
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 March 2025
Sec. Atherosclerosis and Vascular Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1538466
This article is part of the Research TopicMechanisms and Management of Inflammation-driven Cardiovascular Risk: from Obesity and Diabetes to Autoimmunity and CancerView all 9 articles
 Giancarlo Pesce1*
Giancarlo Pesce1* Gaelle Gusto2
Gaelle Gusto2 Pierre Johansen3
Pierre Johansen3 Artak Khachatryan4
Artak Khachatryan4 Bernabe Lopez-Ledesma5
Bernabe Lopez-Ledesma5 Jelena Vukmirica6
Jelena Vukmirica6 Aleix Cases7
Aleix Cases7
Introduction: Systemic inflammation is recognised as a critical driver of atherosclerotic cardiovascular disease (ASCVD), especially in patients with comorbid chronic kidney disease (CKD). This study aims to assess the prevalence of systemic inflammation in the ASCVD population in Spain.
Methods: Outpatient electronic medical records from The Health Improvement Network (THIN®) database were used to identify patients with ASCVD and a C-reactive protein (CRP) measurement ≥1 between January 2014 and July 2023 in Spain. The proportion of patients with systemic inflammation (defined as CRP ≥ 2 mg/L) was estimated at the first CRP measurement (index date) and at the end of the study. The patients' characteristics, comorbidities, and drug dispensation in the prior 12 months were reported by systemic inflammation status at the index date.
Results: Overall, 15,798 patients with ASCVD were included in the study (mean age: 71.1 years; 57% men), of whom 34% had CKD. The proportion of patients with systemic inflammation at the index date was 58% (65% among CKD patients) and 56% (62% among CKD patients) at the end of the study. Patients with systemic inflammation were more frequently smokers, obese, with comorbidities, and had higher low-density lipoprotein cholesterol and triglycerides levels than patients without systemic inflammation. Overall, patients with ASCVD and systemic inflammation used statins and aspirin less frequently compared to patients without systemic inflammation, while they used antibiotics, anticoagulants, and antihypertensives more frequently.
Conclusion: Systemic inflammation prevalence is high among patients with ASCVD in Spain, especially among patients with comorbid CKD. Therapeutic strategies focused on targeting systemic inflammation may have beneficial effects in reducing the burden of ASCVD.
• Atherosclerotic cardiovascular disease (ASCVD) affects ∼10% of the Spanish population, contributing to 28% of all deaths.
• Despite optimal therapy, patients with ASCVD remain at high risk of recurrent major adverse cardiovascular events; systemic inflammation has been identified as an independent driver of residual cardiovascular risk.
• In Spain, systemic inflammation prevalence is high among patients with ASCVD, especially among patients with comorbid CKD.
• Patients with systemic inflammation also had higher prevalences of several comorbidities and atherogenic dyslipidaemia.
• This study highlights the need for novel strategies that target the reduction of inflammation for the secondary prevention of cardiovascular events and mortality in patients with ASCVD and systemic inflammation.
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of mortality in developed countries, posing a substantial burden in terms of morbidity and utilisation of healthcare resources (1). Globally, over half a billion people suffer from cardiovascular diseases, with nearly 20 million deaths annually (2), and approximately three-quarters of these cases are attributed to atherosclerotic diseases, particularly ischemic heart disease and stroke (2, 3). In Spain, ASCVD affects nearly 10% of the population, contributing to 28% of deaths and 13% of hospital admissions (4, 5).
Current guidelines for the management of patients with established ASCVD focus on lifestyle optimisation and more stringent control of classical modifiable cardiovascular risk factors, including diabetes, hypertension, and low-density lipoprotein cholesterol (LDL-C) (6). Despite optimal therapy, patients with ASCVD remain at high risk of recurrent major adverse cardiovascular events.
In recent years, there has been a growing recognition of systemic inflammation as an independent driver of residual cardiovascular risk (6–8). Increasing evidence has highlighted the pivotal role of systemic inflammation in the pathogenesis of ASCVD (9). Systemic inflammation can promote ASCVD progression during clinically stable phases, exacerbate the disease by advancing atherosclerosis, trigger atherosclerotic plaque destabilisation and acute coronary syndromes, and respond to cardiomyocyte necrosis in myocardial infarction (MI) (10). C-reactive protein (CRP) is a biomarker of systemic inflammation. In healthy women, high levels of CRP were predictive of incident cardiovascular events during a 30-year period (11). Systemic inflammation, defined by a CRP level equal to or greater than 2 mg/L, has been associated with a poor prognosis in patients with ASCVD, including an increased risk of cardiovascular events and higher overall mortality (7, 8, 12).
Chronic kidney disease (CKD) is associated with a state of systemic inflammation that contributes to faster renal deterioration and disease progression (13, 14) and is independently associated with an increased incidence of ASCVD and risk of MI and stroke (15–18).
Targeting systemic inflammation in patients with ASCVD, especially in those with comorbid CKD, may improve these high-risk patients' outcomes (19–23). Despite emerging evidence on the role of systemic inflammation as a risk factor in the progression of ASCVD (24–27), the epidemiology of systemic inflammation in patients with ASCVD within the general population has not been thoroughly investigated.
This study aims to generate real-world evidence to understand the prevalence and characteristics of systemic inflammation in patients with ASCVD and CKD in Spain, as it is crucial to better understand the residual cardiovascular risk, clinical characteristics, and unmet needs of patients with ASCVD in the real-world setting to inform healthcare providers and deliver optimised patient care to prevent recurrent cardiovascular events and mortality.
The specific objectives of the study are as follows: (1) to estimate the prevalence of systemic inflammation in patients with ASCVD (overall and by CKD status); (2) to describe the characteristics of patients with ASCVD by systemic inflammation status, including demographics, clinical parameters, comorbidities, and medication use.
This was a population-based, retrospective, observational cohort study. Data were collected from the Spanish arm of The Health Improvement Network (THIN®) database (https://www.the-health-improvement-network.com/). THIN® is a secondary, irreversible anonymised database from primary care and ambulatory specialist care, linked at the patient level, with data recorded by general practitioners (GPs) during routine clinical practice. In Spain, THIN® data are routinely derived from electronic health records and include data on patient characteristics, prescriber information, and patient medical history, including clinical diagnostics (diagnosis recorded using the international classification of diseases, version 9: ICD-9), prescriptions (drugs and procedures), and laboratory test results. THIN® holds clinical data for ∼3 million individuals living in Spain, including approximately 1.2 million active patients with at least one visit during the previous 12 months (i.e., ∼3% of the Spanish general population). The database is updated monthly, with 1–3 months of lag time for the availability of the most recent data.
This study was carried out following the recommendations for clinical investigations included in the Declaration of Helsinki of the World Medical Association. The data used in this study are derived from a secondary database, where patients had previously provided informed consent at the source before the irreversible anonymisation of the database. For the processing and transfer of personal data to the THIN® Spain database, informed and unequivocal consent is obtained from both patients and their clinical practices. If any individual exercises their right to revoke consent for data processing, the patient's information is removed from the database prior to anonymisation. Since the database was anonymised at its origin and prior informed consent was ensured, ethical approval was not required for this study.
Adult patients (age ≥18 years) with ASCVD and at least one eligible CRP measurement recorded between 1 January 2014 and 31 July 2023 (latest data available at the time of data extraction) were included in the study (Supplementary Table S1). ASCVD was defined by ICD-9 records for atherosclerotic coronary events (e.g., MI, angina, other ischemic conditions, or undergoing revascularisation), cerebrovascular events (e.g., occlusion of cerebral arteries or transient cerebral ischemia), peripheral arterial disease, and other atherosclerotic vascular diseases (e.g., generalised and unspecified atherosclerosis), as presented in the Appendix. Patients without an eligible CRP measurement were excluded from the study.
Systemic inflammation was defined as a CRP measurement ≥2 mg/L (10). CRP measurements were not considered eligible for the present study and excluded if they were recorded: (i) before first diagnosis of ASCVD; (ii) concomitantly with active infections (i.e., all CRP measurements >20 mg/L; CRP test taken within 2 months after or 7 days before antibiotic/antiviral prescriptions); (iii) under the effect of immunosuppressants (i.e., CRP tests taken less than 3 months after the prescription of immunosuppressant drugs); (iv) in patients with active cancer (i.e., CRP test taken less than 3 years after a diagnosis code for a malignancy, except non-melanoma skin cancer). In the case of multiple eligible CRP measurements taken within 3 months, they were grouped and averaged using a geometric mean. Notably, information about the type of CRP testing (standard or high-sensitivity) is not recorded in the THIN® database.
CKD was defined as a prior record of stage 3 or higher CKD diagnosis, estimated glomerular filtration rate [eGFR, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2021 formula] <60 ml/min/1.73 m2, or a urinary albumin-to-creatinine ratio (UACR) ≥ 30 mg/g (28, 29). Patients' comorbidities were defined using primary care diagnoses, and drug prescriptions were classified using the anatomical therapeutic chemical (ATC) classification system (codes provided in the Appendix).
Categorical variables were reported using percentages and continuous variables were reported using mean ± standard deviation or median [interquartile range] according to a normal or skewed distribution, respectively.
The demographic [e.g., sex, age, smoking status, and socioeconomic status (SES)] and clinical characteristics of the patients were those reported at the time of the first eligible CRP measurement, which was set as the index date, or the most recent information available prior to the index date. Low SES was defined as residents with minimum earnings, a drug reimbursement rate of 100%, or non-retired patients with a drug reimbursement rate ≥90%.
For laboratory measurements [i.e., eGFR, UACR, haemoglobin level, total cholesterol, high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides] and clinical measurements [body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP)], the most recent information available within 18 months before the index date was considered. The history of comorbidities was defined using the presence of ICD-9 diagnosis codes recorded at any time before the index date. The use of medications was defined by dispensation with selected ATC codes in the 12 months before the index date. The dispensation of medications in the 12 months after the index date was also reported.
The proportion of systemic inflammation in patients with ASCVD was assessed at three different time points:
(i) at first eligible CRP measurement, i.e., the number of patients with first eligible CRP measurement ≥ 2 mg/L divided by the total number of patients included in the study;
(ii) at the latest available date (point prevalence), i.e., the proportion of patients with systemic inflammation among the patients alive at the data cutoff date (31 July 2023) and with at least one eligible CRP measurement in the prior 18 months;
(iii) over the entire study period, i.e., the proportion of patients with systemic inflammation at any time during the study period among the overall study population.
A diagram illustrating how patients were defined in terms of systemic inflammation at each time point is shown in Supplementary Figure S2. Analyses were conducted for all patients with ASCVD included in the study and separately for the subgroup of patients with ASCVD and CKD at first CRP measurement. Within the patient population with CKD, the proportion of patients with systemic inflammation was further assessed by CKD stage.
As systemic inflammation is elevated with chronic inflammatory conditions, the proportion of patients with systemic inflammation was further assessed after the exclusion of patients with ASCVD and comorbid inflammatory and rheumatoid diseases, as defined in the Appendix.
Overall, 76,423 patients with a primary care diagnosis of ASCVD between 1 January 2014 and 31 July 2023 were identified in the THIN-Spain database (Figure 1). After the exclusion of patients aged <18 years or with chronic infection diseases, 34% of the adult patients had at least one CRP measurement during the study period (n = 26,082) of whom n = 15,798 had at least one eligible CRP measurement and were included in the analyses. Overall, 32% (n = 5,111) of the included patients with ASCVD had CKD diagnosed at the time of their first eligible CRP measurement, 62% (n = 9,712) had no prior diagnosis of CKD, and a CKD status was not assigned in 6% of patients who did not have any eGFR measurement or diagnoses of CKD. A majority of the included patients had a diagnosis of coronary artery disease (54.5%), 42.3% had been diagnosed with a cerebrovascular atherosclerotic event, 7.7% had peripheral artery disease (PAD), and 9.9% suffered from other atherosclerotic vascular diseases.
Most of the patients (67%) had only one eligible CRP measurement during the study period, while 33% had two or more measurements. The mean and median values of eligible CRP measurements were 3.7 mg/L (standard deviation: 3.8) and 2.4 mg/L (interquartile range: 1.2–4.8), respectively. Overall, 58% of the patients with ASCVD had systemic inflammation at their first eligible CRP measurement (Figure 2). The proportion was higher in the patients with CKD compared to the patients without CKD (65% vs. 55%, respectively). The point prevalence of systemic inflammation at the data cutoff date (31 July 2023) was 56% among alive patients with at least one CRP measurement in the prior 18 months, and it was greater among the patients with ASCVD and CKD (62%) than in the patients with ASCVD without CKD (52%). Overall, 64% of the patients had at least 1 CRP measurement greater than 2 mg/L during the entire study period. Among the patients with ASCVD and CKD, the proportion of patients with systemic inflammation was greater among the patients with stage 4 CKD or stage 5 CKD compared to patients with a lower CKD stage (Supplementary Table S1).
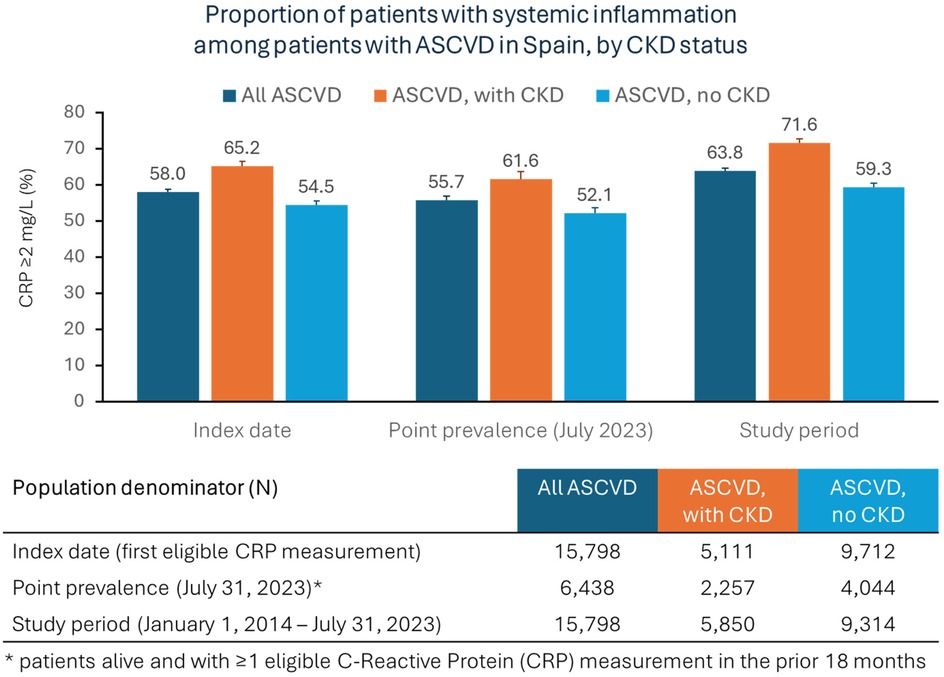
Figure 2. Prevalence of systemic inflammation among patients with ASCVD in Spain, overall and by CKD status (central illustration).
The proportion of patients with systemic inflammation at the index date was slightly higher in women than men (59% vs. 57%, respectively), both among all patients with ASCVD and among patients with comorbid CKD (Supplementary Figure S3). Overall, patients with ASCVD older than 75 years had a higher proportion of systemic inflammation than younger age groups at the index date (56%, 57%, and 60% among patients aged <65, 65–74, and ≥75 years, respectively). In contrast, among the patients with ASCVD and comorbid CKD, the proportion of systemic inflammation was higher in the patients <65 years old than in older age groups (69%, 64%, and 65% among patients aged <65, 65–74, and ≥75 years, respectively) (Supplementary Figure S4).
The mean age of the overall ASCVD study population was 71 years and 57% were men (Table 1). Compared to the patients without systemic inflammation, the patients with systemic inflammation were older, included more women and smokers and had a lower SES and higher BMIs. The patients with systemic inflammation had a higher prevalence of CKD and other comorbidities including diabetes, hypertension, PAD, heart failure, chronic obstructive pulmonary disease (COPD), and atrial fibrillation. Furthermore, the patients with systemic inflammation had lower baseline eGFR, haemoglobin, and HDL-C levels and higher LDL-C and triglycerides levels than the patients without systemic inflammation.
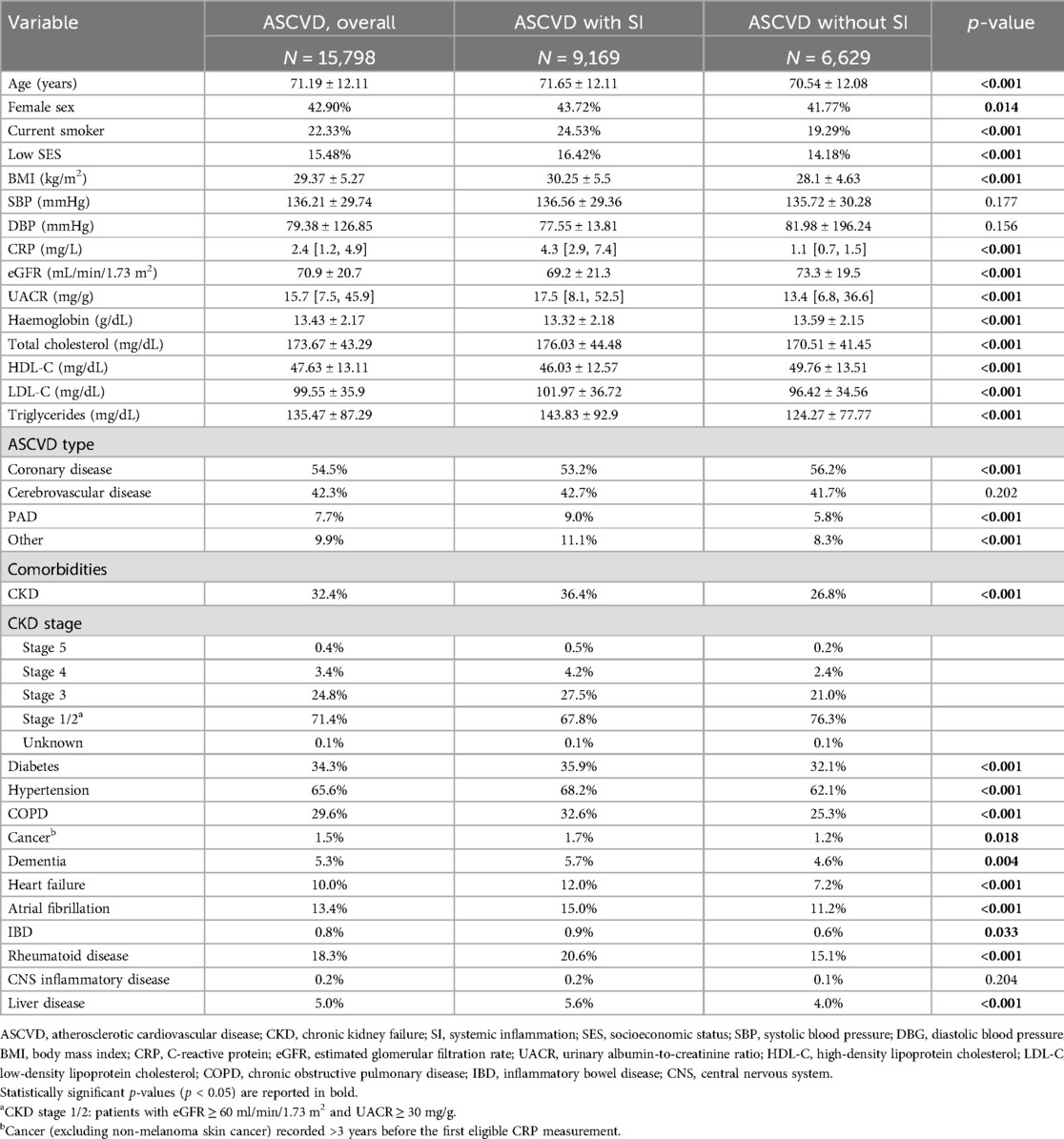
Table 1. Demographic and clinical characteristics of patients with ASCVD by systemic inflammation (SI) status. Data expressed as mean ± standard deviation, median [IQR], or percentage.
Among the patients with ASCVD and CKD, those with systemic inflammation were more frequently smokers and had higher BMIs; lower eGFR, haemoglobin, and HDL-C levels; and higher levels of LDL-C and triglycerides. The patients with ASCVD, CKD, and inflammation had a higher prevalence of COPD, heart failure, atrial fibrillation, rheumatoid disease, and PAD than the patients with ASCVD and CKD but without systemic inflammation (Table 2).
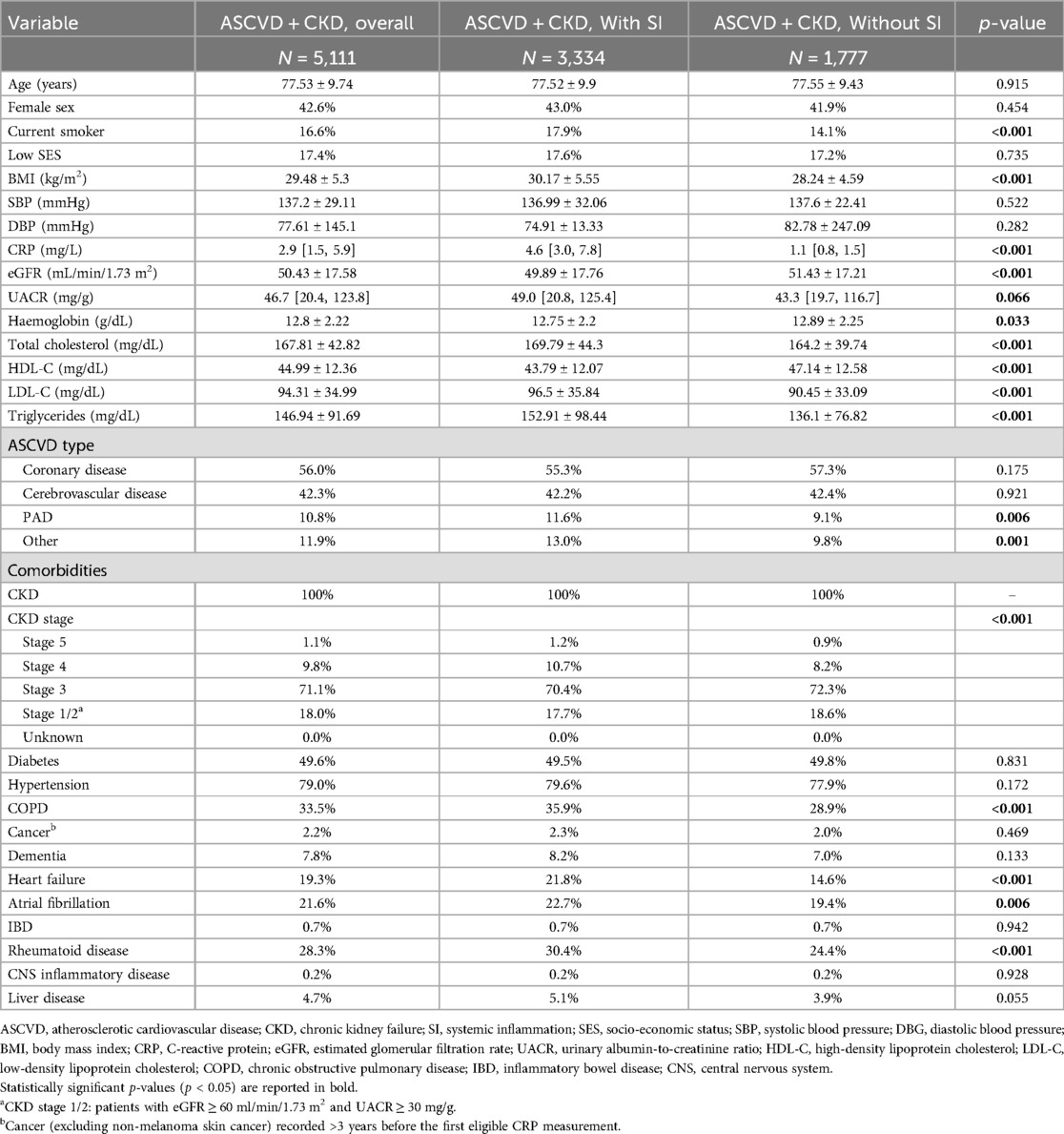
Table 2. Demographic and clinical characteristics of patients with ASCVD with CKD by SI status. Data expressed as mean ± standard deviation, median [IQR], or percentage.
Drug utilisation in the 12 months prior to the first eligible CRP measurement in patients with ASCVD is summarised in Table 3. Compared to the patients without systemic inflammation, those with systemic inflammation generally had a greater utilisation of most medications, including angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEis/ARBs), mineralocorticoid receptor antagonists (MRAs), diuretics and anticoagulants, except for aspirin and antiplatelet agents, statins, and other lipid-lowering agents that were less prescribed in the patients with systemic inflammation.
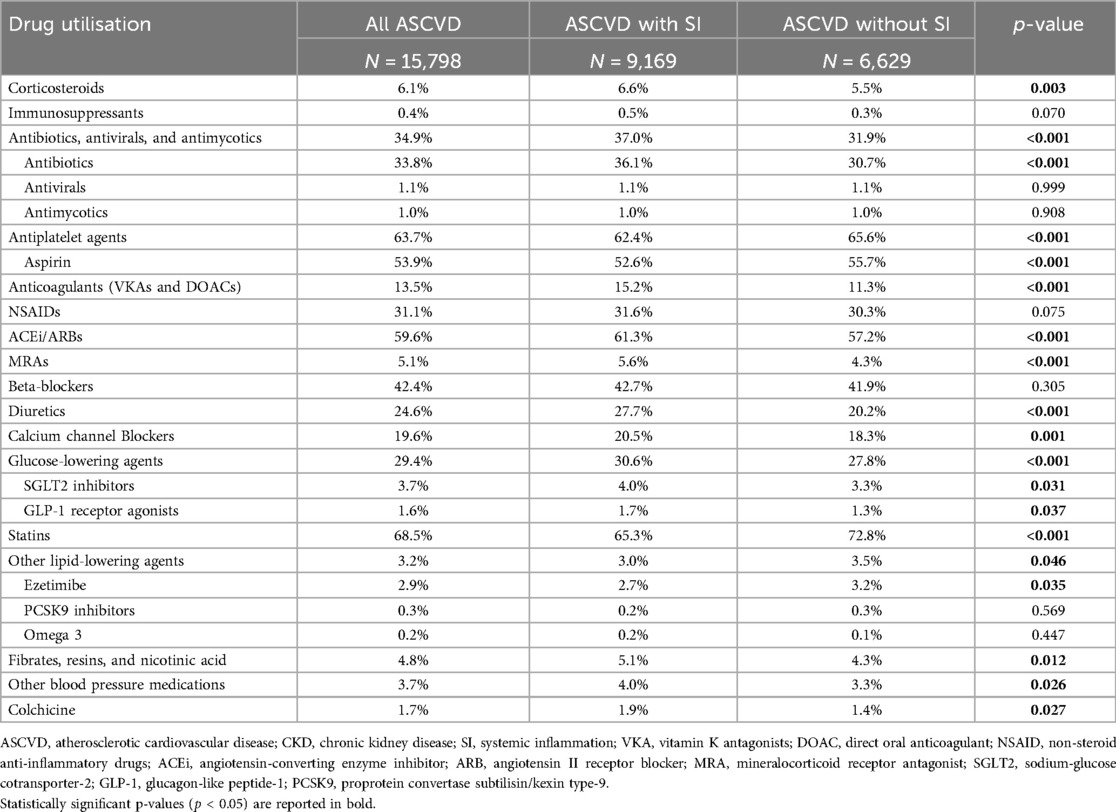
Table 3. Drug utilisation in patients with ASCVD, by SI status. The table reports the percentage of patients using each pharmacological group in the 12 months prior to the first eligible CRP measurement.
Among the patients with ASCVD and CKD, those with systemic inflammation used more antibiotics, anticoagulants, MRAs, and diuretics but they had a lower utilisation of antiplatelet agents (including aspirin) and statins in the 12 months before the index date than the patients without systemic inflammation (Table 4). Similar drug utilisation was observed during the 12 months after the index date in patients with ASCVD overall and in those with comorbid CKD (Supplementary Tables S2 and S3).
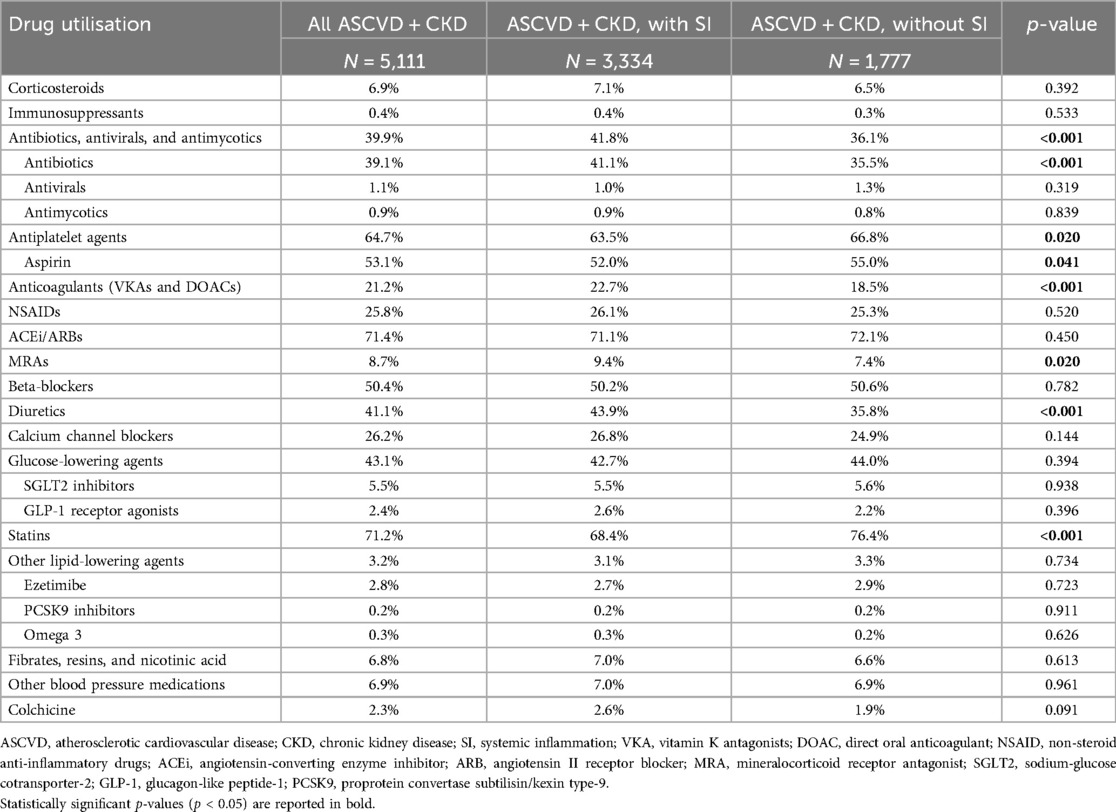
Table 4. Drug utilisation in patients with ASCVD with CKD, by SI status. The table reports the percentage of patients using each pharmacological group in the 12 months prior to the first eligible CRP measurement.
This study investigated the prevalence of systemic inflammation in patients with ASCVD using electronic medical records from a nationwide, population-based primary care database in Spain and reported the demographic and clinical characteristics of the patients. The main findings of the study are as follows:
• In Spain, 56% of patients with ASCVD had systemic inflammation, and the proportion increased to 62% among those with comorbid CKD.
• Systemic inflammation was more frequent in women; smokers; patients with obesity and comorbidities, including PAD, hypertension, diabetes, heart failure, atrial fibrillation, and non-cardiovascular comorbidities; and patients with a more atherogenic risk profile (higher LDL-C and triglyceride levels and lower HDL-C level).
• The patients with ASCVD and systemic inflammation used statins and aspirin less frequently and antibiotics, anticoagulants, MRAs, and diuretics more frequently compared to the patients without systemic inflammation.
The analyses highlighted that most of the Spanish patients with ASCVD had evidence of systemic inflammation, and the proportion was 62% among the patients with comorbid CKD. Considering that nearly 5 million patients in Spain live with ASCVD (5), our estimates suggest that nearly 2.5 million patients with ASCVD have systemic inflammation, of whom approximately 1 million have comorbid CKD.
Systemic inflammation has been increasingly recognised as a significant risk factor for cardiovascular events. Clinical trials have shown that anti-inflammatory strategies may reduce the incidence of cardiovascular events in patients with ASCVD, underscoring the critical role of inflammation in residual cardiovascular risk (30–32). The phase-3 ZEUS trial is currently testing whether targeting systemic inflammation with ziltivekimab, a monoclonal antibody that targets interleukin-6, can reduce the risk of recurrent cardiovascular events in patients with established ASCVD, CKD, and systemic inflammation (33).
This is one of the first studies investigating the prevalence of systemic inflammation in patients with ASCVD from the general population, and the first one carried out in Spain. To our knowledge, only two other studies have reported the prevalence of systemic inflammation in representative samples of patients with ASCVD. A recent study using the Stockholm's healthcare database in Sweden showed that 59% of adult patients with ASCVD and 69% of patients with ASCVD and CKD had systemic inflammation at their first high-sensitivity CRP measurement (12). In the US, a study using the National Health and Nutrition Examination Survey (NHANES) data reported that 55% of adult patients with ASCVD and 62% of patients with ASCVD and stage 3 or 4 CKD had systemic inflammation (34). These estimates for systemic inflammation among the ASCVD populations in Sweden and the US are largely aligned with our findings in the THIN-Spain database. Another recent publication on ∼24,000 participants in three randomised, placebo-controlled phase-3 trials investigating the efficacy of semaglutide (SELECT, SOUL, and FLOW) reported that systemic inflammation was present in 56% of patients with ASCVD and CKD, as compared to 47% of patients with ASCVD and without CKD (35).
CRP is currently the most relevant biomarker for the assessment of residual inflammatory risk (36), and a cutoff of 2 mg/L has been adopted in several clinical trials, including CANTOS, JUPITER, RESCUE, and ZEUS (21, 33, 37, 38) to identify a subgroup of patients with ASCVD at greater risk of atheroprogression and mortality (7).
Systemic inflammation was more prevalent in patients with ASCVD and CKD, with greater prevalence at more advanced stages of the disease. In fact, CKD is associated with a proinflammatory milieu (17). Furthermore, inflammation is a critical factor in the progression of CKD, and persistent systemic inflammation has been associated with a faster decline of renal function and hypertension and increased mortality and cardiovascular events in this population (14).
In our study, the patients with ASCVD and systemic inflammation also had a greater prevalence of several comorbidities (including higher BMI, hypertension, diabetes, PAD, heart failure, and atrial fibrillation) than the patients without systemic inflammation. This finding is in line with current medical literature, as systemic inflammation has been reported to play a role in the onset and progression of several diseases such as diabetes (39), hypertension (40), Alzheimer's disease and dementia (41, 42), atrial fibrillation (43), rheumatoid arthritis (44), and liver complications such as non-alcoholic fatty liver disease and cirrhosis (45). CRP, moreover, has been identified as a physiological marker of multimorbidity (46), suggesting that systemic inflammation may negatively affect multiple organs simultaneously and increase the risk of comorbidities.
In our study, greater proportions of smokers were observed among the patients with ASCVD and systemic inflammation compared to those without systemic inflammation. The association of tobacco smoking with increased levels of inflammation markers, including CRP, is well-acknowledged (47).
The patients with ASCVD and systemic inflammation had higher BMIs compared to those without systemic inflammation. The link between obesity and systemic chronic inflammation is well-established (48). Adipose tissue can activate the innate immune system and the release of inflammatory cytokines, sustaining systemic inflammation and causing an increase in CRP levels; in turn, sustained systemic inflammation may induce tissue fibrosis and necrosis and ultimately cause significant organ damage (48). Atherogenic dyslipidaemia, which is another independent risk factor for future cardiovascular events, has been observed in a higher proportion of patients with ASCVD and systemic inflammation (49). In patients with ASCVD treated with statins, however, systemic inflammation was a greater driver of recurrent cardiovascular events than increased LDL-C levels (19).
In our study, patients with ASCVD and CRP ≥ 2 mg/L more frequently used drugs such as antibiotics, oral anticoagulants, MRAs, and diuretics than patients with lower CRP levels. This likely reflects worse health conditions and a greater proportion of comorbidities in patients with systemic inflammation (46). In contrast, patients with ASCVD but without systemic inflammation more frequently used aspirin and statins compared to those with systemic inflammation. The lower utilisation of aspirin may be due to the increased use of anticoagulants in the latter. However, the reduced use of lipid-lowering therapies, particularly statins, in patients with ASCVD and systemic inflammation is difficult to explain, especially given their generally more atherogenic profile. As a result, those patients with higher cardiovascular risk may not be achieving strict target lipid levels in secondary prevention.
The use of medications to control classical cardiovascular risk factors (e.g., ACEi/ARBs, statins and lipid-lowering therapies) in patients with ASCVD, remains suboptimal, both in patients with or without systemic inflammation and with or without CKD. Furthermore, mean LDL-C levels were significantly higher than the values recommended by the current guidelines (50), suggesting there is room for stricter control of known cardiovascular risk factors and further improvements in preventing recurrent ASCVD outcomes.
The strengths of this study include the use of a large population-based and nationwide sample: over 15,000 patients with ASCVD were characterised for CRP levels, demographic and clinical characteristics, treatment patterns, and a set of biomarkers routinely monitored in clinical practice. However, a limitation of this study was that the data analysis was restricted to information routinely documented in the THIN-Spain database. Inpatient diagnoses and procedures are not recorded in the THIN-Spain database, which may result in lower sensitivity in detecting ASCVD. Incomplete or erroneous documentation of diagnoses, prescriptions, and comorbidities cannot be entirely excluded since there was no case ascertainment through medical records. Another limitation of this study is that the THIN-Spain database did not record whether the CRP tests were performed using regular or high-sensitivity assays. This, however, may have impacted the results to a minor extent, as high-sensitivity CRP tests can measure CRP levels in the low-sensitivity range (i.e., below 1 mg/L) and do not denote a more specific differential diagnosis of systemic inflammation (51). Moreover, the proportion of patients with systemic inflammation in our study was similar to the proportions observed in the studies conducted using the Stockholm's healthcare database and NHANES data, in which all CRP assays were performed using high-sensitivity CRP methods (12, 34). Some CRP measurements may be affected by underlying infections or other inflammatory chronic conditions. To minimise this type of confounding, patients with chronic infections (e.g., HIV, tuberculosis, and viral hepatitis) and CRP measurements taken within 3 months of an acute infection diagnosis, the use of antibiotics or antivirals, concomitantly with immunosuppressants, or in patients with a recent cancer diagnosis were excluded from the analyses. A sensitivity analysis was performed by excluding patients with chronic inflammatory diseases, which gave a similar prevalence of systemic inflammation as the main analysis (Supplementary Table S4). However, residual confounding due to undiagnosed infections or inflammatory diseases may still be present.
Another limitation is that CRP measurement is not routinely performed in clinical practice, and only a subgroup of patients with ASCVD had systemic inflammation tested in our sample. In fact, despite systemic inflammation being acknowledged as a risk factor for incident cardiovascular events, only in the 2024 ESC guidelines is CRP testing recommended for risk stratification in secondary cardiovascular prevention (6). Without clinically available targeted treatments for systemic inflammation, the diagnosis of systemic inflammation does not currently lead to optimal management and improved outcomes in patients with ASCVD. As such, the demand for CRP measurements from cardiologists, nephrologists, and general practitioners is currently low. In the THIN-Spain database, only one-third of patients with diagnosed ASCVD had at least one CRP measurement performed during the study period, and only one-fifth had an eligible measurement after the exclusion of CRP measurements taken during concurrent acute or chronic infections, concomitantly with the use of immunosuppressants, or after a diagnosis of cancer. This may limit the generalisability of our estimates to the entire ASCVD population, as the characteristics of patients with CRP measurements may be different compared to patients with ASCVD for whom a CRP measurement was not available. In the THIN-Spain database, the patients with ASCVD and CRP measurements had similar age and body mass indexes but different proportions of women, smokers, and concomitant CKD than the patients with ASCVD and without a CRP measurement (Supplementary Table S5).
Further, some variables, such as BMI, UACR, and laboratory values, were not recorded for all the patients included in the THIN® database, and therefore results might be biased by some selection (e.g., GPs might record weight more frequently in patients with weight issues compared to patients with regular weights).
Finally, when interpreting the results of this study, it should be considered that the study design did not allow us to assess causality or chronological order in the link between systemic inflammation and comorbidities and concomitant treatments (e.g., if systemic inflammation occurs before or after the onset of the associated diseases), and the observed directions may have been affected by the presence of confounding factors.
Systemic inflammation is highly prevalent among Spanish patients with ASCVD, particularly those with comorbid CKD, highlighting a subgroup of patients at increased risk of cardiovascular events and death despite statin therapy. However, most of the patients with ASCVD had never had their CRP levels assessed. Novel strategies that aim to reduce systemic inflammation may offer significant benefits for secondary prevention of cardiovascular events and mortality in a substantial proportion of patients with ASCVD and systemic inflammation. Routine CRP assessment in all ASCVD patients is crucial for identifying patients at higher risk and delivering optimal, targeted treatment.
The data analyzed in this study is subject to the following licenses/restrictions: Data were collected from the Spanish arm of The Health Improvement Network (THIN®) database. Requests to access these datasets should be directed to https://www.the-health-improvement-network.com.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
GP: Conceptualization, Formal Analysis, Investigation, Methodology, Project Administration, Validation, Visualization, Writing – original draft, Writing – review & editing. GG: Data Curation, Formal Analysis, Investigation, Visualization, Writing – review & editing. PJ: Funding Acquisition, Resources, Supervision, Validation, Writing – review & editing. AK: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. BL-L: Supervision, Validation, Writing – review & editing. JV: Resources, Supervision, Validation, Writing – review & editing. AC: Supervision, Validation, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by Novo Nordisk.
GP, GG, and AK report that their institution (Certara) received funding from Novo Nordisk for the study and medical writing during the conduct of the study.
PJ, BL-L, and JV are employees of Novo Nordisk and hold stock in the company during the conduct of the study.
AC received payments or honoraria for consultancy from Novo Nordisk during the conduct of the study. Outside of the submitted work, AC has received research grants from CSL Vifor, consultancy fees from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, GSK, Lilly, Novo Nordisk, Otsuka, and CSL Vifor, and lecture fees from Astellas, AstraZeneca, Amgen, Bayer, Medscape, Novo Nordisk, Sanofi (Mexico), and CSL Vifor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1538466/full#supplementary-material
1. Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. (2015) 46(5):328–38. doi: 10.1016/j.arcmed.2015.06.006
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Nedkoff L, Briffa T, Zemedikun D, Herrington S, Wright FL. Global trends in atherosclerotic cardiovascular disease. Clin Ther. (2023) 45(11):1087–91. doi: 10.1016/j.clinthera.2023.09.020
4. Martinez Lopez I, Gomez Cerezo JF, Gamez JM, Egocheaga Cabello I, Castellanos M, Campuzano Ruiz R, et al. Post-event follow-up costs in patients with atherosclerotic cardiovascular disease in Spain. Front Cardiovasc Med. (2024) 11:1324537. doi: 10.3389/fcvm.2024.1324537
6. Vrints C, Andreotti F, Koskinas KC, Rossello X, Adamo M, Ainslie J, et al. 2024 ESC guidelines for the management of chronic coronary syndromes. Eur Heart J. (2024) 45(36):3415–537. doi: 10.1093/eurheartj/ehae177
7. Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. (2010) 375(9709):132–40. doi: 10.1016/S0140-6736(09)61717-7
8. Ridker PM. Targeting residual inflammatory risk: the next frontier for atherosclerosis treatment and prevention. Vascul Pharmacol. (2023) 153:107238. doi: 10.1016/j.vph.2023.107238
9. Liberale L, Badimon L, Montecucco F, Luscher TF, Libby P, Camici GG. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. (2022) 79(8):837–47. doi: 10.1016/j.jacc.2021.12.017
10. Lawler PR, Bhatt DL, Godoy LC, Luscher TF, Bonow RO, Verma S, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. (2021) 42(1):113–31. doi: 10.1093/eurheartj/ehaa099
11. Ridker PM, Moorthy MV, Cook NR, Rifai N, Lee IM, Buring JE. Inflammation, cholesterol, lipoprotein(a), and 30-year cardiovascular outcomes in women. New Engl J Med. (2024) 391(22):2087–97. doi: 10.1056/NEJMoa2405182
12. Mazhar F, Faucon AL, Fu EL, Szummer KE, Mathisen J, Gerward S, et al. Systemic inflammation and health outcomes in patients receiving treatment for atherosclerotic cardiovascular disease. Eur Heart J. (2024) 45(44):4719–30. doi: 10.1093/eurheartj/ehae557
13. Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, et al. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol. (2016) 11(9):1546–56. doi: 10.2215/CJN.13121215
14. Kadatane SP, Satariano M, Massey M, Mongan K, Raina R. The role of inflammation in CKD. Cells. (2023) 12(12):1581. doi: 10.3390/cells12121581
15. Amdur RL, Feldman HI, Dominic EA, Anderson AH, Beddhu S, Rahman M, et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis. (2019) 73(3):344–53. doi: 10.1053/j.ajkd.2018.09.012
16. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. (2005) 293(14):1737–45. doi: 10.1001/jama.293.14.1737
17. Speer T, Dimmeler S, Schunk SJ, Fliser D, Ridker PM. Targeting innate immunity-driven inflammation in CKD and cardiovascular disease. Nat Rev Nephrol. (2022) 18(12):762–78. doi: 10.1038/s41581-022-00621-9
18. Writing Group for the CKDPC, Grams ME, Coresh J, Matsushita K, Ballew SH, Sang Y, et al. Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. (2023) 330(13):1266–77. doi: 10.1001/jama.2023.17002
19. Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. (2023) 401(10384):1293–301. doi: 10.1016/S0140-6736(23)00215-5
20. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. (2011) 473(7347):317–25. doi: 10.1038/nature10146
21. Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (justification for the use of statins in prevention-an intervention trial evaluating Rosuvastatin) trial. J Am Coll Cardiol. (2010) 55(12):1266–73. doi: 10.1016/j.jacc.2010.01.020
22. Ridker PM, Tuttle KR, Perkovic V, Libby P, MacFadyen JG. Inflammation drives residual risk in chronic kidney disease: a CANTOS substudy. Eur Heart J. (2022) 43(46):4832–44. doi: 10.1093/eurheartj/ehac444
23. Batra G, Ghukasyan Lakic T, Lindback J, Held C, White HD, Stewart RAH, et al. Interleukin 6 and cardiovascular outcomes in patients with chronic kidney disease and chronic coronary syndrome. JAMA Cardiol. (2021) 6(12):1440–5. doi: 10.1001/jamacardio.2021.3079
24. Jeppesen J, Hansen TW, Olsen MH, Rasmussen S, Ibsen H, Torp-Pedersen C, et al. C-reactive protein, insulin resistance and risk of cardiovascular disease: a population-based study. Eur J Cardiovasc Prev Rehabil. (2008) 15(5):594–8. doi: 10.1097/HJR.0b013e328308bb8b
25. Leening MJG, Cook NR, Franco OH, Manson JE, Lakshminarayan K, LaMonte MJ, et al. Comparison of cardiovascular risk factors for coronary heart disease and stroke type in women. J Am Heart Assoc. (2018) 7(19):e007514. doi: 10.1161/JAHA.117.007514
26. Liu Y, Guan S, Xu H, Zhang N, Huang M, Liu Z. Inflammation biomarkers are associated with the incidence of cardiovascular disease: a meta-analysis. Front Cardiovasc Med. (2023) 10:1175174. doi: 10.3389/fcvm.2023.1175174
27. Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, et al. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol. (2013) 33(7):1728–33. doi: 10.1161/ATVBAHA.112.301174
28. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385(19):1737–49. doi: 10.1056/NEJMoa2102953
29. KDIGO. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105(4S):S117–314. doi: 10.1016/j.kint.2023.10.018
30. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. New Engl J Med. (2020) 383(19):1838–47. doi: 10.1056/NEJMoa2021372
31. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl J Med. (2017) 377(12):1119–31. doi: 10.1056/NEJMoa1707914
32. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. New Engl J Med. (2019) 381(26):2497–505. doi: 10.1056/NEJMoa1912388
33. Ridker PM. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc Res. (2021) 117(11):e138–e40. doi: 10.1093/cvr/cvab231
34. Nanna MS L, Faurby M, Husemoen LLN, Tombak G, Yerragolam D, Sattar N. Abstract 11398: prevalence and characteristics of systemic inflammation in adults with atherosclerotic cardiovascular disease and chronic kidney disease: results from the national health and nutrition examination survey. Circulation. (2022) 146:A11398. doi: 10.1161/circ.146.suppl_1.11398
35. Marx N, Deanfield JE, Gerward S, Hovingh GK, Plunde O, Pratley RE, et al. Prevalence of systemic inflammation in individuals with atherosclerotic cardiovascular disease: baseline characteristics from the SELECT, SOUL and FLOW phase 3 trials of semaglutide. Eur Heart J. (2023) 44(2):ehad655.2751. doi: 10.1093/eurheartj/ehad655.2751
36. Amezcua-Castillo E, Gonzalez-Pacheco H, Saenz-San Martin A, Mendez-Ocampo P, Gutierrez-Moctezuma I, Masso F, et al. C-Reactive protein: the quintessential marker of systemic inflammation in coronary artery disease-advancing toward precision medicine. Biomedicines. (2023) 11(9):2444. doi: 10.3390/biomedicines11092444
37. Thompson PL, Nidorf SM. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: lessons from the CANTOS trial. J Thorac Dis. (2018) 10(2):695–8. doi: 10.21037/jtd.2018.01.119
38. Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. (2021) 397(10289):2060–9. doi: 10.1016/S0140-6736(21)00520-1
39. Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. (2019) 14(1):50–9. doi: 10.15420/ecr.2018.33.1
40. Leon-Pedroza JI, Gonzalez-Tapia LA, del Olmo-Gil E, Castellanos-Rodriguez D, Escobedo G, Gonzalez-Chavez A. Low-grade systemic inflammation and the development of metabolic diseases: from the molecular evidence to the clinical practice. Cir Cir. (2015) 83(6):543–51. doi: 10.1016/j.circir.2015.05.041
41. Walker KA, Ficek BN, Westbrook R. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem Neurosci. (2019) 10(8):3340–2. doi: 10.1021/acschemneuro.9b00333
42. Xie J, Van Hoecke L, Vandenbroucke RE. The impact of systemic inflammation on Alzheimer’s disease pathology. Front Immunol. (2022) 12:796867. doi: 10.3389/fimmu.2021.796867
43. Nso N, Bookani KR, Metzl M, Radparvar F. Role of inflammation in atrial fibrillation: a comprehensive review of current knowledge. J Arrhythm. (2021) 37(1):1–10. doi: 10.1002/joa3.12473
44. Jeppesen J. Low-grade chronic inflammation and vascular damage in patients with rheumatoid arthritis: don't forget “metabolic inflammation”. J Rheumatol. (2011) 38(4):595–7. doi: 10.3899/jrheum.110006
45. Petrescu M, Vlaicu SI, Ciumarnean L, Milaciu MV, Marginean C, Florea M, et al. Chronic inflammation-A link between nonalcoholic fatty liver disease (NAFLD) and dysfunctional adipose tissue. Medicina. (2022) 58(5):641. doi: 10.3390/medicina58050641
46. Ferreira GD, Simoes JA, Senaratna C, Pati S, Timm PF, Batista SR, et al. Physiological markers and multimorbidity: a systematic review. J Comorb. (2018) 8(1):2235042X18806986. doi: 10.1177/2235042X18806986
47. Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. (2014) 106(11):dju294. doi: 10.1093/jnci/dju294
48. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. (2017) 127(1):1–4. doi: 10.1172/JCI92035
49. Mortensen MB, Dzaye O, Botker HE, Jensen JM, Maeng M, Bentzon JF, et al. Low-density lipoprotein cholesterol is predominantly associated with atherosclerotic cardiovascular disease events in patients with evidence of coronary atherosclerosis: the western Denmark heart registry. Circulation. (2023) 147(14):1053–63. doi: 10.1161/CIRCULATIONAHA.122.061010
50. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European association of preventive cardiology (EAPC). Rev Esp Cardiol. (2022) 75(5):429. doi: 10.1093/eurheartj/ehab484
51. Nehring SM, Goyal A, Patel BC. C reactive protein. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2025). https://www.ncbi.nlm.nih.gov/books/NBK441843/
Keywords: atherosclerotic cardiovascular disease, chronic kidney disease, systemic inflammation, C-reactive protein, undertreatment
Citation: Pesce G, Gusto G, Johansen P, Khachatryan A, Lopez-Ledesma B, Vukmirica J and Cases A (2025) Systemic inflammation prevalence in patients with atherosclerotic cardiovascular disease and chronic kidney disease: a population-based study using a nationwide primary care database in Spain. Front. Cardiovasc. Med. 12:1538466. doi: 10.3389/fcvm.2025.1538466
Received: 2 December 2024; Accepted: 4 February 2025;
Published: 4 March 2025.
Edited by:
Xi-Ming Yuan, Linköping University, SwedenReviewed by:
Alexandr Ceasovschih, Grigore T. Popa University of Medicine and Pharmacy, RomaniaCopyright: © 2025 Pesce, Gusto, Johansen, Khachatryan, Lopez-Ledesma, Vukmirica and Cases. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giancarlo Pesce, Z2lhbmNhcmxvLnBlc2NlQGNlcnRhcmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.