- 1Department of Cardiovascular Surgery, University of Medicine and Pharmacy, Ho Chi Minh City, Vietnam
- 2Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
Transcatheter aortic valve replacement (TAVR) has become a leading treatment for aortic stenosis, but managing thromboembolic and bleeding risks post-procedure remains challenging. This review examines current evidence on antithrombotic therapy after TAVR. Subclinical leaflet thrombosis is observed in 10%–20% of patients, though its clinical significance remains uncertain. Clinical valve thrombosis is rare. Current guidelines favor single antiplatelet therapy for patients without indications for long-term anticoagulation, as dual antiplatelet therapy increases bleeding risk without improving outcomes. For patients requiring long-term anticoagulation, monotherapy with direct oral anticoagulants or vitamin K antagonists is recommended to minimize bleeding. Ongoing trials aim to clarify optimal antithrombotic regimens and strategies for preventing subclinical leaflet thrombosis. Individualized therapy based on patient risk profiles is likely needed to improve the efficacy and safety of antithrombotic treatment post-TAVR.
1 Introduction
Aortic stenosis (AS) is one of the most prevalent valvular heart diseases, especially among older adults, and is associated with significant morbidity and mortality if left untreated. Traditionally, surgical aortic valve replacement has been the standard treatment for severe symptomatic AS. However, the advent of transcatheter aortic valve replacement (TAVR) has revolutionized the treatment landscape, offering a minimally invasive alternative with favorable outcomes, particularly for patients at high or prohibitive surgical risk (1, 2). Since its introduction by Cribier et al. in 2002, the use of TAVR has expanded to include lower-risk populations because of technical advances, reduced complications, and favorable outcomes (3). However, the risk of thromboembolic events, myocardial infarction (MI), and both subclinical and clinical valve thrombosis remain critical issues requiring optimal postprocedural management. Balancing the prevention of thromboembolic events against the risk of bleeding is central to managing patients after TAVR (4, 5). Selection of appropriate antithrombotic therapy, including single antiplatelet therapy (SAPT), dual antiplatelet therapy (DAPT), or anticoagulation, remains a subject of debate. This review comprehensively assesses current evidence in the field, focusing on key trials and clinical recommendations to provide insights into the optimal antithrombotic regimen after TAVR.
2 Need for antithrombotic therapy after TAVR
Patients undergoing TAVR have an increased risk of thrombotic and bleeding events in the periprocedural period and during long-term follow-up post TAVR because of their older age and comorbidities (4, 5). The possibility of cardiovascular embolic events, such as stroke and MI, and valve thrombosis require the use of preventive antithrombotic therapy after TAVR. However, the older and often frail patient population undergoing TAVR is also at high risk for bleeding, which complicates the choice of therapy. Antithrombotic regimens must be tailored to individual risk profiles that consider various factors, including the presence of atrial fibrillation (AF), coronary artery disease, and previous stroke.
2.1 Cardiovascular embolic events
2.1.1 Stroke and transient ischemic attack
Stroke remains a significant complication post TAVR, even though 30-day stroke rates have decreased from 4∼6% in early trials to below 1% in recent studies including PARTNER 3 (6–13). Furthermore, the risk of stroke persists beyond the periprocedural period, with rates ranging between 0.2% and 7.8% at 1 year after TAVR. The mechanisms contributing to periprocedural and delayed strokes differ. Periprocedural strokes are often related to embolization of calcified aortic debris during valve deployment. Although cerebral embolic protection devices may appear to reduce disabling stroke in the PROTECTED TAVR trial, none of the randomized controlled trials, including PROTECTED TAVR, SENTINEL, and REFLECT II, have demonstrated a significant reduction in cerebral ischemic events with these devices, and their adoption remains limited (14–17). Post-TAVR strokes are often associated with new-onset AF or valve-related thrombus formation. Subclinical leaflet thrombosis has been observed in up to 22% of patients undergoing TAVR and is associated with a more than 3-fold increase in stroke risk (18, 19).
2.1.2 Myocardial infarction
Though less common than stroke, MI can also occur during or after TAVR. The risk of MI associated with TAVR ranges from 0% to 2.8% at 30 days and from 0.4% to 3.5% at 1 year (6–13). MI is more likely in patients with preexisting coronary artery disease and those undergoing valve-in-valve procedures, which may result in obstruction of the coronary ostia by the implanted prosthetic valve. Postprocedural coronary ischemia can also result from impaired coronary flow dynamics secondary to interactions between the prosthetic valve and the native aortic anatomy. In rare instances, MI may be caused by coronary embolism. Nonetheless, coronary artery disease is a very common comorbidity in patients undergoing TAVR, and MI is often the result of disease progression.
2.2 Valve thrombosis
Valve thrombosis encompasses a spectrum ranging from subclinical leaflet thrombosis to clinically apparent valve thrombosis (20). Subclinical leaflet thrombosis is characterized by hypoattenuated leaflet thickening on computed tomography (CT) (Figure 1) and may progress to reduced leaflet motion. Although subclinical leaflet thrombosis is detected in 10%–20% of patients within the first year after TAVR, clinical valve thrombosis occurs much less frequently, with an incidence of approximately 1.2% (20–22).
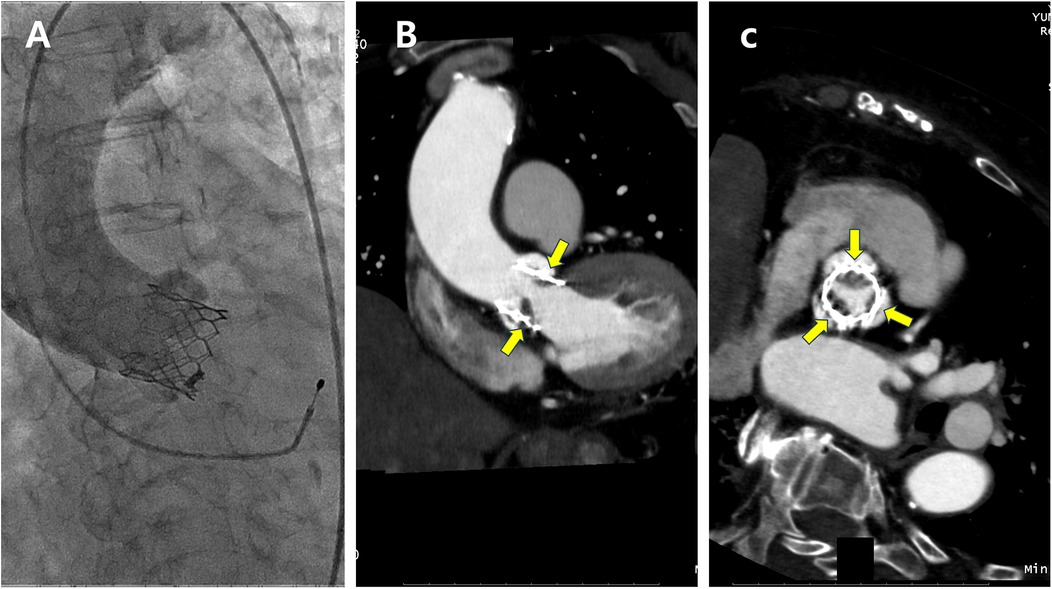
Figure 1. A patient with subclinical leaflet thrombosis detected on computed tomography (CT) at 3-month follow-up. (A) Aortography immediately after transcatheter aortic valve replacement. (B,C) Implanted aortic valve with hypoattenuated leaflet thickening (yellow arrows) on CT suggestive of leaflet thrombosis (B, longitudinal view; C, short axis view).
The exact mechanisms leading to valve thrombosis following TAVR are not fully understood. However, factors such as hypercoagulability at the bioprosthetic surface, leaflet surface damage during device deployment, and blood flow disturbances around the valve may contribute to thrombus formation (20). Subclinical leaflet thrombosis may progress over time to clinical valve thrombosis, which can manifest as valve dysfunction, with an increased transvalvular gradient, overt heart failure, or thromboembolic events (23). However, the clinical significance of subclinical leaflet thrombosis remains uncertain. Several meta-analyses reported conflicting conclusions regarding whether subclinical leaflet thrombosis or reduced leaflet motion is associated with an increased risk of stroke or structural valve deterioration (18, 22, 24–26).
2.3 Preexisting and new-onset atrial fibrillation
Atrial fibrillation (AF) and aortic stenosis (AS) share common risk factors, such as advanced age and hypertension (27). AS further increases the risk of AF by elevating pressure overload in the left ventricle and left atrium. AF is observed in 33%–44% of patients undergoing TAVR, reflecting the high-risk profile of this population (28). The prevalence of new-onset AF after TAVR ranges from 6.8% to 9.9%, depending on the study population (29, 30). Risk factors for developing new-onset AF include higher Society of Thoracic Surgeons score, transapical access, pulmonary hypertension, chronic kidney disease, peripheral vascular disease, and severe mitral regurgitation (30). Hemodynamic instability, myocardial injury during the procedure, and the subsequent systemic inflammatory response are thought to contribute to its development during and after TAVR (29, 30).
Both preexisting and new-onset AF are associated with increased cardiovascular complications, including stroke, mortality, and bleeding (29). Preexisting AF primarily increases the risk of late stroke and mortality, while new-onset AF is linked to higher rates of stroke, mortality, and bleeding in the early phase (within 30 days) following TAVR (27, 28, 30). However, the impact of new-onset AF on long-term outcomes, such as late stroke and mortality, remains inconsistent. Although early new-onset AF resolves in approximately 83% of cases, anticoagulation therapy is often underutilized in these patients, leaving them at an elevated risk of cardiovascular embolic events (31). Current guidelines do not specifically address monitoring for new-onset AF or recommend antithrombotic therapy tailored to the type of AF. This highlights an unmet need for individualized antithrombotic strategies in patients with AF undergoing TAVR.
Furthermore, left atrial appendage (LAA) occlusion may be a viable option for patients with AF undergoing TAVR, particularly those at high risk of bleeding. A recent multicenter randomized trial compared concomitant TAVR with LAA occlusion to TAVR combined with medical therapy in patients with AF (32). This study found that the combined procedures were safe and non-inferior to TAVR with medical therapy alone. However, the potential benefits of such combined procedures require further investigation in future clinical trials.
3 Bleeding risk after TAVR
Bleeding is a common and serious complication following TAVR, with major bleeding events occurring in 2.2%–41.7% of patients within 30 days and in 2.8%–46.1% within 1 year, depending on the patient's bleeding risk and the valve system used (6–13, 33). According to the recent CENTER 2 trial, a pooled, large-scale patient-level database from 10 clinical trials, the major bleeding rate decreased from 11.95 in 2007–2010 to 5.1% in 2019–2022 (34). Early bleeding (within the first 30 days post procedure) accounts for the majority of bleeding events after TAVR and is often related to procedural and technical factors, such as access site complications. The introduction of smaller delivery systems and the increased use of transfemoral access has led to a reduced incidence of major early bleeding, although the risk remains substantial, especially in patients with a high bleeding risk. Later bleeding events (beyond the first 30 days post TAVR) are typically unrelated to the access site and reflect the combined effects of antithrombotic therapy and patient-related factors.
Bleeding risk in patients undergoing TAVR is influenced by multiple factors, including patient age, frailty, and comorbidities (e.g., chronic kidney disease, anemia) as well as the type and duration of antithrombotic therapy. Older adults with frailty or significant comorbidities are especially vulnerable to gastrointestinal or neurologic bleeding and therefore require close monitoring. Patients with intermediate and high surgical risk experience higher rates of major bleeding compared to those with low surgical risk (34). However, surgical risk scores, such as the Society of Thoracic Surgeons (STS) predicted risk for mortality and the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II, do not reliably correlate with bleeding risks or clinical outcomes in TAVR patients (35). Recently, the Valve Academic Research Consortium for High Bleeding Risk (VARC-HBR) has established specific criteria to identify patients at high risk for bleeding during and after TAVR (33, 36). According to these criteria, over 90% of TAVR patients are considered high bleeding risk. For these individuals, aggressive antithrombotic therapy can result in life-threatening bleeding, emphasizing the need for careful risk stratification and tailored therapeutic strategies. The incorporation of additional bleeding risk categories, such as moderate, high, and very high, could facilitate an individualized, risk-based approach to minimize bleeding complications while optimizing patient outcomes (37).
4 Recent trials regarding antithrombotic therapy after TAVR
4.1 Patients with no indications for anticoagulation
4.1.1 Dual vs. single antiplatelet therapy
Based on clinical practice after percutaneous coronary intervention, antithrombotic therapy after TAVR initially consisted of 1–6 months of DAPT, followed by lifelong SAPT with aspirin. Two randomized controlled trials evaluated the benefits and risks of SAPT vs. DAPT after TAVR in patients with no indications for anticoagulation. The ARTE (Aspirin Versus Aspirin and Clopidogrel Following Transcatheter Aortic Valve Implantation) trial was the first study comparing SAPT (aspirin) with DAPT (aspirin plus clopidogrel) in these patients (Table 1) (38). The primary endpoint was the composite of death, MI, stroke or transient ischemic attack, or major or life-threatening bleeding (according to VARC-2 definitions) within the first 3 months post TAVR. The study was stopped prematurely after enrolling 222 participants (74% of the planned cohort) because of slow recruitment and lack of continued financial support. The primary composite endpoint tended to occur more frequently in the DAPT group than in the SAPT group at 3-month follow-up, although the difference was not statistically significant (15.3% vs. 7.2%, p = 0.065). When each outcome was evaluated separately, the rates of all thromboembolic events were similar between groups, but the rate of major or life-threatening bleeding was significantly higher in the DAPT group than in the SAPT group (10.8% vs. 3.6%, p = 0.038).
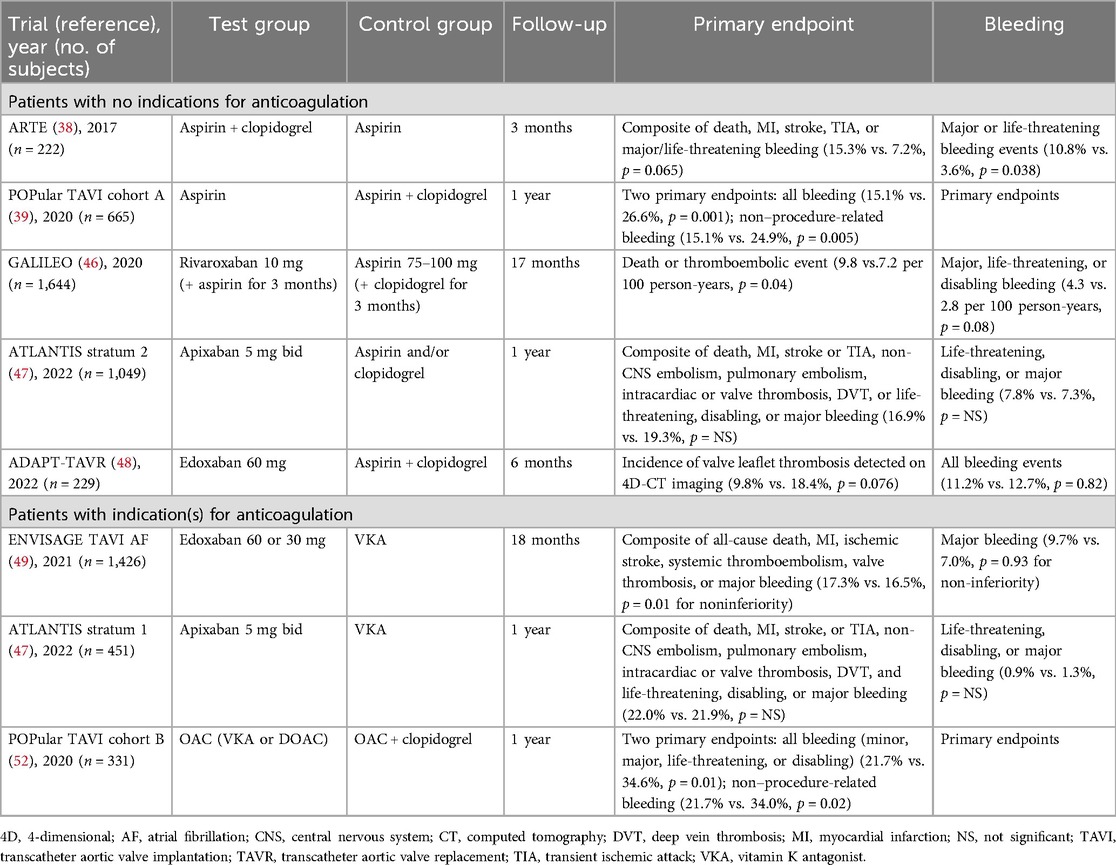
Table 1. Major outcomes of randomized clinical trials of antithrombotic therapy after transcatheter aortic valve replacement for patients with or without indications for chronic anticoagulation.
The POPular TAVI (Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation) trial also compared SAPT (aspirin) and DAPT (aspirin plus clopidogrel) (cohort A) and found that DAPT administered for 3 months did not reduce post-TAVR ischemic events but significantly increased the risk of all bleeding (26.6% vs. 15.1%, p = 0.001) and non–procedure-related bleeding (24.9% vs. 15.1%, p = 0.005) at the 12-month follow-up (39). Several meta-analyses confirmed that DAPT increases the risk of bleeding events without reducing thromboembolism or mortality rates compared with SAPT (40–45).
4.1.2 Direct oral anticoagulants vs. antiplatelet therapy
The GALILEO (Global Study Comparing a Rivaroxaban-based Antithrombotic Strategy to an Antiplatelet-based Strategy after Transcatheter Aortic Valve Replacement to Optimize Clinical Outcomes) trial compared rivaroxaban 10 mg (plus aspirin 75–100 mg for the first 3 months) with aspirin 75–100 mg (plus clopidogrel 75 mg for the first 3 months) but was terminated prematurely because of higher rates of thromboembolic complications, bleeding, and mortality in the rivaroxaban group than in the antiplatelet therapy group (46).
In stratum 2 of the ATLANTIS (Anti-Thrombotic Strategy to Lower All Cardiovascular and Neurologic Ischemic and Hemorrhagic Events after Trans-Aortic Valve Implantation for Aortic Stenosis) trial, patients with no indications for anticoagulation received apixaban or antiplatelet therapy with aspirin and/or clopidogrel (as SAPT or DAPT) (47). Apixaban provided no net clinical benefit over antiplatelet therapy. Rates of mortality and the composite outcome of death, any stroke or TIA, or systemic embolism were significantly higher in the apixaban group than in the antiplatelet group, although bleeding rates were similar between groups.
The ADAPT-TAVR (Anticoagulation Versus Dual Antiplatelet Therapy for Prevention of Leaflet Thrombosis and Cerebral Embolization After Transcatheter Aortic Valve Replacement) trial compared the effectiveness of edoxaban vs. DAPT (aspirin plus clopidogrel) for preventing leaflet thrombosis at 6 months post TAVR (48). The incidence of leaflet thrombosis was lower with edoxaban than with DAPT, although the difference between groups was not statistically significant. There were also no significant between-group differences with regard to deaths, thrombo-ischemic events, or bleeding events. No significant association was observed between the presence or extent of leaflet thrombosis and new cerebral lesions or changes in neurologic or neurocognitive function.
Based on these clinical trials, SAPT is currently considered the first-line post-TAVR therapy for patients with no indications for anticoagulation. DAPT may be appropriate for patients who recently underwent coronary stenting or other endovascular procedures. Nevertheless, it is currently unclear which antiplatelet agent is preferable for SAPT. Although a retrospective study reported that clopidogrel monotherapy was associated with a lower incidence of cardiovascular death after TAVR compared with aspirin monotherapy, data are limited regarding this issue. Future studies are required to determine the optimal antiplatelet regimen(s) after TAVR.
4.2 Patients with indications for anticoagulation
4.2.1 Direct oral anticoagulants vs. vitamin K antagonists
In the ENVISAGE AF-TAVI (Edoxaban vs. Standard of Care and Their Effects on Clinical Outcomes in Patients Having Undergone Transcatheter Aortic Valve Implantation–Atrial Fibrillation) trial, edoxaban was compared to a vitamin K antagonist (VKA) in patients with AF after TAVR (Table 1) (49). Although the primary composite outcome of thromboembolic events was similar between groups, the incidence of major bleeding (mainly gastrointestinal bleeding) was higher with edoxaban than with a VKA. However, in stratum 1 (patients with indications for anticoagulation) of the ATLANTIS trial, no differences were observed for any of the outcomes between apixaban and a VKA (47). Furthermore, a meta-analysis of five studies including a total of 2,569 patients found no significant differences in all-cause mortality, major and/or life-threatening bleeding, or stroke between direct oral anticoagulants (DOACs) and VKAs in patients undergoing TAVI with concomitant indications for oral anticoagulation (50).
By contrast, a study from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry (including a total of 21,131 patients) found that in patients with AF, DOAC use was associated with a comparable risk of stroke but a lower incidence of any bleeding, intracranial hemorrhage, or death at 1 year after TAVR compared with VKA therapy (51). Therefore, although DOACs and VKAs appear to have comparable efficacy for preventing stroke in this patient population, there are discrepancies in the literature regarding the relative bleeding risks of these anticoagulation regimens.
4.2.2 Anticoagulation vs. antiplatelet therapy plus anticoagulation
In cohort B of the POPular TAVI trial, patients with an indication for long-term anticoagulation (approximately 95% of whom had AF) received either an oral anticoagulant (OAC) alone (either a VKA or a DOAC) or a combination of an OAC plus clopidogrel after TAVR (52). At the 12-month follow-up, major bleeding as defined by VARC-2 was observed less frequently in the OAC alone group than in the OAC plus clopidogrel group. Two meta-analyses of patients requiring long-term anticoagulation also demonstrated that the risk of major and life-threatening bleeding was lower with an OAC regimen than with an OAC plus an antiplatelet agent, without affecting stroke rates (44, 53).
4.3 Antithrombotic therapy for the prevention and treatment of leaflet thrombosis
The LRT 2.0 (Strategies to Prevent Transcatheter Heart Valve Dysfunction in Low Risk Transcatheter Aortic Valve Replacement) trial was the first randomized trial to compare aspirin monotherapy vs. warfarin plus aspirin for the prevention of bioprosthetic valve dysfunction at 30 days after TAVR in low-risk patients (54). The rate of hypoattenuated leaflet thickening was 16.3% for aspirin and 4.7% for warfarin plus aspirin [p = 0.07; odds ratio, 4.0 (95% confidence interval, 0.8–20.0)]. There was no excess bleeding at 30 days in the patients who received warfarin and aspirin.
A substudy of the GALILEO trial showed that rivaroxaban was more effective than antiplatelet therapy for preventing subclinical leaflet thrombosis (detected on 4D-CT) at the 3-month follow-up after TAVR, but the increased risk of bleeding associated with rivaroxaban reported in the main GALILEO trial has limited its use in this setting (48, 55). In the ADAPT-TAVR trial comparing edoxaban vs. DAPT for the prevention of leaflet thrombosis at 6 months post TAVR, the incidence of leaflet thrombosis was lower with edoxaban, but the difference between groups was not statistically significant (48). Of note, subclinical leaflet thrombosis may appear and resolve multiple times with anticoagulation therapy, including DOACs or VKAs (18, 56). Given the poorly understood natural history and clinical implications of subclinical leaflet thrombosis, the need for preventive treatment remains unclear.
5 Guidelines
The latest guidelines from the European Society of Cardiology and the American College of Cardiology/American Heart Association provide specific recommendations for antithrombotic therapy after TAVR, tailored to patients with or without preexisting indications for anticoagulation (Table 2) (1, 2). Both sets of guidelines recommend aspirin monotherapy as the standard of care for most patients with no indications for long-term anticoagulation after TAVR. In patients requiring anticoagulation (such as those with AF), both guidelines favor oral anticoagulation alone and advise against routine combination therapy because of the increased risk of bleeding. Although the use of DAPT may still be considered in select patients, both guidelines have moved away from recommending it broadly after TAVR, prioritizing SAPT to mitigate bleeding complications.
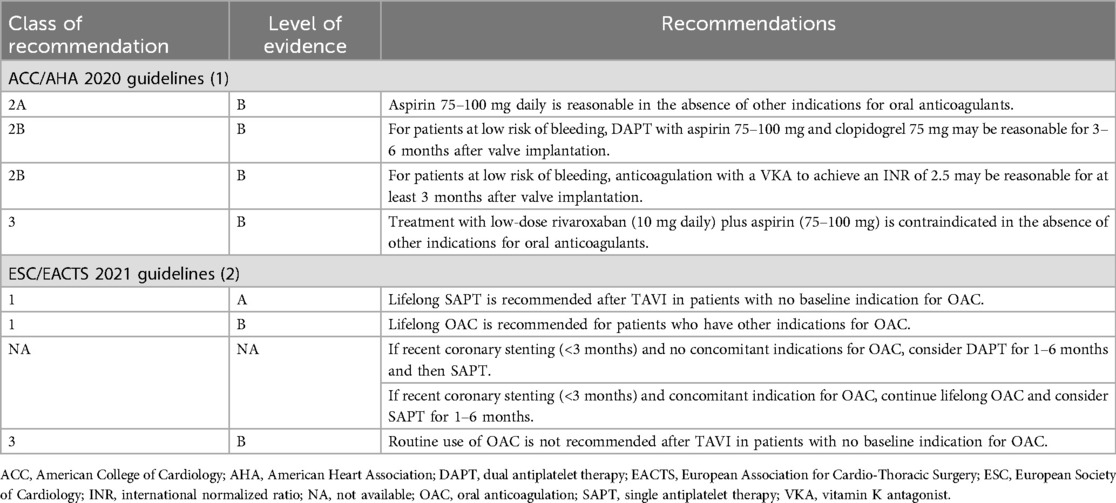
Table 2. Current US and European guidelines for the management of antithrombotic therapies in patients undergoing transcatheter aortic valve replacement.
6 Ongoing trials and future directions
Several ongoing trials are investigating alternative antithrombotic strategies to refine post-TAVR management strategies. For example, in the ACLO-TAVR (Aspirin Versus Clopidogrel for Leaflet Thrombosis Prevention in Patients Undergoing Transcatheter Aortic Valve Replacement) trial, a planned total of 230 patients will first receive 4 weeks of DAPT (aspirin 100 mg and clopidogrel 75 mg) after TAVR and then be randomized to receive monotherapy with either aspirin or clopidogrel. The study will evaluate the incidence of leaflet thrombosis at 3 months post TAVI using cardiac CT and transthoracic echocardiography (NCT05493657). In the AVATAR (Anticoagulation Alone Versus Anticoagulation and Aspirin Following Transcatheter Aortic Valve Interventions) trial, OAC (DOAC or VKA) monotherapy is compared with OAC plus aspirin combination therapy post-TAVR (NCT02735902), and in the ACASA-TAVI (AntiCoagulation Versus AcetylSalicylic Acid After Transcatheter Aortic Valve Implantation) trial, a DOAC is compared with aspirin for the prevention of valve thrombosis after TAVR (NCT05035277). The POPular PAUSE TAVI (Periprocedural Continuation Versus Interruption of Oral Anticoagulant Drugs During Transcatheter Aortic Valve Implantation) trial compares the effects of pausing vs. maintaining OAC use perioperatively (NCT04437303). In the POPular ATLANTIS (Personalized, CT-guided Antithrombotic Therapy Versus Lifelong Single Antiplatelet Therapy to Reduce Thromboembolic and Bleeding Events in Non-atrial Fibrillation Patients After Transcatheter Aortic Valve Implantation) trial, variable antithrombotic treatment (based on the presence of thrombus on CT) is compared with lifelong SAPT following TAVR in patients with no indications for anticoagulation (NCT06168370). Furthermore, the NAPT (Non-antithrombotic Therapy After Transcatheter Aortic Valve Implantation) trial compares non-antithrombotic strategies with SAPT after TAVR in patients with a high risk of bleeding (NCT06007222) (57). Future clinical investigations should focus on a tailored approach based on both patient thromboembolic and bleeding risk profiles to improve the efficacy and safety of antithrombotic treatment post-TAVR.
7 Conclusion
The optimal antithrombotic regimen after TAVR is highly patient-specific, requiring careful balancing of the risks of thromboembolism and the risks of bleeding. For patients with no indications for anticoagulation, SAPT is favored over DAPT because of its lower risk of bleeding and comparable protection against ischemic/thromboembolic events. In patients requiring anticoagulation, monotherapy with a VKA or DOAC is sufficient, with the addition of antiplatelet therapy conferring no additional benefit but increasing the risk of bleeding.
Management of subclinical leaflet thrombosis and clinical valve thrombosis remains a complex issue, with ongoing studies expected to provide further clarity. Future research should focus on improving risk stratification and tailoring antithrombotic therapy to individual patient characteristics to optimize outcomes.
Author contributions
NT: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. MH: Investigation, Supervision, Writing – original draft, Writing – review & editing. YK: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a MEF Fellowship conducted as part of “Education and Research capacity building project at University of Medicine and Pharmacy at Ho Chi Minh City” implemented by the Korea International Cooperation Agency (KOICA) in 2023. (No. 2021-00020-3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 143(5):e72–227. doi: 10.1161/CIR.0000000000000923
2. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
3. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106(24):3006–8. doi: 10.1161/01.cir.0000047200.36165.b8
4. Mangieri A, Montalto C, Poletti E, Sticchi A, Crimi G, Giannini F, et al. Thrombotic versus bleeding risk after transcatheter aortic valve replacement: JACC review topic of the week. J Am Coll Cardiol. (2019) 74(16):2088–101. doi: 10.1016/j.jacc.2019.08.1032
5. Saito Y, Nazif T, Baumbach A, Tchetche D, Latib A, Kaple R, et al. Adjunctive antithrombotic therapy for patients with aortic stenosis undergoing transcatheter aortic valve replacement. JAMA Cardiol. (2020) 5(1):92–101. doi: 10.1001/jamacardio.2019.4367
6. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363(17):1597–607. doi: 10.1056/NEJMoa1008232
7. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364(23):2187–98. doi: 10.1056/NEJMoa1103510
8. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. (2014) 370(19):1790–8. doi: 10.1056/NEJMoa1400590
9. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374(17):1609–20. doi: 10.1056/NEJMoa1514616
10. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376(14):1321–31. doi: 10.1056/NEJMoa1700456
11. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380(18):1706–15. doi: 10.1056/NEJMoa1816885
12. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380(18):1695–705. doi: 10.1056/NEJMoa1814052
13. Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. (2015) 65(20):2184–94. doi: 10.1016/j.jacc.2015.03.014
14. Kapadia SR, Makkar R, Leon M, Abdel-Wahab M, Waggoner T, Massberg S, et al. Cerebral embolic protection during transcatheter aortic-valve replacement. N Engl J Med. (2022) 387(14):1253–63. doi: 10.1056/NEJMoa2204961
15. Kapadia SR, Kodali S, Makkar R, Mehran R, Lazar RM, Zivadinov R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. (2017) 69(4):367–77. doi: 10.1016/j.jacc.2016.10.023
16. Nazif TM, Moses J, Sharma R, Dhoble A, Rovin J, Brown D, et al. Randomized evaluation of TriGuard 3 cerebral embolic protection after transcatheter aortic valve replacement: REFLECT II. JACC Cardiovasc Interv. (2021) 14(5):515–27. doi: 10.1016/j.jcin.2020.11.011
17. Jimenez Diaz VA, Kapadia SR, Linke A, Mylotte D, Lansky AJ, Grube E, et al. Cerebral embolic protection during transcatheter heart interventions. EuroIntervention. (2023) 19(7):549–70. doi: 10.4244/EIJ-D-23-00166
18. Bogyi M, Schernthaner RE, Loewe C, Gager GM, Dizdarevic AM, Kronberger C, et al. Subclinical leaflet thrombosis after transcatheter aortic valve replacement: a meta-analysis. JACC Cardiovasc Interv. (2021) 14(24):2643–56. doi: 10.1016/j.jcin.2021.09.019
19. Rashid HN, Gooley RP, Nerlekar N, Ihdayhid AR, McCormick LM, Nasis A, et al. Bioprosthetic aortic valve leaflet thrombosis detected by multidetector computed tomography is associated with adverse cerebrovascular events: a meta-analysis of observational studies. EuroIntervention. (2018) 13(15):e1748–55. doi: 10.4244/EIJ-D-17-01062
20. Chitturi KR, Aladin AI, Braun R, Al-Qaraghuli AK, Banerjee A, Reddy P, et al. Bioprosthetic aortic valve thrombosis: definitions, clinical impact, and management: a state-of-the-art review. Circ Cardiovasc Interv. (2024) 17(7):e014143. doi: 10.1161/CIRCINTERVENTIONS.123.014143
21. Garcia S, Fukui M, Dworak MW, Okeson BK, Garberich R, Hashimoto G, et al. Clinical impact of hypoattenuating leaflet thickening after transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2022) 15(3):e011480. doi: 10.1161/CIRCINTERVENTIONS.121.011480
22. Rheude T, Pellegrini C, Stortecky S, Marwan M, Xhepa E, Ammon F, et al. Meta-analysis of bioprosthetic valve thrombosis after transcatheter aortic valve implantation. Am J Cardiol. (2021) 138:92–9. doi: 10.1016/j.amjcard.2020.10.018
23. Jose J, Sulimov DS, El-Mawardy M, Sato T, Allali A, Holy EW, et al. Clinical bioprosthetic heart valve thrombosis after transcatheter aortic valve replacement: incidence, characteristics, and treatment outcomes. JACC Cardiovasc Interv. (2017) 10(7):686–97. doi: 10.1016/j.jcin.2017.01.045
24. Sannino A, Hahn RT, Leipsic J, Mack MJ, Grayburn PA. Meta-analysis of incidence, predictors and consequences of clinical and subclinical bioprosthetic leaflet thrombosis after transcatheter aortic valve implantation. Am J Cardiol. (2020) 132:106–13. doi: 10.1016/j.amjcard.2020.07.018
25. Makki N, Ghao X, Whitson B, Shreenivas S, Crestanello J, Lilly S. Slope of left ventricular filling as an index of valvular and paravalvular regurgitation in native and prosthetic aortic valves. Catheter Cardiovasc Interv. (2018) 92(7):1397–403. doi: 10.1002/ccd.27684
26. Chakravarty T, Sondergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. (2017) 389(10087):2383–92. doi: 10.1016/S0140-6736(17)30757-2
27. Mojoli M, Gersh BJ, Barioli A, Masiero G, Tellaroli P, D’Amico G, et al. Impact of atrial fibrillation on outcomes of patients treated by transcatheter aortic valve implantation: a systematic review and meta-analysis. Am Heart J. (2017) 192:64–75. doi: 10.1016/j.ahj.2017.07.005
28. Nso N, Emmanuel K, Nassar M, Bhangal R, Enoru S, Iluyomade A, et al. Impact of new-onset versus pre-existing atrial fibrillation on outcomes after transcatheter aortic valve replacement/implantation. Int J Cardiol Heart Vasc. (2022) 38:100910. doi: 10.1016/j.ijcha.2021.100910
29. Petronio AS, Giannini C. Atrial fibrillation after transcatheter aortic valve replacement: which came first, the chicken or the egg? JACC Cardiovasc Interv. (2022) 15(6):614–7. doi: 10.1016/j.jcin.2022.02.010
30. Ryan T, Grindal A, Jinah R, Um KJ, Vadakken ME, Pandey A, et al. New-onset atrial fibrillation after transcatheter aortic valve replacement: a systematic review and meta-analysis. JACC Cardiovasc Interv. (2022) 15(6):603–13. doi: 10.1016/j.jcin.2022.01.018
31. Shahim B, Malaisrie SC, George I, Thourani VH, Biviano AB, Russo M, et al. Postoperative atrial fibrillation or flutter following transcatheter or surgical aortic valve replacement: PARTNER 3 trial. JACC Cardiovasc Interv. (2021) 14(14):1565–74. doi: 10.1016/j.jcin.2021.05.026
32. Kapadia SR, Krishnaswamy A, Whisenant B, Potluri S, Iyer V, Aragon J, et al. Concomitant left atrial appendage occlusion and transcatheter aortic valve replacement among patients with atrial fibrillation. Circulation. (2024) 149(10):734–43. doi: 10.1161/CIRCULATIONAHA.123.067312
33. Avvedimento M, Nuche J, Farjat-Pasos JI, Rodes-Cabau J. Bleeding events after transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 81(7):684–702. doi: 10.1016/j.jacc.2022.11.050
34. van Nieuwkerk AC, Aarts HM, Hemelrijk KI, Canton T, Tchetche D, de Brito FS Jr., et al. Bleeding in patients undergoing transfemoral transcatheter aortic valve replacement: incidence, trends, clinical outcomes, and predictors. JACC Cardiovasc Interv. (2023) 16(24):2951–62. doi: 10.1016/j.jcin.2023.10.011
35. Tarantini G, Lefevre T, Terkelsen CJ, Frerker C, Ohlmann P, Mojoli M, et al. One-year outcomes of a European transcatheter aortic valve implantation cohort according to surgical risk. Circ Cardiovasc Interv. (2019) 12(1):e006724. doi: 10.1161/CIRCINTERVENTIONS.118.006724
36. Capodanno D, Collet JP, Dangas G, Montalescot G, Ten Berg JM, Windecker S, et al. Antithrombotic therapy after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2021) 14(15):1688–703. doi: 10.1016/j.jcin.2021.06.020
37. Avvedimento M, Cepas-Guillen P, Ternacle J, Urena M, Alperi A, Cheema A, et al. Validation of the valve academic research consortium high bleeding risk definition in patients undergoing TAVR. Circ Cardiovasc Interv. (2025) 18(1):e014800. doi: 10.1161/CIRCINTERVENTIONS.124.014800
38. Rodes-Cabau J, Masson JB, Welsh RC, Garcia Del Blanco B, Pelletier M, Webb JG, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (aspirin versus aspirin+clopidogrel following transcatheter aortic valve implantation) randomized clinical trial. JACC Cardiovasc Interv. (2017) 10(13):1357–65. doi: 10.1016/j.jcin.2017.04.014
39. Brouwer J, Nijenhuis VJ, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, et al. Aspirin with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med. (2020) 383(15):1447–57. doi: 10.1056/NEJMoa2017815
40. Zhu Y, Zou Z, Huang Y, Zhang L, Chen H, Li Y, et al. Comparative efficacy and safety of antithrombotic therapy for transcatheter aortic valve replacement: a systematic review and network meta-analysis. Eur J Cardiothorac Surg. (2020) 57(5):965–76. doi: 10.1093/ejcts/ezz335
41. Brouwer J, Nijenhuis VJ, Rodes-Cabau J, Stabile E, Barbanti M, Costa G, et al. Aspirin alone versus dual antiplatelet therapy after transcatheter aortic valve implantation: a systematic review and patient-level meta-analysis. J Am Heart Assoc. (2021) 10(8):e019604. doi: 10.1161/JAHA.120.019604
42. Al Halabi S, Newman J, Farkouh ME, Fortuin D, Leya F, Sweeney J, et al. Meta-analysis of studies comparing dual- versus mono-antiplatelet therapy following transcatheter aortic valve implantation. Am J Cardiol. (2018) 122(1):141–8. doi: 10.1016/j.amjcard.2018.03.019
43. Ahmad Y, Howard JP, Madhavan MV, Leon MB, Makkar RR. Single versus dual antiplatelet therapy after transcatheter aortic valve replacement: a meta-analysis of randomized clinical trials. Cardiovasc Revasc Med. (2022) 34:46–53. doi: 10.1016/j.carrev.2021.01.016
44. Navarese EP, Grisafi L, Spinoni EG, Mennuni MG, Rognoni A, Ratajczak J, et al. Safety and efficacy of different antithrombotic strategies after transcatheter aortic valve implantation: a network meta-analysis. Thromb Haemost. (2022) 122(2):216–25. doi: 10.1055/a-1496-8114
45. Alkhalil M, Edwards R, Puri R, Kalra A, Zaman A, Das R. Aspirin versus dual antiplatelet therapy in patients undergoing trans-catheter aortic valve implantation, updated meta-analysis. Cardiovasc Drugs Ther. (2022) 36(2):279–83. doi: 10.1007/s10557-021-07146-6
46. Dangas GD, Tijssen JGP, Wohrle J, Sondergaard L, Gilard M, Mollmann H, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med. (2020) 382(2):120–9. doi: 10.1056/NEJMoa1911425
47. Collet JP, Van Belle E, Thiele H, Berti S, Lhermusier T, Manigold T, et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: the ATLANTIS trial. Eur Heart J. (2022) 43(29):2783–97. doi: 10.1093/eurheartj/ehac242
48. Park DW, Ahn JM, Kang DY, Kim KW, Koo HJ, Yang DH, et al. Edoxaban versus dual antiplatelet therapy for leaflet thrombosis and cerebral thromboembolism after TAVR: the ADAPT-TAVR randomized clinical trial. Circulation. (2022) 146(6):466–79. doi: 10.1161/CIRCULATIONAHA.122.059512
49. Van Mieghem NM, Unverdorben M, Hengstenberg C, Mollmann H, Mehran R, Lopez-Otero D, et al. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N Engl J Med. (2021) 385(23):2150–60. doi: 10.1056/NEJMoa2111016
50. Ueyama H, Kuno T, Ando T, Briasoulis A, Fox J, Hayashida K, et al. Meta-analysis comparing direct oral anticoagulants versus vitamin K antagonists after transcatheter aortic valve implantation. Am J Cardiol. (2020) 125(7):1102–7. doi: 10.1016/j.amjcard.2019.12.039
51. Tanawuttiwat T, Stebbins A, Marquis-Gravel G, Vemulapalli S, Kosinski AS, Cheng A. Use of direct oral anticoagulant and outcomes in patients with atrial fibrillation after transcatheter aortic valve replacement: insights from the STS/ACC TVT registry. J Am Heart Assoc. (2022) 11(1):e023561. doi: 10.1161/JAHA.121.023561
52. Nijenhuis VJ, Brouwer J, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, et al. Anticoagulation with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med. (2020) 382(18):1696–707. doi: 10.1056/NEJMoa1915152
53. Yokoyama Y, Briasoulis A, Takagi H, Kuno T. Anticoagulation with or without antiplatelet therapy following transcatheter aortic valve replacement for patients with atrial fibrillation: a meta-analysis. Cardiovasc Revasc Med. (2021) 24:42–7. doi: 10.1016/j.carrev.2020.08.011
54. Rogers T, Shults C, Torguson R, Shea C, Parikh P, Bilfinger T, et al. Randomized trial of aspirin versus warfarin after transcatheter aortic valve replacement in low-risk patients. Circ Cardiovasc Interv. (2021) 14(1):e009983. doi: 10.1161/CIRCINTERVENTIONS.120.009983
55. De Backer O, Dangas GD, Jilaihawi H, Leipsic JA, Terkelsen CJ, Makkar R, et al. Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med. (2020) 382(2):130–9. doi: 10.1056/NEJMoa1911426
56. Adrichem R, Rodes Cabau J, Mehran R, Park DW, Ten Berg JM, de Backer O, et al. Treatment of transcatheter aortic valve thrombosis: JACC review topic of the week. J Am Coll Cardiol. (2024) 84(9):848–61. doi: 10.1016/j.jacc.2024.05.064
Keywords: aortic valve stenosis, transcatheter aortic valve replacement, thrombosis, antithrombotic agents, hemorrhage
Citation: Thanh NVT, Hong M-K and Ko Y-G (2025) Optimal antithrombotic therapy after transcatheter aortic valve replacement: a comprehensive review. Front. Cardiovasc. Med. 12:1528071. doi: 10.3389/fcvm.2025.1528071
Received: 14 November 2024; Accepted: 24 February 2025;
Published: 10 March 2025.
Edited by:
Sunil Mankad, Mayo Clinic, United StatesReviewed by:
Kesavan Sankaramangalam, East Carolina University, United StatesSteven Yakubov, OhioHealth, United States
Copyright: © 2025 Thanh, Hong and Ko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young-Guk Ko, eWdrb0B5dWhzLmFj
 Nguyen Van Thai Thanh
Nguyen Van Thai Thanh Myeong-Ki Hong
Myeong-Ki Hong Young-Guk Ko
Young-Guk Ko