
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 25 March 2025
Sec. Hypertension
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1549878
 Yingying Zhang1,2,3,†
Yingying Zhang1,2,3,† Xintian Cai1,2,3,†
Xintian Cai1,2,3,† Shuaiwei Song1,2,3
Shuaiwei Song1,2,3 Junli Hu1,2,3,4,5,6
Junli Hu1,2,3,4,5,6 Pan Zhou1,2,3
Pan Zhou1,2,3 Kangxin Cai1,2,3
Kangxin Cai1,2,3 Rui Ma1,2,3
Rui Ma1,2,3 Huimin Ma1,2,3
Huimin Ma1,2,3 Di Shen1,2,3
Di Shen1,2,3 Wenbo Yang1,2,3
Wenbo Yang1,2,3 Delian Zhang1,2,3,4,5,6
Delian Zhang1,2,3,4,5,6 Qin Luo1,2,3,4,5,6
Qin Luo1,2,3,4,5,6 Jing Hong1,2,3,4,5,6
Jing Hong1,2,3,4,5,6 Nanfang Li1,2,3,4,5,6*
Nanfang Li1,2,3,4,5,6*
Objectives: To investigate the relationship between plasma aldosterone concentration (PAC) and the prevalence of peripheral artery disease (PAD) in hypertensive patients and to determine any potential threshold effects.
Methods: This cross-sectional study analyzed data from 13,157 hypertensive individuals from the People's Hospital of Xinjiang Uygur Autonomous Region, China. PAD was diagnosed based on an ankle-brachial index (ABI) of ≤0.90. A multivariate logistic regression model was utilized to evaluate the association between PAC and PAD, and a generalized additive model (GAM) was employed to explore non-linear relationships.
Results: The fully adjusted logistic regression model revealed a significant positive association between PAC and PAD, with an odds ratio (OR) [95% confidence interval (CI)] of 1.06 (1.04, 1.08) per unit increase in PAC. The GAM identified a critical threshold at 17.00 ng/dl for PAC, above which the prevalence of PAD increased by 9% for each unit increase in PAC, with an OR (95% CI) of 1.09 (1.06, 1.11). Sensitivity and subgroup analyses confirmed the robustness of these findings.
Conclusion: This study establishes a non-linear relationship between PAC and the prevalence of PAD in hypertensive patients, with a critical threshold at 17.00 ng/dl. These findings underscore the importance of aldosterone homeostasis in vascular health and the need for further large-scale, prospective studies to validate these results and explore their clinical implications.
Ranking as the third most prevalent atherosclerotic condition globally, peripheral artery disease (PAD) is surpassed only by coronary heart disease (CHD) and stroke in terms of incidence (1). The global burden of PAD is substantial, with an estimated 202 million individuals affected, highlighting its emergence as a significant public health challenge with considerable economic implications (2, 3). PAD not only imposes limb-threatening complications, such as intermittent claudication, ischemic rest pain, ulceration, gangrene, and significant functional decline, but also leads to a significant increase in cardiovascular morbidity and mortality (4–6). Hypertension, a preventable risk factor, plays a crucial role in the development of PAD (1, 7–9). A nationally representative cross-sectional health survey has indicated that the prevalence of PAD is disproportionately higher by 68% in untreated hypertensive individuals compared to the general population, with an odds ratio (OR) and 95% confidence intervals (CIs) of 1.68 (1.13, 2.50). However, for people with treated but uncontrolled hypertension, the prevalence of PAD was 95% higher than for the general population, with an OR of 1.95 (1.40, 2.72) (10). Furthermore, the research by Korhonen et al. has underscored the particular vulnerability of individuals with resistant hypertension to PAD, exhibiting a higher risk than those with well-managed hypertension (11). Therefore, identifying and mitigating potential risk factors for PAD in hypertensive individuals is critical to prevent adverse cardiovascular outcomes and limb-related sequelae.
Aldosterone, a mineralocorticoid hormone produced by the zona glomerulosa of the adrenal cortex, is crucial for the regulation of arterial blood pressure and maintenance of fluid and electrolyte balance (12). Primary aldosteronism (PA) is characterized by the autonomous and excessive secretion of aldosterone by the adrenal cortex (13). This hormonal imbalance is associated with more severe arterial wall damage compared to essential hypertension (14, 15). Numerous studies has consistently demonstrated that aldosterone promotes the local production of vasoconstrictive agents, including endothelin (ET), and angiotensin II (Ang II), thereby contributing to endothelial dysfunction (16). There is a significant correlation between elevated plasma aldosterone concentrations (PAC) and the presence of subclinical atherosclerosis, inflammation, oxidative stress, and endothelial dysfunction (17, 18). As a recognized fundamental pathophysiological mechanism of vascular disease, atherosclerosis is also a major pathological manifestation of PAD (19). These associations suggest that elevated PAC may actively promote atherosclerosis, inflammation, oxidative stress, and endothelial dysfunction, thereby contributing to the development or progression of PAD (20, 21).
We hypothesize that aldosterone may serve as a modifiable risk factor for PAD, particularly among hypertensive patients. To explore this hypothesis, we conducted a cross-sectional study to investigate the correlation between PAC and the prevalence of PAD within this specific demographic. Our findings could potentially identify a novel therapeutic target for the intervention of PAD in hypertensive individuals.
The present cross-sectional study leveraged data extracted from the electronic medical records at the People's Hospital of Xinjiang Uygur Autonomous Region, China, conducted over a three-year interval from December 2020 to December 2023. The study encompassed an initial pool of 15,984 subjects, from which of 13,157 hypertensive patients was selected for the final analysis, as depicted in Figure 1. Eligibility was determined for individuals (18-to-75 years old) with a diagnosis of hypertension. Exclusion criteria were as follows: 1. Participants with incomplete data for ankle-brachial index (ABI) and PAC; 2. Individuals with an ABI greater than 1.4 were excluded due to the potential for arterial wall calcification, which can falsely elevate ABI values (22, 23); 3. Participants with renal impairment, severe hepatic disease, autoimmune disorders, or malignancy; 4. Individuals with a history of myocardial infarction, coronary revascularization, heart failure, or stroke. This study, conducted in alignment with the ethical principles of the Declaration of Helsinki, received ethical approval from the institutional review board, reference number KY2024072536. Informed consent was secured from all participants prior to their inclusion in the study.
We extracted a comprehensive dataset from the electronic medical records of patients at the People's Hospital of Xinjiang Uygur Autonomous Region, China. The data included demographic data, anthropometric measures, and biochemical profiles. Demographic data comprised age, gender, smoking habits, and alcohol consumption. Anthropometric indices included body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Biochemical assays encompassed fasting plasma glucose (FPG), lipid profiles (high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], total cholesterol [TC], and triglycerides [TG]), serum uric acid (SUA), blood urea nitrogen (BUN), serum creatinine (Scr), liver enzymes [aspartate aminotransferase [AST] and alanine aminotransferase [ALT]], serum potassium (K+), and homocysteine (Hcy). Comorbidities such as diabetes mellitus, hyperlipidemia, and CHD were recorded, along with medication use, specifically antihypertensives, lipid-lowering agents, antidiabetics, and antiplatelet drugs.
PAC was measured using the DSL-8600 ACTIVE Aldosterone Coated Tube Radioimmunoassay Kit, adhering to clinical guidelines and our prior research (24–30). Plasma renin activity (PRA) was quantified using an iodine angiotensin I radioimmunoassay kit. The aldosterone/renin ratio (ARR) was calculated as the ratio of plasma aldosterone to PRA. Supplementary Material provides further details on data collection protocols and definitions.
Participants rested in a controlled-temperature setting for a minimum of 10 min, with limbs exposed to standardize conditions. Consumption of stimulants was prohibited 30 min prior to measurement to minimize vasoactive effects. ABI was measured using the Omron Colin BP-203RPE III device (Omron Health Care, Kyoto, Japan) by trained technicians, who obtained simultaneous SBP readings from the brachial and ankle arteries in the supine position, in accordance with the manufacturer's guidelines (31). ABI was calculated as the ratio of ankle SBP to brachial SBP, with the device's software automatically computing bilateral values for accuracy and reproducibility. The lower ABI value was used for each participant in analysis. PAD was diagnosed if the ABI was ≤0.9 in either lower limb, following standard diagnostic criteria (23, 32).
Participants were stratified into tertiles by PAC levels. Multicollinearity was assessed using variance inflation factors, detailed in Supplementary Table S1. Univariate logistic regression analysis was performed to assess the impact of the clinical and biochemical indicators on the risk of PAD (Supplementary Table S2). We applied multivariate logistic regression to calculate ORs and 95% CIs for the association between PAC tertiles and PAD prevalence. A generalized additive model (GAM) was used to explore nonlinear relationships between PAC and PAD, with a recursive partitioning algorithm identifying inflection points for nonlinearity. Subgroup analyses were conducted to uncover additional risk factors that could modulate the PAC-PAD association. Sensitivity analyses were conducted to ascertain the robustness of our findings. The Supplementary Material includes a full description of the statistical methods. All tests were two-tailed, with significance set at α = 0.05, and analyses were conducted using R software (version 4.1.1).
Table 1 presents the demographic and clinical characteristics of the study population, categorized by PAC tertiles. Participants in the upper PAC tertiles were predominantly female and younger, with higher levels of SBP, DBP, FPG, HDL-C, BUN, Scr, PRA, and ARR. There was also a higher prevalence of calcium channel blocker and diuretic use in these groups. In contrast, a lower proportion of individuals in the upper PAC tertiles were current smokers or drinkers. No statistically significant differences were found across PAC tertiles for BMI, TC, TG, SUA, LDL-C, AST/ALT, Hcy, hyperlipidemia, antiplatelet drugs, lipid-lowering drugs, or comorbidities. The distribution of PAC into tertiles facilitated an analysis of PAD prevalence across these groups, as shown in Figure 2. The prevalence rates were 1.23% for the first tertile (T1), 1.12% for the second tertile (T2), and 2.27% for the third tertile (T3).
We constructed four logistic regression models to assess the correlation between PAC and PAD (Table 2). The crude model revealed an OR of 1.07 (1.05, 1.08), suggesting a 7% increase in PAD prevalence for each unit increase in PAC. After full adjustment, model 3 indicated a 6% increase in PAD prevalence per unit increase in PAC. Table 2 illustrates the positive association between PAC and PAD prevalence, which attenuated but remained significant with progressive adjustment. To conduct a trend analysis, PAC was categorized into tertiles. The ORs (95% CI) for the T2 and T3 compared with the T1 were 0.91 (0.62, 1.35) and 1.68 (1.18, 2.38), respectively. There was a consistent, statistically significant increase in PAD prevalence across PAC tertiles, with the highest tertile showing the greatest OR compared to the lowest (P for trend <0.05).
Figure 3 depicts the nonlinear relationship between PAC and PAD prevalence. Utilizing the GAM, we identified a nonlinear correlation and determined the inflection point at 17.00 ng/dl. Above this threshold, there was a 9% increase in PAD prevalence for each unit increase in PAC, with an OR of 1.09 (1.06, 1.11). Below the threshold, the association between PAC and PAD did not reach statistical significance, with an OR of 0.98 (0.92, 1.04) as detailed in Table 3.
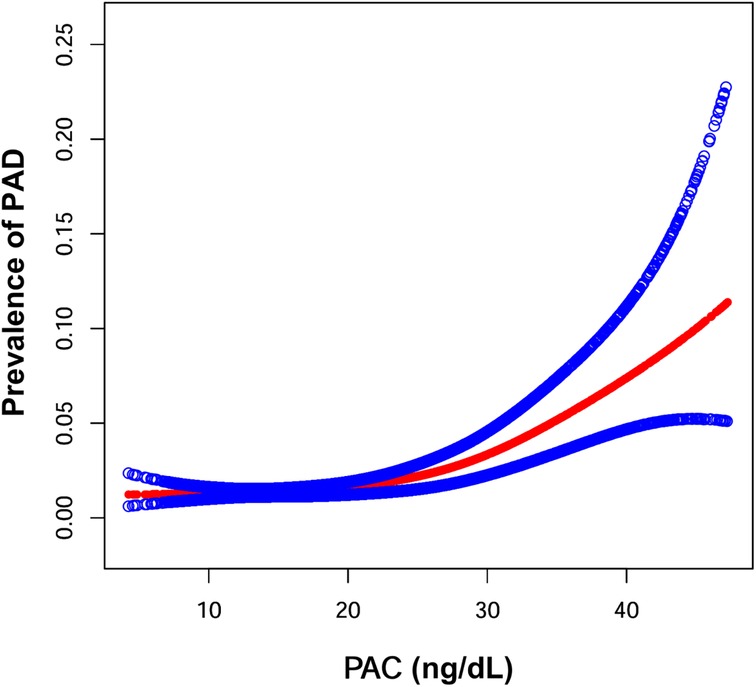
Figure 3. Generalized additive model were performed to examine the shape of the associations between PAC and the prevalence of PAD. Age, sex, smoking state, drinking state, diabetes, coronary heart disease, hyperlipidemia, BMI, SBP, DBP, FPG, HDL-C, LDL-C, TC, TG, SUA, BUN, Scr, AST/ALT, Hcy, K+, ACEIs/ARBs, beta blockers, calcium channel blockers, diuretics, lipid-lowering drugs, antidiabetic drugs, antiplatelet drugs were adjusted.
Sensitivity analyses were performed by excluding individuals suspected of having PA, and the stability of our findings was confirmed (Supplementary Table S3). To address the potential impact of outliers, we removed data points for PAC values outside the 1st and 99th percentiles, and the results remained consistent (Supplementary Table S4). To further ensure the robustness of our statistical findings, we excluded participants with incomplete covariate data, and the results were found to be consistent (Supplementary Table S5).
To assess the association between PAC and PAD across diverse populations, we conducted subgroup analyses stratified by age, sex, smoking status, drinking status, hyperlipidemia, diabetes, CHD, BMI, and PRA (Table 4). However, none of the interaction tests between PAC and any of the stratified subgroups were statistically significant. Additionally, recognizing that medications may influence PAC levels and thereby indirectly affect our findings, we conducted a subgroup analysis based on the use of various drugs. The results across all subgroups were consistent with the overall findings. Consequently, this analysis strengthens our conclusion that elevated PAC increases the risk of PAD in hypertensive patients, and this association is not influenced by the use of these medications (Supplementary Table S6).
In this cross-sectional investigation, a significant correlation between PAC and the prevalence of PAD was elucidated within a sample of hypertensive patients. Employing non-linear regression analysis, we discerned an inflection point at a PAC threshold of 17.00 ng/dl, delineating distinct patterns of association on either side of this threshold. PAC exhibited a positive correlation with PAD prevalence to the right of the inflection point, whereas no such correlation was observed to the left. The identification of this inflection point signifies a critical threshold above which the incidence of PAD appears to augment, implying that excessive aldosterone may exert a substantial impact on vascular integrity. These findings advocate for further elucidation of the intricate role of aldosterone in vascular pathophysiology and its clinical ramifications, particularly in the management of PAD associated with hypertension.
The relationship between antihypertensive therapies, aldosterone levels, and PAD outcomes is complex. A meta-analysis of 7 randomized controlled trials (RCTs) involving 71,971 patients revealed that calcium channel blockers (CCB) reduced the odds of PAD development in hypertensive patients compared to placebo or active treatment, with an OR of 0.70 (0.58, 0.86) (33). While these medications may offer protective effects against PAD, their impact on limb-specific outcomes remains inconsistent. For instance, Khan et al. found no significant reduction in major amputations with the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) (OR 1.04, 95% CI: 0.91, 1.18), whereas Elsayed et al. reported a modest improvement [hazard ratio (HR) 0.93, 95% CI: 0.87, 0.99] in a propensity-matched cohort (34, 35). This inconsistency may be attributed to varying rates of aldosterone “escape” across studies, a phenomenon where chronic inhibition of the renin-angiotensin-aldosterone system (RAAS) triggers compensatory aldosterone elevation through non-ACE pathways, potentially offsetting the vascular protective effects in PAD (36, 37). Furthermore, a review of studies involving 47,612 participants highlighted a significant association between diuretic use and increased amputation risk in PAD patients, with a meta-analysis showing an OR of 1.75 (1.53, 1.99) (38). To better understand the differential effects of drug classes, we conducted subgroup analyses stratified by ACEIs/ARBs, beta-blockers, calcium channel blockers (CCB), diuretics, lipid-lowering drugs, antidiabetic drugs, and antiplatelet drugs. The results remained consistent, reinforcing the stability of our findings. This study provides new insights into the interplay between antihypertensive drugs, PAC, and PAD outcomes.
The intricate relationship between PAC and PAD in hypertensive populations is not yet fully delineated. Aldosterone is posited to exert deleterious effects on the vasculature through various mechanisms, including the promotion of atherosclerosis, endothelial dysfunction, vascular inflammation, and vascular remodeling (39, 40). PAD is initiated by the pathological deposition of lipid and fibrous substances within the arterial walls of the lower limbs (41), with subclinical atherosclerosis identified as a significant risk factor for PAD (17). A prospective study by Marieke et al. (42) involving 2,758 patients with established coronary artery disease revealed that baseline aldosterone levels were positively correlated with the involvement of multiple vascular territories and the presence of carotid artery stenosis, and negatively associated with ABI. These findings suggest a link between aldosterone signaling and atherosclerotic processes. However, to date, no studies have directly assessed the association between PAC and PAD in hypertensive populations.
Aldosterone excess has been implicated in endothelial cell (EC) and vascular smooth muscle cell (VSMC) dysfunction (17, 18, 43). Current research supports the role of increased plasma aldosterone levels in the pathogenesis of impaired vascular relaxation and vascular stiffness. The intricate interplay between ECs and VSMCs is crucial for the modulation of vascular function and tone. ECs exert their vasodilatory effects on VSMCs principally through the production of nitric oxide (NO). NO production by ECs inhibits VSMC proliferation and migration, thereby preventing vascular stiffening (44, 45). Nishizaka et al. (46). demonstrated that elevated PAC is correlated with impaired flow-mediated vasodilation in hypertensive patients. NO, released by the endothelium, induces vasodilation, reduces vascular resistance, enhances regional blood flow, and lowers blood pressure (47). Conversely, high aldosterone concentrations can suppress NO synthesis and release (46, 48), potentially increasing EC stiffness and adversely affecting vascular function (49). Chronic exposure to elevated aldosterone levels may exacerbate endothelial dysfunction, contributing to the development of PAD (50).
Inflammation is another critical factor. Aldosterone overproduction can stimulate the local production of Ang II, a key mediator in the process involving transforming growth factor-beta 1 (TGF-β1) (16, 51). TGF-β1 and highly pro-inflammatory interleukins such as IL-1β and IL-18 can stimulate excessive extracellular matrix fibroblasts to differentiate into active myofibroblasts (52, 53). Myofibroblasts promote excessive fibrosis of the vascular wall, reducing vascular elasticity, and leading to thickening and hardening of the vascular wall (54, 55). It is noteworthy that the renin-angiotensin system (RAS) and TGF-β1 are not only key mediators of cardiac adaptations to hemodynamic overload but also critically involved in the pathogenesis of cardiac hypertrophy and failure. Ang II upregulates TGF-β1 expression via activation of the angiotensin type 1 (AT1) receptor in cardiac myocytes and fibroblasts, and induction of this cytokine is absolutely required for Ang II-induced cardiac hypertrophy in vivo. This interplay between Ang II and TGF-β1 may provide insight into the potential mechanisms by which aldosterone influences PAD pathogenesis. In conclusion, the multifaceted effects of aldosterone on the vasculature suggest a potential role in the development and progression of PAD. Further research is warranted to elucidate the direct relationship between PAC and PAD in hypertensive populations, which could have significant implications for the management and treatment of these conditions.
The present study is bolstered by its large sample size, which facilitates robust statistical analysis and enhances the precision of our estimates. The implementation of rigorous inclusion criteria has effectively minimized selection bias, ensuring a more accurate representation of the hypertensive population under study. Additionally, our research introduces a novel perspective by uncovering a nonlinear association between PAC and the prevalence of PAD, a finding that could significantly inform future investigative and clinical endeavors. Despite these strengths, our study is not without limitations that warrant consideration. Firstly, the cross-sectional design limits our ability to infer causality, a shortcoming inherent to this type of observational research. Longitudinal studies are needed to confirm the temporality and directionality of the observed associations. Secondly, the measurement of PAC at a single time point may not fully capture the dynamic fluctuations in aldosterone levels that occur over time, potentially leading to misestimation of the true exposure-risk relationship. Thirdly, while we adjusted for the use of antihypertensive medications (e.g., diuretics, ACEIs/ARBs, beta-blockers) in our analyses, detailed data on drug dosages, duration of use, and combination therapies were unavailable. This limitation introduces the possibility of residual confounding, as these factors may modulate aldosterone levels and obscure or exaggerate the observed associations. Fourthly, the exclusive use of ABI for PAD diagnosis, while practical, may not capture the full spectrum of PAD cases, particularly in the early stages of the disease. The incorporation of more sensitive diagnostic tools, such as computed tomography angiography (CTA), could enhance the accuracy of PAD detection (56–61). Lastly, the study's focus on a hypertensive patient population limits the generalizability of our findings to other demographic groups. Future research should aim to include diverse populations to ascertain the broader applicability of our observations regarding the relationship between PAC and PAD.
In conclusion, our large cross-sectional analysis establishes a significant nonlinear association between PAC and the prevalence of PAD in hypertensive patients, with a critical threshold at 17.00 ng/dl. Above this threshold, each unit increase in PAC is associated with a 9% increase in PAD prevalence. These findings highlight the potential role of aldosterone in vascular pathology and warrant further investigation into its clinical implications for PAD management in hypertensive individuals.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study design was approved by the Clinical Ethics Committee of the People’s Hospital of Xinjiang Uyghur Autonomous Region (KY2024072536).
YZ: Writing – original draft, Writing – review & editing. XC: Writing – original draft, Writing – review & editing, Data curation, Formal analysis. SS: Data curation, Methodology, Writing – review & editing. JH: Data curation, Writing – review & editing. PZ: Data curation, Methodology, Writing – review & editing. KC: Formal analysis, Methodology, Writing – review & editing. RM: Data curation, Formal analysis, Writing – review & editing. HM: Project administration, Supervision, Writing – review & editing. DS: Data curation, Investigation, Writing – review & editing. WY: Data curation, Resources, Writing – review & editing. DZ: Investigation, Methodology, Writing – review & editing. QL: Project administration, Supervision, Writing – review & editing. JH: Data curation, Validation, Writing – review & editing. NL: Data curation, Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Tianshan Talent Training Program-Science and Technology Innovation Team (2023TSYCTD0016).
The authors acknowledge the contribution of all the staff who participated in this study as well as the study participants who shared their time with us.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1549878/full#supplementary-material
1. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. (2013) 382(9901):1329–40. doi: 10.1016/S0140-6736(13)61249-0
2. Malyar N, Fürstenberg T, Wellmann J, Meyborg M, Lüders F, Gebauer K, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J. (2013) 34(34):2706–14. doi: 10.1093/eurheartj/eht288
3. Déruaz-Luyet A, Raabe C, Garry EM, Brodovicz KG, Lavery LA. Incidence of lower extremity amputations among patients with type 1 and type 2 diabetes in the United States from 2010 to 2014. Diabetes Obes Metab. (2020) 22(7):1132–40. doi: 10.1111/dom.14012
4. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. (2021) 128(12):1818–32. doi: 10.1161/CIRCRESAHA.121.318535
5. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. (2015) 116(9):1509–26. doi: 10.1161/CIRCRESAHA.116.303849
6. Das JR, Eberhardt RT. Contemporary risk assessment and cardiovascular outcomes in peripheral arterial disease. Cardiovasc Hematol Disord Drug Targets. (2013) 13(3):185–96. doi: 10.2174/1871529X1303140129154241
7. Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham offspring study. Am Heart J. (2002) 143(6):961–5. doi: 10.1067/mhj.2002.122871
8. Emdin CA, Anderson SG, Callender T, Conrad N, Salimi-Khorshidi G, Mohseni H, et al. Usual blood pressure, peripheral arterial disease, and vascular risk: cohort study of 4.2 million adults. Br Med J. (2015) 351:h4865. doi: 10.1136/bmj.h4865
9. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the national health and nutrition examination survey, 1999–2000. Circulation. (2004) 110(6):738–43. doi: 10.1161/01.CIR.0000137913.26087.F0
10. Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the national health and nutrition examination survey 1999–2004. J Am Geriatr Soc. (2007) 55(4):583–9. doi: 10.1111/j.1532-5415.2007.01123.x
11. Korhonen PE, Kautiainen H, Kantola I. Patients with resistant hypertension have more peripheral arterial disease than other uncontrolled hypertensives. J Hum Hypertens. (2015) 29(1):46–9. doi: 10.1038/jhh.2014.65
12. Wright FS, Giebisch G. Renal potassium transport: contributions of individual nephron segments and populations. Am J Physiol. (1978) 235(6):F515–27. doi: 10.1152/ajprenal.1978.235.6.F515
13. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2008) 93(9):3266–81. doi: 10.1210/jc.2008-0104
14. Holaj R, Zelinka T, Wichterle D, Petrák O, Strauch B, Widimský J Jr. Increased intima-media thickness of the common carotid artery in primary aldosteronism in comparison with essential hypertension. J Hypertens. (2007) 25(7):1451–7. doi: 10.1097/HJH.0b013e3281268532
15. Strauch B, Petrák O, Wichterle D, Zelinka T, Holaj R, Widimský J Jr. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am J Hypertens. (2006) 19(9):909–14. doi: 10.1016/j.amjhyper.2006.02.002
16. Nguyen Dinh Cat A, Jaisser F. Extrarenal effects of aldosterone. Curr Opin Nephrol Hypertens. (2012) 21(2):147–56. doi: 10.1097/MNH.0b013e32834fb25b
17. Ferreira NS, Tostes RC, Paradis P, Schiffrin EL. Aldosterone, inflammation, immune system, and hypertension. Am J Hypertens. (2021) 34(1):15–27. doi: 10.1093/ajh/hpaa137
18. Concistrè A, Petramala L, Bisogni V, Mezzadri M, Olmati F, Saracino V, et al. Subclinical atherosclerosis due to increase of plasma aldosterone concentrations in essential hypertensive individuals. J Hypertens. (2019) 37(11):2232–9. doi: 10.1097/HJH.0000000000002170
19. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5(1):56. doi: 10.1038/s41572-019-0106-z
20. Campia U, Gerhard-Herman M, Piazza G, Goldhaber SZ. Peripheral artery disease: past, present, and future. Am J Med. (2019) 132(10):1133–41. doi: 10.1016/j.amjmed.2019.04.043
21. Mandaglio-Collados D, Marín F, Rivera-Caravaca JM. Peripheral artery disease: update on etiology, pathophysiology, diagnosis and treatment. Med Clin (Barc). (2023) 161(8):344–50. doi: 10.1016/j.medcli.2023.06.005
22. Wassel CL, Berardi C, Pankow JS, Larson NB, Decker PA, Hanson NQ, et al. Soluble P-selectin predicts lower extremity peripheral artery disease incidence and change in the ankle brachial index: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. (2015) 239(2):405–11. doi: 10.1016/j.atherosclerosis.2015.01.022
23. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American heart association. Circulation. (2012) 126(24):2890–909. doi: 10.1161/CIR.0b013e318276fbcb
24. Song S, Cai X, Hu J, Zhu Q, Shen D, Ma H, et al. Effectiveness of spironolactone in reducing osteoporosis and future fracture risk in middle-aged and elderly hypertensive patients. Drug Des Devel Ther. (2024) 18:2215–25. doi: 10.2147/DDDT.S466904
25. Song S, Cai X, Hu J, Zhu Q, Shen D, Heizhati M, et al. Correlation between plasma aldosterone concentration and bone mineral density in middle-aged and elderly hypertensive patients: potential impact on osteoporosis and future fracture risk. Front Endocrinol (Lausanne). (2024) 15:1373862. doi: 10.3389/fendo.2024.1373862
26. Hu J, Cai X, Zhu Q, Heizhati M, Wen W, Luo Q, et al. Relationship between plasma aldosterone concentrations and non-alcoholic fatty liver disease diagnosis in patients with hypertension: a retrospective cohort study. Diabetes Metab Syndr Obes. (2023) 16:1625–36. doi: 10.2147/DMSO.S408722
27. Shen D, Cai X, Hu J, Song S, Zhu Q, Ma H, et al. Associating plasma aldosterone concentration with the prevalence of MAFLD in hypertensive patients: insights from a large-scale cross-sectional study. Front Endocrinol (Lausanne). (2024) 15:1451383. doi: 10.3389/fendo.2024.1451383
28. Cai X, Song S, Hu J, Zhu Q, Shen D, Yang W, et al. Association of the trajectory of plasma aldosterone concentration with the risk of cardiovascular disease in patients with hypertension: a cohort study. Sci Rep. (2024) 14(1):4906. doi: 10.1038/s41598-024-54971-4
29. Cai X, Li N. Association between use of spironolactone and risk of stroke in hypertensive patients: a cohort study. Pharmaceuticals (Basel). (2022) 16(1):57. doi: 10.3390/ph16010057
30. Zhou P, Cai X, Song S, Hu J, Zhu Q, Ma H, et al. Association of plasma aldosterone concentration with arterial stiffness progression in hypertensive patients: insights from a longitudinal analysis. Postgrad Med. (2025) 137:164–73. doi: 10.1080/00325481.2025.2460417
31. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2017) 135(12):e790. doi: 10.1161/CIR.0000000000000471
32. Felício JS, Koury CC, Abdallah Zahalan N, de Souza Resende F, Nascimento de Lemos M, Jardim da Motta Corrêa Pinto R, et al. Ankle-brachial index and peripheral arterial disease: an evaluation including a type 2 diabetes mellitus drug-naïve patients cohort. Diab Vasc Dis Res. (2019) 16(4):344–50. doi: 10.1177/1479164119829385
33. Shetty S, Malik AH, Feringa H, El Accaoui R, Girotra S. Meta-Analysis evaluating calcium channel blockers and the risk of peripheral arterial disease in patients with hypertension. Am J Cardiol. (2020) 125(6):907–15. doi: 10.1016/j.amjcard.2019.12.026
34. Khan SZ, O'Brien-Irr MS, Rivero M, Blochle R, Cherr GS, Dryjski ML, et al. Improved survival with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic limb-threatening ischemia. J Vasc Surg. (2020) 72(6):2130–8. doi: 10.1016/j.jvs.2020.02.041
35. Elsayed N, Straus SL, Clouse D, Motaganahalli RL, Malas M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with improved amputation-free survival in chronic limb-threatening ischemia. J Vasc Surg. (2025) 81(1):229–234.e1. doi: 10.1016/j.jvs.2024.09.008
36. Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. (2007) 3(9):486–92. doi: 10.1038/ncpneph0575
37. Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. (2007) 49(4):839–45. doi: 10.1161/01.HYP.0000259805.18468.8c
38. Ba K, Sow MA, Magne J, Salle L, Lacroix P, Chastaingt L, et al. Risk of amputation under diuretics in patients with or at risk of lower extremity arterial disease: a systematic review and meta-analysis. Arch Cardiovasc Dis. (2023) 116(6-7):357–63. doi: 10.1016/j.acvd.2023.04.002
39. Dinh QN, Young MJ, Evans MA, Drummond GR, Sobey CG, Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. (2016) 1637:146–53. doi: 10.1016/j.brainres.2016.02.034
40. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. (2010) 6(5):261–73. doi: 10.1038/nrneph.2010.30
41. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0
42. Hillaert MA, Lentjes EG, Kemperman H, van der Graaf Y, Nathoe HM, Beygui F, et al. Aldosterone, atherosclerosis and vascular events in patients with stable coronary artery disease. Int J Cardiol. (2013) 167(5):1929–35. doi: 10.1016/j.ijcard.2012.05.034
43. Chen ZW, Tsai CH, Pan CT, Chou CH, Liao CW, Hung CS, et al. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci. (2019) 20(20):5214. doi: 10.3390/ijms20205214
44. Hill MA, Jaisser F, Sowers JR. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovasc Res. (2022) 118(1):130–40. doi: 10.1093/cvr/cvaa326
45. Vasan RS, Short MI, Niiranen TJ, Xanthakis V, DeCarli C, Cheng S, et al. Interrelations between arterial stiffness, target organ damage, and cardiovascular disease outcomes. J Am Heart Assoc. (2019) 8(14):e012141. doi: 10.1161/JAHA.119.012141
46. Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. (2004) 109(23):2857–61. doi: 10.1161/01.CIR.0000129307.26791.8E
47. Toda N, Nakanishi S, Tanabe S. Aldosterone affects blood flow and vascular tone regulated by endothelium-derived NO: therapeutic implications. Br J Pharmacol. (2013) 168(3):519–33. doi: 10.1111/j.1476-5381.2012.02194.x
48. Hashikabe Y, Suzuki K, Jojima T, Uchida K, Hattori Y. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol. (2006) 47(4):609–13. doi: 10.1097/01.fjc.0000211738.63207.c3
49. Li H, Förstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol. (2013) 13(2):161–7. doi: 10.1016/j.coph.2013.01.006
50. Fels J, Oberleithner H, Kusche-Vihrog K. Ménage à trois: aldosterone, sodium and nitric oxide in vascular endothelium. Biochim Biophys Acta. (2010) 1802(12):1193–202. doi: 10.1016/j.bbadis.2010.03.006
51. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. (2018) 281(1):8–27. doi: 10.1111/imr.12621
52. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. (2008) 214(2):199–210. doi: 10.1002/path.2277
53. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. (2016) 12(6):325–38. doi: 10.1038/nrneph.2016.48
54. Oberleithner H, Ludwig T, Riethmüller C, Hillebrand U, Albermann L, Schäfer C, et al. Human endothelium: target for aldosterone. Hypertension. (2004) 43(5):952–6. doi: 10.1161/01.HYP.0000123572.45556.a5
55. Rizzoni D, Paiardi S, Rodella L, Porteri E, De Ciuceis C, Rezzani R, et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with primary aldosteronism. J Clin Endocrinol Metab. (2006) 91(7):2638–42. doi: 10.1210/jc.2006-0101
56. Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. (2003) 163(8):884–92. doi: 10.1001/archinte.163.8.884
57. Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA. (1969) 207(10):1869–74. doi: 10.1001/jama.1969.03150230083009
58. Ouriel K, Zarins CK. Doppler Ankle pressure: an evaluation of three methods of expression. Arch Surg. (1982) 117(10):1297–300. doi: 10.1001/archsurg.1982.01380340031008
59. Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg. (1969) 56(9):676–9. doi: 10.1002/bjs.1800560910
60. Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation. (1968) 37(4):624–37. doi: 10.1161/01.CIR.37.4.624
Keywords: plasma aldosterone concentration, peripheral vascular disease, ankle-brachial index, hypertension, non-linear relationship, cross-sectional study
Citation: Zhang Y, Cai X, Song S, Hu J, Zhou P, Cai K, Ma R, Ma H, Shen D, Yang W, Zhang D, Luo Q, Hong J and Li N (2025) Association of plasma aldosterone concentration with peripheral artery disease in hypertensive patients: insights from a large cross-sectional analysis. Front. Cardiovasc. Med. 12:1549878. doi: 10.3389/fcvm.2025.1549878
Received: 22 December 2024; Accepted: 12 March 2025;
Published: 25 March 2025.
Edited by:
Bertram Pitt, University of Michigan, United StatesReviewed by:
Fabrizio Buffolo, University of Turin, ItalyCopyright: © 2025 Zhang, Cai, Song, Hu, Zhou, Cai, Ma, Ma, Shen, Yang, Zhang, Luo, Hong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanfang Li, bG5hbmZhbmcyMDE2QHNpbmEuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.