- 1Universidad UTE, Facultad de Ciencias de la Salud Eugenio Espejo, Centro de Investigación Genética y Genómica, Quito, Ecuador
- 2Universidad UTE, Facultad de Ciencias de la Salud Eugenio Espejo, Quito, Ecuador
Cardiovascular diseases (CVDs) are the leading global cause of mortality, with South America reflecting similar trends. Among congenital heart diseases (CHDs), atrioventricular (AV) block is included. AV block is a condition defined by abnormal electrical signal transmission between the atria and ventricles. Advances in Next-Generation Sequencing (NGS) have facilitated the identification of genetic variants associated with cardiac disorders, such as AV block. Notably, the transcription factor NKX2-5 plays a crucial role in heart development and function, and mutations in this gene have been linked to bradycardia and AV block. This article describes the case report of a young Ecuadorian child diagnosed with AV block and bradycardia. Furthermore, by performing NGS, a missense variant, p.(Tyr274Ser) substitution, in the NKX2-5 gene has been identified and classified as a variant of uncertain significance (VUS). Ancestral analysis has shown a genetic background of 16.5% African, 45.9% European, and 37.6% Native American. These findings suggest a potential association between the identified NKX2-5 variant and the patient's phenotype, highlighting the importance of integrating genomic and ancestral analyses to advance personalized diagnostics and therapeutics in diverse populations, such as the mestizo population.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of mortality worldwide, and the situation in South America mirrors this trend (1, 2). CVDs encompass a range of disorders affecting the heart and blood vessels, including congenital heart diseases (CHD), which affect the functioning and development of cardiac structures (3). Among CHDs, atrioventricular (AV) block is one of the most common disorders (4), characterized by impaired transmission of electrical signals from the atria to the ventricles (5). A common sign of AV block is bradycardia, defined as a heart rate below the age-specific lowest normal threshold (6, 7).
Genomic screenings are fundamental in diagnosing CVDs and developing personalized treatment strategies (1, 8–10). In this context, Next-Generation Sequencing (NGS) has been established as a valuable tool for identifying genetic variants associated to various cardiac conditions (11–13). Notably, numerous genes have been implicated in bradycardia and AV block (7), including the NKX2-5 gene, a key transcription factor involved in normal heart development and function (14, 15).
Ethnicity and ancestry have also been linked to the prevalence of cardiac conditions (16). For instance, Black adults exhibit the highest prevalence of hypertension, a condition strongly associated with multiple cardiac diseases (17).
This report describes a novel NKX2-5 variant in an Ecuadorian child with AV block and bradycardia.
Case presentation
In June 2019, a 6-month-old male infant was referred to a cardiologist for evaluation. Upon examination, an arrhythmia associated with bradycardia was detected in the absence of other signs or symptoms. Based on these findings, an electrocardiogram (ECG) was requested for further assessment.
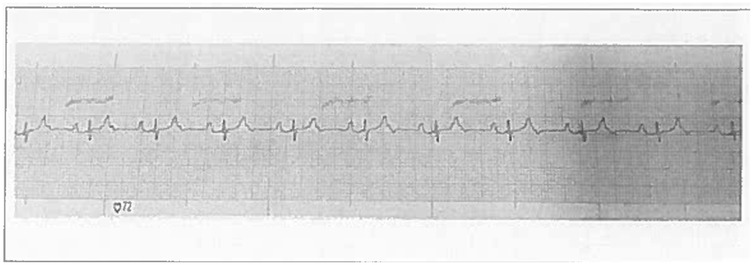
The ECG revealed a heart rate of 68 beats per minute (bpm) with a narrow QRS complex measuring 60 ms in duration. An R wave in V1 and an S wave in V6 were observed, without electrocardiographic evidence of significant right ventricular enlargement. The PR interval was prolonged at 24 ms, indicating the presence of a 2:1 AV block (Figure 1).
An echocardiogram was performed, revealing no apparent structural or functional abnormalities. The patient exhibited situs solitus with AV concordance. Both atria were of normal size, with an intact interatrial septum. The tricuspid valve demonstrated normal morphology and function, and the right ventricle was of normal size with preserved systolic performance. The interventricular septum was classified as Type I. The left ventricle also exhibited normal size and function, with both the left and right ventricular outflow tracts appearing structurally and functionally normal. The aortic and pulmonary valves, as well as the pulmonary trunk and its branches, were morphologically and functionally normal.
In September 2019, a 20-h and 30-min Holter monitoring was performed. The report indicated a mean heart rate of 70 bpm, with a maximum peak of 91 bpm. No ST segment abnormalities were detected, and no significant heart rate variations were observed compared to the resting electrocardiogram. Consequently, periodic medical follow-up was recommended.
In October 2020, at 1 year and 10 months of age, the patient was evaluated by a specialist for follow-up. The medical history was negative for syncopal episodes; however, brief episodes of perioral cyanosis during crying were reported. Psychomotor development was appropriate, and growth parameters were within normal limits. Cardiac examination revealed a grade II holosystolic murmur, an accentuated second heart sound (R2), and a hyperdynamic precordium. Based on these findings the specialist requested another electrocardiogram and echocardiogram.
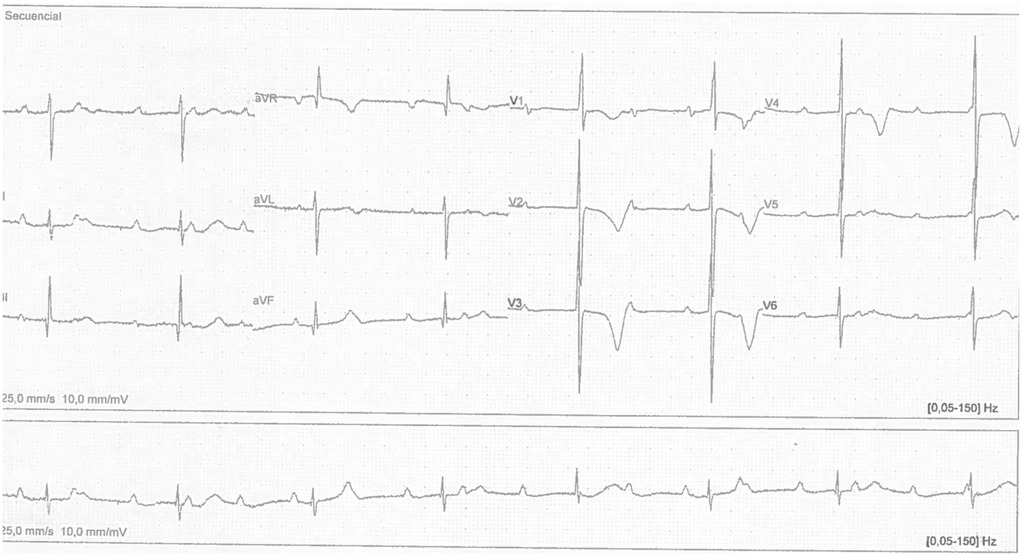
The electrocardiogram revealed a heart rate of 46 beats per minute (bpm), a narrow QRS complex, an RV1 pattern and an SV6 wave indicative of right ventricular enlargement. In addition, a variable PR interval, right axis deviation and evidence of complete AV block (third-degree) were observed (Figure 2).
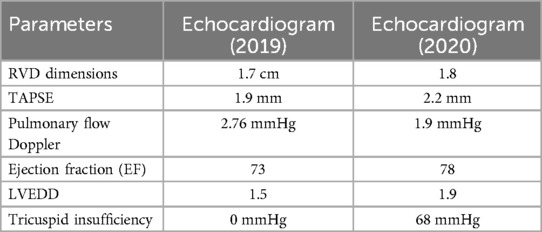
The echocardiogram performed during this evaluation revealed mild tricuspid regurgitation, dilation of both the right and left ventricles, and an estimated pulmonary pressure of 55 mmHg, confirming the diagnosis of moderate to severe pulmonary hypertension (Table 1). Given that the patient remained asymptomatic with preserved ventricular function, conservative management with enalapril was initiated.
A timeline of the relevant episodes of care is depicted in Figure 3.
Furthermore, the patient had a significant family history of cardiovascular disease. His father was diagnosed with tachycardia at 18 years of age and underwent catheter ablation. The paternal grandfather had a history of hypertension, and the paternal great-grandmother had a medical history of heart disease and suffered a stroke. A detailed pedigree illustrating the family history is provided in Figure 4.
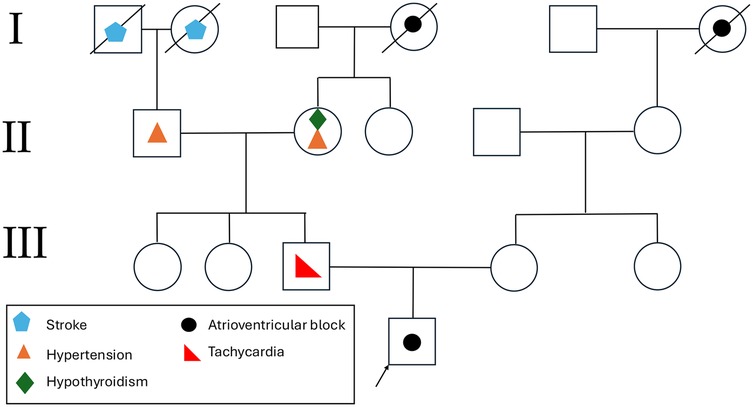
Figure 4. Representation of the proband's pedigree. Affected family members are marked with disease-specific symbols, with a legend in the bottom left explaining the symbols used.
Materials and methods
Ethical considerations
For participation in this study, the legal representative provided written informed consent, and the patient gave informed assent.
DNA extraction and next generation sequencing (NGS)
DNA extraction was extracted from peripheral blood using the PureLinkTM Genomic DNA Mini Kit. Quantification and quality assessment were performed using spectrophotometric and fluorometric methods. NGS was performed at the Center for Genetic and Genomic Research (CIGG) following the manufacturer's protocol for the TruSight™ Cardio (TSC) sequencing panel (Illumina MiSeq). The TSC sequencing panel includes 174 genes related to 17 inherited cardiovascular diseases.
Bioinformatics analyses were performed using DRAGEN Enrichment v3.9.5, Annotation Engine v3.15, PolyPhen, SIFT, and Variant Interpreter v2.16.1.300 platforms were used.
Ancestry analysis
Ancestry analysis was conducted using 46 ancestry-informative INDEL markers (AIMs) through a multiplex polymerase chain reaction (PCR), following the protocol described by Zambrano et al. (15). Fragment detection was performed using the Genetic Analyzer 3500 (Applied Biosystems, USA) was used to detect fragments. Data collection and analysis were conducted using Gene Mapper v.5 and Data Collection v.3.3. Ancestry composition was determined using the STRUCTURE v.2.3.4 software (18).
Results
Genomic analysis of the patient was performed using NGS. The results revealed that 98.42% of the target regions in the TSC Sequencing Panel had a coverage of ≥20×. A variant of uncertain significance (VUS) was identified, potentially associated with the patient's cardiac condition. The detected missense variant, c.821A >C, resulted in a p.(Tyr274Ser) substitution in the NKX2-5 gene.
Moreover, an ancestral composition analysis was performed, and the results revealed 16.5% African, 45.9% European, and 37.6% Native American components.
Discussion
In the present study, a novel Tyr274Ser mutation in the NKX2-5 gene was identified in a patient with bradycardia caused by an AV block. The cardiac homeobox protein NKX2-5 is essential for multiple stages of cardiac development. Numerous studies have indicated that mutations in this gene lead to a wide range of congenital cardiac malformations, including AV conduction disorders, and atrial septal defects (ASD), with variable expressivity and penetrance (19, 20).
Several studies have reported that mutations in the NKX2-5 gene, in addition to potentially leading to ASD-II, could also result to the development of AV block (21–25). Briggs et al. demonstrated that NKX2-5 is crucial for cardiac conduction and contraction by regulating the expression of several ion channel genes, including SCN5A, T-type Ca2+ channels (α1G and α1H), and RyR2. These findings suggest that NKX2-5 is not only involved in atrial septum formation but is also important in AV node development and the regulated expression of ion channels involved in cellular contraction and conduction (26).
Schott et al. analyzed the NKX2-5 gene in four families with ASD-II and AV block, identifying three distinct heterozygous mutations. Among 33 affected individuals, 27 had ASD, and all those with available clinical data exhibited AV conduction defects, suggesting that some variant carriers may experience conduction disturbances without ASD. Additionally, eight patients had other structural heart defects, including ventricular septal defect (VSD), tetralogy of Fallot (TOF), aortic subvalvular stenosis, left ventricular hypertrophy, pulmonary atresia, and redundant mitral leaflets with fenestrations (27).
Two of the mutations were predicted to impair NKX2-5 binding to target DNA, resulting in haploinsufficiency, while the third mutation could potentially enhance binding to target DNA. These findings indicate that NKX2-5 is crucial for the regulation of septation during cardiac morphogenesis and for the maturation and maintenance of AV node function throughout life (27).
The 324-residue NKX2-5 protein has three conserved domains: Tinman (TN), homeobox (HD), and NK2-specific domain (NK2-SD). While numerous NKX2-5 mutations have been reported across its sequence, this is the first time a missense mutation at residue 274 has been identified (28).
In this study, a well-conserved, aromatic, and hydrophobic tyrosine at position 274 in the NKX2-5 amino acid sequence, was substituted by the neutral, polar amino acid serine (Tyr274Ser). Various computational platforms were used to assess the potential impact of this variant. The Variant Interpreter platform classified Tyr274Ser as a VUS. Additionally, the Combined Annotation-Dependent Depletion (CADD) tool, which predicts the deleteriousness of genomic variants, assigned this variant a score of 22.8, suggesting a “quite likely deleterious” effect (29). In contrast, predictions from AlphaMissense and Evolutionary Scaled Model (ESM-1b) classified the variant as benign (30, 31). Based on these results, this variant has been catalogued as a VUS using the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) guidelines (32, 33).
To further investigate the conservation of this region, a protein BLAST analysis utilizing the NCBI tool identified the Tyr 274 residue across multiple mammalian species. Moreover, sequence alignments from UniRef90, analyzed with the ScoreCons algorithm, yielded a conservation score of 0.46, classifying this position as “moderate conservation” (34).
Interestingly, the subject in this study does not have ASD-II, but has a progressive AV block that has now advanced to third-degree AV block. Similarly, Morlanes-Gracia et al. described a family carrying an NKX2-5 mutation in which not all family members exhibited the same phenotype. Instead, individuals presented various cardiac disturbances, including ASD, conduction disorders, and tachyarrhythmias. This underscores the variable penetrance and expressivity of NKX2-5 mutations and their association with diverse cardiac phenotypes (35). Likewise, Jhaveri et al. presented a case involving two male siblings carrying a NKX2-5 mutation, concluding that children and young adults with NKX2-5 mutations may present a broad spectrum of cardiac disturbances, affecting both electrical conduction as well as structural integrity (36).
Although the discovered mutation alters the amino acid sequence of NKX2-5 at position 274, the HD and NK2-SD domains remain intact. This suggests that the observed phenotype is unllikely due to decreased DNA-binding activity, as the homeodomain remains unchanged. Instead, this mutation may exert dominant-negative effects, potentially altering the protein's affinity for transcriptional partners (19). These findings could provide new insights into the underlying genetic mechanisms of AV block and could contribute to the development of novel therapeutic approaches.
Furthermore, Kalayinia et al. conducted a population study to assess NKX2-5 mutation prevalence. Among 105 identified mutations, the highest prevalence was observed in European populations (24.1%), while the Latin American populations exhibited a lower prevalence (4.1%) (37). Interestingly, despite being Ecuadorian, a country with a high Native American ancestral component, the patient in this case report has a higher proportion of European ancestry, which may be associated with an increased susceptibility to NKX2-5 mutations.
The limitations in this case report derive from the inherent challenges associated with NGS. These include the requirement for specialized equipment, the necessity of expertise in this technology, and the reliance on advanced bioinformatic tools for data analysis. Furthermore, NGS is relatively more expensive than other technologies, which limits its access particularly in countries like Ecuador (8, 11, 13).
Despite these constraints, NGS offers significant advantages over other available techniques, such as Sanger sequencing. Notable strengths of NGS include its ability to achieve higher genomic coverage, analyze multiple genes simultaneously, and provide results with greater confidence (8).
Conclusion
This case report describes a novel NKX2-5 mutation, Tyr274Ser, identified in a patient with progressive AV block. The study contributes to the growing body of evidence on the role of NKX2-5 variants in cardiac conduction disorders. Notably, this is the first time a missense mutation at residue 274 has been reported, emphasizing the significance of genetic analysis in uncovering new pathogenic variants.
The findings further highlight the variable expressivity and incomplete penetrance associated with NKX2-5 mutations. While many carriers of NKX2-5 variants present with ASD, the patient in this study exhibited an isolated conduction defect, reinforcing the heterogeneous phenotypic manifestations of these mutations. This underscores the need for comprehensive clinical and genetic evaluations to better characterize genotype-phenotype correlations.
Moreover, ancestry determination played a crucial role in this study, as the patient, despite being from Ecuador showed a higher proportion of European ancestry, which has been linked to an increased prevalence of NKX2-5 mutations. This observation suggests that population-specific genetic backgrounds may influence the susceptibility to congenital heart diseases, an aspect that warrants further investigation.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1238311/, accession number: PRJNA1238311.
Ethics statement
The studies involving humans were approved by Comité de Ética de Investigación en Seres Humanos Universidad UTE. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
VR-P: Writing – original draft, Writing – review & editing, Formal analysis, Methodology. SC-U: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. EP-C: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. RT-T: Methodology, Writing – original draft, Writing – review & editing. PG-R: Methodology, Writing – original draft, Writing – review & editing. PO-R: Writing – original draft, Writing – review & editing. AZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Publication of this article was funded by Universidad UTE.
Acknowledgments
The authors are grateful to Universidad UTE for all their support. OpenAI (Name: ChatGPT, Model: GPT-4, Version: Turbo, Source: OpenAI) was used to enhance the English language of the document to improve clarity, precision, and grammar.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. OpenAI (Name: ChatGPT, Model: GPT-4, Version: Turbo, Source: OpenAI) was used to enhance the English language of the document to improve clarity, precision, and grammar.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tamayo-Trujillo R, Paz-Cruz E, Cadena-Ullauri S, Guevara-Ramirez P, Ruiz-Pozo VA, Ibarra-Castillo R, et al. Exploring atrial fibrillation: understanding the complex relation between lifestyle and genetic factors. J Med Cases. (2024) 15(8):186–94. doi: 10.14740/jmc4250
2. Lopez-Jaramillo P, Joseph P, Lopez-Lopez JP, Lanas F, Avezum A, Diaz R, et al. Risk factors, cardiovascular disease, and mortality in South America: a PURE substudy. Eur Heart J. (2022) 43(30):2841–51. doi: 10.1093/eurheartj/ehac113
3. World Health Organization. Cardiovascular Diseases (CVDs). Geneva: World Health Organization (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (Accessed December 19, 2024).
4. Shan R, Ning Y, Ma Y, Liu S, Wu J, Fan X, et al. Prevalence and risk factors of atrioventricular block among 15 million Chinese health examination participants in 2018: a nation-wide cross-sectional study. BMC Cardiovasc Disord. (2021) 21(1):289. doi: 10.1186/s12872-021-02105-3
5. Kashou AH, Goyal A, Nguyen T, Chhabra L, Kukuc LG. Atrioventricular block (nursing). StatPearls (2021). Available online at: http://www.ncbi.nlm.nih.gov/pubmed/33760517 (cited December 19, 2024).
6. Sidhu S, Marine JE. Evaluating and managing bradycardia. Trends Cardiovasc Med. (2020) 30(5):265–72. doi: 10.1016/j.tcm.2019.07.001
7. Baruteau AE, Pass RH, Thambo JB, Behaghel A, Le Pennec S, Perdreau E, et al. Congenital and childhood atrioventricular blocks: pathophysiology and contemporary management. Eur J Pediatr. (2016) 175(9):1235. doi: 10.1007/s00431-016-2748-0
8. Cadena-Ullauri S, Guevara-Ramírez P, Ruiz-Pozo VA, Tamayo-Trujillo R, Paz-Cruz E, Simancas-Racines D, et al. Genomic analysis of an Ecuadorian individual carrying an SCN5A rare variant. BMC Cardiovasc Disord. (2024) 24(1):388. doi: 10.1186/s12872-024-04049-w
9. Tamayo-Trujillo R, Ibarra-Castillo R, Laso-Bayas JL, Guevara-Ramirez P, Cadena-Ullauri S, Paz-Cruz E, et al. Identifying genomic variant associated with long QT syndrome type 2 in an Ecuadorian mestizo individual: a case report. Front Genet. (2024) 15:1395012. doi: 10.3389/fgene.2024.1395012
10. Morton SU, Quiat D, Seidman JG, Seidman CE. Genomic frontiers in congenital heart disease. Nat Rev Cardiol. (2021) 19(1):26–42. doi: 10.1038/s41569-021-00587-4
11. Cadena-Ullauri S, Guevara-Ramirez P, Ruiz-Pozo V, Tamayo-Trujillo R, Paz-Cruz E, Sánchez Insuasty T, et al. Case report: genomic screening for inherited cardiac conditions in Ecuadorian mestizo relatives: improving familial diagnose. Front Cardiovasc Med. (2022) 9:1037370. doi: 10.3389/fcvm.2022.1037370
12. Paz-Cruz E, Ruiz-Pozo VA, Cadena-Ullauri S, Guevara-Ramirez P, Tamayo-Trujillo R, Ibarra-Castillo R, et al. Associations of MYPN, TTN, SCN5A, MYO6 and ELN mutations with arrhythmias and subsequent sudden cardiac death: a case report of an Ecuadorian individual. Cardiol Res. (2023) 14(5):409–15. doi: 10.14740/cr1552
13. Guevara-Ramírez P, Cadena-Ullauri S, Ibarra-Castillo R, Laso-Bayas JL, Paz-Cruz E, Tamayo-Trujillo R, et al. Genomic analysis of a novel pathogenic variant in the gene LMNA associated with cardiac laminopathies found in Ecuadorian siblings: a case report. Front Cardiovasc Med. (2023) 10:1141083. doi: 10.3389/fcvm.2023.1141083
14. Yamada Y, Yasuda K, Hata Y, Nishida N, Hirono K. A novel NKX2–5 variant in a child with left ventricular noncompaction, atrial septal defect, atrioventricular conduction disorder, and syncope. J Clin Med. (2022) 11(11):3171. doi: 10.3390/jcm11113171
15. Cao C, Li L, Zhang Q, Li H, Wang Z, Wang A, et al. Nkx2.5: a crucial regulator of cardiac development, regeneration and diseases. Front Cardiovasc Med. (2023) 10:1270951. doi: 10.3389/fcvm.2023.1270951
16. Ogunniyi MO, Commodore-Mensah Y, Ferdinand KC. Race, ethnicity, hypertension, and heart disease: JACC focus seminar 1/9. J Am Coll Cardiol. (2021) 78(24):2460–70. doi: 10.1016/j.jacc.2021.06.017
17. Cleveland Clinic. Heart Disease Risk: How Race and Ethnicity Play a Role. Cleveland: Cleveland Clinic (2022). Available online at: https://my.clevelandclinic.org/health/articles/23051-ethnicity-and-heart-disease (cited December 19, 2024).
18. Zambrano AK, Gaviria A, Cobos-Navarrete S, Gruezo C, Rodríguez-Pollit C, Armendáriz-Castillo I, et al. The three-hybrid genetic composition of an Ecuadorian population using AIMs-InDels compared with autosomes, mitochondrial DNA and Y chromosome data. Sci Rep. (2019) 9:1–8. doi: 10.1038/s41598-019-45723-w
19. Stallmeyer B, Fenge H, Nowak-Göttl U, Schulze-Bahr E. Mutational spectrum in the cardiac transcription factor gene NKX2.5 (CSX) associated with congenital heart disease. Clin Genet. (2010) 78(6):533–40. doi: 10.1111/j.1399-0004.2010.01422.x
20. Sarkozy A, Conti E, Neri C, D’Agostino R, Digilio MC, Esposito G, et al. Spectrum of atrial septal defects associated with mutations of NKX2.5 and GATA4 transcription factors. J Med Genet. (2005) 42(2):e16. doi: 10.1136/jmg.2004.026740
21. Nouira S, Kamoun I, Ouragini H, Charfeddine C, Mahjoub H, Ouechtati F, et al. Clinical and genetic investigation of atrial septal defect with atrioventricular conduction defect in a large consanguineous Tunisian family. Arch Med Res. (2008) 39(4):429–33. doi: 10.1016/j.arcmed.2008.01.002
22. Gutierrez-Roelens I, Sluysmans T, Gewillig M, Devriendt K, Vikkula M. Progressive AV-block and anomalous venous return among cardiac anomalies associated with two novel missense mutations in the CSX/NKX2-5 gene. Hum Mutat. (2002) 20(1):75–6. doi: 10.1002/humu.9041
23. Gutierrez-Roelens I, De Roy L, Ovaert C, Sluysmans T, Devriendt K, Brunner HG, et al. A novel CSX/NKX2-5 mutation causes autosomal-dominant AV block: are atrial fibrillation and syncopes part of the phenotype? Eur J Hum Genet. (2006) 14(12):1313–6. doi: 10.1038/sj.ejhg.5201702
24. Bjørnstad PG, Leren TP. Familial atrial septal defect in the oval fossa with progressive prolongation of the atrioventricular conduction caused by mutations in the NKX2.5 gene. Cardiol Young. (2009) 19(1):40–4. doi: 10.1017/S1047951108003387
25. Rifai L, Maazouzi W, Sefiani A. Novel point mutation in the NKX2-5 gene in a Moroccan family with atrioventricular conduction disturbance and an atrial septal defect in the oval fossa. Cardiol Young. (2007) 17(1):107–9. doi: 10.1017/S1047951106001338
26. Briggs LE, Takeda M, Cuadra AE, Wakimoto H, Marks MH, Walker AJ, et al. Perinatal loss of Nkx2-5 results in rapid conduction and contraction defects. Circ Res. (2008) 103(6):580–90. doi: 10.1161/CIRCRESAHA.108.171835
27. Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science (1979). (1998) 281(5373):108–11. doi: 10.1126/science.281.5373.108
28. Kolomenski JE, Delea M, Simonetti L, Fabbro MC, Espeche LD, Taboas M, et al. An update on genetic variants of the NKX2-5. Hum Mutat. (2020) 41(7):1187–208. doi: 10.1002/humu.24030
29. Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. (2019) 47(D1):D886–94. doi: 10.1093/nar/gky1016
30. Frazer J, Notin P, Dias M, Gomez A, Min JK, Brock K, et al. Disease variant prediction with deep generative models of evolutionary data. Nature. (2021) 599(7883):91–5. doi: 10.1038/s41586-021-04043-8
31. Cheng J, Novati G, Pan J, Bycroft C, Žemgulyte A, Applebaum T, et al. Accurate proteome-wide missense variant effect prediction with AlphaMissense. Science. (2023) 381(6664):eadg7492. doi: 10.1126/science.adg7492
32. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
33. Harrison SM, Biesecker LG, Rehm HL. Overview of specifications to the ACMG/AMP variant interpretation guidelines. Curr Protoc Hum Genet. (2019) 103(1):e93. doi: 10.1002/cphg.93
34. Valdar WS, Thornton JM. Protein-protein interfaces: analysis of amino acid conservation in homodimers. Proteins. (2001) 4(1):108–24. doi: 10.1002/1097-0134(20010101)42:1%3C108::AID-PROT110%3E3.0.CO;2-O
35. Morlanes-Gracia P, Antoniutti G, Alvarez-Rubio J, Torres-Juan L, Heine-Suñer D, Ripoll-Vera T. Case report: a novel NKX2-5 mutation in a family with congenital heart defects, left ventricular non-compaction, conduction disease, and sudden cardiac death. Front Cardiovasc Med. (2021) 8:691203. doi: 10.3389/fcvm.2021.691203
36. Jhaveri S, Aziz PF, Saarel E. Expanding the electrical phenotype of NKX2-5 mutations: ventricular tachycardia, atrial fibrillation, and complete heart block within one family. HeartRhythm Case Rep. (2018) 4(11):530–3. doi: 10.1016/j.hrcr.2018.08.001
Keywords: case report, cardiovascular disease, genetics, genomics, healthcare
Citation: Ruiz-Pozo VA, Cadena-Ullauri S, Paz-Cruz E, Tamayo-Trujillo R, Guevara-Ramirez P, Onofre-Ruiz P and Zambrano AK (2025) Identification of a novel NKX2-5 variant in a young Ecuadorian patient with atrioventricular block and bradycardia: a case report. Front. Cardiovasc. Med. 12:1552423. doi: 10.3389/fcvm.2025.1552423
Received: 28 December 2024; Accepted: 19 March 2025;
Published: 4 April 2025.
Edited by:
Neil Morgan, University of Birmingham, United KingdomReviewed by:
Stefan Kurath-Koller, Medical University Graz, AustriaLeonel Diaz-Gonzalez, University Hospital La Paz, Spain
Copyright: © 2025 Ruiz-Pozo, Cadena-Ullauri, Paz-Cruz, Tamayo-Trujillo, Guevara-Ramirez, Onofre-Ruiz and Zambrano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Karina Zambrano, YW5hemFtYnJhbm8xN0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Viviana A. Ruiz-Pozo
Viviana A. Ruiz-Pozo Santiago Cadena-Ullauri
Santiago Cadena-Ullauri Elius Paz-Cruz
Elius Paz-Cruz Rafael Tamayo-Trujillo
Rafael Tamayo-Trujillo Patricia Guevara-Ramirez
Patricia Guevara-Ramirez Paul Onofre-Ruiz
Paul Onofre-Ruiz Ana Karina Zambrano
Ana Karina Zambrano