
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 18 February 2025
Sec. Coronary Artery Disease
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1523352
Ischemia with no obstructive coronary arteries (INOCA) is an increasingly recognized condition in patients presenting with angina and positive stress tests but without significant coronary artery stenosis. This review addresses the pathophysiology, diagnostic approaches, and management strategies associated with INOCA, emphasizing epicardial coronary spasms and coronary microvascular dysfunction (CMD) as underlying mechanisms and myocardial bridging (MB) as a risk factor. Diagnostic modalities include both non-invasive techniques and invasive procedures, such as acetylcholine provocation testing, to differentiate vasospasm from microvascular causes. The paper discusses a potential interference between vasodilators used in trans-radial access and coronary spasm testing. Long-term management approaches for INOCA patients, including pharmacologic therapies and lifestyle interventions, are reviewed.
Patients with coronary artery disease (CAD) experience several signs and symptoms, including episodes of angina and shortness of breath. Obstructive CAD is well documented and can be readily detected by conventional coronary angiograms. Patients presenting with angina with a positive stress test who end up having a left heart catheterization showing normal coronary arteries or stenosis of <50% of the artery's diameter are increasing in number (1). This presentation of anginal symptoms without hemodynamically significant stenotic lesions is termed angina with non-obstructed coronary arteries (ANOCA), which is suggestive of ischemia with non-obstructive coronary arteries (INOCA), but can also occur independently due to a lack of clear evidence of ischemia or inadequate testing (2). Myocardial infarction with non-obstructive coronary arteries (MINOCA) shows myocardial necrosis and is characterized by elevated troponin without obstructive CAD or any other non-coronary etiology. Several risk factors contribute to INOCA, including but not limited to the traditional CAD risk factors and the presence of myocardial bridging (MB).
Functional assessment of coronary arteries using invasive or non-invasive methods is needed to detect the underlying pathology of INOCA. Commonly performed non-invasive diagnostic modalities are transthoracic Doppler echocardiography (TTDE), cardiac positron emission tomography (PET), and cardiac magnetic resonance (CMR). However, these modalities often do not offer a complete comprehensive assessment given the limitations of evaluating coronary spasms. Invasive approaches involve guidewire-based assessment of the coronary circulation allowing for a more comprehensive evaluation. The invasive functional study assesses the coronary blood flow during resting and hyperemic states induced by pharmacologic agents such as adenosine or papaverine. In addition, spasm provocation testing using acetylcholine can be performed for diagnosing vasospasms. Several indices, including the fractional flow reserve (FFR), coronary flow reserve (CFR), and index of microvascular resistance (IMR), are indicative of the coronary circulation function and are obtained from different functional tests.
In this paper, we review the underlying pathologies, clinical presentation, prevalence, and risk factors for ANOCA and/or INOCA. Among different risk factors, we focus on myocardial bridging and its association with angina. Functional assessment of the coronary arteries is discussed along with a potential limitation of invasive testing through trans-radial access (TRA), which is radial artery spasms (RAS). RAS can be minimized by the administration of anti-spasmolytic vasodilators. However, this raises a concern about an interaction between the different vasodilators utilized for RAS prevention and acetylcholine administration during coronary spasm provocation testing, potentially masking spasms and reducing the sensitivity of the test. We discuss the efficacy of different vasodilating agents for RAS prevention and a reasonable approach to minimize this interaction by using vasodilators with short half-lives. Long-term management of INOCA patients comprising pharmacological disease-modifying or symptomatic therapies and non-pharmacological treatments is also reviewed.
The most common underlying cause of INOCA is dysfunction of the coronary vasculature, which mainly manifests as epicardial coronary spasms, coronary microvascular dysfunction (CMD), or both combined (3). Ischemia caused by CMD presents clinically as microvascular angina (MVA), previously called cardiac syndrome X, and ischemia due to epicardial coronary spasms presents as vasospastic angina (VSA), caused by hyperconstriction of large coronary arteries with vasoconstrictor stimuli (4). The presence of INOCA in patients presenting with ANOCA can be readily detected by ECG stress testing with high specificity (5).
As shown in Figure 1, CMD traditionally encompasses coronary microvascular spasms or vasodilation impairment and is comprised of two basic endotypes. These are endothelium-dependent dysfunction or endothelium-independent dysfunction. The endothelium-independent pathway is tested using adenosine while the endothelium-dependent pathway is evaluated via administration of acetylcholine. Other descriptors of microvascular dysfunction include structural and functional dysfunction. Structural dysfunction is thought to be caused by inward remodeling of coronary arterioles or capillary rarefaction (6). This reduces vasodilation capacity in coronary microcirculation and increases the sensitivity to vasoconstrictor stimuli (7). Functional dysfunction might be caused by the presence of endothelium dysfunction impairing vasodilation and causing vasoconstriction along the coronary circulation (8). CMD increases mortality by fourfold and increases major adverse cardiovascular events (MACE) by fivefold as demonstrated in a systematic review assessing the association of CMD and outcomes (9). CMD was found to have a role across different cardiovascular diseases other than non-obstructive CAD, including Takotsubo syndrome, angina post-PCI or CABG, obstructive CAD, heart failure, diabetic cardiomyopathy, aortic stenosis, and infiltrative heart disease (10). Moreover, a prospective study on 56 patients comparing CMD in heart failure with preserved or reduced ejection fraction (HFpEF or HFrEF) demonstrated different characteristics of CMD in both subtypes with a lower rate of recovery at follow-up in HFrEF, represented by reduced left ventricular reverse remodeling (11). These findings propose a prognostic and pathophysiological role of CMD across different cardiovascular diseases (10, 11).
According to a systematic review and meta-analysis that included 56 studies (12) investigating the provenance of CMD and vasospastic angina in patients without obstructive coronary arteries, the prevalence of CMD, coronary spasm, or a combination of both was reported as 0.41, 0.49, and 0.23, respectively. Therefore, approximately half of the population presenting without obstructive coronary arteries will have either CMD or coronary spasms. Furthermore, the prevalence of INOCA varies between different ethnicities. According to a study (13), comparing the prevalence of coronary vasomotion disorders between Japanese and Caucasian populations, CMD was more common among Caucasians, and epicardial spasms were significantly higher in Japanese patients. Another study (14) involving different ethnic groups with heart failure demonstrated that South Asians had significantly lower endothelium-mediated microvascular response to acetylcholine than Caucasians and African Caribbeans. The prevalence of non-obstructive CAD and INOCA among Middle Eastern patients is poorly investigated, and further studies are required in this region.
An established risk factor for INOCA is female gender. The Woman Ischemia Syndrome Evaluation (WISE) study recruited women with angina or suspected myocardial infraction undergoing a clinically indicated invasive coronary angiogram and other functional tests if required to diagnose ischemic heart disease. The study indicated that approximately two-thirds of the recruited women were found to have non-obstructive CAD (15, 16). Traditional risk factors contribute to INOCA, but this relationship is not well established. Also, risk factors vary for different underlying pathologies of INOCA. Older age, cigarette smoking, obesity, dyslipidemia, and hypertension are positively associated with INOCA (3, 17). Another risk factor for developing INOCA, and specifically coronary spasm, which is not well investigated is myocardial bridging (MB) (18).
Myocardial bridging (MB) occurs when coronary arteries run through the myocardium rather than their normal surface position (19). MB has been thought of as a benign condition as it appears harmless in most patients (20). Since coronary arteries fill during diastole, the compression of a tunneled artery during systole, known as the “milking effect,” should not affect myocardial perfusion (21). However, cardiovascular complications have been reported in some cases of MB, including vasospastic angina and acute myocardial infarctions. Moreover, several studies found a significant association between MB and myocardial ischemia (18).
Intravascular ultrasounds assessing MB demonstrated delayed compression release of the affected artery until early diastole, which can potentially precipitate ischemia (22). Persistent lumen diameter reduction during mid-diastole was also reported (23). Consequently, conditions that shorten the diastolic time, such as tachycardia, will contribute to ischemia. Another underlying mechanism of ischemia in MB is endothelial dysfunction due to systolic kinking of arteries, causing less production of vasoactive substances like nitric oxide (NO) and endothelin (24). Vascular dysfunction caused by MB contributes to the occurrence of vasospasms (25). Atherosclerosis proximally to a tunneled artery has been reported as a complication to MB due to decreased shear stress and increased vasoactive substances in that segment (24).
Variable prevalence of MB was reported among different studies and based on the diagnostic modality. A 2017 meta-analysis reported an overall 19% prevalence of MB (26). However, it was reported as high as 42% on autopsy despite a 6% prevalence of coronary angiography. Furthermore, computed tomography studies revealed a prevalence of 22% (26). Myocardial bridging contributes to INOCA through several mechanisms. A retrospective study of 62 patients with INOCA reported the presence of MB in 15 patients (24%). Coronary spasms were more common among patients with MB. However, coronary microvascular function was similar in both patient groups. This study demonstrates that MB predisposes patients with INOCA to coronary spasms but has a limited effect on coronary microvascular dysfunction (CMD) (27).
MB was associated with increased occurrence of coronary spasm and MINOCA in a prospective study on 310 participants with stable non-obstructive CAD or MINOCA. The percentage of patients with a positive acetylcholine test was 59% (183 patients), and compared with patients with a negative test, a higher prevalence of MINOCA (53.6% vs 33.9%) and MB was reported (23% vs 8.7%). Moreover, the overall prevalence of MB among the study participants was 17.1%, who presented with a higher rate of major adverse cardiac events (MACE) and decreased Seattle Angina Questionnaire scores compared with patients without MB. Overall, coexistent MB and positive acetylcholine test reported the poorest clinical outcome (28). However, more studies are needed to further investigate the relationship between INOCA and MB.
Conventional angiography or coronary computed tomographic angiography (CCTA) demonstrating non-obstructive coronary artery disease in a patient with INOCA/ANOCA necessitates further evaluation through invasive or non-invasive functional assessment for CMD. Despite the advantages of non-invasive testing, such as fewer procedural complications, an invasive approach can identify vasospasm using provocation testing, which is not clinically feasible through non-invasive methods (29). The CorMicA randomized controlled trial showed that tailored stratified medical therapy through interventional diagnostic procedures or functional tests in patients with ANOCA improved patient outcomes (30).
The most common approach for invasively assessing hemodynamics is through a coronary wire equipped with pressure and temperature sensors allowing for complete coronary physiologic assessment such as the PressureWire X Guidewire by Abbott Laboratories (31). The combination of a pressure and temperature sensor allows for both pressure changes and flow characteristics. This approach relies on thermodilution by either bolus or continuous infusions of saline to assess the coronary microvasculature. Continuous thermodilution is a novel method that directly measures absolute coronary blood flow and microvascular resistance (32). Compared to bolus thermodilution, continuous thermodilution was found more precise and demonstrated a significant decrease in variability (33, 34). Another approach includes Doppler-wire assessment measuring Doppler velocity coronary flow (35).
The most studied index and gold standard for evaluating stenotic epicardial arteries is the fractional flow reserve (FFR) (36). FFR is defined as the ratio of pressure distal and proximal to stenosis measured by the ratio of pressure distal to the lesion and the pressure in the aorta under maximal hyperemia (37). A ratio of >0.80 rules out hemodynamically and functionally significant stenosis (38). However, FFR alone does not assess the microcirculation. Subsequently, the coronary flow reserve (CFR) and the index of microvascular resistance (IMR) are used. The administration of a hyperemia-inducing agent such as adenosine is required to measure CFR. Alternatively, papaverine or regadenoson can be used. This allows for the evaluation of the endothelium-independent pathway. CFR is a measure of blood flow increase that the coronary circulation can yield in response to stress (39). It reflects the vasodilator capacity of both the epicardial and microvascular circulation with an abnormal value defined as <2–2.5 (40). When hemodynamically significant stenosis of the epicardial vessel is ruled out, CFR can then serve as an indicator of the microcirculation's ability to augment flow under hyperemic states. CFR, reflecting CMD, and major adverse cardiovascular events (MACE) are inversely related, as MACE occurred more frequently and earlier with lower CFR values as shown in Figure 2 (41). IMR, which is specific to the microcirculation with an abnormal value of >25, is calculated by measuring distal coronary pressure at maximal hyperemia and the average period of time that blood spends in a vessel (39). The microvascular resistance reserve (MRR) is a novel index specific to the coronary microvasculature, operator-independent, and unaffected by coronary autoregulation, epicardial resistance, or myocardial mass (42). MRR is defined as the microvascular resistance at rest when the epicardial artery is normally divided by microvascular resistance under maximal hyperemia and can be measured by dividing CFR by FFR when obtained through continuous thermodilution (42). Furthermore, unlike CFR, MRR is independent of epicardial resistance as CFR was shown to decrease in response to increasing epicardial resistance separating from MRR (42). Other than continuous thermodilution, MRR can also be obtained through several modalities including bolus thermodilution, Doppler velocity, or non-invasively through CT, MRI, or PET (33, 42). However, continuous thermodilution has shown superiority in precision when compared with bolus thermodilution, and does not require a hyperemia-inducing agent (33, 43). The ILIAS study on the prognosis of patients without obstructive coronary artery disease and with impaired CFR and microvascular resistance values demonstrated an increase in major adverse cardiac events (MACE) and target vessel failure (TVF) at 5 years with impaired CFR, but not with abnormal microvascular resistance (44). This demonstrates the poor prognostic value of microvascular resistance compared with CFR.
Complete functional evaluation can proceed to assess the endothelium-dependent microvascular pathway through provocation testing most commonly using intracoronary acetylcholine or ergonovine following a fixed protocol. Angina with a reduction of >90% in the diameter of epicardial vessels and ECG abnormalities indicate epicardial vasospastic angina (45, 46). While still being heavily researched, the accepted definition of microvascular angina includes the induction of angina and ischemic ECG changes without a >90% reduction of the epicardial arteries. This would indicate endothelium-dependent microvascular spasm (47). Coexistence of both epicardial and microvascular spasms occurs with undetermined frequency due to its identification difficulty (48). A novel method to detect coexistent microvascular and epicardial spasms is the acetylcholine rechallenge test, where coronary arteries are rechallenged with acetylcholine after administering an anti-vasospastic agent such as nitroglycerin. A study (48) on patients with epicardial spasms demonstrated that 48% had coexisting microvascular spasms. In addition, microvascular spasms were diagnosed at lower doses of acetylcholine, while epicardial spasms were detected with higher doses (48). Of note, acetylcholine might cause vasoconstriction, affecting adenosine studies by disrupting the resting hemodynamic state. Hence, starting with adenosine testing might be a better approach.
Several non-invasive modalities can be utilized to diagnose INOCA/ANOCA. Transthoracic doppler echocardiography (TTDE) can non-invasively measure CFR by measuring flow velocity in the LAD artery to identify CMD (49–51). However, it poorly differentiates epicardial from microvascular dysfunction and is affected by intra-operator variability (46). Positron emission tomography (PET) is the most reliable and widely preferred modality for the evaluation of microvascular disease (52). PET measures MBF and myocardial flow reserve (MFR), which is obtained from MBF values at rest and hyperemia. An MFR value of <1.5 suggests microvascular disease (53). MBF can be also evaluated through cardiac MRI to assess for microvascular dysfunction. The myocardial perfusion reserve (MPR) index can be obtained from myocardial perfusion at rest and hyperemia with an abnormal value of <1.5 (54). ECG stress testing conventionally represents obstructive coronary but has shown accurate prediction of non-obstructive coronary artery disease in recent studies. Stress ECG was found to be 100% specific for identifying ischemia underlined by CMD in patients with ANOCA (5). However, functional assessment invasively remains superior to non-invasive stress ECG to identify microvascular disease as shown to significantly decrease the false discovery rate in the UZ clear study (55).
In line with the 2024 ESC guidelines (46), Class 1 recommendations state that invasive functional testing through a coronary guidewire to obtain CFR and IMR should be considered to assess patients presenting with coronary microvascular angina and persistent symptoms without stenosis or a change in FFR/iFR. Conversely, non-invasive testing for INOCA through modalities, such as transthoracic Doppler or stress echocardiograph, cardiac MRI, and PET to assess CFR, was listed as a Class 2b recommendation. Additionally, Class 1 recommendations for the diagnosis of vasospastic angina indicate invasive assessment with an intracoronary provocation test to identify spasms in the absence of obstruction on angiography.
Trans-radial access (TRA) has several advantages over trans-femoral access (TFA), including decreased hemorrhage risk, shorter recovery time, higher patient satisfaction, and decreased procedural costs (56). However, a major limiting factor to TRA is the risk of developing radial artery spasms (RAS) and thus the administration of anti-spasmodic medications or the use of hydrophilic long radial sheaths (57, 58) is commonly practiced to overcome RAS. Common spasmolytic agents used during trans-radial access are nitroglycerin, verapamil, or a combination of vasodilators in a cocktail, mostly including nitroglycerin. Other used agents are demonstrated in Table 1. The optimal agent is debated, and the preferred choice varies in clinical practice (59). It is unknown whether or not using vasodilators during radial access potentially impacts vasoreactivity results due to the pre-administration of anti-spasmodic medications in the radial artery prior to intracoronary acetylcholine administration. Given the short procedural time and the opposed effects of acetylcholine and vasodilators, using agents with short half-lives (or no anti-spasmatic agents at all) into the radial artery to limit the occurrence of any interaction would seem prudent. Alternatively, the use of hydrophilic long radial sheaths has shown efficacy in overcoming RAS (57, 58). Nitroglycerin has the shortest half-life compared with other vasodilators used in this setting such as verapamil (3 min vs 3–5 h), thus offering the advantage of less potential for overlapping with the desired acetylcholine effect (60, 61). Given the half-life of nitroglycerin, we recommend waiting at least 10 min prior to the initiation of vasoreactivity testing from the last dose of intra-arterial nitroglycerin administration to ensure multiple half-live have passed. This also applies if FFR and/or CFR testing is to be performed prior to acetylcholine testing as typically intracoronary nitroglycerin is administered prior to FFR and/or CFR testing.
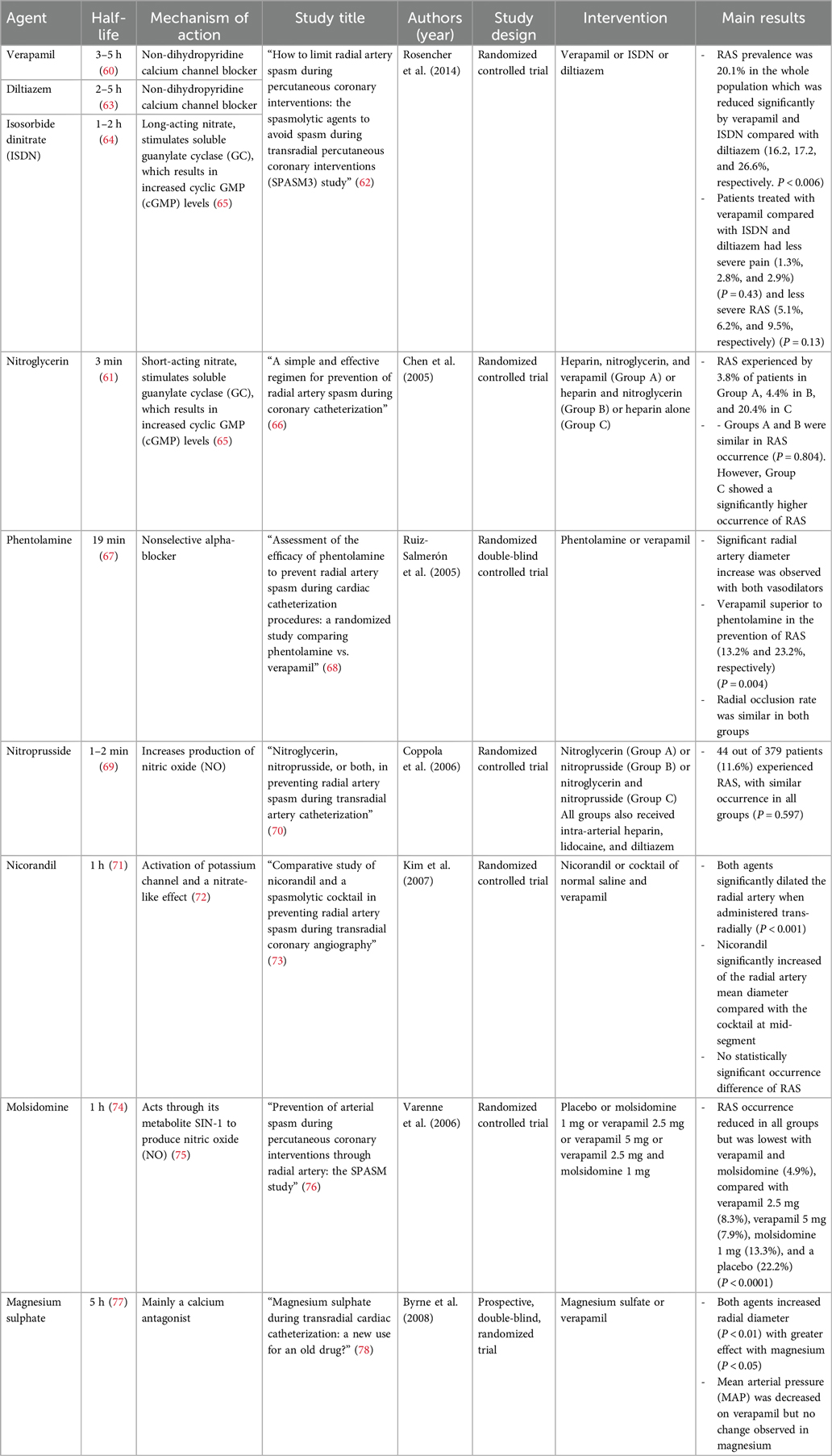
Table 1. Half-lives, mechanisms of action, and studies assessing different agents for the prevention of radial artery spasms (RAS).
Pharmacological therapeutic approaches for ANOCA and/or INOCA include disease-modifying therapies or symptomatic treatments. Non-pharmacological management is also essential and involves lifestyle modifications and risk factor management.
Disease-modifying therapies include statins, angiotensin-converting enzyme (ACE) inhibitors, and low-dose aspirin. Statins can potentially enhance endothelial function and reduce blood viscosity and shear stress, improving CFR and the microcirculation's function (79). A study (80) on 68 patients with microvascular angina, randomized into three groups receiving fluvastatin, diltiazem, or a combination of both, demonstrated improved CFR in all groups after 90 days. However, a combination of fluvastatin and diltiazem was the most effective (80). The beneficial effect of these drugs might be attributed to increasing nitric oxide and reducing endothelin-1 (80). ACE inhibitors significantly improve CFR, exercise tolerance, and angina symptoms (81, 82). A randomized placebo-controlled trial on 45 patients with ANOCA evaluated the effect of a combination of statins and ACE inhibitors on the antioxidant enzyme superoxide dismutase (SOD), reflecting oxidative stress, and on exercise capacity and Seattle Angina Questionnaire (SAQ) scores, reflecting quality of life. At 6 months of treatment, the intervention group receiving atorvastatin and ramipril showed significant reduction (P = 0.001) in SOD compared with placebo. In addition, increased exercise duration by 23.46% and SAQ score by 64.1% were observed with the treatment, indicating improved quality of life (82).
Anti-anginal treatments vary for different underlying mechanisms of INOCA, so tailored treatment plans are required as demonstrated in the CorMicA study where stratified medical therapy improved outcomes (83). The first-line treatment for patients with microvascular angina is beta-blockers, but they are contraindicated for coronary vasospasm (84). In patients with coronary vasospasm, calcium channel blockers (CCB) are considered first-line therapy and highly effective, as demonstrated in a double-blind placebo-controlled study, where 21 patients with angina at rest were randomized to a placebo, isosorbide-5-mononitrate, or nifedipine (85). Results showed that both drugs significantly reduced spontaneous and induced ischemic attacks (85). Both dihydropyridine and non-dihydropyridine calcium channel blockers have a well-established efficacy in relieving angina as demonstrated in many studies. However, choosing an agent has to be patient-tailored taking factors such as specific adverse reactions to each drug into consideration (86). In a meta-analysis of four studies on Japanese vasospastic angina patients, 1,349 patients who tested positive on the coronary spasm provocation test received one of four CCBs, which are benidipine, amlodipine, nifedipine, and diltiazem. Patients treated with benidipine showed significantly fewer major adverse cardiovascular events (MACE), which demonstrated a more beneficial prognostic effect of benidipine compared with amlodipine, nifedipine, and diltiazem (87). Furthermore, the EDIT-CMD randomized clinical trial (88) assessed the efficacy of diltiazem compared with placebo in patients identified with either vasospasms or microvascular dysfunction on coronary function testing. On a second coronary function test 6 weeks after treatment, coronary vasomotor dysfunction, symptoms, and quality of life were similar between the patients treated with diltiazem and placebo. However, epicardial spams were significantly reduced with diltiazem. CCBs are also effective for CMD, thus in cases where the underlying mechanism of INOCA is unknown or treatment with beta-blockers for CMD is not sufficient, CCBs are recommended. Long-acting nitrates can be considered second-line therapy for vasospastic angina but are ineffective for microvascular angina with the potential to aggravate the symptoms (89–91).
Nicorandil acts as a potassium channel activator and a nitrate-like factor with established efficacy for both CMD and coronary spasms and can be used as a third-line treatment for both endotypes (92–94). A randomized controlled trial on 13 patients with microvascular angina showed that nicorandil improved exercise-induced myocardial ischemia without modification of cardiac autonomic activity, which demonstrates a direct vasodilatory effect of nicorandil on the microcirculation (94). Another study assessing the effect of nicorandil on 10 patients with spontaneous and ergonovine-evoked coronary spasms showed complete spasm relief in all patients (92).
Studies demonstrated conflicting results on the efficacy of ranolazine for the treatment of CMD. A randomized controlled crossover trial on 20 females with ANOCA and ischemia treated with ranolazine demonstrated higher Seattle Angina Questionnaire (SAQ) scores and improved myocardial ischemia among women with low CFR (95). Moreover, ranolazine significantly increased CFR in a double-blind, placebo-controlled trial on 58 patients with angina and INOCA (96). Several studies showed improved outcomes with the use of ranolazine (95–97). However, it was found generally ineffective in one trial (98).
The ChaMP-CMD randomized controlled study (99) assessed the effect of amlodipine and ranolazine on treadmill exercise time and Seattle Angina Questionnaire summary score in patients with CMD defined as CFR <2.5 compared with patients with CFR >2.5. Patients with CMD showed prolonged treadmill exercise time on both drugs compared with the control group. However, the Seattle Angina Questionnaire score was improved in CMD patients taking ranolazine but not in response to amlodipine. The study suggested that only patients with decreased CFR might respond to anti-ischemic therapy emphasizing the importance of stratified medical therapy through measurement of CFR.
Other treatments can be used alternatively or in combination with the therapies mentioned above and demonstrated their efficacy in some studies. These include imipramine (100), ivabradine (97), aminophylline (101), L-arginine (102), phosphodiesterase (PDE) inhibitors (103), metformin (104), trimetazidine (105), and Rho-kinase inhibitors (106). However, the benefits of these therapies are not well established. The mechanisms of action and efficacy studies for the different agents used for the long-term management of INOCA are discussed in Table 2. Figure 3 summarizes the management plan for patients with INOCA of different underlying pathologies.
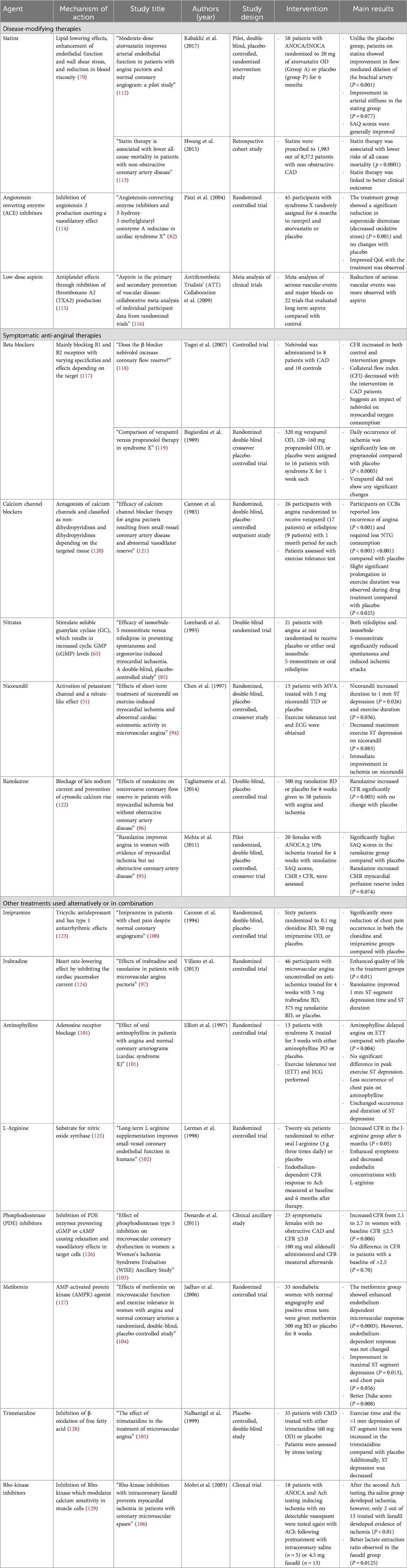
Table 2. Mechanisms of action and studies assessing different agents for the long-term management of INOCA.
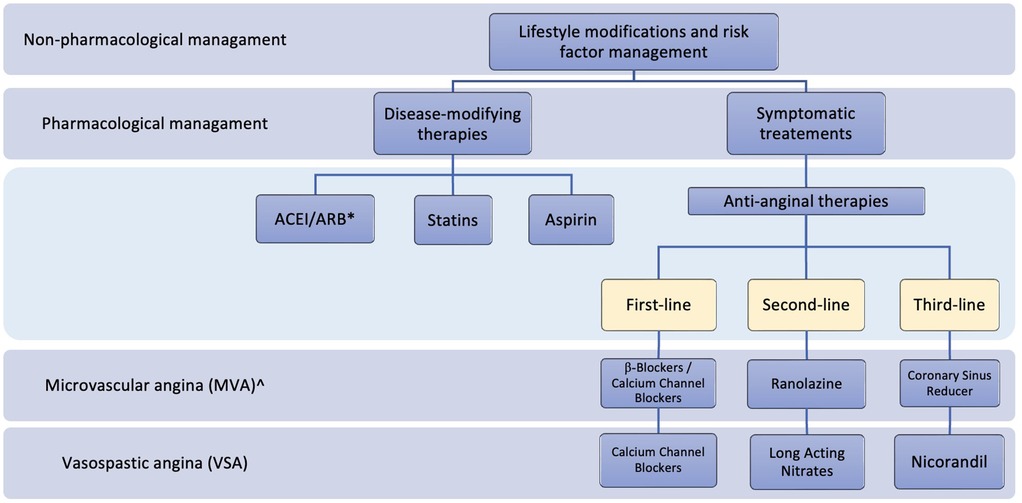
Figure 3. Summary of the management plan for patients with INOCA. *ACEI, Angiotensin-Converting Enzyme Inhibitors; ARB, angiotensin receptor blockers; ^, endothelium-independent.
Non-pharmacological approaches have a crucial role in managing ANOCA and INOCA. Cardiac rehabilitation which includes exercise training is beneficial in patients with ANOCA. A systematic review (107) of eight studies including 218 participants with exertional chest pain and absence of obstructive CAD who underwent cardiac rehabilitation including exercise programs with variable intensities concluded that exercise training improved exercise capacity and quality of life in most studies. Moreover, a pilot study on 16 patients with ANOCA who underwent an aerobic high-intensity training program for 3 months showed increased coronary flow velocity reserve (CFVR) and flow-mediated vasodilation (FMD) with exercise (108).
The use of a coronary sinus reducer is another non-pharmacologic novel approach to managing non-obstructive CAD. The device is an hourglass-shaped balloon expandable stent, which is implanted percutaneously in the coronary sinus and works by increasing the pressure in the sinus relieving angina (109). In a randomized clinical trial (109), 104 patients with myocardial ischemia and Canadian Cardiovascular Society (CCS) Class III or IV angina underwent a reducer implant surgery or a sham procedure. Results showed two CSS classes improvement in 35% of the treatment group compared with 15% of the control group at 6 months. Moreover, the quality of life significantly improved in patients undergoing reducer implantation according to the Seattle Angina Questionnaires scores. This has a potential use for patients with microvascular dysfunction.
Another non-pharmacological approach to the treatment of angina is enhanced external counterpulsation (EECP) which is non-invasive. It works by placing external inflatable cuffs on the lower extremities to increase the blood flow to the heart during diastole, which is followed by deflation of the cuffs during systole. A study (110) on the effect of EECP on patients with ANOCA and refractory angina assessed weekly anginal episodes before and after EECP treatment in 101 participants. Post-EECP treatment, CCS angina class, 6 min walk test, Duke Activity Status Index (DASI), Seattle Angina Questionnaire 7 (SAQ7), and weekly anginal episodes improved significantly. Moreover, a systematic review of 18 studies demonstrated the efficacy of EECP in the treatment of patients with refractory angina irresponsive to therapy (111).
Clinical experience would suggest management of epicardial vasospasm is usually responsive to medical therapy with good symptomatic relief. CMD, however, by experience, is a challenging entity for good symptomatic relief and often requires a combination and titration of medications tailored to the individual for relief. Invasive testing allows for some insight to help guide the appropriate therapy. Furthermore, a notable limitation of the current therapeutic approaches is the lack of extensive clinical trials, which necessitates the need for further research to guide clinical practice.
The prevalence of ANOCA and/or INOCA is increasing due to increased recognition and diagnosis, with CMD and epicardial coronary vasospasms as the most common underlying pathologies. Several risk factors are involved in the development of ANOCA and/or INOCA, including myocardial bridging, which is not well investigated and has been thought of as a benign condition. The prevalence of ANOCA and/or INOCA is not well investigated in the middle east, and further studies are needed in the region. Functional assessment of the coronary circulation is required to detect INOCA and can be done non-invasively or invasively, the latter being more complete. Moreover, trans-radial access is preferred. An interaction between acetylcholine used in spasm provocation testing and vasodilators to prevent RAS might be a concern and should be further investigated. Using vasodilators with the shortest half-life for RAS management, such as intra-arterial nitroglycerin, is a reasonable approach to minimizing the occurrence of any interaction and might lead to more accurate outcomes. The long-term management of INOCA involves pharmacological and non-pharmacological therapies and should be tailored to different underlying pathologies to achieve maximum benefit.
MB: Writing – original draft, Writing – review & editing. RS: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing. WM: Writing – original draft, Writing – review & editing. BA: Supervision, Writing – original draft, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Merz CNB. Ischemia and no obstructive coronary artery disease (INOCA): what is the risk? J Am Heart Assoc. (2018) 7(17):e008868. doi: 10.1161/JAHA.118.008868
2. Merz CNB, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study. J Am Coll Cardiol. (2006) 47(3_Supplement):S21–S9. doi: 10.1016/j.jacc.2004.12.084
3. Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, et al. Ischemia and no obstructive coronary artery disease. Circ: Cardiovasc Interventions. (2019) 12(12):e008126. doi: 10.1161/CIRCINTERVENTIONS.119.008126
4. Kaski JC, Crea F, Meran D, Rodriguez L, Araujo L, Chierchia S, et al. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. (1986) 74(6):1255–65. doi: 10.1161/01.CIR.74.6.1255
5. Sinha A, Dutta U, Demir OM, De Silva K, Ellis H, Belford S, et al. Rethinking false positive exercise electrocardiographic stress tests by assessing coronary microvascular function. J Am Coll Cardiol. (2024) 83(2):291–9. doi: 10.1016/j.jacc.2023.10.034
6. Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. (2015) 36(45):3134–46. doi: 10.1093/eurheartj/ehv100
7. Sorop O, Merkus D, Beer V, Houweling B, Pistea A, McFalls EO, et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res. (2008) 102(7):795–803. doi: 10.1161/CIRCRESAHA.108.172528
8. Sorop O, van den Heuvel M, van Ditzhuijzen NS, de Beer VJ, Heinonen I, van Duin RW, et al. Coronary microvascular dysfunction after long-term diabetes and hypercholesterolemia. Am J Physiol Heart Circ Physiol. (2016) 311(6):H1339–h51. doi: 10.1152/ajpheart.00458.2015
9. Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc. (2020) 9(9):e014954. doi: 10.1161/JAHA.119.014954
10. Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 78(13):1352–71. doi: 10.1016/j.jacc.2021.07.042
11. Paolisso P, Gallinoro E, Belmonte M, Bertolone DT, Bermpeis K, De Colle C, et al. Coronary microvascular dysfunction in patients with heart failure: characterization of patterns in HFrEF versus HFpEF. Circ Heart Fail. (2024) 17(1):e010805. doi: 10.1161/CIRCHEARTFAILURE.123.010805
12. Mileva N, Nagumo S, Mizukami T, Sonck J, Berry C, Gallinoro E, et al. Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: systematic review and meta-analysis. J Am Heart Assoc. (2022) 11(7):e023207. doi: 10.1161/JAHA.121.023207
13. Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. (1999) 33(6):1442–52. doi: 10.1016/S0735-1097(99)00073-X
14. Shantsila E, Wrigley B, Shantsila A, Tapp LD, Blann AD, Gill PS, et al. Ethnic differences in macrovascular and microvascular function in systolic heart failure. Circ Heart Fail. (2011) 4(6):754–62. doi: 10.1161/CIRCHEARTFAILURE.111.962365
15. Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the women’s ischemia syndrome evaluation study and the St James Women Take Heart Project. Arch Intern Med. (2009) 169(9):843–50. doi: 10.1001/archinternmed.2009.50
16. Merz CN B, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, et al. The women’s ischemia syndrome evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. (1999) 33(6):1453–61. doi: 10.1016/S0735-1097(99)00082-0
17. Wessel TR, Arant CB, McGorray SP, Sharaf BL, Reis SE, Kerensky RA, et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women's Ischemia Syndrome Evaluation (WISE). Clin Cardiol. (2007) 30(2):69–74. doi: 10.1002/clc.19
18. Hostiuc S, Rusu MC, Hostiuc M, Negoi RI, Negoi I. Cardiovascular consequences of myocardial bridging: a meta-analysis and meta-regression. Sci Rep. (2017) 7(1):14644. doi: 10.1038/s41598-017-13958-0
19. Kim SY, Lee YS, Lee JB, Ryu JK, Choi JY, Chang SG, et al. Evaluation of myocardial bridge with multidetector computed tomography. Circ J. (2010) 74(1):137–41. doi: 10.1253/circj.CJ-09-0407
20. Hayashi T, Ishikawa K. Myocardial bridge: harmless or harmful. Internal Medicine. (2004) 43(12):1097–8. doi: 10.2169/internalmedicine.43.1097
21. Gould KL, Johnson NP. Myocardial bridges: lessons in clinical coronary pathophysiology∗. JACC. (2015) 8(6):705–9. doi: 10.1016/j.jcmg.2015.02.013
22. Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Görge G, et al. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. (1994) 89(4):1725–32. doi: 10.1161/01.CIR.89.4.1725
23. Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional characteristics of myocardial bridging: a combined angiographic and intracoronary Doppler flow study. Eur Heart J. (1997) 18(3):434–42. doi: 10.1093/oxfordjournals.eurheartj.a015263
24. Masuda T, Ishikawa Y, Akasaka Y, Itoh K, Kiguchi H, Ishii T. The effect of myocardial bridging of the coronary artery on vasoactive agents and atherosclerosis localization. J Pathol. (2001) 193(3):408–14. doi: 10.1002/1096-9896(2000)9999:9999%3C::AID-PATH792%3E3.0.CO;2-R
25. Teragawa H, Fukuda Y, Matsuda K, Hirao H, Higashi Y, Yamagata T, et al. Myocardial bridging increases the risk of coronary spasm. Clin Cardiol. (2003) 26(8):377–83. doi: 10.1002/clc.4950260806
26. Hostiuc S, Negoi I, Rusu MC, Hostiuc M. Myocardial bridging: a meta-analysis of prevalence. J Forensic Sci. (2018) 63(4):1176–85. doi: 10.1111/1556-4029.13665
27. Teragawa H, Oshita C, Uchimura Y. The impact of myocardial bridging on the coronary functional test in patients with ischaemia with non-obstructive coronary artery disease. Life (Basel). (2022) 12(10):6–8. doi: 10.3390/life12101560
28. Montone RA, Gurgoglione FL, Del Buono MG, Rinaldi R, Meucci MC, Iannaccone G, et al. Interplay between myocardial bridging and coronary spasm in patients with myocardial ischemia and non-obstructive coronary arteries: pathogenic and prognostic implications. J Am Heart Assoc. (2021) 10(14):e020535. doi: 10.1161/JAHA.120.020535
29. Benenati S, Campo G, Seitun S, Caglioni S, Leone AM, Porto I. Ischemia with non-obstructive coronary artery (INOCA): non-invasive versus invasive techniques for diagnosis and the role of #FullPhysiology. Eur J Intern Med. (2024) 127:15–24. doi: 10.1016/j.ejim.2024.07.017
30. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. (2018) 72(23, Part A):2841–55. doi: 10.1016/j.jacc.2018.09.006
31. De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. (2001) 104(17):2003–6. doi: 10.1161/hc4201.099223
32. Belmonte M, Gallinoro E, Pijls NHJ, Bertolone DT, Keulards DCJ, Viscusi MM, et al. Measuring absolute coronary flow and microvascular resistance by thermodilution: JACC review topic of the week. J Am Coll Cardiol. (2024) 83(6):699–709. doi: 10.1016/j.jacc.2023.12.014
33. Gallinoro E, Bertolone DT, Mizukami T, Paolisso P, Bermpeis K, Munhoz D, et al. Continuous vs bolus thermodilution to assess microvascular resistance reserve. JACC Cardiovasc Interv. (2023) 16(22):2767–77. doi: 10.1016/j.jcin.2023.09.027
34. Gallinoro E, Bertolone DT, Fernandez-Peregrina E, Paolisso P, Bermpeis K, Esposito G, et al. Reproducibility of bolus versus continuous thermodilution for assessment of coronary microvascular function in patients with ANOCA. EuroIntervention. (2023) 19(2):e155–e66. doi: 10.4244/EIJ-D-22-00772
35. Feenstra RGT, Seitz A, Boerhout CKM, de Winter RJ, Ong P, Beijk MAM, et al. Reference values for intracoronary Doppler flow velocity-derived hyperaemic microvascular resistance index. Int J Cardiol. (2023) 371:16–20. doi: 10.1016/j.ijcard.2022.09.054
36. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI). Eur Heart J. (2014) 35(37):2541–619. doi: 10.1093/eurheartj/ehu278
37. Benenati S, De Maria GL, Scarsini R, Porto I, Banning AP. Invasive “in the cath-lab” assessment of myocardial ischemia in patients with coronary artery disease: when does the gold standard not apply? Cardiovasc Revasc Med. (2018) 19(3, Part B):362–72. doi: 10.1016/j.carrev.2018.01.005
38. De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, et al. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med. (2012) 367(11):991–1001. doi: 10.1056/NEJMoa1205361
39. Fearon WF, Kobayashi Y. Invasive assessment of the coronary microvasculature. Circulation. (2017) 10(12):e005361. doi: 10.1161/CIRCINTERVENTIONS.117.005361
40. Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. (2019) 140(22):1805–16. doi: 10.1161/CIRCULATIONAHA.119.041595
41. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72(21):2625–41. doi: 10.1016/j.jacc.2018.09.042
42. De Bruyne B, Pijls NHJ, Gallinoro E, Candreva A, Fournier S, Keulards DCJ, et al. Microvascular resistance reserve for assessment of coronary microvascular function: JACC technology corner. J Am Coll Cardiol. (2021) 78(15):1541–9. doi: 10.1016/j.jacc.2021.08.017
43. Mangiacapra F, Viscusi MM, Verolino G, Paolucci L, Nusca A, Melfi R, et al. Invasive assessment of coronary microvascular function. J Clin Med. (2022) 11(1):2–11. doi: 10.3390/jcm11010228
44. Boerhout CKM, de Waard GA, Lee JM, Mejía-Rentería H, Lee SH, Jung J-H, et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. EuroIntervention. (2022) 18(9):719–28. doi: 10.4244/EIJ-D-22-00043
45. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. (2017) 38(33):2565–8. doi: 10.1093/eurheartj/ehv351
46. Vrints C, Andreotti F, Koskinas KC, Rossello X, Adamo M, Ainslie J, et al. 2024 ESC guidelines for the management of chronic coronary syndromes: developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2024) 45(36):3415–537. doi: 10.1093/eurheartj/ehae177
47. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. (2018) 250:16–20. doi: 10.1016/j.ijcard.2017.08.068
48. Seitz A, Feenstra RGT, Konst RE, Martínez Pereyra V, Beck S, Beijk MAM, et al. Acetylcholine rechallenge: a first step toward tailored treatment in patients with coronary artery spasm. JACC Cardiovascular Interventions. (2022) 15(1):65–75. doi: 10.1016/j.jcin.2021.10.003
49. Fu B, Wei X, Lin Y, Chen J, Yu D. Pathophysiologic basis and diagnostic approaches for ischemia with non-obstructive coronary arteries: a literature review. Front Cardiovasc Med. (2022) 9:2–10. Available online at: https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2022.731059
50. Carbone A, D'Andrea A, Sperlongano S, Tagliamonte E, Mandoli GE, Santoro C, et al. Echocardiographic assessment of coronary microvascular dysfunction: basic concepts, technical aspects, and clinical settings. Echocardiography. (2021) 38(6):993–1001. doi: 10.1111/echo.15059
51. Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, et al. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation. (1998) 97(16):1557–62. doi: 10.1161/01.CIR.97.16.1557
52. Florian A. Diastolic dysfunction in women with ischemia and non-obstructive coronary arteries (INOCA) – could non-invasive imaging reveal the missing piece of the puzzle? Int J Cardiol. (2021) 334:21–3. doi: 10.1016/j.ijcard.2021.04.024
53. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. (2016) 23(5):1187–226. doi: 10.1007/s12350-016-0522-3
54. Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, et al. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. (2000) 101(12):1379–83. doi: 10.1161/01.CIR.101.12.1379
55. Vandeloo B, Andreini D, Brouwers S, Mizukami T, Monizzi G, Lochy S, et al. Diagnostic performance of exercise stress tests for detection of epicardial and microvascular coronary artery disease: the UZ clear study. EuroIntervention. (2023) 18(13):e1090–e8. doi: 10.4244/EIJ-D-22-00270
56. Fischman AM, Swinburne NC, Patel RS. A technical guide describing the use of transradial access technique for endovascular interventions. Tech Vasc Interv Radiol. (2015) 18(2):58–65. doi: 10.1053/j.tvir.2015.04.002
57. Rathore S, Stables RH, Pauriah M, Hakeem A, Mills JD, Palmer ND, et al. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention. JACC Cardiovascular Interventions. (2010) 3(5):475–83. doi: 10.1016/j.jcin.2010.03.009
58. Challa AS, Luther E, Burks J, Saini V, Abecassis J, Silva M, et al. Radial long sheath angioplasty for proximal severe flow-limiting radial artery spasm using the dotter technique. World Neurosurg. (2022) 160:16–21. doi: 10.1016/j.wneu.2022.01.025
59. Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: incidence, predisposing factors, prevention, and management. Cardiovasc Revasc Med. (2012) 13(3):193–5. doi: 10.1016/j.carrev.2011.11.003
60. Follath F, Ha HR, Schütz E, Bühler F. Pharmacokinetics of conventional and slow-release verapamil. Br J Clin Pharmacol. (1986) 21(Suppl 2):149s–53. doi: 10.1111/j.1365-2125.1986.tb02864.x
61. McNiff EF, Yacobi A, Young-Chang FM, Golden LH, Goldfarb A, Fung H-L. Nitroglycerin pharmacokinetics after intravenous infusion in normal subjects. J Pharm Sci. (1981) 70(9):1054–8. doi: 10.1002/jps.2600700923
62. Rosencher J, Chaïb A, Barbou F, Arnould MA, Huber A, Salengro E, et al. How to limit radial artery spasm during percutaneous coronary interventions: the spasmolytic agents to avoid spasm during transradial percutaneous coronary interventions (SPASM3) study. Catheter Cardiovasc Interv. (2014) 84(5):766–71. doi: 10.1002/ccd.25163
63. Hermann P, Rodger SD, Remones G, Thenot JP, London DR, Morselli PL. Pharmacokinetics of diltiazem after intravenous and oral administration. Eur J Clin Pharmacol. (1983) 24(3):349–52. doi: 10.1007/BF00610053
64. Platzer R, Reutemann G, Galeazzi RL. Pharmacokinetics of intravenous isosorbide-dinitrate. J Pharmacokinet Biopharm. (1982) 10(6):575–86. doi: 10.1007/BF01062541
65. Kukovetz WR, Holzmann S, Romanin C. Mechanism of vasodilation by nitrates: role of cyclic GMP. Cardiology. (1987) 74(Suppl 1):12–9. doi: 10.1159/000174258
66. Chen C-W, Lin C-L, Lin T-K, Lin C-D. A simple and effective regimen for prevention of radial artery spasm during coronary catheterization. Cardiology. (2005) 105(1):43–7. doi: 10.1159/000089246
67. DAILYMED. Label: phentolamine mesylate injection, powder, for solution: national library of medicine. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ed6c506c-5535-4b7c-ae1a-d63f09d796c9 (updated December 12, 2018).
68. Ruiz-Salmerón RJ, Mora R, Masotti M, Betriu A. Assessment of the efficacy of phentolamine to prevent radial artery spasm during cardiac catheterization procedures: a randomized study comparing phentolamine vs. verapamil. Catheter Cardiovasc Interv. (2005) 66(2):192–8. doi: 10.1002/ccd.20434
69. Friederich JA, Butterworth JF. Sodium nitroprusside: twenty years and counting. Anesth Analg. (1995) 81(1):152–62. doi: 10.1213/00000539-199507000-00031
70. Coppola J, Patel T, Kwan T, Sanghvi K, Srivastava S, Shah S, et al. Nitroglycerin, nitroprusside, or both, in preventing radial artery spasm during transradial artery catheterization. J Invasive Cardiol. (2006) 18(4):155–8. Available online at: https://pubmed.ncbi.nlm.nih.gov/16729400/16729400
71. Frydman AM, Chapelle P, Diekmann H, Bruno R, Thebault JJ, Bouthier J, et al. Pharmacokinetics of nicorandil. Am J Cardiol. (1989) 63(21):J25–33. doi: 10.1016/0002-9149(89)90201-4
72. Kukovetz WR, Holzmann S, Pöch G. Molecular mechanism of action of nicorandil. J Cardiovasc Pharmacol. (1992) 20(Suppl 3):S1–7. doi: 10.1097/00005344-199206203-00002
73. Kim SH, Kim EJ, Cheon WS, Kim M-K, Park WJ, Cho G-Y, et al. Comparative study of nicorandil and a spasmolytic cocktail in preventing radial artery spasm during transradial coronary angiography. Int J Cardiol. (2007) 120(3):325–30. doi: 10.1016/j.ijcard.2006.10.008
74. Rosenkranz B, Winkelmann BR, Parnham MJ. Clinical pharmacokinetics of molsidomine. Clin Pharmacokinet. (1996) 30(5):372–84. doi: 10.2165/00003088-199630050-00004
76. Varenne O, Jégou A, Cohen R, Empana JP, Salengro E, Ohanessian A, et al. Prevention of arterial spasm during percutaneous coronary interventions through radial artery: the SPASM study. Catheter Cardiovasc Interv. (2006) 68(2):231–5. doi: 10.1002/ccd.20812
77. Okusanya BO, Oladapo OT, Long Q, Lumbiganon P, Carroli G, Qureshi Z, et al. Clinical pharmacokinetic properties of magnesium sulphate in women with pre-eclampsia and eclampsia. BJOG. (2016) 123(3):356–66. doi: 10.1111/1471-0528.13753
78. Byrne J, Spence M, Haegeli L, Fretz E, Della Siega A, Williams M, et al. Magnesium sulphate during transradial cardiac catheterization: a new use for an old drug? J Invasive Cardiol. (2008) 20(10):539–42. Available online at: https://pubmed.ncbi.nlm.nih.gov/18829998/18829998
79. Eshtehardi P, McDaniel MC, Dhawan SS, Binongo JNG, Krishnan SK, Golub L, et al. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J Interv Cardiol. (2012) 24(10):522. Available online at: https://pubmed.ncbi.nlm.nih.gov/23043036/
80. Zhang X, Li Q, Zhao J, Li X, Sun X, Yang H, et al. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron Artery Dis. (2014) 25(1):40–4. doi: 10.1097/MCA.0000000000000054
81. Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. (2011) 162(4):678–84. doi: 10.1016/j.ahj.2011.07.011
82. Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac syndrome X. Circulation. (2004) 109(1):53–8. doi: 10.1161/01.CIR.0000100722.34034.E4
83. Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv. (2020) 13(1):33–45. doi: 10.1016/j.jcin.2019.11.001
84. Robertson RM, Wood AJ, Vaughn WK, Robertson D. Exacerbation of vasotonic angina pectoris by propranolol. Circulation. (1982) 65(2):281–5. doi: 10.1161/01.CIR.65.2.281
85. Lombardi M, Morales MA, Michelassi C, Moscarelli E, Distante A, L'Abbate A. Efficacy of isosorbide-5-mononitrate versus nifedipine in preventing spontaneous and ergonovine-induced myocardial ischaemia. A double-blind, placebo-controlled study. Eur Heart J. (1993) 14(6):845–51. doi: 10.1093/eurheartj/14.6.845
86. Singh J, Elton A, Kwa M. Comparison of various calcium antagonist on vasospastic angina: a systematic review. Open Heart. (2023) 10(1):e002179. doi: 10.1136/openhrt-2022-002179
87. Nishigaki K, Inoue Y, Yamanouchi Y, Fukumoto Y, Yasuda S, Sueda S, et al. Prognostic effects of calcium channel blockers in patients with vasospastic angina–a meta-analysis. Circ J. (2010) 74(9):1943–50. doi: 10.1253/circj.CJ-10-0292
88. Jansen TPJ, Konst RE, Ad V, Paradies V, Teerenstra S, Oord S, et al. Efficacy of diltiazem to improve coronary vasomotor dysfunction in ANOCA. JACC: Cardiovascular Imaging. (2022) 15(8):1473–84. doi: 10.1016/j.jcmg.2022.03.012
89. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. (2013) 34(38):2949–3003. doi: 10.1093/eurheartj/eht296
90. Wu M, Villano A, Russo G, Di Franco A, Stazi A, Lauria C, et al. Poor tolerance and limited effects of isosorbide-5-mononitrate in microvascular angina. Cardiology. (2015) 130(4):201–6. doi: 10.1159/000370027
91. Russo G, Di Franco A, Lamendola P, Tarzia P, Nerla R, Stazi A, et al. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc Drugs Ther. (2013) 27(3):229–34. doi: 10.1007/s10557-013-6439-z
92. Aizawa T, Ogasawara K, Nakamura F, Hirosaka A, Sakuma T, Nagashima K, et al. Effect of nicorandil on coronary spasm. Am J Cardiol. (1989) 63(21):J75–J9. doi: 10.1016/0002-9149(89)90210-5
93. Yamabe H, Namura H, Yano T, Fujita H, Kim S, Iwahashi M, et al. Effect of nicorandil on abnormal coronary flow reserve assessed by exercise 201Tl scintigraphy in patients with angina pectoris and nearly normal coronary arteriograms. Cardiovasc Drugs Ther. (1995) 9(6):755–61. doi: 10.1007/BF00879868
94. Jaw-Wen C, Wen-Lieng L, Nai-Wei H, Shing-Jong L, Chih-Tai T, Shih-Pu W, et al. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. Am J Cardiol. (1997) 80(1):32–8. doi: 10.1016/S0002-9149(97)00279-8
95. Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. (2011) 4(5):514–22. doi: 10.1016/j.jcmg.2011.03.007
96. Tagliamonte E, Rigo F, Cirillo T, Astarita C, Quaranta G, Marinelli U, et al. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography. (2015) 32(3):516–21. doi: 10.1111/echo.12674
97. Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. (2013) 112(1):8–13. doi: 10.1016/j.amjcard.2013.02.045
98. Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. (2015) 37(19):1504–13. doi: 10.1093/eurheartj/ehv647
99. Sinha A, Rahman H, Douiri A, Demir OM, De Silva K, Clapp B, et al. ChaMP-CMD: a phenotype-blinded, randomized controlled, cross-over trial. Circulation. (2024) 149(1):36–47. doi: 10.1161/CIRCULATIONAHA.123.066680
100. Cannon RO, Quyyumi AA, Mincemoyer R, Stine AM, Gracely RH, Smith WB, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. (1994) 330(20):1411–7. doi: 10.1056/NEJM199405193302003
101. Elliott PM, Krzyzowska-Dickinson K, Calvino R, Hann C, Kaski JC. Effect of oral aminophylline in patients with angina and normal coronary arteriograms (cardiac syndrome X). Heart. (1997) 77(6):523–6. doi: 10.1136/hrt.77.6.523
102. Lerman A, Burnett JC, Higano ST, McKinley LJ, Holmes DR. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. (1998) 97(21):2123–8. doi: 10.1161/01.CIR.97.21.2123
103. Denardo SJ, Wen X, Handberg EM, Bairey Merz CN, Sopko GS, Cooper-DeHoff RM, et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a women’s ischemia syndrome evaluation (WISE) ancillary study. Clin Cardiol. (2011) 34(8):483–7. doi: 10.1002/clc.20935
104. Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. (2006) 48(5):956–63. doi: 10.1016/j.jacc.2006.04.088
105. Nalbantgil S, Altintiğ A, Yilmaz H, Nalbantgil II, Önder R. The effect of trimetazidine in the treatment of microvascular angina. Int J Angiol. (1999) 8(1):40–3. doi: 10.1007/BF01616842
106. Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. (2003) 41(1):15–9. doi: 10.1016/S0735-1097(02)02632-3
107. Kissel CK, Nikoletou D. Cardiac rehabilitation and exercise prescription in symptomatic patients with non-obstructive coronary artery disease-a systematic review. Curr Treat Options Cardiovasc Med. (2018) 20(9):78. doi: 10.1007/s11936-018-0667-2
108. Larsen AI, Sæland C, Vegsundvåg J, Skadberg MS, Nilsen J, Butt N, et al. Aerobic high-intensity interval exercise training in patients with angina and no obstructive coronary artery disease: feasibility and physiological effects. Eur Heart J Open. (2023) 3(2):oead030. doi: 10.1093/ehjopen/oead030
109. Verheye S, Jolicœur EM, Behan MW, Pettersson T, Sainsbury P, Hill J, et al. Efficacy of a device to narrow the coronary Sinus in refractory angina. N Engl J Med. (2015) 372(6):519–27. doi: 10.1056/NEJMoa1402556
110. Ashokprabhu ND, Fox J, Henry TD, Schmidt CW, Tierney D, Gallatin J, et al. Enhanced external counterpulsation for the treatment of angina with nonobstructive coronary artery disease. Am J Cardiol. (2024) 211:89–93. doi: 10.1016/j.amjcard.2023.10.061
111. Zhang C, Liu X, Wang X, Wang Q, Zhang Y, Ge Z. Efficacy of enhanced external counterpulsation in patients with chronic refractory angina on Canadian cardiovascular society (CCS) angina class: an updated meta-analysis. Medicine (Baltimore). (2015) 94(47):e2002. doi: 10.1097/MD.0000000000002002
112. Kabaklić A, Fras Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: a pilot study. Arch Med Sci. (2017) 4(4):827–36. doi: 10.5114/aoms.2017.68238
113. Hwang IC, Jeon JY, Kim Y, Kim HM, Yoon YE, Lee SP, et al. Statin therapy is associated with lower all-cause mortality in patients with non-obstructive coronary artery disease. Atherosclerosis. (2015) 239(2):335–42. doi: 10.1016/j.atherosclerosis.2015.01.036
114. Herman LL, Padala SA, Ahmed I, Bashir K. Angiotensin-Converting Enzyme Inhibitors (ACEI). Treasure Island, FL: StatPearls Publishing (2023).
115. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. (2011) 72(4):619–33. doi: 10.1111/j.1365-2125.2011.03943.x
116. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) 373(9678):1849–60. doi: 10.1016/S0140-6736(09)60503-1
118. Togni M, Vigorito F, Windecker S, Abrecht L, Wenaweser P, Cook S, et al. Does the β-blocker nebivolol increase coronary flow reserve? Cardiovasc Drugs Ther. (2007) 21(2):99–108. doi: 10.1007/s10557-006-0494-7
119. Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. (1989) 63(5):286–90. doi: 10.1016/0002-9149(89)90332-9
120. McKeever RG, Hamilton RJ. Calcium Channel Blockers. Treasure Island, FL: StatPearls Publishing LLC (2023).
121. Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. (1985) 56(4):242–6. doi: 10.1016/0002-9149(85)90842-2
122. Ghosh GC, Ghosh RK, Bandyopadhyay D, Chatterjee K, Aneja A. Ranolazine: multifaceted role beyond coronary artery disease, a recent perspective. Heart Views. (2018) 19(3):88–98. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_18_18
124. Tse S, Mazzola N. Ivabradine (corlanor) for heart failure: the first selective and specific I f inhibitor. Pharmacy and Therapeutics. (2015) 40(12):810–4. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4671466/#:∼:text=MECHANISM%20OF%20ACTION,hence%20regulates%20the%20heart%20rate26681903
125. Böger RH, Bode-Böger SM. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol. (2001) 41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79
126. Padda IS, Tripp J. Phosphodiesterase Inhibitors. Treasure Island, FL: StatPearls Publishing LLC (2023).
127. Bu Y, Peng M, Tang X, Xu X, Wu Y, Chen AF, et al. Protective effects of metformin in various cardiovascular diseases: clinical evidence and AMPK-dependent mechanisms. J Cell Mol Med. (2022) 26(19):4886–903. doi: 10.1111/jcmm.17519
128. Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. (2000) 86(5):580–8. doi: 10.1161/01.RES.86.5.580
Keywords: INOCA, ANOCA, microvascular resistance, microvascular dysfunction, CMD, functional testing, myocardial bridge (MB), radial artery spasm
Citation: Al Bitar M, Shantouf R, Al Azzoni A, Al Mahmeed W and Atallah B (2025) Ischemia with no obstructed coronary arteries and microvascular testing procedures: a review of utility, pharmacotherapy, and current challenges. Front. Cardiovasc. Med. 12:1523352. doi: 10.3389/fcvm.2025.1523352
Received: 5 November 2024; Accepted: 3 February 2025;
Published: 18 February 2025.
Edited by:
Emanuele Gallinoro, OLV Aalst, BelgiumReviewed by:
Stefano Benenati, San Martino Hospital (IRCCS), ItalyCopyright: © 2025 Al Bitar, Shantouf, Al Azzoni, Al Mahmeed and Atallah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bassam Atallah, YXRhbGxhYkBjbGV2ZWxhbmRjbGluaWNhYnVkaGFiaS5hZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.