity analysis further confirmed that excluding any single study did not alter the statistical outcomes (Supplementary Table S10).
There was no statistically significant difference in the incidence of major bleeding between the MAG and SAG. However, the RA vs. SAG subgroup showed a reduced risk of bleeding with low heterogeneity (HR = 0.32, 95% CI = 0.10–0.90, p = 0.032, I2 = 35.2%; Supplementary Figure S4). No publication bias was evident in funnel plot analysis (Supplementary Figure S2G). Sensitivity analysis revealed that, upon excluding the study by Thuijs (25), the result became statistically significant (HR = 0.57, 95% CI = 0.32–0.99, p = 0.047; Supplementary Table S10).
The outcomes of the as-treated analysis were similar to those of the intention-to-treat analysis (Supplementary Tables S7, S8, and S11).
Discussion
CABG, as an effective surgical treatment for coronary heart disease, has always been a key focus in clinical practice regarding the selection of vascular grafts. MAG and SAG, as two commonly used methods of vascular grafting, each have their unique advantages and disadvantages. This meta-analysis compared the outcomes of MAG and SAG, and showed that MAG was associated with higher survival from all-cause and cardiac mortality, lower risk of postoperative MI, and lower need for repeat revascularization, regardless of intention-to-treat or as-treated data. However, the absence of significant differences were found in the postoperative outcomes such as sternal wound infection complications, major bleeding or stroke between MAG and SAG surgical revascularization.
Observational studies consistently favour MAG over SAG for multivessel coronary artery disease, showing better survival (8, 27–29). A study included over 1 million patients and found that MAG had a survival benefit over a 10-year follow-up period (30). This superiority could be explained by the greater patency rates of durable arterial grafts (31) or unmeasured confounders (32). Surprisingly the largest RCT addressing this issue to date, ART, had neutral outcomes (5). The possible explanation for this that an arterial graft (most commonly the LITA) is anastomosed to the most important vessel (commonly the LAD). Occlusion of a graft in a coronary artery other than the LAD artery may not have a survival effect (33).
Our findings are consistent with those of previous observational studies on mortality. In comparison with previous RCTs, our meta-analysis had a much larger sample size (5,047 MAG and 5,096 SAG). Our study included patients with RITA and RA MAG, whereas Gaudino et al. (534 with MAG, 502 with SAG) included only patients with RA (34). In addition, the follow-up period in our meta-analysis ranged from 0.5 to 12.6 years. A long follow-up period may yield a survival advantage in patients with MAG. This was evident in our subgroup analysis in which the benefits were pronounced in the subgroup with a follow-up period of >10 years. Sensitivity analysis showed that excluding the 1990 Morris study yielded statistically significant results, confirming this point. The MAG group showed a trend towards reduced cardiac mortality compared to the SAG group (p = 0.05). After excluding the studies by Goldman and Petrovic, the results were considered statistically significant. A potential reason could be the risk of competitive coronary flow on arterial grafts. To minimise competitive flow, the RA is currently grafted to coronary arteries with stenosis of 90% or more of the vessel diameter (35). But Goldman et al. and Petrovic et al. used >70% and >80% proximal stenosis, respectively, as the entry criterion for a study vessel to receive an RA graft This could explain why there was no significant reduction in cardiac mortality in the MAG group.
MAG grafts have been shown to have superior angiographic patency compared to vein grafts (92% vs. 80% at 5 years) (34). Our result is consistent with the meta-analysis of Gaudino et al. (34), the risk of MI and repeat revascularization was lower with MAG than with SAG, in which RA MAG had lower MI (HR = 0.72, 95% CI = 0.53–0.99, p = 0.04) and decreased repeat revascularization rate (HR = 0.50, 95% CI = 0.40–0.63, p < 0.001) than that of SAG. The increased rates of postoperative MI and revascularization in the SAG group may be associated with the lower patency rate of the SVG. The average lifespan of an SVG is approximately ten years (36), and this effect becomes more pronounced as the follow-up period is extended.
MAG is not the primary approach in CABG because of the higher complexity of the surgical technique and concerns about postoperative SWIs. Francisca et al.'s study indicated that there was a nearly 2-fold increased risk of SWIs caused by MAG with BITA grafting compared with other grafting approaches whereas the RA grafting is not associated with an increased risk of SWIs (10). However, the post hoc analysis from the EXCEL trial reported neutral outcomes regarding the risk of SWIs caused by BITA vs. SITA grafting (25). The incidence of wound complications did not reach statistical significance in our study, although in most studies evaluating the rate of SWIs in the RITA and RITA/RA group, many of the confidence intervals cross one. This might be due to the improvements in surgical techniques and surgeons' understanding of skeletonized vessels (37, 38) have decreased the incidence of adverse events, including SWIs. The type of ITA was reported in four of 15 studies that evaluated SWIs (6, 12, 21, 23). Two studies used skeletonized harvesting methods (6, 12). The differences in vessel collection may be the reason for the insignificant effect size. Although we cannot determine the true direction of effect statistically, we need to pay attention to wound complications caused by MAG, and the use of skeletonized BITA can potentially reduce the incidence of SWIs.
Lower stroke rates in the MAG may be related to less aortic manipulation. A meta-analysis of observational studies conducted by Buttar et al. showed fewer cerebrovascular accidents with the RITA MAG than with the SAG (1.3% vs. 2.9%; p = 0.0003) (39). But the risk of stroke in our study was similar between the MAG and SAG groups. This finding may be attributed to the inclusion of a larger amount of RA data in our study. Furthermore, we found that the MAG did not increase the risk of major bleeding, particularly in the RA group, and that the risk of bleeding was significantly decreased. This may be related to characteristics such as the thicker arterial wall being less prone to injury and the arterial wall fitting snugly at the anastomotic site.
Although our research has demonstrated the survival benefits of MAG compared to SAG, there are still some limitations and uncertainties. Firstly, the studies included in our analysis vary in terms of trial design, sample size, and follow-up duration, which may lead to bias in the results. The ART study was the main contributor to the outcomes of this meta-analysis (weight of 39.61% for all-cause mortality) (11). However, the high crossover rates and the modification according to surgeon volume in the ART may influence the analysis of intention-to-treat data. To overcome the limitations of the ART, the ROMA trial (7), which is underway, will compare all MAG approaches and SAG without imposing on the surgeon which graft configuration should be adopted. Moreover, the EXCEL trial (25) and SYNTAX trail (3) included in our study were designed randomly to compare the effectiveness of PCI and CABG in patients with left main CAD, but SAG or MAG revascularization in the CABG group was chosen at the surgeon's discretion, and patients were not randomized, potentially leading to selection bias. For example, our meta-analysis revealed that MAG effectively reduced all-cause mortality (p = 0.004). Within the post hoc subgroup, MAG demonstrated a significant reduction in all-cause mortality (p = 0.012). However, in the subgroup of pure RCTs, MAG showed a trend towards decreasing all-cause mortality, but the results did not achieve statistical significance (p = 0.053). This outcome aligns with our expectation that the inclusion of post hoc analyses may have slightly inflated our effect size, because the post hoc analysis data is non-randomized in nature and may introduce selection bias to some extent. Consequently, future studies should adopt more stringent inclusion criteria and methodologies.
Except for Muneretto et al. did not report the patient randomization method (20), other studies (12, 14, 16) may have introduced selection bias by excluding high-risk patients with shorter life expectancies and those requiring emergency surgeries. In the study by Damgaard et al. (18), revascularization was performed by seven surgeons using different surgical techniques, which could lead to intervention bias. Furthermore, Kim et al. (13) found that eight patients in the SV group required a third limb conduit to lengthen the graft for complete revascularization, compared to 39 patients in the RITA group, and this difference could confound the outcomes. Muneretto included a high proportion of diabetic patients (40.5%) (21), and diabetes was an independent predictor of cardiac-related events and mortality, potentially leading to reporting bias. Thus, the selection criteria differed across studies and may have been based on the patients' clinical attributes and status, potentially leading to selection bias, and our results were not adjusted for biases.
Most RCTs that compared MAG and SAG were underpowered for long-term survival estimation, focused on angiographic primary outcomes, had small sample sizes (except for EXCEL and ART, all included less than 1,000 patients), and limited follow-up periods or rates. For instance, the CARRPO trial (18) and the study by Goldman et al. (17) estimated 5- year and 1-year graft patency, respectively, and both evaluated 1-year survival (3 and 16 deaths, respectively); the weight of these studies in this meta-analysis was 0.28% and 1.61%, respectively. Kim et al. (13) reported 5-year clinical outcomes but assessed 1-year angiographic patency. Song et al. (16) and Le et al. (15) estimated 1-year and 6-month graft patency, and the weight of these studies was 0.15% and 0.10%, respectively. The studies of Kim et al. (13), Damgaard et al. (18), and Le et al. (15) reported incomplete angiographic follow-up rates of 15%, 17%, and 24%, respectively. Missing data may introduce outcome bias.
Secondly, the results demonstrated the superiority in MAG compared to SAG but may have been affected by unmeasured confounders. For example, the EXCEL trail included perioperative MI (PMI) in the definition of major adverse cardiovascular and cerebrovascular events (MACCEs), garnering extensive controversy for its definition of MI and its powering (40, 41), in contrast to the NOBLE trial (42). The definition and detection of PMI varied across trials, limiting the interpretation of the results. The inclusion of PMI may overestimate the incidence of MI, as minor myocardial injuries detected in routine tests would be classified as MI, although these injuries are not necessarily clinically significant adverse events. Furthermore, the design of the EXCEL trial may tend to demonstrate the superiority of CABG, especially in long-term follow-up (43). These differences in trial design may have influenced the interpretation of the results.
Either BITA (8) or SITA+RA (44) grafting has a survival benefit over SITA grafting. Due to the lack of clear evidence, the potentially increased sternal wound complication rate, and the perceived technical complexity when using bilateral internal thoracic arteries often results in the RA as the preferred second conduit of choice. The proportion of patients receiving BITA grafts tends to decrease as the RA becomes more frequently utilized as the second conduit, although BITA grafts have better long-term patency and survival rates (45). The effect size of the subgroup of patients receiving RA/RITA grafting in our study was insignificant. This result may be attributed to the widespread use of the RA in this subgroup (data not provided), limiting the potential benefits of BITA grafts. Moreover, Lytle et al. suggested that sufficient follow-up time is needed to clarify the unequivocal long-term survival benefits of BITA (46).
Thirdly, our study failed to fully evaluate the differences in response to MAG and SAG among specific patient subgroups, which limits the generalizability of our findings. A higher mortality in females who have undergone CABG compared to males has been well-documented in many studies (47, 48). Female sex is an independent predictor of operative and long-term mortality after CABG (48). This phenomenon may be related to sex differences in biology and baseline risk characteristics, as well as smaller coronary artery diameter and body surface area in women (49). In addition, some widely recognized risk factors include older age, poorer renal function, lower BMI, and the comorbidity of diabetes mellitus, hypertension, peripheral arterial disease, or COPD (50, 51). Observational studies focused on the comparison between MAG and SAG, which patients receiving MAG had a low percentage of females, and fewer comorbidities, including diabetes, peripheral vascular disease, and extensive CAD etc. (8). These factors could explain the better outcomes of MAG. However, in our study, the sex distribution and the proportion of preoperative complications such as hypertension, diabetes mellitus, previous MI and COPD was similar between the two groups. The MAG group has a lower prevalence of obesity and peripheral arterial disease, but a higher incidence of hyper lipidaemia. Although the included studies provide limited data, we must acknowledge that these factors may confound the results. Thus, future studies should perform subgroup analyses of MAG outcomes by sex, complications, and other variables to determine the patient groups more likely to benefit from MAG.
In conclusion, compared with SAG, MAG was associated with higher survival, lower risk of MI, and lower need for repeat revascularization, and the benefits increasing as the follow-up time prolongs. Nonetheless, there were no significant differences in the incidence of sternal wound infections, major bleeding, and stroke between MAG and SAG.
In the future, additional meta-analyses are necessary to investigate the impact of baseline characteristics, including sex, BMI and comorbidities etc, on the choice of conduit; and independently analysis are needed regarding non-survival-based outcomes to comprehensively understand the safety of MAG. Therefore, we recommend that the decision to use MAG or SAG should be individualised.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Supplementary Table S1) (52, 53), we conducted a systematic search of PubMed, Google, and Web of Science databases from inception until December 2024 with English language without geographical restrictions. We defined MeSH terms and free text terms to define each component of the PICO expression: P) Population, coronary artery disease adult patients submit ted to CABG procedure; I) Intervention, multiple arterial grafts; C) Comparison, single arterial graft and O) Outcomes, death, survival, MACCEs, MI, repeat revascularization, and postoperative complications (SWIs, stroke, and bleeding). The search queries are shown in Supplementary Tables S2–S4.
Duplicates, reviews, meta-analysis, cross-sectional and case-control studies, case series, case reports, abstracts conference presentations, editorials and expert opinions, and studies that did not report HRs or RRs were excluded. The inclusion criteria were (1) studies on adults (age ≥18 years), (2) studies published in English, (3) RCTs or post hoc analyses, (4) studies that evaluated the outcomes of MAG and SAG; and 5) studies with follow-up periods of at least 6 months. The flowchart of study selection is shown in Figure 1.
Data extraction was independently conducted by two authors (Q.D. and Q.Z.), and any discrepancies were resolved through discussion and adjudication by a senior author (M.G.). The demographic data and study characteristics were recorded. The assessed clinical outcomes included primary endpoints: all-cause mortality, cardiac mortality; secondary endpoints: MI, repeat revascularization; and postoperative complications: stroke, sternal wound complications, major bleeding. The outcomes were evaluated during the maximum follow-up period.
The studies were divided into three subgroups based on the arterial grafts used in the MAG group: (1) RITA MAG: The first arterial graft was LITA and second arterial graft used was RITA. (2) RA MAG: The first and second arterial grafts were the LITA and RA, respectively. (3) RITA/RA MAG: the first arterial graft was the LITA, and the second arterial graft was either the RITA or RA. All SVGs were used in addition to and after the arterial grafts were used. The SAG group consistently had one arterial graft in the LAD and additional venous grafts in the other coronary vessels.
The RCT quality was assessed using the Revised Cochrane Risk-of-Bias Tool for Randomised Trials (RoB2) (54). The pooled HR or RR was calculated for the outcomes using the generic inverse variance method. Tests of heterogeneity of the HRs across studies were estimated using the chi-square and I-square (I2) tests, with fixed-effects or random-effects models based on the criteria of P > 0.10 and I2 < 50%. Funnel plots were used to assess publication bias, and sensitivity analysis was applied to measure the influence of each study. Statistical significance was defined as a two-tailed P-value <0.05. All data were analysed using Stata (version 17.0; Stata Corp., College Station, TX, USA).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
QD: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. QZ: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. LL: Data curation, Formal analysis, Methodology, Writing – original draft. XC: Project administration, Resources, Validation, Visualization, Writing – review & editing. MG: Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from National Science Foundation of China (No. 82400942).
Acknowledgments
We thank all the researchers for their contribution and support for making this study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1555242/full#supplementary-material
References
1. Boylan MJ, Lytle BW, Loop FD, Taylor PC, Borsh JA, Goormastic M, et al. Surgical treatment of isolated left anterior descending coronary stenosis. Comparison of left internal mammary artery and venous autograft at 18–20 years of follow-up. J Thorac Cardiovasc Surg. (1994) 107(3):657–62.8127094
2. Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. (1986) 314(1):1–6. doi: 10.1056/nejm198601023140101
3. Thuijs D, Davierwala P, Milojevic M, Deo SV, Noack T, Kappetein AP, et al. Long-term survival after coronary bypass surgery with multiple versus single arterial grafts. Eur J Cardiothorac Surg. (2022) 61(4):925–33. doi: 10.1093/ejcts/ezab392
4. Buxton BF, Hayward PA, Raman J, Moten SC, Rosalion A, Gordon I, et al. Long-term results of the RAPCO trials. Circulation. (2020) 142(14):1330–8. doi: 10.1161/circulationaha.119.045427
5. Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B, et al. Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med. (2019) 380(5):437–46. doi: 10.1056/NEJMoa1808783
6. Nasso G, Coppola R, Bonifazi R, Piancone F, Bozzetti G, Speziale G. Arterial revascularization in primary coronary artery bypass grafting: direct comparison of 4 strategies–results of the stand-in-Y mammary study. J Thorac Cardiovasc Surg. (2009) 137(5):1093–100. doi: 10.1016/j.jtcvs.2008.10.029
7. Gaudino MFL, Taggart DP, Fremes SE. The ROMA trial: why it is needed. Curr Opin Cardiol. (2018) 33(6):622–6. doi: 10.1097/hco.0000000000000565
8. Buttar SN, Yan TD, Taggart DP, Tian DH. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart. (2017) 103(18):1419–26. doi: 10.1136/heartjnl-2016-310864
9. Magouliotis DE, Fergadi MP, Zotos PA, Rad AA, Xanthopoulos A, Bareka M, et al. Differences in long-term survival outcomes after coronary artery bypass grafting using single vs multiple arterial grafts: a meta-analysis with reconstructed time-to-event data and subgroup analyses. Gen Thorac Cardiovasc Surg. (2023) 71(2):77–89. doi: 10.1007/s11748-022-01891-7
10. Saraiva FA, Leite-Moreira JP, Barros AS, Lourenço AP, Benedetto U, Leite-Moreira AF. Multiple versus single arterial grafting in coronary artery bypass grafting: a meta-analysis of randomized controlled trials and propensity score studies. Int J Cardiol. (2020) 320:55–63. doi: 10.1016/j.ijcard.2020.08.001
11. Taggart DP, Gaudino MF, Gerry S, Gray A, Lees B, Dimagli A, et al. Effect of total arterial grafting in the arterial revascularization trial. J Thorac Cardiovasc Surg. (2022) 163(3):1002–9.e6. doi: 10.1016/j.jtcvs.2020.03.013
12. Fomenko MS, Schneider YA, Tsoi VG, Pavlov AA, Shilenko PA. Left or bilateral internal mammary artery employment in coronary artery bypass grafting: midterm results. Asian Cardiovasc Thorac Ann. (2021) 29(8):758–62. doi: 10.1177/0218492321990764
13. Kim MS, Hwang HY, Kim JS, Oh SJ, Jang MJ, Kim KB. Saphenous vein versus right internal thoracic artery as a Y-composite graft: five-year angiographic and clinical results of a randomized trial. J Thorac Cardiovasc Surg. (2018) 156(4):1424–33.e1. doi: 10.1016/j.jtcvs.2018.04.123
14. Petrovic I, Nezic D, Peric M, Milojevic P, Djokic O, Kosevic D, et al. Radial artery vs saphenous vein graft used as the second conduit for surgical myocardial revascularization: long-term clinical follow-up. J Cardiothorac Surg. (2015) 10:127. doi: 10.1186/s13019-015-0331-9
15. Le J, Baskett RJ, Buth KJ, Hirsch GM, Brydie A, Gayner R, et al. A pilot randomized controlled trial comparing CABG surgery performed with total arterial grafts or without. J Cardiothorac Surg. (2015) 10(1). doi: 10.1186/s13019-014-0203-8
16. Song SW, Sul SY, Lee HJ, Yoo KJ. Comparison of the radial artery and saphenous vein as composite grafts in off-pump coronary artery bypass grafting in elderly patients: a randomized controlled trial. Korean Circ J. (2012) 42(2):107–12. doi: 10.4070/kcj.2012.42.2.107
17. Goldman S, Sethi GK, Holman W, Thai H, McFalls E, Ward HB, et al. Radial artery grafts vs saphenous vein grafts in coronary artery bypass surgery: a randomized trial. JAMA. (2011) 305(2):167–74. doi: 10.1001/jama.2010.1976
18. Damgaard S, Wetterslev J, Lund JT, Lilleør NB, Perko MJ, Kelbaek H, et al. One-year results of total arterial revascularization vs. Conventional coronary surgery: CARRPO trial. Eur Heart J. (2009) 30(8):1005–11. doi: 10.1093/eurheartj/ehp048
19. Collins P, Webb CM, Chong CF, Moat NE. Radial artery versus saphenous vein patency randomized trial: five-year angiographic follow-up. Circulation. (2008) 117(22):2859–64. doi: 10.1161/CIRCULATIONAHA.107.736215
20. Muneretto C, Bisleri G, Negri A, Manfredi J, Carone E, Morgan JA, et al. Left internal thoracic artery-radial artery composite grafts as the technique of choice for myocardial revascularization in elderly patients: a prospective randomized evaluation. J Thorac Cardiovasc Surg. (2004) 127(1):179–84. doi: 10.1016/j.jtcvs.2003.08.004
21. Muneretto C, Negri A, Manfredi J, Terrini A, Rodella G, Elqarra S, et al. Safety and usefulness of composite grafts for total arterial myocardial revascularization: a prospective randomized evaluation. J Thorac Cardiovasc Surg. (2003) 125(4):826–35. doi: 10.1067/mtc.2003.154
22. Buxton BF, Raman JS, Ruengsakulrach P, Gordon I, Rosalion A, Bellomo R, et al. Radial artery patency and clinical outcomes: five-year interim results of a randomized trial. J Thorac Cardiovasc Surg. (2003) 125(6):1363–71. doi: 10.1016/S0022-5223(02)73241-8
23. Myers WO, Berg R, Ray JF, Douglas-Jones JW, Maki HS, Ulmer RH, et al. All-artery multigraft coronary artery bypass grafting with only internal thoracic arteries possible and safe: a randomized trial. Surgery. (2000) 128(4):650–9. doi: 10.1067/msy.2000.108113
24. Morris JJ, Smith LR, Glower DD, Muhlbaier LH, Reves JG, Wechsler AS, et al. Clinical evaluation of single versus multiple mammary artery bypass. Circulation. (1990) 82(5 Suppl):Iv214–23.2225407
25. Thuijs D, Head SJ, Stone GW, Puskas JD, Taggart DP, Serruys PW, et al. Outcomes following surgical revascularization with single versus bilateral internal thoracic arterial grafts in patients with left main coronary artery disease undergoing coronary artery bypass grafting: insights from the EXCEL trial†. Eur J Cardiothorac Surg. (2019) 55(3):501–10. doi: 10.1093/ejcts/ezy291
26. Changal K, Masroor S, Elzanaty A, Patel M, Mir T, Khan S, et al. Meta-analysis comparing multiple arterial grafts versus single arterial graft for coronary-artery bypass grafting. Am J Cardiol. (2020) 130:46–55. doi: 10.1016/j.amjcard.2020.06.012
27. Yi G, Shine B, Rehman SM, Altman DG, Taggart DP. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation. (2014) 130(7):539–45. doi: 10.1161/CIRCULATIONAHA.113.004255
28. Rizzoli G, Schiavon L, Bellini P. Does the use of bilateral internal mammary artery (IMA) grafts provide incremental benefit relative to the use of a single IMA graft? A meta-analysis approach. Eur J Cardiothorac Surg. (2002) 22(5):781–6. doi: 10.1016/S1010-7940(02)00470-0
29. Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. (2001) 358(9285):870–5. doi: 10.1016/S0140-6736(01)06069-X
30. Sabik JF 3rd, Mehaffey JH, Badhwar V, Ruel M, Myers PO, Sandner S, et al. Multiarterial vs single-arterial coronary surgery: 10-year follow-up of 1 million patients. Ann Thorac Surg. (2024) 117(4):780–8. doi: 10.1016/j.athoracsur.2024.01.008
31. Tatoulis J, Buxton BF, Fuller JA. Patencies of 2,127 arterial to coronary conduits over 15 years. Ann Thorac Surg. (2004) 77(1):93–101. doi: 10.1016/S0003-4975(03)01331-6
32. Gaudino M, Di Franco A, Rahouma M, Tam DY, Iannaccone M, Deb S, et al. Unmeasured confounders in observational studies comparing bilateral versus single internal thoracic artery for coronary artery bypass grafting: a meta-analysis. J Am Heart Assoc. (2018) 7(1). doi: 10.1161/JAHA.117.008010
33. Gaudino M, Tondi P, Benedetto U, Milazzo V, Flore R, Glieca F, et al. Radial artery as a coronary artery bypass conduit: 20-year results. J Am Coll Cardiol. (2016) 68(6):603–10. doi: 10.1016/j.jacc.2016.05.062
34. Gaudino M, Benedetto U, Fremes S, Biondi-Zoccai G, Sedrakyan A, Puskas JD, et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med. (2018) 378(22):2069–77. doi: 10.1056/NEJMoa1716026
35. Shapira OM. Radial artery as the preferred second conduit for coronary bypass. N Engl J Med. (2018) 378(22):2134–5. doi: 10.1056/NEJMe1804750
36. Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a department of veterans affairs cooperative study. J Am Coll Cardiol. (2004) 44(11):2149–56. doi: 10.1016/j.jacc.2004.08.064
37. Van den Eynde J, Heeren A, Szecel D, Meuris B, Jacobs S, Verbrugghe P, et al. Skeletonisation contributing to a reduction of sternal wound complications: a retrospective study in OPCAB patients. J Cardiothorac Surg. (2019) 14(1):162. doi: 10.1186/s13019-019-0985-9
38. Benedetto U, Codispoti M. Age cutoff for the loss of survival benefit from use of radial artery in coronary artery bypass grafting. J Thorac Cardiovasc Surg. (2013) 146(5):1078–84; discussion 84–5. doi: 10.1016/j.jtcvs.2013.07.025
39. Gaudino M, Bakaeen FG, Benedetto U, Di Franco A, Fremes S, Glineur D, et al. Arterial grafts for coronary bypass: a critical review after the publication of ART and RADIAL. Circulation. (2019) 140(15):1273–84. doi: 10.1161/CIRCULATIONAHA.119.041096
40. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Eur Heart J. (2018) 39(23):2192–207. doi: 10.1093/eurheartj/ehy223
41. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
42. Mäkikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. (2016) 388(10061):2743–52. doi: 10.1016/S0140-6736(16)32052-9
43. Ben-Yehuda O, Chen S, Redfors B, McAndrew T, Crowley A, Kosmidou I, et al. Impact of large periprocedural myocardial infarction on mortality after percutaneous coronary intervention and coronary artery bypass grafting for left main disease: an analysis from the EXCEL trial. Eur Heart J. (2019) 40(24):1930–41. doi: 10.1093/eurheartj/ehz113
44. Gaudino M, Lorusso R, Rahouma M, Abouarab A, Tam DY, Spadaccio C, et al. Radial artery versus right internal thoracic artery versus saphenous vein as the second conduit for coronary artery bypass surgery: a network meta-analysis of clinical outcomes. J Am Heart Assoc. (2019) 8(2):e010839. doi: 10.1161/JAHA.118.010839
45. Benedetto U, Caputo M, Gaudino M, Marsico R, Rajakaruna C, Bryan A, et al. Right internal thoracic artery or radial artery? A propensity-matched comparison on the second-best arterial conduit. J Thorac Cardiovasc Surg. (2017) 153(1):79–88.e4. doi: 10.1016/j.jtcvs.2016.08.060
46. Lytle BW, Blackstone EH, Sabik JF, Houghtaling P, Loop FD, Cosgrove DM. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. (2004) 78(6):2005–12; discussion 12–4. doi: 10.1016/j.athoracsur.2004.05.070
47. Blasberg JD, Schwartz GS, Balaram SK. The role of gender in coronary surgery. Eur J Cardiothorac Surg. (2011) 40(3):715–21. doi: 10.1016/j.ejcts.2011.01.003
48. Blankstein R, Ward RP, Arnsdorf M, Jones B, Lou YB, Pine M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: contemporary analysis of 31 midwestern hospitals. Circulation. (2005) 112(9 Suppl):I323–7. doi: 10.1161/circulationaha.104.525139
49. Johnston A, Mesana TG, Lee DS, Eddeen AB, Sun LY. Sex differences in long-term survival after major cardiac surgery: a population-based cohort study. J Am Heart Assoc. (2019) 8(17):e013260. doi: 10.1161/jaha.119.013260
50. Chua TKT, Gao F, Chia SY, Sin KYK, Naik MJ, Tan TE, et al. Long-term mortality after isolated coronary artery bypass grafting and risk factors for mortality. J Cardiothorac Surg. (2024) 19(1):429. doi: 10.1186/s13019-024-02943-0
51. Ferguson TB J. Mortality in coronary artery bypass grafting: what’s next? Circulation. (2012) 125(20):2409–11. doi: 10.1161/CIRCULATIONAHA.112.106856
52. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
53. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
Keywords: multiple arterial graft (MAG), single arterial graft (SAG), coronary artery bypass graft (CABG), randomized controlled trials (RCTs), survival
Citation: Ding Q, Zhu Q, Lu L, Cheng X and Ge M (2025) Comparison of multiple arterial grafts vs. single arterial graft in coronary artery bypass surgery: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1555242. doi: 10.3389/fcvm.2025.1555242
Received: 3 January 2025; Accepted: 18 March 2025;
Published: 27 March 2025.
Edited by:
Shahzad Raja, Harefield Hospital, United KingdomReviewed by:
Camilla Sofia Rossi, NewYork-Presbyterian, United StatesGeorgia Layton, University of Leicester, United Kingdom
Copyright: © 2025 Ding, Zhu, Lu, Cheng and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Cheng, Y2hlbmd4aWFvZmVuZ19nbHl5QDE2My5jb20=; Min Ge, Z2VtaW4yMDAwQG91dGxvb2suY29t
†These authors have contributed equally to this work
 Qiuju Ding
Qiuju Ding Qingqing Zhu1,†
Qingqing Zhu1,† Xiaofeng Cheng
Xiaofeng Cheng