
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 17 February 2025
Sec. Cardiac Rhythmology
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1534899
Background: Left atrial appendage occlusion (LAAO) serves as an alternative to oral anticoagulation (OAC) for atrial fibrillation (AF) patients at high risk of bleeding. The aim of this study was to compare the peri-procedural safety, complete or incomplete occlusion, the incidence of the peri-device leak (PDL), and device-related thrombosis (DRT) among LAAO, cryoballoon ablation (CBA) combined with LAAO, and radiofrequency catheter ablation (RFCA) combined with LAAO and to explore the risk factors of PDL and incomplete occlusion.
Methods: 382 patients with non-valvular AF who underwent either LAAO alone (n = 117), CBA combined with LAAO (n = 125), or RFCA combined with LAAO (n = 140) were included in the retrospective study. The study assessed peri-procedural complications and imaging results (3 months post-procedure). Multivariable logistic regression was employed to identify risk factors for incomplete occlusion and PDL.
Results: Peri-procedural complication rates were low among all groups, with 2.9% in the RFCA combined with the LAAO group. In contrast, the LAAO alone and CBA combined with LAAO groups reported no major complications (p = 0.347). At the 3-month follow-up, the incidence of DRT was 1.7% in the LAAO group, 2.4% in the CBA combined with the LAAO group, and 2.1% in the RFCA combined with the LAAO group (p = 0.930). Complete occlusion rates were comparable among the groups: 64.8% for CBA combined with LAAO, 62.4% for LAAO alone, and 60.7% for RFCA combined with LAAO (p = 0.794). PDL occurred in 33.3% of LAAO-alone patients, 34.4% of CBA combined with LAAO patients, and 38.6% of RFCA combined with LAAO patients (p = 0.644). Multivariable analysis identified persistent AF and serum creatinine (SCr) as independent predictors of PDL and incomplete occlusion.
Conclusion: Peri-procedural complications, complete occlusion, PDL, and DRT rates were similar across the three treatment strategies. Persistent AF and SCr were significant risk factors for incomplete occlusion and PDL. These findings highlight the importance of individualized treatment strategies based on patient-specific risk factors for optimizing outcomes.
Atrial fibrillation (AF) is a common cardiac arrhythmia, and one of its most severe complications is stroke (1). Thrombus formation is the primary cause of stroke, with over 90% of thrombi in AF patients originating from the left atrial appendage (LAA) (2, 3). Traditionally, oral anticoagulants (OACs) have been the mainstay for stroke prevention in patients with AF. However, the bleeding risk associated with long-term use of anticoagulants has prompted clinicians to explore alternative preventive strategies (4, 5). Consequently, left atrial appendage occlusion (LAAO) has emerged as an effective alternative to anticoagulant therapy for AF patients at high risk of bleeding or those who are unwilling to receive anticoagulant therapy or have contraindications to anticoagulant therapy. Several studies have confirmed its clinical efficacy and safety (6, 7).
LAAO reduces the risk of stroke by mechanically sealing the LAA and has become an important therapeutic option in the management of AF. In addition to LAAO alone, recent attention has been given to a “one-stop” approach combining LAAO with catheter ablation (CA). CA primarily involves two techniques: cryoballoon ablation (CBA) and radiofrequency catheter ablation (RFCA). CBA isolates the pulmonary veins using geothermal energy, while RFCA disrupts abnormal atrial electrical conduction pathways through thermal energy. Existing studies suggest that this combined procedure effectively restores sinus rhythm and prevents thrombus formation without significantly increasing the risk of serious adverse events (SAEs) during the peri-procedural period (8). Although most research has focused on evaluating the safety and efficacy of RFCA combined with LAAO, studies on the clinical outcomes of CBA combined with LAAO remain relatively scarce (9–15). Moreover, only a limited number of comparative studies have investigated the effects of combining LAAO with either RFCA or CBA (16). To date, comparative studies on the efficacy of these three surgical approaches are relatively limited. Therefore, this study aimed to compare the outcomes of three different treatment strategies in patients with AF, focusing on peri-procedural complications and post-procedural imaging results, specifically device-related thrombus (DRT), peri-device leak (PDL) and the rate of complete occlusion. By providing a comparative analysis of these outcomes, this study seeks to offer valuable insights into the individualized treatment of AF patients.
This retrospective study included those patients with non-valvular AF who underwent LAAO at the Electrophysiology Center of the affiliated Taizhou People's Hospital of Nanjing Medical University. Patients were included if they were adults with symptomatic non-valvular paroxysmal or persistent AF and were at high risk of stroke (CHA2DS2-VASc score ≥2 for males, ≥3 for females). Additionally, all patients met at least one of the following criteria: (1) electrical isolation of the pulmonary vein during CA; (2) contraindications to OAC; (3) unwillingness or inability to take OAC; (4) a history of stroke while on OAC. Five hundred ninety-five patients were initially screened, 117 of whom received the LAmbre device. After excluding 96 patients who did not undergo coronary computed tomography angiography (CCTA) at 3 months post-LAAO, 382 patients who performed LAAO with the Watchman2.5 device were included in the final analysis (Figure 1).
As we described previously (17), femoral venous access was established under local or general anesthesia, followed by transseptal puncture to gain entry into the left atrium (LA). Subsequently, heparin was administered, and the activated clotting time (ACT) was meticulously monitored to ensure it remained above 300 s. The anatomical structure of the LAA was evaluated using fluoroscopy to confirm the absence of thrombus formation. Based on the LAA anatomy, the appropriate Watchman occluder was selected and delivered into the LAA via a catheter. The device was then adjusted according to the “PASS” criteria (Position, Anchor, Size, Seal) to ensure optimal placement and effective sealing. After deployment, the occluder's position was confirmed with contrast imaging, ensuring any residual leak was less than 5 mm. If the leak exceeded this threshold, the occluder was repositioned or replaced with a larger device.
Following transseptal puncture, intravenous heparin was administered as a bolus, and the ACT was closely monitored to ensure it remained above 300 s. As our previous report (18), after establishing femoral venous access and performing a transseptal puncture to enter the LA, a mapping catheter was used to perform electroanatomic mapping and identify the target ablation areas. Ablation was performed with a power setting of 35–45 watts for 20–30 s per pulmonary vein until all electrical activity in the pulmonary veins was completely isolated. After ablation, LAAO was immediately performed using the Watchman occluder.
CBA was performed through femoral venous access into the LA, followed by administering heparin and close monitoring of the ACT to maintain a value exceeding 300 s. Each pulmonary vein was confirmed using contrast injection via the catheter, and cryoablation was performed for 180–240 s, with temperatures between −45℃ and −55℃. Upon successful ablation, LAAO was performed immediately using the Watchman occluder.
At 3 months post-procedure, all patients underwent CCTA with the Siemens SOMATOM Force 128-slice dual-source CT scanner. A contrast agent was injected at a rate of 4–5 ml/s with 80 ml of non-ionic contrast media to enhance image quality. Scanning parameters were set at 100–120 kV tube voltage and 800–1,235 mA tube current, with a collimation width of 256 × 0.625 mm, 270 ms rotation time, and 0.2 mm slice thickness. Dual-phase scanning was performed, with the second phase initiated 60 s after the first. Radiation dosage was automatically controlled by ECG-triggered prospective gating, with 30% and 80% of the R-R interval exposure. The scanning range extended from 1 cm below the carina to the cardiac diaphragm. All imaging data were processed using the Syngo VB10 workstation (Siemens), with analyses including 3D reconstruction, volume rendering (VR), and maximum intensity projection (MIP)—the procedures described above assessed the incidents of complete or incomplete occlusion, PDL and DRT.
In this study, complete occlusion is defined by an LAA attenuation value of <100 HU or an LAA LA attenuation ratio of ≤0.25 (Figure 2A). Incomplete occlusion is characterized by a CT attenuation value >100 HU or an LAA/LA CT attenuation ratio of >0.25 (Figure 2B). Incomplete occlusion includes trans-fabric leaks, PDL, and mixed leaks. Trans-fabric leak refers to contrast leakage through the fabric structure of the device into the LAA, rather than from the edge of the device. PDL refers to contrast leakage around the occlusion device. DRT is defined as thrombus formation on or around the device, typically detected by imaging, and is characterized by nodular or mass-like areas of enhancement defects, with regions of pronounced low attenuation thickening.
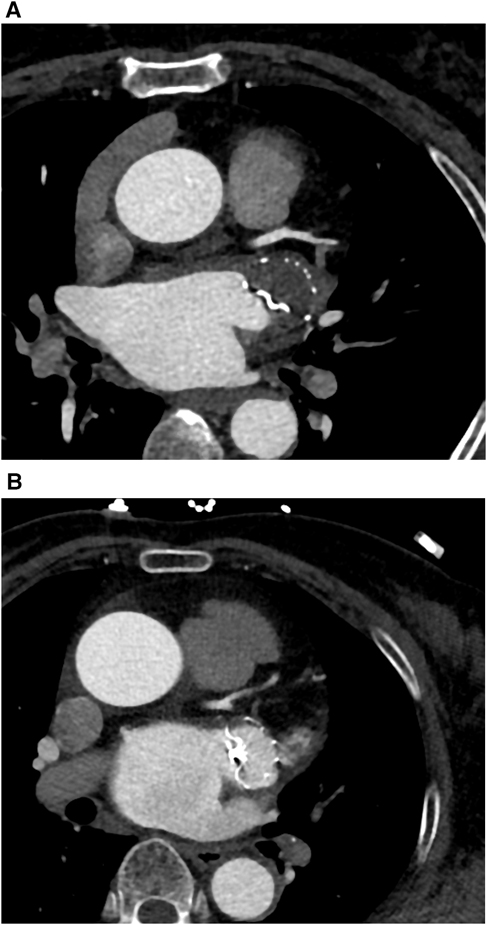
Figure 2. (A) One case (a 63-year-old woman with persistent atrial fibrillation) for complete occlusion at 3 months follow-up after LAAO. LAA CTA showed no residual contrast agent flow inside the LAA. (B) One case (a 68-year-old man with persistent atrial fibrillation) for incomplete occlusion at 3 months follow-up after LAAO. LAA CTA showed residual contrast agent flow inside the LAA.
Continuous variables with a normal distribution were expressed as mean ± standard deviation (SD), while non-normally distributed variables were presented as median and interquartile range (IQR). Categorical variables were described as numbers and percentages. Differences in continuous variables were assessed using the T-test or Mann–Whitney U test, and differences in categorical variables were analyzed using the Pearson chi-square test or Fisher's exact test. Univariate logistic regression analysis was performed to evaluate the risk factors, with variables showing a p-value of <0.05 included in the multivariate analysis. Multivariate logistic regression was used to further adjust for confounding factors. All statistical analyses were conducted using SPSS 27.0 software, and a two-sided p-value of <0.05 was considered statistically significant.
A total of 382 patients who successfully underwent Watchman occluder implantation and completed CCTA three months post-procedure were included in the study. Of these, 125 patients were in the CBA combined with the LAAO group, 117 in the LAAO alone group, and 140 in the RFCA combined with the LAAO group. Baseline characteristics are summarized in Table 1. There were no significant differences in age, sex, AF type, or comorbidities [including heart failure, hypertension, diabetes, coronary artery disease (CAD), prior stroke, vascular disease, abnormal renal function, smoking, or alcohol consumption] among the three groups (p > 0.05). Furthermore, no significant differences were found in the CHA2DS2-VASc score, HAS-BLED score, NT-proBNP levels, left atrial diameter (LAD), left ventricular ejection fraction (LVEF), or anticoagulation therapy regimen among the groups (p > 0.05). However, a significant difference was observed in the LAA ostial diameter among the three groups (p = 0.006), with a value of 22.4 ± 3.4 mm in the CBA combined with the LAAO group, 23.3 ± 3.5 mm in the RFCA combined with LAAO group, and 23.8 ± 3.7 mm in the LAAO alone group.
As shown in Table 2, the overall incidence of peri-procedural complications was low across all groups. No adverse events were reported in the CBA combined with LAAO or LAAO-alone groups. In contrast, four adverse events (2.9%) occurred in the RFCA combined with the LAAO group, including 1 case of severe bleeding and 3 cases of pericardial effusion. No embolization or pseudoaneurysm was observed in any of the groups. There were no statistically significant differences in the overall incidence of complications among the groups (p = 0.347).
The imaging results at 3 months post-procedure are shown in Figure 3. The overall incidence of DRT was 3.2% across all patients. Among the groups, the incidence of DRT was 2.4% in the CBA combined with LAAO group, 1.7% in the LAAO-alone group, and 2.1% in the RFCA combined with LAAO group, with no statistically significant differences (p = 0.930).
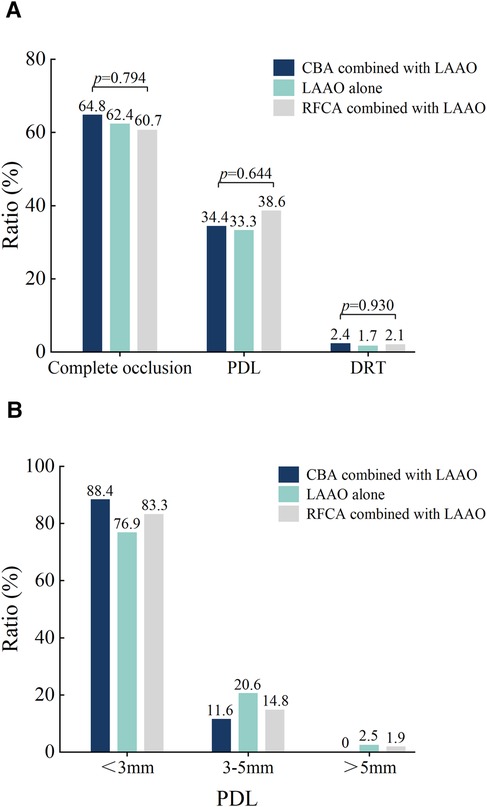
Figure 3. Imaging results 3-month post-procedure. (A) The ratios of DRT, Complete occlusion, and PDL for the three different procedures. (B) Comparison of PDL among three different procedures after three months. DRT, device-related thrombus; PDL, peri-device leak; CBA, cryoballoon ablation; RFCA, radiofrequency ablation; LAAO, left atrial appendage occlusion.
Complete occlusion was achieved in 62.7% of the overall cohort. Specifically, complete occlusion rates were 64.8% in the CBA combined with the LAAO group, 62.4% in the LAAO-alone group, and 60.7% in the RFCA combined with the LAAO group, showing no significant differences among the groups (p = 0.794). The overall incidence of PDL was 35.1%. The incidence rates of PDL by group were 34.4% in the CBA combined with LAAO group, 33.3% in the LAAO-alone group, and 38.6% in the RFCA combined with LAAO group, with no statistically significant differences (p = 0.644). In terms of leak size distribution, the majority of PDLs were <3 mm, with 88.4% of leaks in the CBA combined with the LAAO group, 76.9% in the LAAO-alone group, and 83.3% in the RFCA combined with the LAAO group. The proportion of leaks measuring between 3 mm and 5 mm was 11.6% in the CBA combined with the LAAO group, 20.6% in the LAAO-alone group, and 14.8% in the RFCA combined with the LAAO group. Larger leaks (>5 mm) were rare, observed in only 1 patient (2.5%) in the LAAO-alone group and 1 patient (1.9%) in the RFCA combined with LAAO group, with no such cases in the CBA combined with LAAO group.
Tables 3, 4 show that univariate and multivariate binary logistic regression analyses were conducted to predict incomplete occlusion and PDL. Due to the small number of cases, regression analysis was not performed for DRT and trans-fabric leaks.
In the analysis of predictors for incomplete occlusion, univariate analysis revealed that persistent AF, LAD, occluder size, LAA ostia diameter and serum creatinine (SCr) were potential influencing factors for IDE. In the multivariate analysis, persistent AF (OR = 0.527, 95% CI: 0.330–0.842, p = 0.007), and SCr (OR = 0.968, 95% CI: 0.956–0.979, p < 0.001) were identified as independent predictors.
The univariate analysis of PDL showed similar results to those of incomplete occlusion. In the multivariate analysis, persistent AF (OR = 2.078, 95% CI: 1.291–3.347, p = 0.003), and SCr (OR = 1.033, 95% CI: 1.021–1.046, p < 0.001) remained independent predictors.
This study evaluated the safety of three treatment approaches in patients with non-valvular AF: CBA combined with LAAO, RFCA combined with LAAO, and LAAO alone. The findings demonstrated that the peri-procedural safety, complete or incomplete occlusion, PDL, and DRT outcomes were comparable across all three approaches. Multivariate analysis revealed that persistent AF and elevated SCr levels were associated with incomplete occlusion and PDL.
Regarding peri-procedural complications, our study showed that the complication rates were relatively low in both the combined ablation and LAAO-alone groups. No significant differences were observed in the incidence of adverse events (e.g., pericardial tamponade, major bleeding, or pseudoaneurysm), indicating good short-term safety for all three approaches. Notably, although three cases of pericardial tamponade occurred in the RFCA combined with the LAAO group, the difference was not statistically significant. This finding was consistent with the results of Ma et al., who also reported low peri-procedural complication rates in combined procedures, further supporting the clinical safety of this approach (16). Additionally, the study by Fassini et al. (14) reported that none of the 35 patients who underwent the “one-stop” procedure experienced major SAEs during a 24-month follow-up, with only 1 case of stroke observed during a longer follow-up period. These findings suggest that the combined procedure is not only safe in the short term but also maintains good safety over long-term follow-up. These data provide further support for the clinical use of the combined ablation and LAAO procedure, although larger prospective studies are needed to confirm its long-term safety and efficacy.
Although this study did not directly compare the efficacy of different postoperative anticoagulation strategies, current clinical guidelines recommend routine anticoagulation and antiplatelet therapy postoperatively (19). Baseline data indicate that most patients were treated with direct oral anticoagulants (DOACs) after surgery, with rivaroxaban and dabigatran used in 80% and 23.2% of cases, respectively. In comparison, warfarin was used in only 3.2% of cases. These results reflect the current clinical practice trend, where DOACs have gradually replaced warfarin due to their lower bleeding risks and the absence of the need for routine monitoring. A systematic review and network meta-analysis published in JACC (20) suggested that novel oral anticoagulants (NOACs) may be the optimal initial anticoagulation strategy after LAAO, as they offer a higher potential for reducing thromboembolic events and major bleeding risks. Further analysis indicated that NOACs were significantly superior to warfarin in reducing the risk of all-cause mortality (OR = 0.39).
A recent study, the OPTION trial (21), further explored the safety and efficacy of LAAO vs. OACs after ablation for AF during long-term follow-up (3 years). This study showed that the incidence of bleeding events in the LAAO group was significantly lower than that in the oral anticoagulant group (8.5% vs. 18.1%, p < 0.0001). However, the incidence of death from any cause, stroke, or systemic embolism was similar between the two groups (5.3% vs. 5.8%, p < 0.0001). These findings suggest that catheter-based AF ablation combined with LAAO was a non-inferior method for preventing stroke when compared with oral anticoagulants. In the present study, catheter-based AF ablation combined with LAAO, whether using RFCA or CBA, demonstrated both safety and effectiveness. However, this study primarily evaluated the short-term safety of different LAAO strategies.
Our study revealed no significant differences in complete and incomplete occlusion rates among the three groups; however, the CBA combined with the LAAO group exhibited a higher rate of complete occlusion than the RFCA combined with LAAO and LAAO-alone groups. Complete occlusion is a crucial factor for long-term device stability and thrombus prevention, and it may be influenced by the local inflammatory response triggered by ablation. RFCA induces thermal injury, leading to coagulative necrosis of myocardial tissue and more extensive tissue disruption. In contrast, CBA utilizes cold temperatures, which preserves cellular ultrastructure, thus reducing endothelial damage and minimizing the risk of thrombus formation. This difference in ablation mechanisms may contribute to the superior occlusion outcomes observed in the CBA combined with the LAAO group (22, 23). Previous studies also supported this conclusion. For example, Khairy et al. (24) found that RFCA was more likely to induce thrombus formation compared to CBA, likely due to the broader inflammatory response and more extensive tissue injury associated with RFCA. In contrast, the relatively milder inflammatory response triggered by CBA may facilitate vascular endothelial cell proliferation and repair, thereby accelerating the endothelialization process, and, in turn, expediting complete occlusion. Additionally, previous research has demonstrated that combining AF ablation with LAAO can improve electrophysiological function and hemodynamics in AF patients (25). These mechanisms may provide a plausible explanation for the observed difference in complete occlusion rates between the CBA combined with LAAO and RFCA combined with LAAO groups in this study. However, the difference did not reach statistical significance.
Incomplete occlusion is closely associated with the risk of DRT. Studies have shown that incomplete occlusion is more prone to thrombus formation (26). Although no significant differences in DRT incidence were observed among the three groups in this study, the higher complete occlusion rate in the CBA combined with the LAAO group suggests a potential for lower long-term thrombus formation risk. Both Dukkipati et al. (27) and Fauchier et al. (28) reported that patients with DRT had significantly higher rates of ischemic stroke or systemic embolism. Among AF patients undergoing percutaneous LAAO, the stroke rate was 15.4% in the DRT group compared to 3.2% in the non-DRT group. However, Lempereur et al. (29) found no significant association between different types of occlusion devices and cerebrovascular events despite the increased risk of DRT. Therefore, the specific mechanisms by which DRT affects long-term outcomes remain to be elucidated. Due to the limited number of DRT events in this study, it was impossible to conduct a comprehensive statistical analysis. Therefore, further investigation into the risk factors associated with DRT was not pursued.
Univariate analysis in this study identified persistent AF, LAD, LAA ostium size, and occluder size as risk factors significantly associated with incomplete occlusion, consistent with previous literature (30–32). The multivariate analysis identified persistent AF and SCr levels as independent predictors of incomplete occlusion. Persistent AF often leads to pathological remodelling and hemodynamic alterations in the atria, which can negatively impact complete occlusion. SCr, a key indicator of renal function, also reflects the patient's metabolic state and systemic inflammatory response. Elevated SCr levels may increase the risk of incomplete occlusion through several mechanisms. First, elevated SCr levels can indicate mild to moderate renal impairment, even if the condition has not yet reached the clinical threshold for diagnosing “renal insufficiency”. Renal impairment is frequently associated with endothelial dysfunction (33), which can reduce the endothelial cell repair and regeneration capacity, thereby delaying or hindering the complete occlusion process of the occlusion device. Second, systemic inflammation often accompanies renal impairment, which may further disrupt endothelial cell function and compromise the complete occlusion process (34).
PDL is a common complication following LAAO and can increase the risk of thrombus formation if it persists (35). In this study, the incidence of PDL was 33.3% in the LAAO-alone group, lower than in the CBA combined with LAAO group (34.4%) and RFCA combined with LAAO group (38.6%). However, the differences among the groups were not statistically significant (p = 0.644). Clinically, PDL is a critical concern and may be influenced by several factors, including LAA anatomy, procedural technique, operator experience, and device positioning (36). Zhao et al. (37) demonstrated that the maximum diameter of the LAA orifice can independently predict the occurrence of postoperative PDL following LAAO, which aligns with the findings of our study.
Multivariate analysis further revealed that persistent AF and elevated SCr levels are independent risk factors for PDL. As discussed previously, elevated SCr reflects impaired renal function and systemic inflammation, which may hinder the healing process of the occluder and contribute to the development of PDL. A single-centre retrospective study involving 172 patients by Chen et al. (38) found that patients without PDL had a faster rate of endothelialization. In contrast, PDL was identified as an independent risk factor for delayed endothelialization. Saw et al. (39) highlighted several mechanisms contributing to PDL, including off-axis device placement, misalignment between the LAA landing zone and the occluder, insufficient expansion of the LAA landing zone, resulting in gaps around the device, and incomplete device endothelialization (IDE). The presence of IDE and PDL will ultimately lead to incomplete occlusion.
Given the significant overlap in risk factors for PDL and incomplete occlusion, preoperative evaluation of SCr levels could be an important predictor of postoperative complications. For patients with elevated SCr, close postoperative monitoring of device healing and adjustments to anticoagulant therapy is recommended based on the patient's metabolic status. This approach may mitigate potential risks and improve long-term outcomes by promoting more effective device integration and complete occlusion.
The results of this study demonstrated no significant differences in peri-procedural complications, Complete or incomplete occlusion, PDL, or DRT rates among the three surgical approaches. However, persistent AF and elevated SCr levels were identified as independent risk factors for PDL and incomplete occlusion. These findings suggest that individualized treatment strategies should be developed for high-risk AF patients based on their clinical characteristics to optimize both peri-procedural and post-procedural outcomes.
This study has several limitations. First, it was a single-centre, retrospective observational study. Despite using multivariate analysis to minimize the impact of confounding factors, selection bias may still be present. Additionally, the follow-up period was relatively short (only 3 months post-procedure), limiting the ability to assess long-term clinical outcomes. Another potential limitation is the lack of detailed classification within incomplete occlusion (including PDL, Trans-fabric leak, and both), which may have influenced the results to some extent.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Taizhou People's Hospital, affiliated with Nanjing Medical University. Since this was a retrospective, observational study, written informed consent was waived. (Approval No. KY 2024-141-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
X-hJ: Writing – original draft, Software, Visualization, Methodology, Data curation. Y-jT: Software, Resources, Methodology, Data curation, Writing – review & editing. R-zW: Resources, Methodology, Data curation, Writing – review & editing. Z-bR: Writing – review & editing, Supervision, Funding acquisition. LZ: Supervision, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Taizhou Science and Technology Support Program Project (Grant No.TS202215).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. (1991) 22:983. doi: 10.1161/01.str.22.8.983
2. Lip GY, Hammerstingl C, Marin F, Cappato R, Meng IL, Kirsch B, et al. Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J. (2016) 178:126–34. doi: 10.1016/j.ahj.2016.05.007
3. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—an update. Europace. (2020) 22:184. doi: 10.1093/europace/euz258
4. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2021) 42:546–7. doi: 10.1093/eurheartj/ehaa945
5. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. (2018) 39:1330. doi: 10.1093/eurheartj/ehy136
6. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. (2019) 74:104. doi: 10.1016/j.jacc.2019.01.011
7. Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation. JAMA. (2014) 312:1988. doi: 10.1001/jama.2014.15192
8. Su F, Gao C, Liu J, Ning Z, He B, Liu Y, et al. Periprocedural outcomes associated with use of a left atrial appendage occlusion device in China. JAMA Netw Open. (2022) 5:e2214594. doi: 10.1001/jamanetworkopen.2022.14594
9. Phillips KP, Walker DT, Humphries JA. Combined catheter ablation for atrial fibrillation and watchman® left atrial appendage occlusion procedures: five-year experience. J Arrhythm. (2016) 32:119. doi: 10.1016/j.joa.2015.11.001
10. Phillips KP, Pokushalov E, Romanov A, Artemenko S, Folkeringa RJ, Szili-Torok T, et al. Combining watchman left atrial appendage closure and catheter ablation for atrial fibrillation: multicentre registry results of feasibility and safety during implant and 30 days follow-up. Europace. (2018) 20:949. doi: 10.1093/europace/eux183
11. Wintgens L, Romanov A, Phillips K, Ballesteros G, Swaans M, Folkeringa R, et al. Combined atrial fibrillation ablation and left atrial appendage closure: long-term follow-up from a large multicentre registry. Europace. (2018) 20:1783. doi: 10.1093/europace/euy025
12. Phillips KP, Romanov A, Artemenko S, Folkeringa RJ, Szili-Torok T, Senatore G, et al. Combining left atrial appendage closure and catheter ablation for atrial fibrillation: 2-year outcomes from a multinational registry. Europace. (2020) 22:225–31. doi: 10.1093/europace/euz286
13. Chen M, Sun J, Wang QS, Zhang PP, Li W, Zhang R, et al. Long-term outcome of combined catheter ablation and left atrial appendage closure in atrial fibrillation patients. Int J Cardiol. (2022) 368:41–8. doi: 10.1016/j.ijcard.2022.08.007
14. Fassini G, Gasperetti A, Italiano G, Riva S, Moltrasio M, Dello Russo A, et al. Cryoballoon pulmonary vein ablation and left atrial appendage closure combined procedure: a long-term follow-up analysis. Heart Rhythm. (2019) 16:1320. doi: 10.1016/j.hrthm.2019.03.022
15. Ren Z, Zhang J, Wang S, Jia P, Li X, Zhang J, et al. Two-Year outcome from combining cryoballoon ablation and left atrial appendage closure: CLACBAC study. Front Cardiovasc Med. (2021) 7:610537. doi: 10.3389/fcvm.2020.610537
16. Ma Y, Guo L, Hu M, Yan Q, Liu H, Yi F. Left atrial appendage occlusion combined with cryoballoon or radiofrequency ablation: one-year follow-up comparison. Front Cardiovasc Med. (2023) 10:1153158. doi: 10.3389/fcvm.2023.1153158
17. Ruan ZB, Li W, Jin K, Ding XW, Chen GC, Zhu JG, et al. A preliminary study of minimal left atrial appendage occlusion using Watchman under the guidance of fluoroscopy. Catheter Cardiovasc Interv. (2024) 103:119–28. doi: 10.1002/ccd.30838
18. Ruan ZB, Liang HX, Wang F, Chen GC, Zhu JG, Ren Y, et al. Influencing factors of recurrence of nonvalvular atrial fibrillation after radiofrequency catheter ablation and construction of clinical nomogram prediction model. Int J Clin Pract. (2022) 2022:8521735. doi: 10.1155/2022/8521735
19. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2024) 149:1. doi: 10.1161/CIR.0000000000001193
20. Carvalho PEP, Gewehr DM, Miyawaki IA, Nogueira A, Felix N, Garot P, et al. Network meta-analysis of initial antithrombotic regimens after left atrial appendage occlusion. J Am Coll Cardiol. (2023) 82:1765. doi: 10.1016/j.jacc.2023.08.010
21. Wazni OM, Saliba WI, Nair DG, Marijon E, Schmidt B, Hounshell T, et al. Left atrial appendage closure after ablation for atrial fibrillation. N Engl J Med. (2024). doi: 10.1056/NEJMoa2408308
22. Wei Y, Bao Y, Lin C, Xie Y, Luo Q, Zhang N, et al. Early recurrence after cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation: mechanism and implication in long-term outcome. BMC Cardiovasc Disord. (2022) 22:400. doi: 10.1186/s12872-022-02816-1
23. Aupperle H, Doll N, Walther T, Ullmann C, Schoon HA, Wilhelm Mohr F. Histological findings induced by different energy sources in experimental atrial ablation in sheep. Interact Cardiovasc Thorac Surg. (2005) 4:450. doi: 10.1510/icvts.2005.109413
24. Khairy P, Chauvet P, Lehmann J, Lambert J, Macle L, Tanguay JF, et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. (2003) 107:2045. doi: 10.1161/01.Cir.0000058706.82623.A1
25. Murray MI, Arnold A, Younis M, Varghese S, Zeiher AM. Cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation: a meta-analysis of randomized controlled trials. Clin Res Cardiol. (2018) 107:658–69. doi: 10.1007/s00392-018-1232-4
26. Prosperi-Porta G, Schnell G, Colbert J, Franko A, Wilton SB, Kuriachan VP. Multiple thromboembolic events from a left atrial appendage occlusion device. Can J Cardiol. (2018) 34:342.e13. doi: 10.1016/j.cjca.2017.12.017
27. Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, et al. Device-Related Thrombus After Left Atrial Appendage Closure. Circulation. (2018) 138:874. doi: 10.1161/CIRCULATIONAHA.118.035090
28. Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, et al. Device-Related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. (2018) 71:1528. doi: 10.1016/j.jacc.2018.01.076
29. Lempereur M, Aminian A, Freixa X, Gafoor S, Kefer J, Tzikas A, et al. Device-associated thrombus formation after left atrial appendage occlusion: a systematic review of events reported with the watchman, the amplatzer cardiac plug and the amulet. Catheter Cardiovasc Interv. (2017) 90:5. doi: 10.1002/ccd.26903
30. Sharma SP, Singh D, Nakamura D, Gopinathannair R, Lakkireddy D. Incomplete endothelialization of WatchmanTM device: predictors and implications from two cases. J Atr Fibrillation. (2019) 11:5. doi: 10.4022/jafib.2162
31. McIvor F, Wall D. Who watches the WATCHMAN™? A case of incomplete endothelialization at 3 years after device implantation. Eur J Cardiothorac Surg. (2019) 56:1194. doi: 10.1093/ejcts/ezz135
32. Xu J, Chen CZ, Xing J, Wang L, Tao YR, Yang B, et al. Clinical relevance of incomplete device endothelialization after left atrial appendage closure. Int J Cardiovasc Imaging. (2023) 39:451. doi: 10.1007/s10554-022-02721-w
33. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. (2013) 9:1057. doi: 10.7150/ijbs.7502
34. Perticone F, Maio R, Tripepi G, Zoccali C. Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation. (2004) 110:821. doi: 10.1161/01.CIR.0000138745.21879.27
35. Bai Y, Xue X, Duenninger E, Muenzel M, Jiang L, Keil T, et al. Real-world survival data of device-related thrombus following left atrial appendage closure: 4-year experience from a single center. Heart Vessels. (2019) 34:1360. doi: 10.1007/s00380-019-01364-7
36. Lakkireddy D, Nielsen-Kudsk JE, Windecker S, Thaler D, Price MJ, Gambhir A, et al. Mechanisms, predictors, and evolution of severe peri-device leaks with two different left atrial appendage occluders. Europace. (2023) 25:9. doi: 10.1093/europace/euad237
37. Zhao MZ, Chi RM, Yu Y, Wang QS, Sun J, Li W, et al. Value of detecting peri-device leak and incomplete endothelialization by cardiac CT angiography in atrial fibrillation patients post watchman LAAC combined with radiofrequency ablation. J Cardiovasc Electrophysiol. (2021) 32:2655. doi: 10.1111/jce.15222
38. Chen T, Lu X, Wang X, Chen Q, Zhao R, Zhang W, et al. Peri-device leakage and delayed endothelialization of the watchman device: a computed tomography study. Eur Radiol. (2024) 34:7285–96. doi: 10.1007/s00330-024-10778-5
Keywords: atrial fibrillation, left atrial appendage occlusion, complete occlusion, peri-device leak, device related thrombosis
Citation: Jiang X-h, Tan Y-j, Wang R-z, Ruan Z-b and Zhu L (2025) Comparison of prognosis and analysis of related risk factors among three different left atrial appendage occlusion procedures in patients with atrial fibrillation. Front. Cardiovasc. Med. 12:1534899. doi: 10.3389/fcvm.2025.1534899
Received: 26 November 2024; Accepted: 28 January 2025;
Published: 17 February 2025.
Edited by:
Massimiliano Marini, Ospedale Santa Chiara, ItalyReviewed by:
Adel Aminian, Centre Hospitalier Universitaire de Charleroi, BelgiumCopyright: © 2025 Jiang, Tan, Wang, Ruan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong-bao Ruan, dHpjYXJkaWFjQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.