
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 April 2025
Sec. Cardiac Rhythmology
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1522154
Background: Non-valvular atrial fibrillation (NVAF) significantly increases ischemic stroke and systemic embolism (SE) risks. Despite the proven efficacy of oral anticoagulants (OAC) in reducing these risks, their underutilization highlights a gap in clinical practice. This study examined OAC utilization patterns within the first year after NVAF diagnosis in patients without prior OAC use and the association between the timing of OAC initiation and the risk of all-cause and stroke/SE-specific hospitalizations.
Methods: A retrospective cohort study was conducted using data from the Premier Healthcare Database and linked claims from 1/1/2017–3/31/2021. Patients newly diagnosed with NVAF, without prior OAC use, were included.
Results: Of 23,148 adults with newly diagnosed NVAF, 11,059 (47.8%) initiated OAC within one year. OAC users predominantly had cardiovascular disease and risk factors, whereas non-OAC users had higher rates of malignancy and dementia. Early OAC initiation (74.9% during the index visit) was linked to lower hospitalization risks compared to those initiating later (29.2% vs. 45.9% for all-cause, p-value < 0.001 and 1.3% vs. 2.6% for stroke/SE-specific, p-value < 0.001). Adjusted odds ratios for all-cause and stroke/SE hospitalization favored early initiation were 0.35 (95% CI: 0.32–0.39) and 0.34 (95% CI: 0.24–0.47), respectively.
Conclusions: This study highlights OAC underutilization in NVAF patients and suggests early initiation may lower hospitalization rates. The findings emphasize the need for further research into real-world compliance with OAC guidelines and call for further research to confirm the benefits of early initiation. Personalized management strategies that consider individual patient profiles are recommended.
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice, significantly increasing the risk of mortality and thromboembolic events, especially ischemic stroke (IS) and systemic embolism (SE) (1, 2). Non-valvular atrial fibrillation (NVAF), defined as AF without mechanical prosthetic heart valves or moderate-to-severe mitral stenosis (3, 4), further amplifies stroke risk, accounting for 20%–30% of all IS worldwide (1, 5). Given the substantial individual and societal implications of stroke, comprehensive management of NVAF is a key component of IS prevention (6).
The United States (U.S.) and European guidelines recommend oral anticoagulants (OAC)—including vitamin K antagonists like Warfarin and direct oral anticoagulants (DOACs, such as dabigatran, rivaroxaban, apixaban, and edoxaban)—to reduce IS and SE risks in patients with NVAF. Although their efficacy and safety have been demonstrated by rigorous randomized clinical trials (RCTs), real-world implementation of these agents frequently encounters hurdles with their notable underutilization (7–10). While recent U.S. studies note an uptrend in OAC use, with 33%–65% of AF patients receiving OACs, substantial variations persist across different populations (11–13). This inconsistency underscores the need for further investigation into OAC utilization patterns among U.S. patients with NVAF.
Moreover, literature regarding the optimal timing for OAC initiation relative to stroke risk in NVAF patients is limited. Although individual studies have reported mixed results (14–17), a recent systematic review and meta-analysis on patients with acute ischemic stroke found that early OAC initiation was associated with a reduced risk of composite outcomes, including ischemic stroke recurrence, intracranial hemorrhage, major bleeding, and all-cause mortality (18). This correlation remains unexplored in patients without prior strokes. In addition, to our knowledge, no prior research has investigated this association in a real-world setting.
This study aimed to describe OAC usage patterns within the first-year post diagnosis of NVAF among patients with no prior OAC use. Additionally, we examined the relationship between the timing of OAC initiation and the one-year risk of all-cause and stroke/SE-specific hospitalizations for patients starting OAC within this timeframe.
This retrospective observational study used data from the Premier Healthcare Database (PHD) and its linked medical and pharmacy insurance claims database. The claims data and PHD data were linked via tokenization. As per US Title 45 Code of Federal Regulations, Part 46, this study of fully deidentified data was exempted from institutional review board approval, as with prior PHD studies (19–22). Researchers followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (Supplementary Table S1).
The index period spanned from January 1, 2017 through March 31, 2021 with a 365-day look-back and follow-up period (Supplementary Figure). Patients were included if they met all of the following criteria: (1) age ≥18 years, (2) had an inpatient visit with a principal or secondary discharge diagnosis of AF as defined by ICD-10-CM diagnosis codes, I48.0, I48.1x, I48.2x, I48.91, or two outpatient visits with a principal or secondary discharge diagnosis of AF during index period, (3) hospitals had continuous data submission during the look-back and follow-up periods, and (4) patients with claims linkage who were eligible for medical and pharmacy benefits during the 6-month look-back period, index visit and follow-up period. Patients were excluded if (1) they had ≥1 visit(s) with a principal or secondary discharge diagnosis of AF during the look-back period, (2) patients with evidence of OAC use during the look-back period, (3) patients with evidence of hip/knee replacement surgery within six weeks before the index date (the admission date of the index visit) due to the potential changes in treatment to prevent venous thromboembolism post-surgery, (4) patients with evidence of valvular heart disease (ICD-10-CM codes: mitral stenosis I05.x, I08.0, I08.8, I08.9, I34.x and tetralogy of Fallot Q21.3), heart valve replacement/transplant, venous thromboembolism, cardiac surgery, pericarditis, hyperthyroidism, thyrotoxicity, or pregnancy anytime during the look-back period or the index visit.
Baseline characteristics assessed at the index visit included demographics (age, sex, race/ethnicity), primary payer, visit characteristics (care setting, admission point of origin, admission type, discharge status), and hospital characteristics (geographic region, urbanicity, teaching status, and number of beds). CHA2DS2-VASc Score for Atrial Fibrillation Stroke Risk was calculated based on age, sex, congestive heart failure, hypertension, stroke/transient ischemic attack/thromboembolism, diabetes, and vascular disease history (23). These conditions were assessed during the look-back period and index visit. HAS-BLED Score was calculated based on hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, elderly, drugs and alcohol. These conditions were assessed during the look-back period and index visit. Similar to other real-world studies (24, 25), the calculation did not include the labile international normalized ratio because of the unavailability of this information in the database. Comorbidities and the Deyo-modified Charlson Comorbidity Index (CCI) (26, 27) were assessed using patient-level data from the index visit and the look-back period (Supplementary Table S2).
Furthermore, the timing of OAC initiation was evaluated. It was categorized based on whether the OAC was initiated at index visit (i.e., filling a prescription for an OAC during their index visit or within a 7-day grace period post-index discharge) or during follow-up (i.e., filling a prescription for an OAC after this 7-day period but within one year of index discharge). The study further assessed the initiation of OAC within specific time frames post-index discharge, specifically within 30, 60, and 90 days for patients who did not initiate OAC at index visit as well as the type of OAC used. All-cause and stroke/SE-specific hospitalizations (i.e., those where the primary diagnosis was stroke or SE) during the follow-up period were assessed. Additionally, the primary diagnosis for all-cause hospitalizations during the follow-up period was evaluated.
Descriptive statistics described demographics and OAC utilization. The association between OAC initiation timing and the one-year risk of hospitalizations was analyzed using descriptive and multivariable adjusted analyses. These analyses compared the hospitalization risks between two cohorts: (1) OAC initiated at index visit, and (2) OAC initiated during follow-up. Multivariable logistic regression adjusted for potential confounders, including patient demographics (age, sex, race, ethnicity), the primary payer, care setting of index visit, hospital characteristics (urban/rural, geographic region, hospital size, teaching status), CCI score, obesity, diabetes, vascular disease history, and the CHA2DS2-VASc score. Sensitivity analyses were conducted by including additional variables, specifically renal disease, dementia, any malignancy, admission type, and discharge status. An additional sensitivity analysis was conducted on patients using DOACs, excluding those treated with warfarin, and by stratifying the results by prior stroke history. Another sensitivity analysis was conducted on patients with at least one refill during follow-up. Collinearity was assessed using variance inflation factor (VIF), where a VIF greater than 5 was considered as collinearity. Continuous variables were presented as means and standard deviations, and compared using t-tests or Wilcoxon Rank Sum test for non-normal distributions. Categorical variables were expressed as counts and percentages, and compared using Chi-square tests. A p-value < 0.05 was considered significant for determining differences between cohorts. Analyses were performed using R 3.6.3 (28).
Between January 1, 2017, and March 31, 2021, we identified 93,251,105 patients aged ≥18. Of these, 23,148 adults with newly diagnosed NVAF were included (Supplementary Table S3). 11,059 (47.8%) received OAC within one year of diagnosis (OAC users), while 12,089 (52.2%) did not (Non-OAC users). The lowest OAC initiation rate (44.5%) was in 2017, and the highest (52.3%) in 2021.
The mean age was 70 ± 14 years (Table 1, median = 70; 62.7% ≥ 65 years), with 46.9% females, and 80.0% white. The major healthcare coverage was Medicare (63.4%), followed by commercial (21.0%), Medicaid (13.2%), and other, uninsured, or unknown (2.4%). The median CCI was 3. Common comorbidities included coronary artery disease (44.2%) and congestive heart failure (39.7%). The median CHA2DS2-VASc score was 4, with 77.8% of males and 85.4% of females having CHA2DS2-VASc scores of ≥2 and ≥3, respectively. The median HAS-BLED score was 3, with 60.4% patients having HAS-BLED score ≥3, and 74.1% of them diagnosed at an inpatient setting. Most patients were admitted from non-healthcare facilities (77.4%), came in for an emergency visit (63.8%), and were discharged to home (64.1%). The characteristics of hospitals, where patients received their NVAF diagnosis, varied (Table 2). Most patients were treated in urban (86.7%), non-teaching hospitals (54.7%), with bed size of 1–299 (38.8%).
When stratified by OAC users and non-OAC users, age, sex, and race distributions were similar between the two groups. Compared to non-OAC users, a lower proportion of OAC users were Hispanic or Latino and on Medicaid, while a higher percentage had commercial payers (p-value < 0.0001). In terms of comorbidities, OAC users generally had a higher prevalence of cardiovascular disease and risk factors than non-OAC users, such as coronary artery disease (46.2% vs. 42.4%), congestive heart failure (45.0% vs. 34.9%), diabetes (40.8% vs. 35.5%), obesity (40.2% vs. 31.4%), vascular disease history (36.2% vs. 34.2%), cerebrovascular disease (24.9% vs. 22.3%), myocardial infarction (21.0% vs. 19.5%), and stroke/SE (13.0% vs. 9.2%). In contrast, non-OAC users had a higher prevalence of malignancy (14.3% vs. 11.5%) and dementia (14.7% vs. 10.7%) than OAC users. The CHA2DS2-VASc Score was higher among OAC users, while the HAS-BLED score was 3 in both groups. A larger proportion of OAC users vs. non-OAC users (were diagnosed with AF in the inpatient setting (79.4% vs. 69.4, respectively, p-value < 0.0001), with higher emergency admissions of (68.2% vs. 59.8%, respectively), and higher elective admissions (18.4% vs. 27.0%, respectively, p-value < 0.0001). Compared to non-OAC users, more OAC users were discharged to home (p-value < 0.0001). Non-OAC users were more frequently discharged to hospice or were documented as expired than OAC users (p-value < 0.0001).
Among OAC users, 74.9% initiated OAC during the index visit, with the remaining 25.1% initiating during the one-year follow-up (Table 3). When stratified by diagnosis visit care setting, 84.3% of patients in an inpatient setting initiated OAC during the index visit, compared to 39.0% of patients in an outpatient setting. Among patients initiating OAC during follow-up, the median time to OAC initiation following diagnosis was 94 days, with no significant differences between those diagnosed in inpatient and those in outpatient settings. Apixaban was the most commonly used OAC (62.2%), followed by rivaroxaban (23.2%), warfarin (15.8%), and dabigatran (3.1%). Throughout this follow-up period, a total of 3,695 (33.4%) OAC users experienced all-cause hospitalization, of which 177 were due to stroke/SE (Table 4). Among those initiating OAC at the index visit, the 12-month risk was 29.2% for all-cause hospitalization and 1.3% for hospitalization due to stroke/SE. In contrast, among those initiating OAC during follow-up, 1,274 (45.9%) and 73 (2.6%) experienced all-cause and stroke/SE hospitalization, respectively. The adjusted odds ratio for all-cause hospitalization was 0.35 (95% CI: 0.32–0.39, p-value < 0.0001) and, for hospitalization due to stroke/SE, it was 0.34 (95% CI: 0.24–0.47, p-value < 0.0001), comparing those initiating OAC at the index visit to those during the follow-up period. When stratified by diagnostic visit care setting, both all-cause hospitalization and hospitalization due to stroke/SE remained lower for those initiating at the index visit compared to during the follow-up period (Inpatient: adjusted OR (95% CI): all-cause hospitalization 0.31 (0.27–0.35); hospitalization due to stroke/SE 0.36 (0.25–0.52); Outpatient: adjusted OR (95% CI): all-cause hospitalization 0.51 (0.42–0.63); hospitalization due to stroke/SE 0.18 (0.05–0.61)). The observed trend of a lower hospitalization rate in those initiating OAC at the index visit compared to during follow-up remained after excluding warfarin users (Supplementary Table S4), stratifying by stroke history (Supplementary Table S5), including additional variables in the model (Supplementary Table S6), and among patients with at least one refill (Supplementary Table S7). Among OAC users, the primary diagnoses leading to all-cause hospitalizations during the follow-up period included atrial fibrillation (20.0%), sepsis (12.3%), hypertensive heart and chronic kidney disease (9.4%), hypertensive heart disease (7.3%), and acute kidney failure (4.5%), as detailed in Table 5.
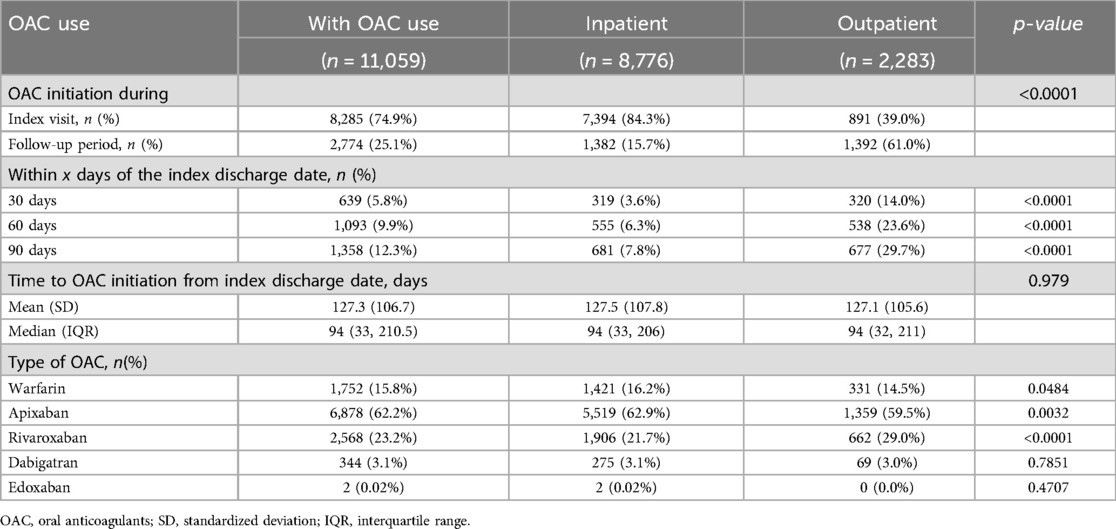
Table 3. OAC initiation, timing and type among OAC users, overall and by diagnosis visit care setting.
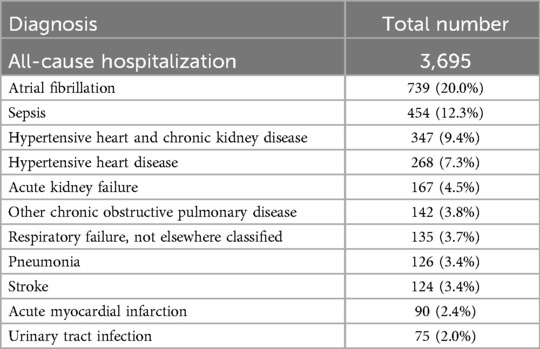
Table 5. Top primary diagnosis of all-cause hospitalization during follow-up period among OAC users.
Using real-world data, this study found that, among patients newly diagnosed with NVAF, 47.8% initiated OAC initiation within one year. It appears that patients with malignancy and dementia were associated with lower odds of initiating OAC within one year post diagnosis. Additionally, for patients who initiated OAC within one year post AF diagnosis, an early initiation was associated with lower risk of all-cause and stroke/SE-specific hospitalization.
The recommendation for using OAC in AF patients has been well-established for years. Both the 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society (4) and the 2020 European Society of Cardiology guidelines (29) have similar recommendations for managing AF, emphasizing the use of the CHA2DS2-VASc score for stroke risk stratification. For patients with higher scores (≥2 for men, ≥3 for women), there is a consensus recommendation to initiate oral anticoagulant (OAC) therapy to mitigate the stroke risk, while for those at intermediate risk (1 for men, 2 for women), OAC prescription remains a consideration. For low-risk patients (0 for men, 1 for women), it is reasonable to omit anticoagulant therapy, given their relatively low risk of stroke. Additionally, both guidelines highlight the importance of balancing potential stroke benefits against the elevated bleeding risk associated with OAC use. Despite these guidelines, OAC use in the AF population remains suboptimal. One study demonstrated a modest increasing trend in OAC initiation from 2010 through 2020 in the Medicare Advantage population, with a peak of 32.9% in 2020 for newly diagnosed AF patients with elevated stroke risk (CHA2DS2-VASc Score ≥2 for men, CHA2DS2-VASc Score ≥3 for women) (11). Another study within the fee-for-service Medicare population between 2013 and 2017 showed a 48.7% OAC utilization rate in patients with a CHA2DS2-VASc Score of ≥2 (12). Furthermore, a study from 2011–2020 in adults aged over 40 with AF across 88 US health systems reported a slight increase in OAC usage over the years, from 56.3%–64.7%. (13) Our study found only 47.8% OAC-naïve NVAF patients initiated OAC within one year following the AF diagnosis, with the rate of OAC initiation among patients with elevated stroke risk (CHA2DS2-VASc Score ≥2 for men, CHA2DS2-VASc Score ≥3 for women) at 50.0%. These findings suggest variation in OAC usage rates across different populations. In addition, despite the general upward trend observed in recent years, these findings highlight the need for enhanced strategies to improve adherence to guideline-recommended OAC use in AF patient, aiming for optimal stroke risk management. Notably, significant disparities in OAC prescription rates have been observed among high-risk AF patients managed by primary care clinicians within the same regional health system (30). Implementing targeted interventions, such as an email-based educational initiative directed at primary care clinicians managing high-risk AF patients, has been shown to effectively increase OAC prescription rates and improve overall stroke prevention in this population (30).
In addition, some patients with low CHA2DS2-VASc scores were also found to be taking OAC (36.1% for men with a CHA2DS2-VASc score of 0, and 31.9% for women with a CHA2DS2-VASc score of 1). It is possible that CHA2DS2-VASc scores are underreported in this study. Our look-back period was 365 days, which may have led to missing vascular events that occurred earlier. Additionally, CHA2DS2-VASc scores were assessed at baseline and could have increased during the follow-up period, during which OAC might have been initiated. Despite these factors, the current finding aligns with previous studies that also reported higher-than-expected OAC use in populations with low CHA2DS2-VASc scores. A study in Australia reported that 25.1% of AF patients with low stroke risk (CHA2DS2-VASc score of 0 for males and 1 for females) had a record of OAC prescription within 60 days of AF diagnosis (31). Another study in the US using the PINNACLE registry found OAC use in 31.1% of AF patients with a CHA2DS2-VASc score of 0 and 34.6% with a CHA2DS2-VASc score of 1 (32). Further research is necessary to better understand benefits and potential risks of OAC use in patients with low CHA2DS2-VASc scores.
Studies have investigated demographic and clinical characteristics influencing the OAC use, reporting that factors such as older age, female, Black race, and certain comorbidities including dementia, frailty, anemia, bleeding were associated with decreased odds of OAC initiation (11, 12). In contrast, histories of cardiovascular diseases, including stroke, SE, and obesity, were associated with increased likelihood of OAC prescription. Interestingly, there was no substantial difference in CHA2DS2-VASc score and HAS-BLED scores between OAC users and non-users. The current study aligns with these observations. Despite similar demographic characteristics between OAC users and non-users, a distinct comorbidity profile was observed: OAC users showed a higher prevalence of cardiovascular disease and risk factors, whereas non-OAC users had a higher prevalence of malignancy and dementia. The CHA2DS2-VASc score was slightly higher among OAC users, with no notable difference in HAS-BLED scores between the groups. These insights underline the complexity of decision-making in anticoagulant therapy and highlight the necessity for a nuanced approach that considers individual patient profiles beyond risk scores alone. This could be pivotal in improving OAC utilization where it is most needed, potentially optimizing therapeutic outcomes in atrial fibrillation management.
The current study emphasized that initiating OAC early might be associated with a decreased risk of all-cause hospitalization and hospitalization due to stroke/SE. Although few studies have explored the association between the timing of OAC initiation and hospitalization risk, this observation is in line with findings from a 3-year follow-up study, which demonstrated that OAC use was associated with a 7% lower risk of all-cause hospitalization compared to non-use among Medicare beneficiaries with AF (33). Although studies specifically examining NVAF patients without prior stroke are limited, there is substantial literature assessing OAC timing for those with NVAF and concurrent stroke (14–17). These studies largely indicated similar safety and effectiveness regardless of whether OAC therapy initiates earlier or later. A meta-analysis comprising six cohort studies and two randomized controlled trials reported no significant difference in the safety and efficacy of OACs initiated within one to two weeks following an acute ischemic stroke (16). Consistent with these findings, the Early vs. Late Initiation of Direct Oral Anticoagulants in Post-ischemic Stroke Patients with Atrial Fibrillation (ELAN) trial showed no significant difference in the combined outcomes, including recurrent ischemic stroke, systemic embolism, major bleeding, symptomatic intracranial hemorrhage, or vascular death, within 30 days of starting early vs. later direct oral anticoagulant (DOAC) administration in patients with NVAF and acute ischemic stroke (OR 0.70, 95% CI 0.44–1.14) (14). Interestingly, a lower risk was observed at the 90-day interval (OR 0.65, 95% CI 0.42–0.99) (14). Other studies, including the TIMING study, a registry-based randomized controlled noninferiority trial, have similarly found no substantial association between the timing of OAC initiation and stroke outcomes following an acute ischemic stroke (15, 17). In the current study, stratified by stroke history, NVAF patients would benefit from initiating OAC earlier regarding stroke/SE with or without stroke history. These collective insights suggest that while the early initiation of OACs might not necessarily amplify adverse outcomes, its advantages, especially in patients without prior stroke, are yet to be conclusively determined. This highlights an evident gap in knowledge and the pressing need for further investigations into the timing of OAC initiation in NVAF patients without a stroke history.
It is noteworthy that the majority of hospitalizations during the follow-up period were not due to stroke or SE. The most prevalent primary diagnosis remains to be atrial fibrillation, followed by sepsis, heart and kidney diseases, and pulmonary diseases. This pattern suggested that patients with NVAF may have a heightened risk of comorbidities and other underlying factors contributing to their overall likelihood of hospitalization. These observations highlight the complexity of the NVAF population and emphasize the urgent need for comprehensive management strategies to effectively mitigate their stroke risk.
This study is not without limitations. Due to its retrospective observational design, it can identify associations but cannot establish causality. There may be residual confounding even after adjustment, such as the subjective nature of anticoagulant selection by physicians, who decide both when and what specific type of OAC to use. Additionally, there is a possibility that patients who started OAC earlier were more health-conscious, which could skew results. Additionally, the reliance on administrative data introduces potential constraints, such as the possibility of coding errors and data incompleteness. For example, HAS-BLED score has labile INR, which is not available in the data, and while NVAF has different forms, their characteristics (e.g., diagnostic modalities and whether it is clinical or subclinical, or with or without symptoms) are not available in this data. Our study utilizes hospital administrative data and closed claims data from a real-world database, which serve as proxies for OAC utilization but may not accurately reflect actual usage or the exact date when the patient started taking the OAC. However, sensitivity analysis among patients with at least one refill of OAC showed similar results, suggesting that the findings are robust among patients likely to be actually taking the OAC. Moreover, this study does not assess OAC discontinuation patterns, such as cessation after successful AF ablation, which may influence outcomes. Despite these limitations, this study, using real-world data, described the recent trends in OAC utilization among newly diagnosed NVAF patients. Moreover, it is the first study to investigate the association between the timing of OAC initiation and the rates of all-cause and stroke/SE-specific hospitalizations, using real-world data. Future studies are needed to further investigate drug adherence, different types of NVAF and their characteristics, and OAC dosing to better assess their impact on the association between OAC and hospitalization in the NVAF population. Future studies should further explore drug adherence, NVAF subtypes and characteristics, and OAC dosing to better understand their impact on hospitalization risks. Additionally, research should evaluate whether healthcare interventions, such as closer follow-up and increased awareness, modify this association. Lastly, assessing follow-up period characteristics, such as fall risk and hemorrhagic events, could provide further insights into patient outcomes across treatment groups.
In conclusion, this study demonstrated the underutilization of OAC among patients with NVAF, despite existing guideline recommendations. Furthermore, our findings suggest that early initiation of OAC therapy could be associated with decreased rates of hospitalization. The findings underscored a compelling need for personalized, comprehensive management strategies for NVAF patients, taking into account not only stroke risk but also their comorbidity profile. The observed correlation between the timing of OAC initiation and reduced hospitalization rates warrants further investigation, especially in patients without stroke history.
The data analyzed in this study was obtained from the Premier Healthcare Database and its linked claims database. The following licenses/restrictions apply: they are proprietary databases that can be accessed by purchasing an applicable license. Requests to access these datasets should be directed to Dr. Chendi Cui (chendi_cui@premierinc.com).
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
CC: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. LC: Formal analysis, Writing – review & editing. NS: Conceptualization, Writing – review & editing. NR: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Bristol Myers Squibb (BMS). The design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, and approval of the manuscript were collaboratively undertaken by the research team, which includes authors from both Premier Applied Sciences and BMS. The decision to submit the manuscript for publication was made collectively by all authors. The involvement of each team member, irrespective of their affiliation, adhered to standard academic practices, ensuring an unbiased and objective approach to the research.
Cate Polacek, MLIS, senior medical writer employed by Premier Applied Sciences, Premier Inc., provided manuscript editing and publication support.
CC, LC, NR were employed by Premier Applied Sciences, Premier Inc. NS is an employee of Bristol Myers Squibb.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1522154/full#supplementary-material
1. Boursier-Bossy V, Zuber M, Emmerich J. Ischemic stroke and non-valvular atrial fibrillation: when to introduce anticoagulant therapy? J Med Vasc. (2020) 45(2):72–80. doi: 10.1016/j.jdmv.2020.01.153
2. Li YG, Pastori D, Lip GYH. Fitting the right non-vitamin K antagonist oral anticoagulant to the right patient with non-valvular atrial fibrillation: an evidence-based choice. Ann Med. (2018) 50(4):288–302. doi: 10.1080/07853890.2018.1460489
3. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. (2018) 39(16):1330–93. doi: 10.1093/eurheartj/ehy136
4. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2024) 149(1):e1–e156. doi: 10.1161/CIR.0000000000001193
5. Benedetti G, Neccia M, Agati L. Direct oral anticoagulants use in elderly patients with non valvular atrial fibrillation: state of evidence. Minerva Cardioangiol. (2018) 66(3):301–13. doi: 10.23736/S0026-4725.17.04553-4
6. Howard PA, Duncan PW. Primary stroke prevention in nonvalvular atrial fibrillation: implementing the clinical trial findings. Ann Pharmacother. (1997) 31(10):1187–96. doi: 10.1177/106002809703101012
7. Deplanque D, Leys D, Parnetti L, Schmidt R, Ferro J, De Reuck J, et al. Stroke prevention and atrial fibrillation: reasons leading to an inappropriate management. Main results of the SAFE II study. Br J Clin Pharmacol. (2004) 57(6):798–806. doi: 10.1111/j.1365-2125.2004.02086.x
8. Deplanque D, Leys D, Parnetti L, Schmidt R, Ferro J, de Reuck J, et al. Secondary prevention of stroke in patients with atrial fibrillation: factors influencing the prescription of oral anticoagulation at discharge. Cerebrovasc Dis. (2006) 21(5-6):372–9. doi: 10.1159/000091546
9. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2014) 45(7):2160–236. doi: 10.1161/STR.0000000000000024
10. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37(38):2893–962. doi: 10.1093/eurheartj/ehw210
11. Ko D, Lin KJ, Bessette LG, Lee SB, Walkey AJ, Cheng S, et al. Trends in use of oral anticoagulants in older adults with newly diagnosed atrial fibrillation, 2010–2020. JAMA Netw Open. (2022) 5(11):e2242964. doi: 10.1001/jamanetworkopen.2022.42964
12. Munir MB, Hlavacek P, Keshishian A, Guo JD, Mallampati R, Ferri M, et al. Oral anticoagulant underutilization among elderly patients with atrial fibrillation: insights from the United States medicare database. J Interv Card Electrophysiol. (2023) 66(3):771–82. doi: 10.1007/s10840-022-01274-1
13. Navar AM, Kolkailah AA, Overton R, Shah NP, Rousseau JF, Flaker GC, et al. Trends in oral anticoagulant use among 436 864 patients with atrial fibrillation in community practice, 2011 to 2020. J Am Heart Assoc. (2022) 11(22):e026723. doi: 10.1161/JAHA.122.026723
14. Fischer U, Koga M, Strbian D, Branca M, Abend S, Trelle S, et al. Early versus later anticoagulation for stroke with atrial fibrillation. N Engl J Med. (2023) 388(26):2411–21. doi: 10.1056/NEJMoa2303048
15. Oldgren J, Åsberg S, Hijazi Z, Wester P, Bertilsson M, Norrving B. Early versus delayed non-vitamin K antagonist oral anticoagulant therapy after acute ischemic stroke in atrial fibrillation (TIMING): a registry-based randomized controlled noninferiority study. Circulation. (2022) 146(14):1056–66. doi: 10.1161/CIRCULATIONAHA.122.060666
16. Palaiodimou L, Stefanou MI, Katsanos AH, Paciaroni M, Sacco S, De Marchis GM, et al. Early anticoagulation in patients with acute ischemic stroke due to atrial fibrillation: a systematic review and meta-analysis. J Clin Med. (2022) 11(17):4981. doi: 10.3390/jcm11174981
17. Wilson D, Ambler G, Banerjee G, Shakeshaft C, Cohen H, Yousry TA, et al. Early versus late anticoagulation for ischaemic stroke associated with atrial fibrillation: multicentre cohort study. J Neurol Neurosurg Psychiatry. (2019) 90(3):320–5. doi: 10.1136/jnnp-2018-318890
18. Palaiodimou L, Stefanou MI, Katsanos AH, De Marchis GM, Aguiar De Sousa D, Dawson J, et al. Timing of oral anticoagulants initiation for atrial fibrillation after acute ischemic stroke: a systematic review and meta-analysis. Eur Stroke J. (2024) 9(4):885–95. doi: 10.1177/23969873241251931
19. Jeong IG, Khandwala YS, Kim JH, Han DH, Li S, Wang Y, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. Jama. (2017) 318(16):1561–8. doi: 10.1001/jama.2017.14586
20. Kadri SS, Gundrum J, Warner S, Cao Z, Babiker A, Klompas M, et al. Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. Jama. (2020) 324(24):2553–4. doi: 10.1001/jama.2020.20323
21. Moon RC, Mackey RH, Cao Z, Emont S, Schott LL, Gayle JA, et al. Is coronavirus disease 2019 (COVID-19) less deadly now? Trends in in-hospital mortality among hospitalized COVID-19 patients in the United States. Clin Infect Dis. (2022) 74(12):2238–42. doi: 10.1093/cid/ciab830
22. Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. (2008) 358(8):771–83. doi: 10.1056/NEJMoa0707571
23. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137(2):263–72. doi: 10.1378/chest.09-1584
24. Alonso A, Norby FL, MacLehose RF, Zakai NA, Walker RF, Adam TJ, et al. Claims-based score for the prediction of bleeding in a contemporary cohort of patients receiving oral anticoagulation for venous thromboembolism. J Am Heart Assoc. (2021) 10(18):e021227. doi: 10.1161/JAHA.121.021227
25. Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. (2018) 49(12):2933–44. doi: 10.1161/STROKEAHA.118.020232
26. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40(5):373–83. doi: 10.1016/0021-9681(87)90171-8
27. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. (1992) 45(6):613–9. doi: 10.1016/0895-4356(92)90133-8
28. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2021). Available online at: https://www.R-project.org/
29. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
30. Dutta R, Ryan JG, Hurley S, Wylie J. A targeted educational intervention increases oral anticoagulation rates in high-risk atrial fibrillation patients. Heart Rhythm O2. (2024) 5(5):294–300. doi: 10.1016/j.hroo.2024.04.005
31. Kefale AT, Bezabhe WM, Peterson GM. Oral anticoagulant use in patients with atrial fibrillation at low risk of stroke and associated bleeding complications. J Clin Med. (2023) 12(19):6182. doi: 10.3390/jcm12196182
32. Katz DF, Maddox TM, Turakhia M, Gehi A, O'Brien EC, Lubitz SA, et al. Contemporary trends in oral anticoagulant prescription in atrial fibrillation patients at low to moderate risk of stroke after guideline-recommended change in use of the CHADS(2) to the CHA(2)DS(2)-VASc score for thromboembolic risk assessment: analysis from the national cardiovascular data registry’s outpatient practice innovation and clinical excellence atrial fibrillation registry. Circ Cardiovasc Qual Outcomes. (2017) 10(5):e003476. doi: 10.1161/CIRCOUTCOMES.116.003476
Keywords: nonvalvular atrial fibrillation, oral anticoagulants, hospitalization, timing of initiation, real-world evidence
Citation: Cui C, Curry L, Singh N and Rosenthal NA (2025) Oral anticoagulant timing and hospitalization in newly diagnosed nonvalvular atrial fibrillation patients. Front. Cardiovasc. Med. 12:1522154. doi: 10.3389/fcvm.2025.1522154
Received: 4 November 2024; Accepted: 10 March 2025;
Published: 4 April 2025.
Edited by:
Uğur Canpolat, Hacettepe University, TürkiyeReviewed by:
Lana Boodhoo, The University of the West Indies St. Augustine, Trinidad and TobagoCopyright: © 2025 Cui, Curry, Singh and Rosenthal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning An Rosenthal, TmluZ19Sb3NlbnRoYWxAcHJlbWllcmluYy5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.