- 1Department of Pediatrics, UCLA, Los Angeles, CA, United States
- 2Doernbecher Children's Hospital, Oregon Health & Science University, Portland, OR, United States
- 3Department of Pediatrics, Santa Barbara Cottage Hospital, Santa Barbara, CA, United States
Intra-atrial baffles of pulmonary and systemic venous flows are relatively rarely used but are important surgical procedures for late-presenting d-transposition of the great arteries (D-TGA) or heterotaxy with anomalous venous connections. Atrial baffle surgery is employed less frequently today due to the advent of the arterial switch procedure. Two children presented with mirror-image venous connections in polysplenic heterotaxy and underwent intra-atrial baffle biventricular repairs. Both cases had ipsilateral pulmonary venous connections, interrupted inferior vena cava with azygous continuation, and concordant ventriculo-arterial connections. Furthermore, a single superior vena cava receiving the azygous and hepatic veins connected to the atrium contralateral to the sub-pulmonary right ventricle was present in both cases. Volumetric cardiac imaging using computed tomography and magnetic resonance imaging allowed for the creation of three-dimensional (3D) model reconstructions for pre-operative surgical planning and postoperative assessment of the atrial baffle pathways. The 3D model reconstructions presented here provide improved visualization and understanding of complex surgical atrial baffles.
Introduction
Intra-atrial baffles of pulmonary and systemic venous flows are relatively rarely used but are important surgical procedures for late-presenting d-transposition of the great arteries (D-TGA) or heterotaxy with anomalous venous connections. Two children presented with mirror-image venous connections in polysplenic heterotaxy with cardiac segments {A,L,I} and {A,D,S} and underwent intra-atrial baffle biventricular repairs. Both cases had ipsilateral pulmonary venous connections, interrupted inferior vena cava (IVC) with azygous continuation, and concordant ventriculo-arterial connections. Furthermore, a single superior vena cava (SVC) receiving the azygous and hepatic veins connected to the atrium contralateral to the sub-pulmonary right ventricle was present in both cases. Volumetric cardiac imaging using computed tomography (CT) and magnetic resonance imaging (MRI) allowed for the creation of three-dimensional (3D) model reconstructions for pre-operative surgical planning and postoperative assessment of the atrial baffle pathways. The 3D model reconstructions presented here provide improved visualization and understanding of complex surgical atrial baffles.
Background
The first successful surgical repair of D-TGA used the atrial baffle (1). This procedure uses the patient's own cardiac tissue to baffle the normally connected systemic veins to the sub-pulmonary left ventricle and the pulmonary veins to the morphologic sub-aortic left ventricle. The procedure was modified by Mustard using external materials. Once the arterial switch, initially performed by Jatene et al., became routine (2), the atrial switch was used much less frequently. However, the atrial switch for D-TGA remains important for D-TGA cases presenting later in life and for those who are not candidates for arterial switch (3, 4). Furthermore, the anomalous venous connections in heterotaxy may require intra-atrial baffling for either a physiologic (morphologic left ventricle) or a full anatomic (sub-pulmonary morphologic right ventricle) repair. Biventricular repair for left isomerism was reported as early as 1995 (5).
An atrial baffle for the venous anatomy in hearts with heterotaxy is not necessarily the same as that in hearts with normal venous anatomy with D-TGA. Anomalous venous connections are common in both asplenia and polysplenia heterotaxy. While specific venous anatomy is common to one or the other, such as interrupted IVC and ipsilateral pulmonary veins in polysplenia, a variety of other arrangements of the superior vena cava, hepatic veins, and total anomalous pulmonary venous drainage are possible. A subset of heterotaxy cases can result in a biventricular repair (6–8). When two adequate ventricles are present, the venous anatomy must be septated to create either a physiologic repair with a morphologic sub-aortic right ventricle or a full anatomic and physiologic repair with a concordant ventriculo-arterial connection.
3D model reconstruction and printing from cardiac MRI and CT have been described for pre-surgical planning to determine the feasibility of biventricular repair, most notably in hearts with a double-outlet right ventricle. The use of 3D printing in surgical planning for complex anomalous venous connections is becoming more commonly reported (8–10), and they include cases requiring various types of atrial baffles (8, 11). While the clinical outcomes of cases using 3D coronary heart disease (CHD) models have been reported, little has been reported on the 3D representations of the post-surgical anatomy.
Herein, we present 3D renderings of the pre-and post-surgical anatomy of two cases of heterotaxy with mirror-image abnormal venous anatomy who underwent atrial baffle repair.
Methods
A retrospective review of two pediatric cases with similar cardiac anatomy was performed. Approval for the case series was obtained from the Institutional Review Board of the University of California, Los Angeles (UCLA). Patient charts and imaging were reviewed for clinical data and included surgical operative notes and pre-and post-surgical advanced axial imaging with CT or MRI.
Imaging
Four-dimensional cardiac magnetic resonance imaging using ferumoxytol, an iron-based contrast agent, on a 3.0 T scanner (Magneton TIM Trio, Siemens Healthineers, Malvern, PA, USA) was used for pre-operative imaging in Case 1 (12). Cardiac non-gated computed tomography was used for pre-operative imaging in Case 2 and electrocardiogram (ECG)-gated volumetric images were obtained on a Siemens Force dual-source CT scanner in both cases for postoperative imaging.
For 3D reconstruction of the images, segmentation of the contrast-enhanced blood pool was first performed using semi-automatic region-growing in the program ITK-SNAP (13). The 3D blood pool representation was then modified and hollowed using the free, online program Meshmixer (Autodesk, Inc., San Francisco, CA, USA). To create the post-operative models, the pulmonary and systemic venous blood pools were segmented separately. For Case 1, the region of the patch was separately segmented up to where it could be visualized intersecting with the atrial walls.
Cases
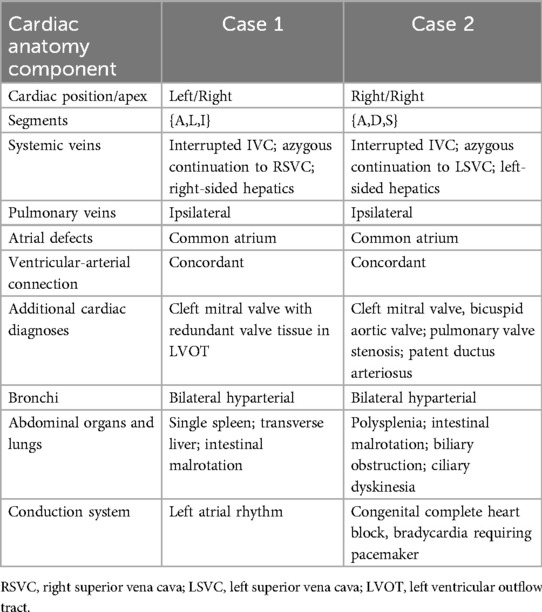
Two pediatric patients, aged 3 years (Case 1) and 5 years (Case 2), presented for surgical repair of heterotaxy with abnormal venous anatomy. The pre-operative anatomy of each case is presented in Table 1 and Figures 1a, 2A. Both cases had dextroposition of the cardiac apex, venous anatomy consisting of an interrupted IVC with azygous continuation to a single SVC that drained into the atrium connected to the morphologic left ventricle, hepatic venous drainage on the same side as the SVC, ipsilateral pulmonary venous drainage, a common atrium, a cleft mitral valve, and ventriculo-arterial concordance. Both patients had baseline 02 saturations of 80%–90%. Each patient underwent cardiopulmonary bypass for atrial baffling to create a physiologic repair with the systemic morphologic left ventricle. Both had a repair of an isolated mitral valve cleft.
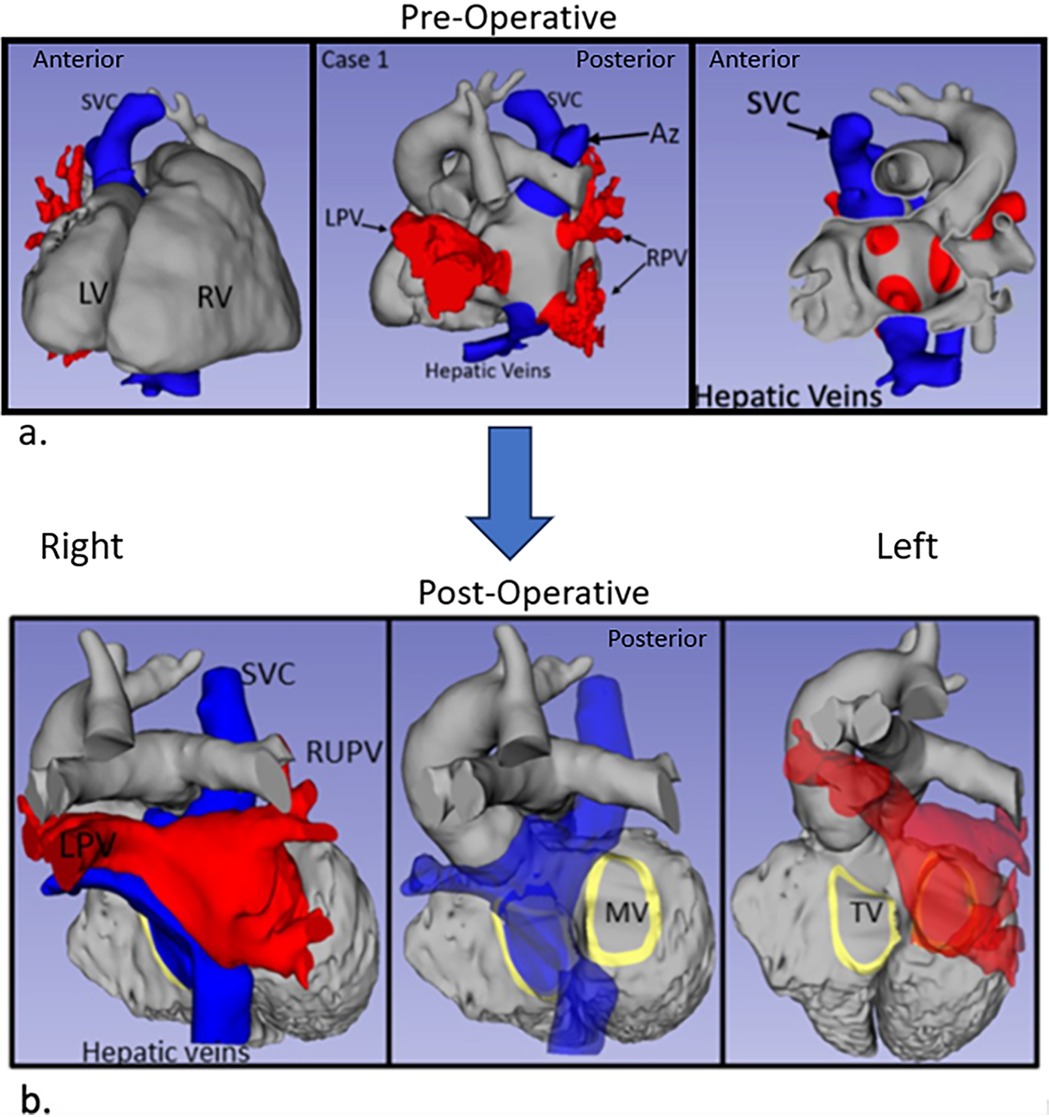
Figure 1. 3D renderings derived from MRI in Case 1. (a) Pre-operative anatomy views shown from left to right: anterior, posterior, and anterior slice through the atria. (b) Post-operative anatomy. Systemic veins are colored blue and pulmonary veins are red. Az, azygous vein; LV, left ventricle; LPV, left pulmonary veins; RPV, right pulmonary veins; RV, right ventricle; SVC, superior vena cava.
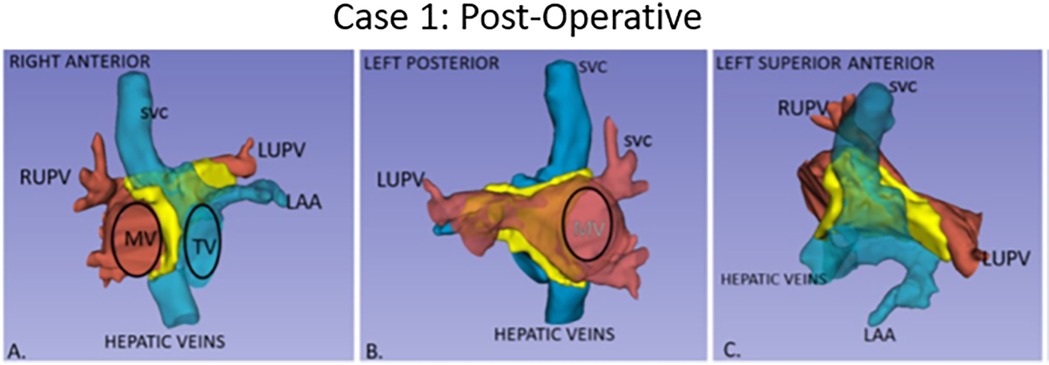
Figure 2. Post-operative anatomy in Case 1 with atrial baffle patch. Three-dimensional segmented blood pools of systemic (blue) and pulmonary (red) anatomy, with the surgical atrial baffle in yellow. (A) Right anterior view with partially transparent systemic veins. (B) Left posterior view with partially transparent pulmonary veins, and right-sided atrium. (C) Left anterior superior view.
Case 1 had cardiac segments {A,L,I}. At surgery, an autologous pericardium was used. To avoid the conduction system in Case 1, care was taken to sew the patch to the left of its expected location in the region of the atrioventricular (AV) valves.
Post-operative transesophageal echocardiography (TEE) demonstrated no baffle obstruction, with a central venous pressure (CVP) of 8 mmHg. The patient was re-admitted 2 weeks post-operatively for left-sided chylothorax, which was treated with drainage and sclerotherapy. There were post-operative arrhythmias or heart blocks. Figure 1 shows the blood pool segmentations derived from the pre- and post-operative imaging. 3D segmentation of the atrial baffle patch separating the pulmonary and systemic venous flows is shown in Figure 2 and Supplementary Video S1.
Case 2 had segments {A,D,S} with surgery consisting of an atrial baffle (Figure 3), pulmonary valvuloplasty, and pacemaker generator change. Post-operatively, the mean echo Doppler gradient was 5 mmHg through the SVC baffle, and the patch and SVC were subsequently augmented, but the mean Doppler gradient remained at 5 mmHg. The central venous pressure in the SVC proximal to the atrium was 6 mmHg.
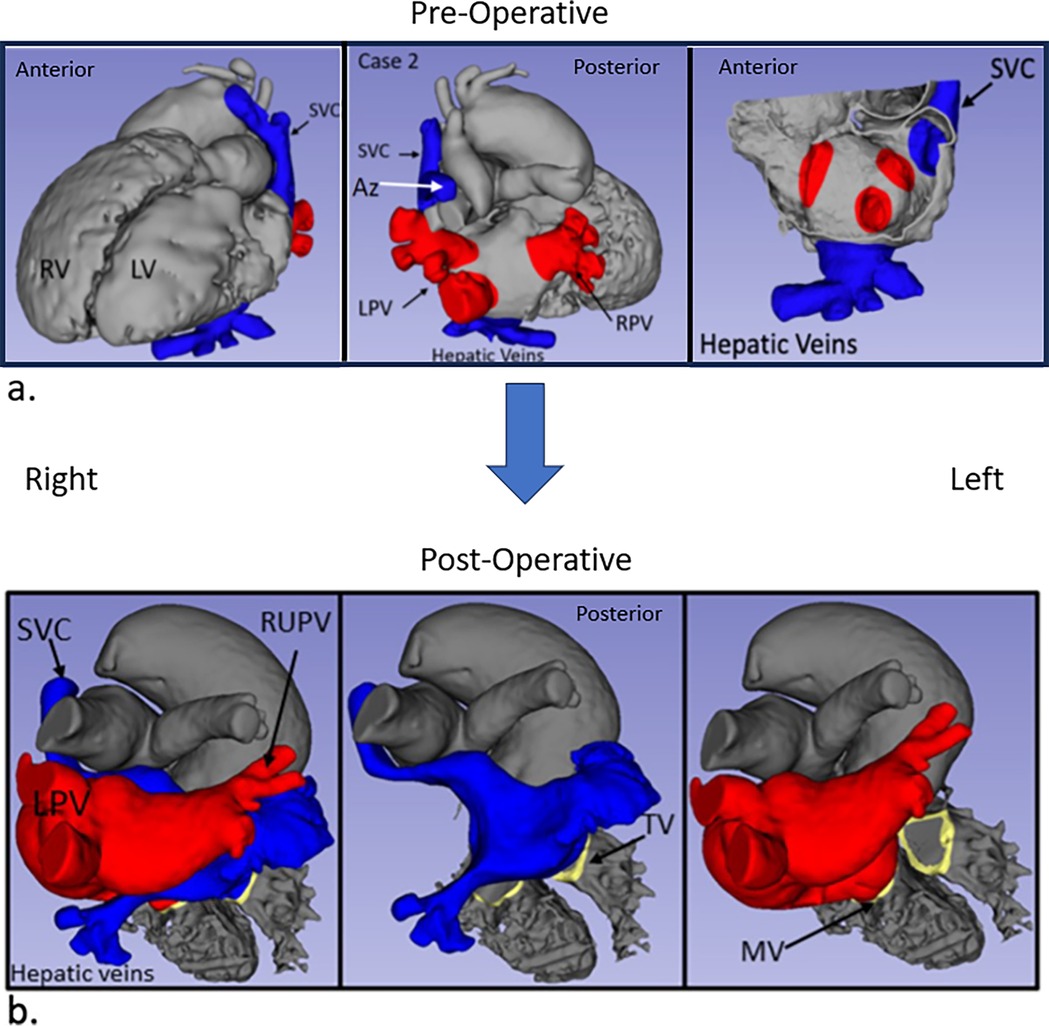
Figure 3. Case 2 imaging derived from CT. Pre-operative (a) and post-operative (b) images are shown with comparable views to Figures 1a,b.
Discussion
Three-dimensional reconstruction and 3D printing from MRI and CT have been demonstrated to assist in pre-procedural planning for complex CHD, often with the goal of a biventricular repair. Many forms of heterotaxy can ultimately undergo atrial and/or ventricular septation, resulting in a biventricular heart. The cases presented here had complex venous anatomy requiring intra-atrial baffles that differed from those classically used for D-TGA.
The two unique anatomic features that needed to be addressed were the increased flow in the SVC from the interrupted IVC and the ipsilateral pulmonary veins. The SVC baffle needed to be larger than a standard Senning or Mustard repair because it included flow from the interrupted IVC to the azygous vein. The 3D models were used to review the possible courses of the SVC flow to the contralateral AV valve. The SVC flow could be baffled inferiorly toward the hepatic vein entry or along the superior aspect of the atrium. In both cases, the SVC flow baffle was baffled superiorly, as shown in Figure 2. Despite surgical augmentation, Case 2 had a mean gradient of 5 mmHg through the SVC baffle, with an acceptable vena caval pressure of 6 mmHg. The flow gradient may have resulted from a relatively increased flow from both the SVC and interrupted IVC through this superior limb of the baffle and some lateral compression from a dilated left pulmonary artery. The CVP remained at 6 mmHg post-operatively and there was no facial or peripheral edema, so no further intervention was performed.
Second, the anomalous lower pulmonary veins that drained to the same side as the systemic veins connected to the atrium were very close to the inferiorly and anteriorly located hepatic veins in both hearts. The atrial baffle patch was extended inferiorly and anteriorly on the side of the systemic veins to separate the flows without obstruction (Figure 3).
Surgical repair of hearts with so-called isolated ventricular inversion, with atrioventricular discordance and ventriculo-arterial concordance, has been described using Senning- or Mustard-type repairs (14–16). Even more rarely, similar anatomies to those presented here, with left atrial isomerism, have been previously described (17, 18). However, imaging of the post-operative anatomy has been limited to 2D echocardiography and illustrations representing the surgical view.
While pre- and post-operative imaging with advanced CT and MRI is not required for surgical correction of left isomerism, axial and three-dimensional imaging does offer a complete view of the anatomy not otherwise visualized by a 2D or 3D echo. Experienced surgical groups have expounded on the usefulness of 3D modeling for congenital heart disease (9–11). The drawbacks of this approach are radiation with CT and, in younger patients, anesthesia.
Limitations
There are limitations to 3D models created from CT and MRI. Most notably, valve leaflet tissue is usually not incorporated into the model unless it is very well imaged. In the cases presented here, the locations of the valve annuli were outlined based on their known locations on the source images. The study is limited in that it only reports a two-patient series. However, this anatomy is rare, and these cases are the only two with this venous anatomy that the authors have encountered at our institution with full-volume advanced imaging available for 3D reconstruction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the University of California, Los Angeles. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GP: Writing – original draft, Writing – review & editing. MS: Conceptualization, Supervision, Writing – review & editing. JF: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1529485/full#supplementary-material
Supplementary Video S1 | Post-operative pulmonary and systemic venous flows and surgical atrial patch baffle.
References
1. Bove EL. Senning's procedure for transposition of the great arteries. Ann Thorac Surg. (1987) 43(6):678–80. doi: 10.1016/s0003-4975(10)60252-4
2. Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, et al. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. (1976) 72:364–70. doi: 10.1016/S0022-5223(19)40063-9
3. Hosny H, Sedky Y, Romeih S, Simry W, Afifi A, Elsawy A, et al. Revival and modification of the Mustard operation. J Thorac Cardiovasc Surg. (2020) 159(1):241–9. doi: 10.1016/j.jtcvs.2019.03.027
4. Schidlow DN, Jenkins KJ, Gauvreau K, Croti UA, Giang DTC, Konda RK, et al. Transposition of the great arteries in the developing world: surgery and outcomes. J Am Coll Cardiol. (2017) 69(1):43–51. doi: 10.1016/j.jacc.2016.10.051
5. Carotti A, Marino B, Oppido G, Marcelletti C. Biventricular repair in patients with left isomerism. J Thorac Cardiovasc Surg. (1995) 110(4 Pt 1):1151–3. doi: 10.1016/s0022-5223(05)80190-4
6. Makhija Z, Marwah A, Mishra S, Kumar J, Goel A, Sharma R. Biventricular repair in heterotaxy patients. World J Pediatr Congenit Heart Surg. (2015) 6(2):195–202. doi: 10.1177/2150135114563772
7. Ortega-Zhindón DB, Calderón-Colmenero J, García-Montes JA, Sandoval JP, Minakata-Quiroga MA, Cervantes-Salazar JL. Cardiac surgery in patients with atrial isomerism: long-term results and outcomes. J Card Surg. (2021) 36(12):4476–84. doi: 10.1111/jocs.15982
8. Najm HK, Karamlou T, Ahmad M, Hassan S, Yaman M, Stewart R, et al. Biventricular conversion in unseptatable hearts: “ventricular switch”. Semin Thorac Cardiovasc Surg. (2021) 33(1):172–80. doi: 10.1053/j.semtcvs.2020.08.010
9. Yoo SJ, Hussein N, Peel B, Coles J, van Arsdell GS, Honjo O, et al. 3D modeling and printing in congenital heart surgery: entering the stage of maturation. Front Pediatr. (2021) 9:621672. doi: 10.3389/fped.2021.621672
10. Yoo SJ, Thabit O, Kim EK, Ide H, Yim D, Dragulescu A, et al. 3D printing in medicine of congenital heart diseases. 3D Print Med. (2015) 2(1):3. doi: 10.1186/s41205-016-0004-x
11. Hussein N, Kasdi R, Coles JG, Yoo SJ. Use of 3-dimensionally printed heart models in the planning and simulation of surgery in patients with Raghib syndrome (coronary sinus defect with left superior vena cava). JTCVS Tech. (2020) 2:135–8. doi: 10.1016/j.xjtc.2020.01.023
12. Nguyen KL, Ghosh RM, Griffin LM, Yoshida T, Bedayat A, Rigsby CK, et al. Four-dimensional multiphase steady-state MRI with ferumoxytol enhancement: early multicenter feasibility in pediatric congenital heart disease. Radiology. (2021) 300(1):162–73. doi: 10.1148/radiol.2021203696
13. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. (2006) 31(3):1116–28. www.itksnap.org doi: 10.1016/j.neuroimage.2006.01.015
14. Pasquini L, Sanders SP, Parness I, Colan S, Keane JF, Mayer JE Jr, et al. Echocardiographic and anatomic findings in atrioventricular discordance with ventriculoarterial concordance. Am J Cardiol. (1988) 62(17):1256–62. doi: 10.1016/0002-9149(88)90270-6
15. Fox LS, Kirklin JW, Pacifico AD, Waldo AL, Bargeron LM Jr. Intracardiac repair of cardiac malformations with atrioventricular discordance. Circulation. (1976) 54(1):123–7. doi: 10.1161/01.cir.54.1.123
16. Ostermeyer J, Bircks W, Krian A, Sievers G, Hilgenberg F. Isolated atrioventricular discordance. Report of two surgical cases with isolated ventricular inversion. J Thorac Cardiovasc Surg. (1983) 86(6):926–9. doi: 10.1016/S0022-5223(19)39071-3
17. McElhinney DB, Reddy VM, Silverman NH, Hanley FL. Intraatrial baffle repair of isolated ventricular inversion with left atrial isomerism. Ann Thorac Surg. (1996) 62(5):1529–32. doi: 10.1016/0003-4975(96)00546-2
Keywords: heterotaxy syndrome, imaging—computed tomography, congenital heart abnormalities, three-dimensional, surgery
Citation: Perens G, Silberbach M and Frazer J (2025) Case Report: Atrial baffles of pulmonary and systemic veins for the anatomic and physiologic repair of left atrial isomerism heterotaxy—pre- and post-operative three-dimensional reconstructions of two mirror-image pediatric hearts. Front. Cardiovasc. Med. 12:1529485. doi: 10.3389/fcvm.2025.1529485
Received: 17 November 2024; Accepted: 27 February 2025;
Published: 20 March 2025.
Edited by:
Inga Voges, University Medical Center Schleswig-Holstein, GermanyReviewed by:
Bruno Marino, Sapienza University of Rome, ItalyGerald Marx, Boston Children's Hospital and Harvard Medical School, United States
Copyright: © 2025 Perens, Silberbach and Frazer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregory Perens, Z3BlcmVuc0BtZWRuZXQudWNsYS5lZHU=
 Gregory Perens
Gregory Perens Michael Silberbach2
Michael Silberbach2