
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 March 2025
Sec. Pediatric Cardiology
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1531754
This article is part of the Research TopicSurgical and Non-Surgical Intervention of Congenital Heart Disease Management in Developing and Developed CountriesView all 10 articles
 Jad Abdul Khalek1
Jad Abdul Khalek1 Christophe El Rassi1,†
Christophe El Rassi1,† Maria Abou Mansour1,†
Maria Abou Mansour1,† Bshara Sleem1
Bshara Sleem1 Issam El Rassi2
Issam El Rassi2 Fadi Bitar3
Fadi Bitar3 Mariam Arabi3*‡
Mariam Arabi3*‡
Background: Cor Triatriatum is a congenital anomaly characterized by the abnormal presence of a fibromuscular junction in one of the atria, as seen on echocardiography. This anomaly can lead to major hemodynamic problems and obstruction of blood flow. This study aims to explore the risk factors, diagnostic modalities, and surgical interventions used to tackle this congenital anomaly at a tertiary care center over an 18-year period.
Materials and methods: Medical records of congenital heart disease patients at the Children's Heart Center at the American University of Beirut Medical Center between 2006 and 2024 were retrospectively reviewed. Data collection included demographic characteristics, clinical outcomes, hospitalization details, and surgical treatment. Ethical approval was obtained, and descriptive statistics were employed for data analysis using SAS 9.4.
Results: At our center, 7 patients were diagnosed with Cor Triatriatum, with a median age of 5 months. 4 of the patients were female, 3 were males, and the median hospital stay was 7 days. All patients were diagnosed with Cor Triatriatum Sinister, and respiratory symptoms were prevalent. Pulmonary vein abnormalities were observed in 4 ouf of 7 (57.1%) patients and atrial septal defects in 2 out of 7 patients (28.5%). Surgery resulted in successful membrane resection for all operated patients, with significant symptom improvement postoperatively.
Conclusion: Cor Triatriatum is a rare congenital anomaly requiring early detection and diagnosis. Surgical intervention remains the mainstay of treatment, with favorable outcomes when performed promptly. Larger studies are recommended to optimize management strategies and improve long-term outcomes for affected patients.
Congenital heart disease is a significant category of congenital problems that is the leading cause of death in infants due to birth defects (1, 2). The treatment and management of congenital heart disease have seen considerable progress over the years (3). John Gibbon achieved a significant milestone in congenital heart disease therapy with the first use of a cardiopulmonary bypass in 195 (4). Advancements in diagnostic methods, especially echocardiography, greatly enhanced our capacity to identify abnormalities over time (5). Nevertheless, complex congenital heart disease continues to present abundant diagnostic and therapeutic challenges.
One such congenital anomaly is Cor Triatriatum, a condition characterized by the presence of an abnormal fibromuscular septum inside one of the atria, dividing it into three chambers instead of the usual two (6). The name “Cor Triatriatum” was coined by Borst in 1905 to describe a septum in the left atrium (7). Figure 1 represents a transthoracic echocardiogram showing the prevalent anatomy of Cor Triatriatum. This anomaly can occur in the left atrium (Cor Triatriatum Sinister) or, less commonly, in the right atrium (Cor Triatriatum Dexter), leading to major hemodynamic turbulence due to obstruction of blood flow (7). The two chambers are typically connected by a small opening, through which the membrane acts as an obstruction to pulmonary venous drainage, ultimately leading to the patient's symptoms. Figure 2 represents a color Doppler echocardiogram demonstrating the turbulent flow across the atrial membrane. Figure 3 highlights the obstruction caused by the triatriatum membrane in a Computed Tomography (CT) image with its associated pulmonary veins. Cor Triatriatum presents as a spectrum of clinical manifestations, from asymptomatic cases detected incidentally to severe cases presenting with heart failure, syncope, and sudden cardiac arrest (8).
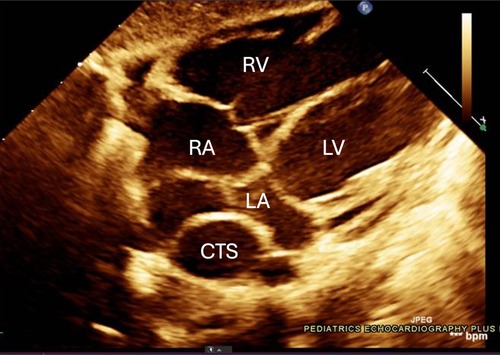
Figure 1. The echocardiogram shows an abnormal membrane or fibromuscular partition that divides the left atrium into two chambers. This partition separates the pulmonary venous inflow, creating a proximal and distal chamber. Notable in this image are the membranous structures and potential flow obstruction, which are typical in Cor Triatriatum cases. The relevant chambers are labeled with *RA: Right Atrium, *RV: Right Ventricle, *LA: Left Atrium, *LV: Left Ventricle, *CTS: Cor Triatriatum Sinister.
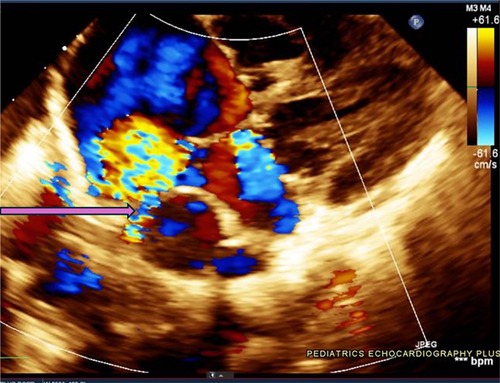
Figure 2. This echocardiogram highlights the turbulent flow pattern within the left atrium, as seen in the color Doppler display. The blue and red color signals indicate a high-velocity flow across the membranous partition within the atrium, characteristic of Cor Triatriatum. The arrow is pointing towards a small restrictive communication present in the membrane, reinforcing the diagnosis and illustrating potential hemodynamic implications.
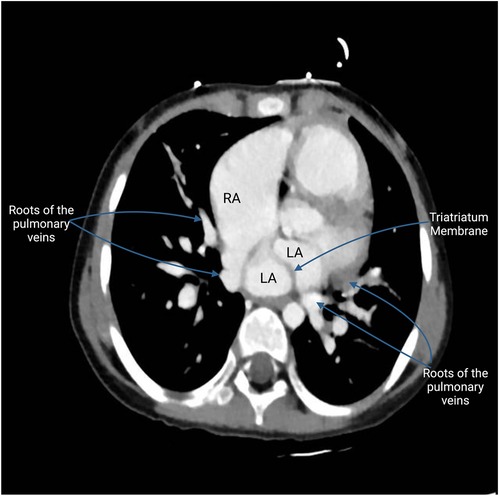
Figure 3. This CT image highlights the fibromuscular membrane dividing the left atrium into two chambers. Visible on the scan is the Cor membrane, which acts as a partition within the atrium, creating a distinct proximal and distal chamber. The proximal chamber receives the pulmonary veins, while the distal chamber channels blood to the mitral valve, leading to the left ventricle. The scan shows the unusual entry points of the pulmonary veins: the roots of two pulmonary veins are depicted entering the roof of the right atrium, an anomalous connection indicative of PAPVR. In contrast, the roots of the two other pulmonary veins are seen entering the left atrium, following the normal anatomical pattern. This difference between normal and abnormal venous connections highlights the unique challenges posed by Cor Triatriatum.
Cor Triatriatum is classified according to various systems, such as the Loeffler classification (9), Lam classification (6), the modified Lucas classification (10), and the newly proposed Mashadi-Narasimhan-Said classification (11). Echocardiography is the diagnostic modality of choice for Cor Triatriatum (12, 13). While cardiac angiography and catheterization were widely used in the past, they are now rarely utilized and largely supplanted by echocardiography, but can still confirm the diagnosis by displaying differential atrial chamber filling (14).
The primary pathophysiological mechanism of Cor Triatriatum involves the obstruction of blood flow due to an abnormal septum in the atria, leading to increased atrial pressures and subsequent pulmonary and systemic complications (6, 15). Table 1 highlights the three prevailing theories on the formation of Cor Triatriatum (16–18). The definitive treatment for Cor Triatriatum involves surgical correction, typically performed under cardiopulmonary bypass (19). The first successful surgical correction of Cor Triatriatum was reported in 1956 by Lam et al., marking a significant milestone in the management of this anomaly (20). Since then, surgical excision of the abnormal septum under cardiopulmonary bypass has become the definitive treatment, with a very low risk of recurrence (10).
Despite advancements in diagnostic and surgical techniques, Cor Triatriatum remains challenging due to its rarity and the potential for delayed diagnosis (21). This study aims to explore the prevalence, risk factors, and possible surgical interventions, as well as multimodal diagnostic modalities for better treatment in pediatric patients with Cor Triatriatum.
This study involved a retrospective chart review of medical records for pediatric patients diagnosed with Cor Triatriatum at the American University of Beirut Medical Center's Children's Heart Center from 2006 to 2024. 7 patients were identified with Cor Triatriatum. Due to the retrospective nature of the study, all patients were deidentified, stored securely, and accessible only to the research team. The study was approved by the Biomedical Institutional Review Board (BIO-2024-0160) and adhered to ethical standards as outlined in the Declaration of Helsinki, the Nuremberg Code, and the Belmont Report.
Data were collected on patient demographics (age, gender, height, and weight), clinical presentations, and diagnostic imaging results, including echocardiography, chest x-rays, and electrocardiograms to assess the morphology of the anomaly and the extent of obstruction. Criteria for diagnosis included the visualization of a membrane dividing the left or right atrium into two distinct chambers with evidence of blood flow obstruction. Additionally, information on surgical interventions and postoperative outcomes, including complications and length of hospital stay was gathered. Loeffler's classification was used for Cor Triatriatum morphology (Figure 4). Data on surgical treatment were highlighted, including type of procedure, intraoperative findings, and immediate postoperative outcomes. Long-term follow-up was also documented highlighting long-term postoperative outcomes and the patient's status during office visits.

Figure 4. (A) Group 1 Loeffler classification of Cor Triatriatum. (B) Group 2 Loeffler classification of Cor Triatriatum. (C) Group 3 Loeffler classification of Cor Triatriatum.
Descriptive statistics were used to showcase continuous variables, which were reported as medians with interquartile ranges based on their distribution. Categorical variables were mostly presented as frequencies. All data analysis was conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
At our center, 7 cases of Cor Triatriatum were diagnosed. The median interquartile range (IQR) age of the identified 7 patients was 5 (4–23.0) months, with a median (IQR) body weight of 14 (9.8–14.0) kg and a median (IQR) height of 92 (80.0–92.75) cm. Of the 7 patients, 4 were females and 3 were males. Table 2 outlines the demographic characteristics of these patients.
The median duration of hospital stay was 7.0 days (5.5–8.0 days) encompassing the time required for both the diagnosis and the surgical intervention of the patients. The cohort included. 4 patients were admitted to the pediatric intensive care unit, and the remaining 3 were managed as floor patients. No patients were admitted to the neonatal intensive care unit. Surgical intervention was performed in 6 out of 7 (85.7%) of the patients. The patients who underwent surgery had successful resections of the obstructing membrane, with significant improvement in clinical symptoms and stabilization of their conditions. Patient outcomes are reported in Table 3.
Laboratory results demonstrated a median white blood cell count of 13,100/cu.mm, indicating a systemic inflammatory response. Furthermore, c-reactive protein levels were elevated, with a median value of 21.4 mg/L, further supporting the presence of an inflammatory state. The hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, median prothrombin time, partial thromboplastin time, and platelet counts were within physiological ranges.
All patients were diagnosed with Cor Triatriatum Sinister (CTS) and the diagnosis was made by echocardiography in all cases. The clinical presentation of patients with Cor Triatriatum was dominated by respiratory symptoms. Dyspnea was a common finding, reported in five patients, with one of these cases also showing signs of pulmonary hypertension. One patient presented with fast and labored breathing. Additionally, one patient had symptoms of an upper respiratory tract infection for two weeks prior, with minimal cough on presentation. Another patient exhibited both dyspnea and congestion. These findings highlight the predominantly respiratory nature of clinical symptoms in patients with Cor Triatriatum.
Atrial septal defects were present in 2 patients. Among the 7 patients with Cor Triatriatum, multiple pulmonary vein abnormalities were documented. One patient exhibited pulmonary veins draining into a chamber posterior to the left auricle, with a connection to the left auricle complicated by obstruction. Another patient showed obstructed venous flow due to the Cor Triatriatum septum itself. Pulmonary veins draining into a distal chamber were observed in one case, while another displayed right pulmonary veins with anomalous drainage into the right atrium, consistent with partial anomalous pulmonary venous return (PAPVR). The finding of PAPVR was also evident in another patient. The remaining 2 patients did not exhibit any associated pulmonary vein abnormalities. Among the 7 patients with Cor Triatriatum, various valvular abnormalities were documented. One patient had both aortic and mitral regurgitation, while another presented with mitral regurgitation alone, with the remaining 5 having normal valves. Radiographic evidence of pulmonary edema was observed in 5 patients.
Table 4 provides a comprehensive overview of each patient's associated anomalies, clinical presentations, atrial communication, and pulmonary vein anomalies, along with hospital stays and outcomes.
6 patients underwent operations for resection of the membrane. Surgical interventions varied across the 6 patients with Cor Triatriatum, each addressing unique structural abnormalities. Cardiopulmonary bypass with aortic cross-clamping was consistently utilized. The cross-clamp time required did not exceed half an hour and the bypass time did not take more than one hour for each individual patient. This step was followed by atriotomies and targeted membrane resections. In three cases, specific approaches were taken to either redirect pulmonary veins by resecting the membrane or reinforcing the mitral valve through mitral valve repair surgery in cases of severe mitral regurgitation. Additionally, some procedures involved the use of autologous pericardial patches for atrial septal repairs and to guide pulmonary venous flow. Each surgery concluded with chest drainage and sternotomy closure, highlighting the individualized yet systematic approach to managing these complex cardiac anomalies. One patient was lost to follow-up.
Cor Triatriatum is a rare congenital disorder comprising 0.1%–0.4% of all congenital heart disorders (15). In particular, CTS presents with a septated left atrium, isolating a proximal part of the atrium with the pulmonary veins' entry from a distal part communicating with the left ventricle through the mitral valve (20, 22). With multiple theories discussed in the literature, the most accepted seems to be an abnormal incorporation of the common pulmonary vein in the left atrium (13, 15). Obstructive symptoms mimicking mitral stenosis and pulmonary vein stenosis may vary greatly depending on the communication level within the now two-chambered left atrium (6, 20, 22, 23). In fact, the Loeffler classification defines three possible presentations of CTS according to the amount of communication provided by the occluding diaphragm (9). A non-communicating diaphragm constitutes group 1 (Figure 4A). The septating membrane presents one or more small openings in group 2 (Figure 4B). Group 3 patients present with a large opening within the diaphragm (Figure 4C) (9, 13, 24). Therefore, a diagnosed case of asymptomatic CTS early in life, which is usually the case in a patient belonging to group 3 CTS, may delay diagnosis till a later age (13, 22). When diagnosed with CTS, regardless of age, patients are sent for surgical resection of the membrane occluding the left atrium, which is a curative management option (13, 25).
In this study, we have compiled data from a cohort of 7 patients, with a median age of 5 months, emphasizing the early presentation typically associated with this anomaly.
The median age at diagnosis for CTS is typically under 1 year, reflecting the early onset of symptoms associated with this congenital abnormality (26). In a Canadian study conducted at a tertiary care center involving 82 patients with CTS, the median age at presentation was 8 months (27). Similarly, an Australian study reported a median age of presentation of 6 months (28). These findings are consistent with those observed in our study. This can be attributed to the fact that the clinical manifestations of CTS, such as respiratory distress and signs of heart failure, often present early in life (26). Furthermore, early diagnosis of CTS is crucial for timely intervention, which may explain the comparable median ages at presentation across various studies (26–29). The physiological impact of CTS largely depends on the size of the opening between the accessory and the main atrial chambers (12). In infants and young children, this restricted flow leads to pronounced symptoms such as respiratory distress and dyspnea (30). This compares to our cohort, where all the patients presented with respiratory symptoms. Conversely, adults are typically asymptomatic when the foramen is large, allowing for normal intra-atrial pressure without significant obstruction (12). During physical examination, the condition may manifest as a diastolic murmur distinct from mitral stenosis by the absence of an opening snap and a distinct loud S1 with a pronounced second heart sound (P2) if pulmonary hypertension develops (31). Our findings demonstrate a nearly equal number of CTS patients in both sexes. These results are consistent with the literature (28). A systematic review by Ullah et al. compiled 235 studies, and the pooled results showed that 54% of the cases were male and 46% were female (32). This consistent observation across different regions suggests that CTS does not have a significant sex predilection, potentially indicating that genetic and congenital factors related to the development of this anomaly are equally likely to occur in both males and females. The patients in our cohort had a median hospital stay of 7 days.
Our results indicate that 100% of our patients had CTS, which aligns with the literature suggesting a higher CTS when compared to Cor Triatriatum Dexter. Specifically, approximately 83% of patients with Cor Triatriatum have CTS, while 17% have Cor Triatriatum Dexter (32). The exclusive finding of CTS in our study may be attributed to the small sample size of 7 patients, which could limit the representation of Cor Triatriatum Dexter cases and highlight the need for larger-scale studies to capture the full spectrum of Cor Triatriatum presentations. Additionally, studies show that the majority of patients with Cor Triatriatum have associated cardiac lesions (12). Our results show that two patients had septal defects, which is notably lower than the 50%-80% range of patients reported in the literature (33). For instance, in a tertiary care center in Toronto, 53% of CTS patients had atrial septal defects and 10% had ventricular septal defects (27). The discrepancy with our findings can be attributed to our sample size, which might not have been sufficient to represent the full range of associated anomalies. Variations in diagnostic criteria or population characteristics may also contribute to this difference, with genetic and environmental factors potentially influencing the clinical presentation of CTS.
In our cohort, pulmonary edema was observed on chest x-ray when performed in 5 patients of our patients. This is expected due to left-sided blood flow obstruction causing an elevated pulmonary venous pressure, eventually leading to capillary leakage and pulmonary edema (34). This finding further indicates the necessity of prompt surgical intervention in dyspneic CTS patients to alleviate the obstruction. Interestingly, 2 patients had no chest x-ray abnormal findings despite having CTS. This can be explained by the variability of CTS and its symptoms, as per Loeffler's classification. Pulmonary hypertension and edema are common CTS findings defined in the literature, with a case series by Ozyuksel et al. reporting it in 8 out of a 15 patient cohort (21, 27, 35–37). Furthermore, Humpl et al. (27) compared the occurrence of pulmonary edema in 3 cohorts: patients who died before intervention, patients who underwent surgical repair, and patients who did not undergo surgical intervention. Pulmonary edema incidence was 55%, 28%, and 0%, respectively, for the 3 groups defined (27). This corroborates the idea that pulmonary edema is a major indicator of the severity of CTS since it was mostly present in the deceased cohort, lesser found in the group requiring surgery, and absent in those not requiring surgery (27). However, it is important to note that normal radiographic findings do not exclude CTS, as shown in the case of pulmonary edema in less severe CTS patients.
In our study, 6 out of 7 (85.7%) of patients with CTS underwent surgical intervention, which is comparable to some findings reported in the literature. Several studies showed that approximately 70% of patients with CTS underwent surgery (27, 32). In a study conducted by the Mayo Clinic, a reported 47% of patients underwent resection of the CTS membrane (38). The variation in surgical rates across different centers may be influenced by differences in the severity of symptoms and institutional practices. The lower surgical rates observed in the Mayo Clinic study may indicate a more conservative management approach or differences in the patient population or severity of presentation (38). In the operated patients, surgery was initiated by median sternotomy and cardiopulmonary bypass, followed by cardioplegic arrest and then resection of the obstructing membrane from an opening in the right atrium. It was finalized by the closure of the atrial septum. Redirection of pulmonary veins in 2 out of the 6 patients undergoing surgery was required as both patients presented with PAPVR. Misplacement and subsequent redirection of pulmonary veins are commonly encountered in the context of CTS repair (39–41). In our cohort, the integrity of the mitral valves was tested, and then regurgitant valves were repaired via commissuroplasty using 5/0 and 6/0 Prolene with pledgets. In a case study of a severe mitral regurgitation co-occurring with CTS, it was reported that this combination is extremely rare and was repaired via annuloplasty (42). The different approaches employed to address the mitral regurgitation are explained by the fact that our patient's mitral valve had a lesion at the posterior commissure, while annuloplasty was used for a “functional regurgitation” due to an annular dilation caused by CTS (42).
A step-by-step protocol for the procedures done in our center is as follows: the procedure is performed under cardiopulmonary bypass and aortic cross clamping. Next, the right atrium is opened and the left atrium is best entered through the interatrial septum, especially if the location of the pulmonary veins and the cor membrane were not accurately identified preoperatively. The anatomy is then meticulously examined, and the position of each pulmonary vein is precisely depicted. Any membrane dividing the left atrium should be excised totally to provide an unobstructed flow between all four pulmonary veins and the mitral valve. The interatrial septal opening is repaired at the end of the procedure. In neonates, a 3–4 mm foramen ovale may be kept open in anticipation of postoperative pulmonary hypertension, especially in cases with obstruction. More details can be seen in Table 5 highlighting individual surgical notes.
Furthermore, our findings indicate that 2 out of 7 (28.6%) patients with CTS had PAPVR. Our study shows no cases of total anomalous pulmonary venous return.
Finally, mortality rates for CTS are also controversial, with some studies reporting rates as high as 23%, while others showing significantly lower rates around 4% (27, 38). The variations in mortality rates between different centers may be due to differences in the presence and severity of associated cardiac anomalies rather than CTS itself. Centers reporting higher mortality rates may often see cases with complex congenital heart defects that significantly impact patient outcomes (27). In contrast, our cohort had no reported mortalities, highlighting potential differences in case characteristics or care approaches.
Our study has several limitations. First, its small sample size (n = 7 patients) may not adequately represent the broader population of patients with CTS, which could affect the generalizability of our findings. Additionally, being a retrospective review, the study may be subject to incomplete or biased data, limiting the accuracy of our conclusions. As the study was conducted at a tertiary care center, there may be referral bias since more complex or severe cases are likely to be overrepresented compared to the general population.
Cor Triatriatum remains a rare yet significant congenital heart anomaly with special challenges in diagnosis and management. This retrospective review of 7 pediatric patients provides valuable insights into the clinical presentation, diagnostic techniques, and therapeutic outcomes. The management of Cor Triatriatum continues to require a tailored approach, considering the anatomical diversity and associated cardiac anomalies. Moreover, the unique classification systems, such as the Loeffler classification, have been instrumental in enhancing our understanding of the anatomical variations within Cor Triatriatum. The findings from this study emphasize the importance of early and accurate diagnosis, timely surgical intervention, and a personalized case-by-case approach.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Institutional Review Board at the American University of Beirut IRB ID for this study: BIO-2024-0160. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The research does not pose more than minimal risk to the included subjects and the waiver of consent will not adversely affect the welfare and rights of the participants. Also, obtaining consent from the subjects is not feasible as not all of the patients are still followed up. "The IRB assessed your request to waive consent for this study and determined based on what you reported in the application that the study fulfills the criteria for waiver of the consenting process under 45 CFR 46.116(d). Therefore, requests to contact these subjects later for follow up data, or the collection of variables that might not be available in the records for this study or for other protocols will not be permissible."
JA: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing, Validation, Conceptualization. CE: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation. MAM: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation. BS: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Project administration. IE: Conceptualization, Software, Visualization, Writing – original draft, Writing – review & editing, Resources. FB: Conceptualization, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MA: Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Investigation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Williams JL, Torok RD, D'Ottavio A, et al. Causes of death in infants and children with congenital heart disease. Pediatr Cardiol. (2021) 42(6):1308–15. doi: 10.1007/s00246-021-02612-2
2. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. (2010) 122(22):2254–63. doi: 10.1161/circulationaha.110.947002
3. Marian AJ. Congenital heart disease: the remarkable journey from the “Post-Mortem Room” to adult clinics. Circ Res. (2017) 120(6):895–7. doi: 10.1161/circresaha.117.310830
4. Nassif M, Abdelghani M, Bouma BJ, et al. Historical developments of atrial septal defect closure devices: what we learn from the past. Expert Rev Med Devices. (2016) 13(6):555–68. doi: 10.1080/17434440.2016.1182860.
5. Nayak S, Patel A, Haddad L, Kanakriyeh M, Varadarajan P. Echocardiographic evaluation of ventricular septal defects. Echocardiography. (2020) 37(12):2185–93. doi: 10.1111/echo.14511.
6. Jha AK, Makhija N. Cor triatriatum: a review. Semin Cardiothorac Vasc Anesth. (2017) 21(2):178–85. doi: 10.1177/1089253216680495.
7. Baxi AJ, et al. Bands, chords, tendons, and membranes in the heart: an imaging overview. Curr Probl Diagn Radiol. (2016) 45(6):380–91. doi: 10.1067/j.cpradiol.2015.08.009.
8. Tran T, et al. Sudden cardiac death as a consequence of cor triatriatum sinistrum in an adult. J Cardiol Cases. (2023) 27(1):4–7. doi: 10.1016/j.jccase.2022.09.004.
9. Loeffler E. Unusual malformation of the left atrium; pulmonary sinus. Arch Pathol (Chic). (1949) 48(5):371–6.18143913
10. Kim D, et al. Surgical outcomes of cor triatriatum sinister: a single-center experience. J Chest Surg. (2022) 55(2):151–7. doi: 10.5090/jcs.21.134.
11. Mashadi AH, et al. The Mashadi-Narasimhan-said classification for cor triatriatum sinister: applicable to all. J Chest Surg. (2023) 56(2):151–3. doi: 10.5090/jcs.22.149.
12. Nassar PN, Hamdan RH. Cor triatriatum sinistrum: classification and imaging modalities. Eur J Cardiovasc Med. (2011) 1(3):84–7. doi: 10.5083/ejcm.20424884.21
13. Butt M, et al. A tale of three chambers: cor triatriatum sinistrum. CASE (Phila). (2024) 8(3Part A):221–5. doi: 10.1016/j.case.2023.12.019
14. Li WW, et al. Cathether-based interventional strategies for cor triatriatum in the adult - feasibility study through a hybrid approach. BMC Cardiovasc Disord. (2015) 15:68. doi: 10.1186/s12872-015-0067-4.
15. Medellin S, Burbano-Vera N, Alfirevic A. Obstructed supramitral inflow: cor triatriatum sinister presentation in adulthood. J Cardiothorac Vasc Anesth. (2024) 38(2):576–80. doi: 10.1053/j.jvca.2023.11.006.
16. Prasad D, Snyder C, Ashwath R. Septum primum malposition defect and inferior sinus venosus defect: a rare association. Cardiol Young. (2015) 25(7):1389–92. doi: 10.1017/S1047951114001899.
17. Webb S, et al. Development of the human pulmonary vein and its incorporation in the morphologically left atrium. Cardiol Young. (2001) 11(6):632–42. doi: 10.1017/S1047951101000993.
18. Thakrar A, et al. Cor triatriatum: the utility of cardiovascular imaging. Can J Cardiol. (2007) 23(2):143–5. doi: 10.1016/S0828-282X(07)70735-3.
19. Bhende VV, et al. Successful repair of cor triatriatum sinistrum in childhood: a single-institution experience of two cases. Cureus. (2022) 14(4):e24579. doi: 10.7759/cureus.24579
20. Işık O, et al. Cor triatriatum sinister: a case series. Turk Kardiyol Dern Ars. (2016) 44(1):20–3. doi: 10.5543/tkda.2015.04780
21. Saxena P, et al. Surgical repair of cor triatriatum sinister: the mayo clinic 50-year experience. Ann Thorac Surg. (2014) 97(5):1659–63. doi: 10.1016/j.athoracsur.2013.12.046.
22. Buchori E, et al. Cardiac MR imaging of cor triatriatum sinister in an elderly man: a rare case report. Radiol Case Rep. (2024) 19(4):1468–71. doi: 10.1016/j.radcr.2024.01.014.
23. Gonzalez TV, Bookwalter CA, François CJ. Multimodality imaging of a mass arising from a cor triatriatum sinister membrane. Radiol Cardiothorac Imaging. (2024) 6(1):e230225. doi: 10.1148/ryct.230225.
25. Al Kindi HN, et al. Cor triatriatum sinister (divided left atrium): histopathologic features and clinical management. Ann Thorac Surg. (2020) 110(4):1380–6. doi: 10.1016/j.athoracsur.2020.01.025.
26. Ather B, Meredith A, Siddiqui WJ. Cor triatriatum. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC (2024). p. 2–3.
27. Humpl T, et al. Cor triatriatum sinistrum in childhood. A single institution’s experience. Can J Cardiol. (2010) 26(7):371–6. doi: 10.1016/S0828-282X(10)70418-9.
28. Alphonso N, et al. Cor triatriatum: presentation, diagnosis and long-term surgical results. Ann Thorac Surg. (2005) 80(5):1666–71. doi: 10.1016/j.athoracsur.2005.04.055.
29. Rodefeld MD, et al. Cor triatriatum: clinical presentation and surgical results in 12 patients. Ann Thorac Surg. (1990) 50(4):562–8. doi: 10.1016/0003-4975(90)90190-H.
30. Kalangos A, et al. Cor triatriatum dexter in children: literature review and case report. JTCVS Tech. (2020) 4:254–8. doi: 10.1016/j.xjtc.2020.08.024.
31. Kokotsakis J, et al. Cor triatriatum presenting as heart failure with reduced ejection fraction: a case report. J Cardiothorac Surg. (2011) 6:83. doi: 10.1186/1749-8090-6-83.
32. Ullah W, et al. A systematic review of a long-forgotten cause of atrial fibrillation and stroke: cor triatriatum. Cureus. (2019) 11(12):e6371. doi: 10.7759/cureus.6371
33. Gheissari A, et al. Cor triatriatum sinistrum: one institution’s 28-year experience. Pediatr Cardiol. (1992) 13(2):85–8. doi: 10.1007/BF00798210.
34. Lemyze M, Mallat J. Understanding negative pressure pulmonary edema. Intensive Care Med. (2014) 40(8):1140–3. doi: 10.1007/s00134-014-3307-7.
35. Ozyuksel A, et al. Surgical correction of cor triatriatum sinister in the paediatric population: mid-term results in 15 cases. Eur J Cardiothorac Surg. (2015) 47(1):e25–8. doi: 10.1093/ejcts/ezu390.
36. Mashadi AH, Narasimhan SL, Said SM. Cor triatriatum sinister: long-term surgical outcomes in children and a proposal for a new classification. J Card Surg. (2022) 37(12):4526–33. doi: 10.1111/jocs.17032.
37. Nagao H, Tanaka T. Mid-term outcomes of cor triatriatum repair: comparison of biventricular physiology and univentricular physiology. Cardiol Young. (2021) 31(2):186–90. doi: 10.1017/S1047951120003595.
38. Fuchs MM, et al. Outcomes in patients with cor triatriatum sinister. Congenit Heart Dis. (2018) 13(4):628–32. doi: 10.1111/chd.12624.
39. Jaschinski C, Uzdenov M, Loukanov T. Cor triatriatum sinister with left anomalous pulmonary venous return to innominate vein. Cardiol Young. (2019) 29(3):428–30. doi: 10.1017/S1047951118002305.
40. Kumar V, et al. Surgical experience with cor triatriatum repair beyond infancy. J Card Surg. (2019) 34(12):1445–51. doi: 10.1111/jocs.14237.
41. Ishiwari K, et al. Cor triatriatum sinister with left anomalous pulmonary venous drainage to innominate vein: what to do with the vertical vein? Gen Thorac Cardiovasc Surg. (2021) 69(4):731–5. doi: 10.1007/s11748-020-01533-w.
Keywords: Cor Triatriatum, echocardiography, congenital heart surgery, congenital heart disease, blood flow obstruction
Citation: Abdul Khalek J, El Rassi C, Abou Mansour M, Sleem B, El Rassi I, Bitar F and Arabi M (2025) Cor Triatriatum: an uncommon congenital anomaly - the experience of a tertiary care center in a developing country. Front. Cardiovasc. Med. 12:1531754. doi: 10.3389/fcvm.2025.1531754
Received: 20 November 2024; Accepted: 18 February 2025;
Published: 4 March 2025.
Edited by:
Corina Maria Vasile, Université de Bordeaux, FranceReviewed by:
Attila Frigy, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaCopyright: © 2025 Abdul Khalek, El Rassi, Abou Mansour, Sleem, El Rassi, Bitar and Arabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariam Arabi, bWE4MUBhdWIuZWR1Lmxi
†These authors have contributed equally to this work and share second authorship
‡ORCID:
Mariam Arabi
orcid.org/0000-0001-6895-1580
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.