
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 25 February 2025
Sec. Heart Failure and Transplantation
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1512742
This article is part of the Research TopicEmerging Techniques in Graft Preservation: Machine Perfusion and Therapeutic Interventions - Volume IIView all articles
Background: Lung transplantation (LTx) is the definitive treatment for end-stage pulmonary diseases, with venoarterial extracorporeal membrane oxygenation (VA-ECMO) used as a common perioperative support. However, it remains unclear if central (cVA-ECMO) or peripheral (pVA-ECMO) cannulation routes yield better outcomes in postoperative prognosis. This study compares the impact of these two cannulation strategies on primary graft dysfunction (PGD) incidence in LTx patients.
Methods: A retrospective analysis was performed on 153 LTx patients supported with VA-ECMO at the Wuxi Lung Transplant Center (January 2019–March 2023). Patients were divided into central (n = 31) and peripheral (n = 91) groups. Data included recipient/donor demographics, preoperative status, and follow-up outcomes. The primary outcome was PGD within 72 h after reperfusion, whereas secondary outcomes included in-hospital mortality, 1-year survival, renal support needs, ventilation duration, intensive care unit (ICU) stay, and biochemical markers.
Results: PGD incidence was significantly higher in the peripheral group, with longer ECMO duration, ventilation, and ICU stay. Central VA-ECMO showed advantages in in-hospital mortality and 1-year survival rates.
Conclusion: Central VA-ECMO cannulation may reduce postoperative complications and improve survival for LTx recipients. Prospective studies are needed to confirm these findings and refine perioperative ECMO management.
Lung transplantation (LTx) represents the definitive treatment for end-stage pulmonary diseases, including chronic obstructive pulmonary disease (COPD) and pulmonary hypertension from various etiologies (1–3). For patients unable to tolerate one-lung ventilation or those who experience hemodynamic disturbances during LTx, cardiopulmonary bypass is essential for perioperative support (1, 4). However, since 2001, advancements have increased the use of extracorporeal membrane oxygenation (ECMO), while most LTx centers have transitioned from CPB to ECMO for mechanical life support since 2008 (5–11). Among ECMO types, venoarterial ECMO (VA-ECMO) showed effective respiratory and circulatory support during LTx.
VA-ECMO supports patients with moderate-to-severe pulmonary hypertension perioperatively and is classified into peripheral and central types based on cannulation location. Peripheral VA-ECMO is less invasive, but it may limit oxygen delivery and hemodynamic stability due to reliance on the patient's lung function. In contrast, central VA-ECMO offers direct, fully oxygenated blood flow, providing better oxygenation and stable circulation, which makes it more suitable for high-risk lung transplant cases despite being more invasive. However, the current lack of high-quality randomized controlled trials and clinical data highlights the potential for selection bias and uncertainty about differences in perioperative management and prognosis between these approaches.
Primary graft dysfunction (PGD) manifests as a spectrum of mild-to-severe lung injury occurring within the first 72 h after LTx, representing a major cause of early morbidity and mortality. PGD is characterized by progressive hypoxemia and alveolar infiltrates on a chest radiography (12). Although no multicenter study revealed a significant correlation between PGD incidence and transplantation surgery (13), some studies still suggested that ECMO support may mitigate the risk of severe PGD and enhance patient survival rates (1, 5).
Therefore, we planned a retrospective analysis of existing data from our center to compare the impact of central (right atrium–ascending aorta) vs. peripheral (femoral artery) venous VA-ECMO cannulation on PGD outcomes.
The Ethics Review Committee of Wuxi People's Hospital approved this study (IRB number: KY21064), which included 153 patients with severe lung disease who underwent LTx with VA-ECMO assistance at the Wuxi Lung Transplant Center from January 1, 2019, to March 31, 2023. Cases with combined organ transplantation, non-first-time LTx, bridging ECMO support before surgery, or loss to follow-up were excluded (Figure 1). Informed consent was waived due to the retrospective nature of the study. Organ donation and transplantation followed the regulations of the Jiangsu Organ Transplantation Committee and the Declaration of Helsinki, with informed consent obtained from donors or their authorized representatives. All cadaveric donors at the Wuxi Organ Transplantation Center have been brain-dead patients since 2015.
Data on recipient variables [gender, age, body mass index BMI), diagnosis, and mean pulmonary artery pressure (MPAP)], donor variables (gender, age, BMI, donation type, oxygenation index, and ventilation days), and operative characteristics [operation duration, cold ischemia time (CIT), mean arterial pressure < map > during surgery and 3 days postoperatively, fluid balance (intake volume−output volume), vasoactive drug dosages, and oxygenation index] were collected. PGD is a clinical syndrome of acute lung injury occurring within the first 72 h after LTx, characterized by hypoxemia and alveolar infiltrates on chest x-ray (CXR) (14, 15). PGD is graded based on the oxygenation index [the PaO2/FiO2 (P/F) ratio] and radiographic findings using a scale from 0 (no disease) to 3 (severe PGD). CIT was defined as the duration from the initiation of ex vivo perfusion of the donor lung to the opening of the pulmonary artery in the transplanted lung, with specific distinctions between single and double lung transplants (16).
The primary outcome measured was the occurrence of PGD grade 3 within 72 h. Post-reperfusion PGD grade was assessed according to the ISHLT2016: PGD is classified as grade 3 when PaO2/FiO2 is <200 with definite radiographic infiltrates of pulmonary edema. Additionally, using extracorporeal life support (ECLS) postoperatively was considered as a possible factor (12, 17).
Secondary outcomes comprised early indicators, such as the need for continuous renal replacement therapy (CRRT), postoperative ventilation duration, and length of stay in the intensive care unit (ICU). In-hospital mortality and 1-year postoperative survival rates were also assessed. Additionally, biomarkers including N-terminal pro-b-type natriuretic peptide (NT-proBNP), troponin T, and albumin levels 72 h after surgery were measured.
Indications for establishing VA-ECMO included (1) hypoxemia; (2) high pulmonary arterial pressure (>50% of systemic blood pressure); (3) right ventricular or biventricular failure; (4) other conditions such as acute chronic heart failure, hypothermia, and cardiac arrest with ongoing cardiopulmonary resuscitation (ECPR) (18–20). The choice between peripheral or central cannulation depended on the surgeon's preference.
The study cohort was divided into two groups based on the ECMO cannulation strategy: (1) the central cannulation group (cVA-ECMO), with inflow through the ascending aorta, and (2) the peripheral group (pVA-ECMO), with inflow through the femoral artery.
All patients underwent radial artery catheterization for blood pressure monitoring before anesthesia induction. General anesthesia was induced using titrated midazolam (0.1 mg/kg), sufentanil (0.5–1 μg/kg), propofol (0.5–1 mg/kg), and rocuronium (0.5–1 mg/kg). A double-lumen endotracheal tube, sized according to the patient's height, was used for intubation. Lung protective ventilation strategies were applied to recipients, utilizing a low tidal volume of ≤6 ml/kg predicted body weight (PBW) for mechanical ventilation, adjusted based on donor characteristics. Intraoperatively, we maintained the minimum FiO₂ necessary to achieve an appropriate PaO₂ > 70 mmHg and hemoglobin oxygen saturation (SpO₂) ≥ 92%, while ensuring PaCO₂ remained within the pre-transplantation range. A Swan-Ganz catheter was placed via the right internal jugular vein after the induction to measure PAP, and cardiac function was assessed using transesophageal echocardiography (TEE). Anesthesia was maintained using propofol, sufentanil, and rocuronium, with vasoactive drugs administered as needed to maintain hemodynamic stability.
The ECMO team, including the anesthesiologist and surgeon, determined ECMO use based on the patient's history and anesthesia assessment. cVA-ECMO involved thoracotomy and cannulation of the right atrium and ascending aorta, while pVA-ECMO was established via the femoral artery and vein cannulation using the Seldinger technique. ECMO was initiated to maintain central venous pressure of >5 mmHg without a significant decrease.
Arterial blood gas and activated clotting time (ACT) were monitored every 30 min, with heparin administered to maintain ACT between 180 and 200 s. TEE was used to assess volume status, and surgery proceeded with ventilation using small tidal volumes (5–6 ml/kg) and low airway pressures (15–25 cm H2O).
After surgery, fluid management and ECMO weaning were based on circulation and oxygenation assessments. VA-ECMO was removed when stable hemodynamics was achieved, the oxygenation index exceeded 300, and echocardiographic evaluations showed adequate left ventricular contractility (aortic velocity-time integral >10 and left ventricular ejection fraction (LVEF) > 30%]. Otherwise, VA-ECMO was maintained, with pVA-ECMO continuing in the ICU or converting cVA-ECMO to pVA-ECMO if needed.
Statistical analysis was conducted using SPSS 25.0 and Stata software to assess the relationship between cannulation strategy and PGD. Demographic and perioperative factors were analyzed. Continuous variables that followed a normal distribution were presented as a mean ± standard deviation (SD). Continuous variables not conforming to a normal distribution were expressed as a median and interquartile range (IQR), whereas categorical variables were presented as percentages (%). The t-test or Mann–Whitney U-test was used for continuous variables, and the chi-square or Fisher's exact test for categorical variables.
A multivariable logistic regression model was applied to adjust for inclusion criteria and assess in-hospital mortality. One-year postoperative mortality was analyzed using Cox regression. Subgroup analysis was used to evaluate survival in the central cannulation group. Incidence rates and Kaplan–Meier survival curves were utilized to compare differences between the two cannulation strategies. A p < 0.05 indicated statistical significance.
A total of 495 patients underwent LTx at our institution from January 2019 to March 2023. We included 122 patients who used VA-ECMO during surgery, excluding those with combined organ transplants, non-first LTx, or patients lost to follow-up. Among these, 91 (74.6%) patients underwent pVA-ECMO, while 31 (25.4%) patients underwent cVA-ECMO.
Table 1 shows patients' demographic characteristics and donor data. No significant differences regarding gender, age, donation types, or general condition were observed between the two groups. The average age of the central group was 57 years, which was slightly older than that of the peripheral group (54 years; P = 0.871). Both groups had normal BMI values, with the central group showing a slightly lower BMI (19.69 vs. 20.94 kg/m2, P = 0.216). Clinical diagnosis demonstrated no statistical difference (P = 0.221), with interstitial pulmonary fibrosis (IPF) being the most common condition.
Table 2 summarizes patients’ clinical characteristics. In the pVA-ECMO group, 65 patients (74.6%) underwent double LTx compared to 28 (90.3%) patients in the central group. Surgeries were performed by three surgeon groups with significant differences in central cannulation rates (P = 0.006). Regarding the incision type, 84 patients underwent thoracotomy, with 79 in the peripheral group and 5 in the central group. The remaining 38 cases were performed using the clamshell approach with a significant difference in distribution (P < 0.0001).
CIT was recorded for each transplanted lung in bilateral cases. No significant difference was found for the first transplanted lung (P = 0.236). However, the CIT for the second lung was significantly longer in the peripheral group (564.45 vs. 498.39 min, P = 0.004), possibly due to different surgical incisions. For single lung transplantation, CITs were similar between the groups (P = 0.509).
PAP was monitored continuously during surgery using a Swan-Ganz catheter. Systolic PAP (SPAP) and MPAP were recorded before and after initiating ECMO. Before ECMO initiation, SPAP and MPAP were similar between the groups, showing no significant differences. After ECMO initiation, both SPAP and MPAP significantly decreased in both groups, with a greater reduction in the central group (SPAP: pVA-ECMO 49.06 vs. cVA-ECMO 34.71 mmHg, P < 0.001; MPAP: pVA-ECMO 35.11 vs. cVA-ECMO 25.26 mmHg, P < 0.001). The central group also had a significantly higher perioperative diversion flow rate (pVA-ECMO 2.17 L/min vs. cVA-ECMO 3.50 L/min, P < 0.001).
Upon the time after the operation (T0), PGD incidence in the peripheral group was significantly higher than that in the central group [pVA-ECMO 54 (59.3%) vs. cVA_ECMO 8 (25.8%), P < 0.001]. Among these patients, there were 9 cases of severe PGD in the peripheral group and 0 cases in the central group (P = 0.477).
The need for postsurgical VA-ECMO support was also assessed. In the peripheral group, 8 patients (8.8%) were weaned off ECMO in the operating room, 73 (80.2%) patients retained pVA-ECMO, 8 (8.8%) patients were switched to VV-ECMO, and 2 patients were switched to VAV-ECMO before transfer to an ICU. In contrast, 23 patients (74.2%) were weaned off ECMO in the operating room, 4 (12.9%) patients were switched to pVA-ECMO, and another 4 (12.9%) patients were switched to VV-ECMO before transfer to an ICU in the central group. The difference in ECMO weaning and support needs between the groups was statistically significant (P < 0.0001).
The postoperative ECMO duration was significantly longer in the peripheral group compared to the central group [pVA-ECMO: 2 (1, 4) days vs. cVA-ECMO: 0.65 (1.49) days, P < 0.0001]. Operation duration, fluid balance, and urine output showed no significant differences between the groups. However, the central group required more albumin infusion (pVA-ECMO: 150 g vs. cVA-ECMO: 269.19 g, P < 0.001).
The incidence and severity of PGD in the ICU were assessed at multiple time points after LTx (Table 3). On day 0, grade 3 PGD was seen in 31 patients (34.1%) in the peripheral group and 8 patients (34.8%) in the central group (P = 0.059). On day 1, 23 patients (25.3%) in the peripheral group and 2 patients (6.5%) in the central group had grade 3 PGD (P = 0.0025). On day 2, grade 3 PGD was observed in 24 patients (26.4%) in the peripheral group and 1 patient (3.2%) in the central group (P = 0.006). Over 72 h, the peripheral group had 57 cases of grade 3 PGD compared to 8 cases in the central group (P < 0.0001).
The oxygenation index and MAP were comparable across all time points (days 0–3). However, the infection rate, including catheter-related and pulmonary infections, was significantly higher in the peripheral group [pVA-ECMO: 41 cases (45.1%) vs. cVA-ECMO: 5 cases (16.1%), P < 0.001]. Additionally, lower limb ischemia occurred in 27 patients in the peripheral group compared to 2 patients in the central group [pVA-ECMO: 27 cases (29.7%) vs. cVA-ECMO: 2 cases (6.5%), P < 0.01].
Postoperative ventilation and ICU stay duration were significantly longer in the peripheral group compared to the central group. The peripheral group had an average ventilation time of 4 days (range: 2–9 days) and an ICU stay of 8 days (range: 5–13 days), while the central group had an average ventilation time of 2 days (range: 1–4 days) and an ICU stay of 4 days (range: 2–7 days; ventilation time: P < 0.001; ICU stay: P < 0.0001). CRRT support demonstrated no significant differences between the groups. Regarding biomarkers, troponin T and NT-proBNP levels were higher 72 h after surgery in the peripheral group (troponin T: 3.73 ng/ml vs. 2.66 ng/ml, p = 0.007; NT-proBNP: 733.57 pg/ml vs. 380.67 pg/ml, P = 0.017). Albumin levels were similar between the groups (P = 0.393).
The 30-day survival rate was comparable between the groups (peripheral: 74.7% vs. central: 87.1%, P = 0.152), as was the 1-year survival rate (peripheral: 65.9% vs. central: 77.4%, P = 0.233). The Kaplan–Meier survival curve showed no statistically significant difference in 30-day and 1-year survival rates between the groups (Figure 2).
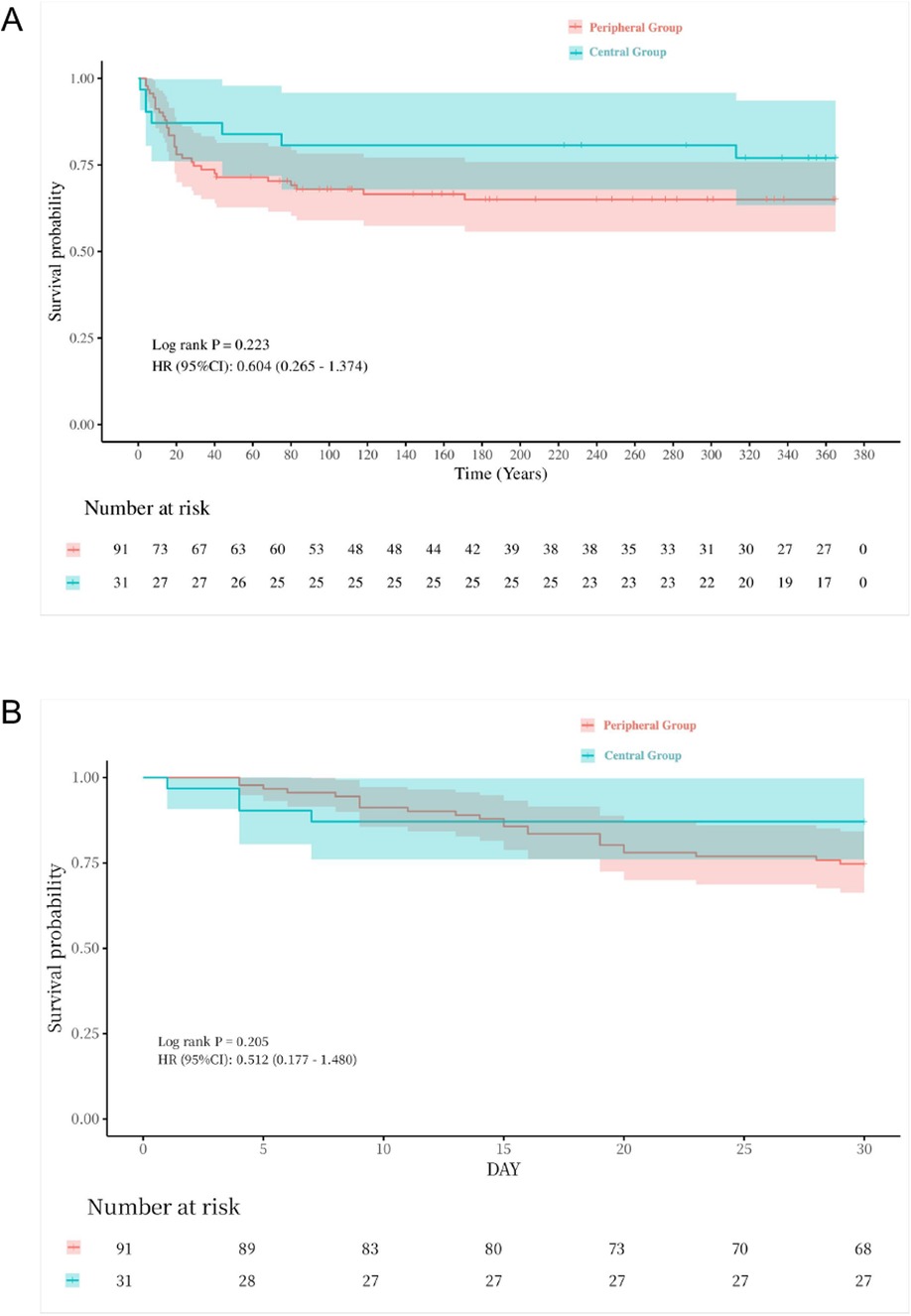
Figure 2. Kaplan–Meier plot for LTx one-year & thirty-day survival for all cases. (A) one-year survival curve, p = 0.233. (B) thirty-day survival curve, p = 0.152.
In the Cox regression model, the hazard of PGD grade 3 was significantly lower in the central group compared to the peripheral group (OR = 0.207, P = 0.001). The risk ratio further decreased to 0.179 (P = 0.001) after adjusting for age, gender, BMI, and bilateral lung transplantation. However, no statistically significant differences in survival rates were observed after adjusting for the surgeon team (Table 4). Regardless of adjustments, the odds ratios for 30-day and 1-year survival rates remained statistically insignificant (Table 4). Additionally, no significant differences in 30-day or 1-year survival rates were found between patients with and without PGD after adjusting for Models 1–3 (Supplementary Table S1).
ECMO is commonly used during cardiothoracic surgeries such as LTx, especially for patients with severe right ventricular dysfunction or hypoxia. PGD contributes significantly to short- and long-term mortality after LTx (21). This study assessed how different VA-ECMO cannulation strategies (central vs. peripheral) influence LTx outcomes, focusing on PGD as the primary measure, with 30-day and 1-year mortality as secondary outcomes. Most patients had severe pulmonary hypertension and were on VA-ECMO, making their condition more critical compared to those on other ECMO types or no extracorporeal support. This caused a more pronounced difference in their prognosis and survival rates.
The choice of VA-ECMO cannulation approach significantly impacted the primary outcome of this study. VA-ECMO stabilizes hemodynamics by bypassing the pulmonary circulation and delivering controlled blood flow to the graft during reperfusion (22). Peripheral VA-ECMO blends blood flow from the patient's lungs with ECMO, with the balance influenced by various factors. In contrast, central cannulation allows direct mixing of fully oxygenated blood with cardiac blood flow, offering stronger respiratory and hemodynamic support without relying on residual cardiac function. Our study found a lower PGD incidence in the central cannulation group compared to the peripheral group. Adjustments for variables revealed differences in PGD severity at different times, indicating that the cannulation method chosen impacts PGD development. Furthermore, the choice of cannulation type during surgery is primarily influenced by the surgeon's preference and the patient's clinical condition, while the postoperative selection is determined by factors such as oxygenation and hemodynamic stability.
A retrospective study identified elevated PAP as an independent risk factor for PGD in LTx patients with IPF, with a 64% increase in risk for every 10 mm Hg rise in PAP. Our study found that cVA-ECMO (with an average transfer flow rate of 3.5 L/min) effectively lowered PAP during pulmonary artery occlusion and reduced lung volume loading during reperfusion compared to pECMO. This reduction mitigated macrophage activation and the inflammatory cascade (23–25), contributing to a lower incidence of severe PGD in the central group, as evidenced by a higher oxygenation index before weaning.
NT-ProBNP is a well-established biomarker of left ventricular overload, commonly used to assess the degree of heart failure (26–28). It presents a significant correlation with pulmonary hypertension and cardiac insufficiency both before and after ECMO utilization according to numerous studies (29, 30). Central cannulation enhances cardiac function more effectively than peripheral ECMO, reducing pulmonary vein return and left atrial congestion. Although the left atrial pressure was not monitored, the central group showed significantly lower postoperative troponin T and NT-ProBNP levels, suggesting that cVA-ECMO may reduce myocardial ischemia-reperfusion injury and protect cardiac function.
The ischemia-reperfusion duration is a critical factor influencing the development of PGD (31). This length of ischemia is influenced by various factors, including incision size, surgeon expertise, and the use of life support technologies. Our institution uses cVA-ECMO, which resulted in shorter CIT for the second lung in bilateral LTx since it avoids the need to close one side before opening the other, thereby conserving time.
High PGD and mortality are also associated with increased risks of inguinal infection, venous thrombosis, and lower limb ischemia (32). Our study identified catheter-related infections despite administering prophylactic antibiotics to prevent bloodstream infections. Immunosuppressive drugs used by transplant patients increase their infection susceptibility. While definitive literature on the impact of central vs. peripheral catheterization on infection rates is scarce, central catheterization offers benefits such as high-flow unidirectional blood flow, which may reduce differential hypoxemia and inguinal infection risks (32). Matthieu et al. reported that the incidence of peripheral VA-ECMO-related infections and bleeding reached 16%, with lower limb ischemia occurring in 12% of cases (32). Reeb et al. suggested that peripheral VA-ECMO should not be considered as the primary ECMO strategy in instances of isolated pulmonary failure (33).The American Association for Thoracic Surgery (AATS) recommends preferring central VA-ECMO during transplantation (34).
Prolonged ECMO is linked to increased complications, longer hospitalization, higher costs, and greater mortality. cVA-ECMO offers an effective solution to address these challenges (35, 36). Our study found that cVA-ECMO was associated with shorter ECMO support, ICU stays, and tracheal tube removal compared to pVA-ECMO, potentially leading to lower mortality rates. However, 30-day and 1-year mortality rates did not differ significantly between the groups (Supplementary Table S1), which may be attributed to several factors. First, the relatively small sample size in the central group may have reduced statistical power, potentially masking differences in survival outcomes. Second, survival is influenced by multiple confounding factors, including the recipient's preoperative condition, donor lung quality, and postoperative infections. Although the central group exhibited a lower risk of primary graft dysfunction (PGD), these patients also had a higher baseline risk, such as a greater proportion undergoing bilateral lung transplantation, which may have offset some of the benefits. Third, PGD is an early postoperative complication, whereas long-term survival is more affected by chronic graft dysfunction and immunosuppressive-related complications, meaning that the advantage of central cannulation in reducing acute injury may not necessarily translate into improved long-term survival. Fourth, variability in surgical team experience may have influenced outcomes, as the distribution of teams across procedural groups was uneven (Table 2, P = 0.006). These findings align with previous studies. Ruszel et al. reported that among lung transplant recipients supported by VA-ECMO, 1-year survival rates were 66% for central cannulation and 50% for peripheral cannulation (37), noting that ECMO is often used not only as planned intraoperative support but also as an unplanned intervention for hemodynamic instability, impaired gas exchange, or right ventricular failure, all of which can impact prognosis (37). Additionally, a large meta-analysis by Biancari et al. indicated higher mortality associated with central ECMO following cardiac surgery and lung transplantation (38). In patients with postcardiotomy cardiogenic shock, Djordjevic et al. found that 30-day survival rates were comparable between central and peripheral ECMO groups (70% vs. 69%), further suggesting no definitive survival advantage based on ECMO configuration alone (39). Factors including excessive bleeding necessitating re-exploration and the administration of a large volume of blood transfusions (38), as well as cardiogenic shock, are more strongly associated with the use of VA-ECMO rather than the specific cannulation strategy employed for ECMO (39). Consequently, further research with larger sample size is needed to explore whether central cannulation provides additional long-term survival benefits compared to peripheral cannulation.
We attribute the lower PGD incidence in the central group primarily to the use of cVA-ECMO during critical periods of LTx. This approach enhances oxygenation and mitigates lung reperfusion issues before ECMO weaning. Central cannulation is particularly beneficial in the operating room for managing pulmonary hypertension and addressing surgical emergencies, such as cardiac arrest, because it can provide immediate and effective support. In contrast, the ICU typically employs a more conservative approach, favoring peripheral ECMO for stability and recovery. Matthieu supports this, showing higher immediate weaning rates in the central group (76%) compared to the peripheral group (35%), with central cannulation maintaining a low weaning rate at 6% (32).
This study has several limitations. First, we relied on the lowest oxygenation index within 72 h after surgery, a static measure that may not reflect dynamic changes in the patient's condition. Shah et al. suggest monitoring PGD fluctuations over time, identifying three phenotypes with different mortality risks (40, 41). Second, the incidence of PGD3 in the central group was higher than the 3.3% rate reported elsewhere, potentially due to the small sample size and mismatched control groups. Thirdly, as a retrospective study, we did not include a control group without ECMO. Although the number of non-ECMO cases was limited, this absence may have influenced the evaluation of ECMO's efficacy. Future prospective studies should consider incorporating a more diverse patient population to comprehensively assess the indications and prognostic outcomes associated with different support strategies. Fourth, although real-time TEE monitoring was performed intraoperatively, the data were not recorded due to limitations in the data collection system. Consequently, this important factor was not fully preserved for further analysis. In future research, we will ensure the comprehensive collection and documentation of echocardiographic data to facilitate a more direct and thorough assessment of cardiac function. Additionally, we recognize the complexity of survival outcomes and the necessity of accounting for multifactorial influences. Our study focuses on 1-year survival and does not address long-term outcomes. Moreover, as a retrospective study, certain data, including echocardiography-derived left ventricular function and end-diastolic dimensions, were not systematically collected. Hence, future randomized controlled trials are needed to validate these findings and address these limitations.
In summary, central cannulation reduces postoperative complications and improves outcomes for LTx recipients. Thus, central cannulation could enhance perioperative care, potentially leading to better recovery and long-term success. However, further prospective studies are needed to confirm these benefits and refine ECMO management protocols, ensuring that LTx patients receive optimal and safe perioperative care.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Review Committee of Wuxi People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because Informed consent was waived for this retrospective study.
XW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. SM: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. TW: Writing – original draft, Writing – review & editing. JC: Writing – original draft, Writing – review & editing. GW: Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing. XZ: Conceptualization, Methodology, Funding acquisition, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by the Science and Technology Achievements Project from Municipal Health Commission of Wuxi (T202202 GL.W), the National Natural Science Foundation of China (82271251 X.Z) and the Wuxi Municipal Commission Fund of Health Planning (M202412, Y.Z).
We would like to express our sincere gratitude to Professor Changhong Miao from Fudan University, for his invaluable guidance and expertise throughout the course of this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1512742/full#supplementary-material
1. Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA, Zacharoulis D. Extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation: a meta-analysis. Gen Thorac Cardiovasc Surg. (2018) 66:38–47. doi: 10.1007/s11748-017-0836-3
2. Cooper JD, Patterson GA, Trulock EP. Results of single and bilateral lung transplantation in 131 consecutive recipients. J Thorac Cardiovasc Surg. (1994) 107:460–71. doi: 10.1016/S0022-5223(94)70091-5
3. Neurohr C, Huppmann P, Zimmermann G, Leuchte H, Baumgartner R, Hatz R, et al. Tacrolimus and mycophenolate mofetil as first line immunosuppression after lung transplantation. Transpl Int. (2009) 22:635–43. doi: 10.1111/j.1432-2277.2009.00843.x
4. Guglin M. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol. (2019) 73:698–716. doi: 10.1016/j.jacc.2018.11.038
5. Ohsumi A, Date H. Perioperative circulatory support for lung transplantation. Gen Thorac Cardiovasc Surg. (2021) 69:631–7. doi: 10.1007/s11748-021-01610-8
6. Ko W, Chen Y, Lee Y. Replacing cardiopulmonary bypass with extracorporeal membrane oxygenation in lung transplantation operations. Artif Organs. (2001) 25:607–12. doi: 10.1046/j.1525-1594.2001.025008607.x
7. Ius F, Kuehn C, Tudorache I, Sommer W, Avsar M, Boethig D, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg. (2012) 144:1510–6. doi: 10.1016/j.jtcvs.2012.07.095
8. Machuca TN, Collaud S, Mercier O, Cheung M, Cunningham V, Kim SJ, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg. (2015) 149:1152–7. doi: 10.1016/j.jtcvs.2014.11.039
9. Winter H, Müller H-H, Meiser B, Neurohr C, Behr J, Guenther S, et al. The Munich lung transplant group: intraoperative extracorporeal circulation in lung transplantation. Thorac Cardiovasc Surg. (2015) 63:706–14. doi: 10.1055/s-0035-1556873
10. Bermudez CA, Shiose A, Esper SA, Shigemura N, D’Cunha J, Bhama JK, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Annals Thorac Surg. (2014) 98:1936–43. doi: 10.1016/j.athoracsur.2014.06.072
11. Biscotti M, Yang J, Sonett J, Bacchetta M. Comparison of extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg. (2014) 148:2410–6. doi: 10.1016/j.jtcvs.2014.07.061
12. Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT working group on primary lung graft dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. (2005) 24:1454–9. doi: 10.1016/j.healun.2004.11.049
13. Cantu E, Diamond JM, Suzuki Y, Lasky J, Schaufler C, Lim B, et al. Quantitative evidence for revising the definition of primary graft dysfunction after lung transplant. Am J Respir Crit Care Med. (2018) 197:235–43. doi: 10.1164/rccm.201706-1140OC
14. Van Slambrouck J, Van Raemdonck D, Vos R, Vanluyten C, Vanstapel A, Prisciandaro E, et al. A focused review on primary graft dysfunction after clinical lung transplantation: a multilevel syndrome. Cells. (2022) 11:745. doi: 10.3390/cells11040745
15. Porteous MK, Lee JC. Primary graft dysfunction after lung transplantation. Clin Chest Med. (2017) 38:641–54. doi: 10.1016/j.ccm.2017.07.005
16. Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report—2017; focus theme: allograft ischemic time. J Heart Lung Transplant. (2017) 36:1047–59. doi: 10.1016/j.healun.2017.07.016
17. Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading—a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. (2017) 36:1097–103. doi: 10.1016/j.healun.2017.07.021
18. Martin AK, Jayaraman AL, Nabzdyk CG, Wilkey BJ, Fritz AV, Kolarczyk L, et al. Extracorporeal membrane oxygenation in lung transplantation: analysis of techniques and outcomes. J Cardiothor Vasc An. (2021) 35:644–61. doi: 10.1053/j.jvca.2020.05.014
19. Wrisinger WC, Thompson SL. Basics of extracorporeal membrane oxygenation. Surg Clin N Am. (2022) 102:23–35. doi: 10.1016/j.suc.2021.09.001
20. Ezad SM, Ryan M, Donker DW, Pappalardo F, Barrett N, Camporota L, et al. Unloading the left ventricle in venoarterial ECMO: in whom, when, and how? Circulation. (2023) 147:1237–50. doi: 10.1161/CIRCULATIONAHA.122.062371
21. Balsara KR, Krupnick AS, Bell JM, Khiabani A, Scavuzzo M, Hachem R, et al. A single-center experience of 1500 lung transplant patients. J Thorac Cardiovasc Surg. (2018) 156:894–905.e3. doi: 10.1016/j.jtcvs.2018.03.112
22. Hashimoto K, Hoetzenecker K, Yeung JC, Jeagal L, Donahoe L, Pierre A, et al. Intraoperative extracorporeal support during lung transplantation in patients bridged with venovenous extracorporeal membrane oxygenation. J Heart Lung Transplant. (2018) 37:1418–24. doi: 10.1016/j.healun.2018.07.003
23. Krishnadasan B, Naidu BV, Byrne K, Fraga C, Verrier ED, Mulligan MS. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. (2003) 125:261–72. doi: 10.1067/mtc.2003.16
24. Eppinger MJ, Jones ML, Deeb GM, Bolling SF, Ward PA. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J Surg Res. (1995) 58:713–8. doi: 10.1006/jsre.1995.1112
25. Fiser SM, Tribble CG, Long SM, Kaza AK, Cope JT, Laubach VE, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. (2001) 121:1069–75. doi: 10.1067/mtc.2001.113603
26. Liu B, Zhang Q, Liang Y, Zhang Y, Yuan X, Ling J, et al. Extracorporeal membrane oxygenation mitigates myocardial injury and improves survival in porcine model of ventricular fibrillation cardiac arrest. Scand J Trauma Resusc Emerg Med. (2019) 27:82. doi: 10.1186/s13049-019-0653-z
27. Huang S-C, Wu E-T, Ko W-J, Lai L-P, Hsu J, Chang C-I, et al. Clinical implication of blood levels of B-type natriuretic peptide in pediatric patients on mechanical circulatory support. Annal Thorac Surg. (2006) 81:2267–72. doi: 10.1016/j.athoracsur.2005.12.061
28. Partridge EA, Hanna BD, Rintoul NE, Herkert L, Flake AW, Adzick NS, et al. Brain-type natriuretic peptide levels correlate with pulmonary hypertension and requirement for extracorporeal membrane oxygenation in congenital diaphragmatic hernia. J Pediatr Surg. (2015) 50:263–6. doi: 10.1016/j.jpedsurg.2014.11.009
29. Avitabile CM, Wang Y, Zhang X, Griffis H, Saavedra S, Adams S, et al. Right ventricular strain, brain natriuretic peptide, and mortality in congenital diaphragmatic hernia. Annals ATS. (2020) 17:1431–9. doi: 10.1513/AnnalsATS.201910-767OC
30. Bo B, Balks J, Gries K, Holdenrieder S, Mueller A, Kipfmueller F. Increased n-terminal pro-B-type natriuretic peptide during extracorporeal life support is associated with poor outcome in neonates with congenital diaphragmatic hernia. J Pediatr. (2022) 241:83–89.e2. doi: 10.1016/j.jpeds.2021.09.034
31. Chen-Yoshikawa TF. Ischemia-reperfusion injury in lung transplantation. Cells. (2021) 10:1333. doi: 10.3390/cells10061333
32. Glorion M, Mercier O, Mitilian D, De Lemos A, Lamrani L, Feuillet S, et al. Central versus peripheral cannulation of extracorporeal membrane oxygenation support during double lung transplant for pulmonary hypertension. Eur J Cardio-thorac. (2018) 54:341–7. doi: 10.1093/ejcts/ezy089
33. Reeb J, Olland A, Renaud S, Lejay A, Santelmo N, Massard G, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis. (2016) 8:S353–63. doi: 10.21037/jtd.2016.04.42
34. Hartwig M, van Berkel V, Bharat A, Cypel M, Date H, Erasmus M, et al. The American association for thoracic surgery (AATS) 2022 expert consensus document: the use of mechanical circulatory support in lung transplantation. J Thorac Cardiovasc Surg. (2023) 165:301–26. doi: 10.1016/j.jtcvs.2022.06.024
35. Weill D. Lung transplantation: indications and contraindications. J Thorac Dis. (2018) 10:4574–87. doi: 10.21037/jtd.2018.06.141
36. Whitson BA, Hayes D. Indications and outcomes in adult lung transplantation. J Thorac Dis. (2014) 6:1018–23. doi: 10.3978/j.issn.2072-1439.2014.07.04
37. Ruszel N, Kiełbowski K, Piotrowska M, Kubisa M, Grodzki T, Wójcik J, et al. Central, peripheral ECMO or CPB? Comparsion between circulatory support methods used during lung transplantation. J Cardiothorac Surg. (2021) 16:341. doi: 10.1186/s13019-021-01719-0
38. Biancari F, Kaserer A, Perrotti A, Ruggieri VG, Cho S-M, Kang JK, et al. Central versus peripheral postcardiotomy veno-arterial extracorporeal membrane oxygenation: systematic review and individual patient data meta-analysis. JCM. (2022) 11:7406. doi: 10.3390/jcm11247406
39. Djordjevic I, Eghbalzadeh K, Sabashnikov A, Deppe A, Kuhn E, Merkle J, et al. Central vs peripheral venoarterial ECMO in postcardiotomy cardiogenic shock. J Card Surg. (2020) 35:1037–42. doi: 10.1111/jocs.14526
40. Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. (2010) 29:1231–9. doi: 10.1016/j.healun.2010.05.013
41. Prekker ME, Nath DS, Walker AR, Johnson AC, Hertz MI, Herrington CS, et al. Validation of the proposed International Society for Heart and Lung Transplantation Grading System for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. (2006) 25:371–8. doi: 10.1016/j.healun.2005.11.436
Keywords: lung transplantation, anesthesia, retrospective study, extracorporeal membrane oxygenation, primary graft dysfunction, survival rate
Citation: Wu X, Miao S, Zhou Y, Wu T, Chen J, Wang G and Zhang X (2025) The comparative impact of central vs. peripheral VA-ECMO cannulation on postoperative graft dysfunction in lung transplantation: a retrospective analysis. Front. Cardiovasc. Med. 12:1512742. doi: 10.3389/fcvm.2025.1512742
Received: 17 October 2024; Accepted: 12 February 2025;
Published: 25 February 2025.
Edited by:
Jamshid Karimov, Cleveland Clinic, United StatesReviewed by:
Mohamed Laimoud, Cairo University, EgyptCopyright: © 2025 Wu, Miao, Zhou, Wu, Chen, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guilong Wang, Z3VpbEBuam11LmVkdS5jbg==; Xin Zhang, eGluemhhbmczQG5qbXUuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.