- 1School of Public Affairs, Zhejiang University, Hangzhou, China
- 2National Institute for Health Innovation, School of Population Health, The University of Auckland, Auckland, New Zealand
- 3Department of Social Medicine and Health Care Management, Fudan University, Shanghai, China
- 4Faculty of Medical and Health Sciences, School of Population Health, The University of Auckland, Auckland, New Zealand
- 5Faculty of Public Administration, School of Law, Hangzhou City University, Hangzhou, China
Background: The exploration of mitochondrial-targeted antioxidants represented a burgeoning field of research with significant implications for cardiometabolic diseases (CMD). The studies reviewed in this scoping analysis collectively highlighted the effect of MitoQ on prevention and management of CMD and underlying mechanisms were discussed, mainly including cardiovascular diseases (CVDs), liver health and others.
Methods: This scoping review aimed to synthesize current research on the health impacts of MitoQ on CMD, focusing primarily on human-based clinical trials. While the primary focus was on human trials, in vivo and in vitro studies were referenced as supplementary material to provide a broader understanding of MitoQ's mechanisms and potential effects.
Results: This scoping review had synthesized the findings that collectively contributed to the understanding of mitochondrial-targeted antioxidants and their role in CMD.
Conclusion: The synthesis of these findings illustrated a broad spectrum of benefits ranging from enhanced insulin secretion to improved lipid profiles and mitochondrial function, yet the path to clinical application required further investigation on appropriate doses and populations.
1 Introduction
Cardiometabolic diseases (CMD) were a group of common but often preventable conditions, including heart attack, stroke, diabetes, insulin resistance and non-alcoholic fatty liver disease (1), representing a significant public health burden globally (2, 3). Cardiovascular diseases (CVDs), liver health and related metabolic disorders were pervasive problems, often linked to mitochondrial dysfunction and oxidative stress (4). MitoQ, a mitochondria-targeted antioxidant, had garnered attention for its potential effects on metabolic health. The role of MitoQ on CMD might have diverse benefits for the prevention and management of CMD (5).
MitoQ was a mitochondria-targeted derivative of Coenzyme Q10 (CoQ10), specifically modified to accumulate within mitochondria more effectively than unmodified CoQ10 (6). The mechanism of action of MitoQ was based on its ability to provide potent antioxidant protection during circulating redox processes while maintaining mitochondrial functional integrity. This property made it a potential agent for the treatment of a wide range of diseases associated with oxidative stress, including cardiovascular diseases, metabolic disorders, neurodegenerative diseases and liver diseases. In detailed, MitoQ reduced mitochondrial oxidative stress, prevented impaired mitochondrial dynamics, and increased mitochondrial turnover by promoting autophagy (mitophagy) and mitochondrial biogenesis, which ultimately contributed to the attenuation of CMD (5), such as fat (7). While MitoQ had been studied in relation to multiple cardiometabolic diseases, the majority of these studies had been conducted at the murine research level rather than in humans.
Recent research emphasized the significance of mitochondrial function in CMD. Previous studies shed light on the role of mitochondria in adipose tissue and the minimal metabolic effects of MitoQ in high-fat-fed mice, respectively (8, 9). These studies indicated that while mitochondrial oxidative stress was intricately linked with metabolic disorders, addressing oxidative stress via mitochondrial targeted antioxidants like MitoQ required deeper exploration. For example, Kim et al. (10) highlighted how obesity exacerbated viral infections in a mouse model, through lipid-induced mitochondrial reactive oxygen species. MitoQ was able to reduce severity of infection and overall mortality, suggesting a broader implication of mitochondrial health in overall metabolic wellness.
CVDs were a significant part of CMD, responsible for an estimated 17.9 million lives annually, which represented 31% of all global deaths (11). These conditions primarily included heart attacks and strokes, often attributed to the accumulation of atherosclerotic plaques in arteries leading to reduced blood flow and oxygen supply (12). Despite advancements in medical therapies, the incidence and impact of CVDs continued to escalate, driven by an aging population and the prevalence of lifestyle-related risk factors such as hypertension, obesity, diabetes, and physical inactivity (13). The pathophysiology of CVD was multifaceted, involving oxidative stress, inflammation, endothelial dysfunction, and altered hemodynamics, necessitating continuous research into novel therapeutic strategies (12).
The liver played a central role in metabolism of lipids and glucose (14); liver disease accounted for two million deaths annually and was responsible for 4% of all deaths (15). Previous studies illustrated that MitoQ could reduce oxidative damage and prevent hepatic fat accumulation (16). MitoQ improved high-fat-induced liver dysfunction by virtue of its antioxidant properties without altering liver fat or mitochondrial bioenergetics (17).
This scoping review would delve into the multifaceted aspects of MitoQ's impact on CMD, especially focusing on metabolic health, CVDs and liver health. By analyzing recent research and data, it aimed to provide a comprehensive overview of the current understanding of mitochondrial-targeted antioxidants in managing CMD, offering insights for future research and potential therapeutic interventions.
2 Materials and methods
The methodology for this scoping review on the health impact of MitoQ on CMD was structured to provide a comprehensive overview of the current literature. It adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for scoping reviews. This methodology aimed to identify, assess, and synthesize research findings from various studies to understand the broader implications of MitoQ on CMD.
2.1 Identifying the research question
The primary research question guiding this scoping review was: “What was the impact of MitoQ on CMD as evidenced in current scientific literature?” This question sought to explore the effects, both positive and negative, of MitoQ on aspects of CMD, including metabolic health, CVDs and liver health.
2.2 Identifying relevant studies
To ensure a comprehensive and relevant synthesis of data, this scoping review adhered to specific criteria for the inclusion and exclusion of studies. These criteria were designed to focus on the most informative and reliable sources of information regarding the cardiometabolic impacts of MitoQ.
2.3 Study selection
The inclusion criteria for the studies were:
(1) Published in peer-reviewed journals. Studies specifically discussed MitoQ and its impact on CMD, including metabolic health, CVDs and liver health.
(2) Studies that provided insight into MitoQ and CMD, such as metabolic health (mitochondrial function, oxidative stress, obesity, and diabetes), CVDs (changes in blood pressure, heart rate variability, endothelial function, and other relevant cardiovascular biomarkers) and liver health in the context of MitoQ use.
(3) Studies published in English.
(4) Studies published within the last 15 years.
The exclusion criteria were:
(1) Non-peer-reviewed literature and grey literature.
(2) Studies not relevant to the direct impact of MitoQ on CMD.
(3) Studies not available in full-text.
2.3.1 Charting the data
Data extraction was performed systematically. Key information extracted from each study included the authors, year of publication, study design, sample size, key findings, and conclusions related to the impact of MitoQ on CMD. This process was crucial for synthesizing and comparing results across different studies.
2.3.2 Collating, summarizing, and reporting the results
The collected data were summarized to provide a narrative synthesis of the findings. The synthesis focused on how MitoQ impacted CMD, drawing on specific aspects such as its role in metabolic health, CVDs and liver health.
2.3.3 Consideration of study quality and bias
The quality of the included studies was assessed based on their methodology, sample size, and the robustness of their findings. Potential biases in the studies were identified and noted, particularly biases related to sample selection, experimental design, and reporting.
2.3.4 Consultation exercise
As part of the scoping review, a consultation exercise with experts in the field of mitochondrial research and CMD was conducted. This exercise provided additional insights and helped validate the findings from the literature search.
2.3.5 Ethical considerations
Given that this scoping review only involved the analysis of published literature and did not involve human participants directly, specific ethical approvals were not required. However, all studies included in the review were assessed for their ethical conduct and approval by respective institutional review boards.
2.4 Search strategy and sources of data
The search strategy for this scoping review was designed to capture a comprehensive range of studies on the effects of MitoQ on CMD. To achieve a thorough and wide-ranging collection of data, the approach combined traditional database searches with referrals from the MitoQ research team, ensuring a holistic overview of available research.
2.4.1 Online database search
A systematic literature search was conducted across several renowned databases, including PubMed, Scopus, Web of Science, and Google Scholar. This diverse selection of databases was chosen to cover a broad spectrum of scientific publications, from biomedical sciences to clinical research. The search strategy employed a combination of keywords and phrases related to MitoQ (“MitoQ”, “mitochondria-targeted ubiquinol”, “mitoquinone”, “mitoquinol”) and cardiovascular health (“cardiovascular”, “heart disease”, “hypertension”, “endothelial function”), MitoQ and metabolic health (“mitochondrial function”, “oxidative stress”, “obesity”, “diabetes”), MitoQ and liver health (“liver”, “liver diseases”). These terms were used in various configurations and combinations to maximize the retrieval of relevant articles. Boolean operators were utilized to refine the search, and filters such as publication date and language were applied to narrow down the results to the most relevant and recent studies.
2.4.2 Referrals from the MitoQ research team
In addition to the database search, direct referrals from the MitoQ research team were also included as a source of data. This approach was adopted to capture any additional studies that might not be readily available in public databases. The MitoQ research team, being at the forefront of research and development in this area, provided valuable insights and references to studies, including ongoing or recently completed trials, which might not yet be published in academic journals.
2.4.3 Hand-searching and cross-referencing
Following the initial collection of studies, hand-searching was employed as a supplementary method. This involved reviewing the reference lists of key articles to identify any additional studies that might have been missed in the database search. Cross-referencing between the studies obtained from the database search and those referred by the MitoQ research team was conducted to ensure comprehensiveness and avoid duplication. By combining these diverse sources of data, the review aimed to provide a robust and inclusive representation of the current state of research on MitoQ's impact on CMD (including metabolic health, cardiovascular health and liver health). This methodology ensured that the review not only relied on published academic literature but also considered insights and data directly from researchers actively working in this field.
2.5 Study selection process
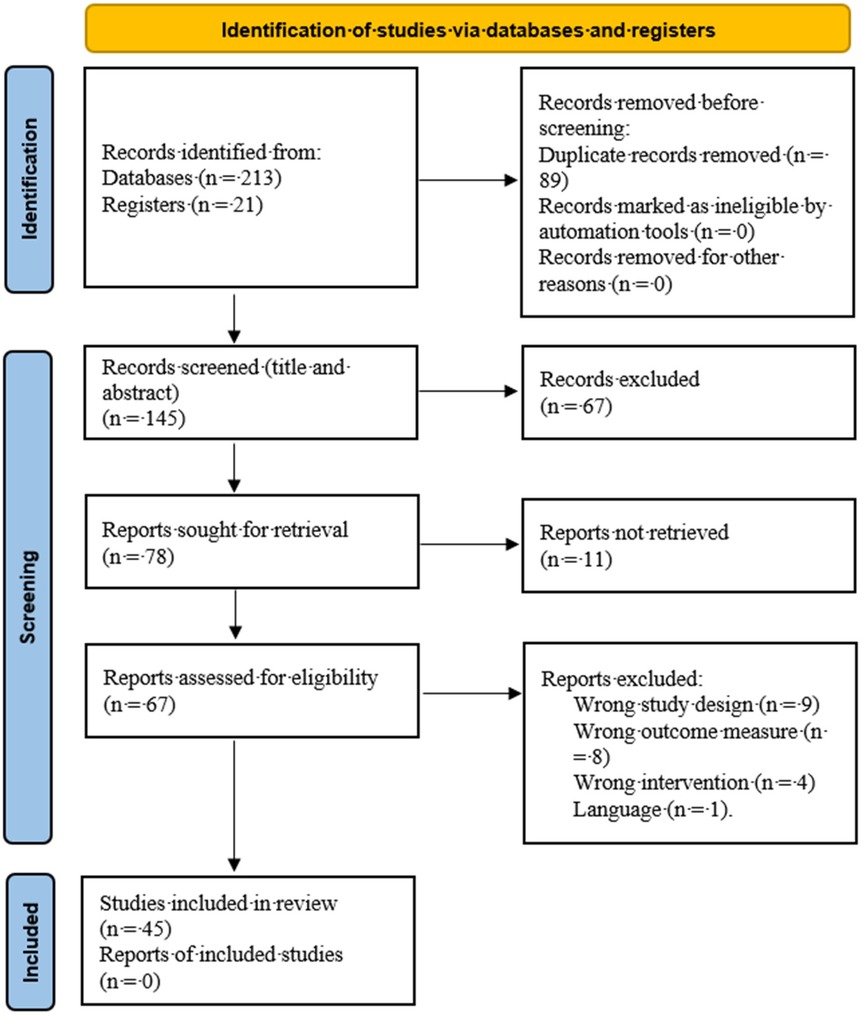
The process of selecting studies for inclusion in this scoping review was systematic and methodical, aimed at ensuring a comprehensive and unbiased overview of research on MitoQ's impact on CMD, Detailed information about selection process was shown in Figure 1.
Step 1: Initial Screening
The first step involved an initial screening of titles and abstracts retrieved from the database searches and referrals. This preliminary screening was crucial to quickly identify studies that were potentially relevant to the review's objectives.
Two reviewers (MP, SW) independently screened the titles and abstracts. This dual-reviewer approach was employed to minimize the risk of bias and ensure a thorough assessment of each study's relevance.
Step 2: Full-Text Review
Studies that passed the initial screening were then subjected to a full-text review. In this phase, the complete articles were obtained and closely examined to ascertain whether they met the inclusion criteria outlined in the methodology.
Discrepancies in opinions between the two reviewers (MP, SW) about the eligibility of specific studies were resolved through discussion. In cases where consensus could not be reached, a third reviewer (JC) was consulted to make the final decision.
Step 3: Cross-Checking with Referrals
In addition to the database-derived articles, the studies referred by the MitoQ research team were also subjected to the same rigorous selection process. This ensured consistency in the application of the inclusion and exclusion criteria across all sources of data.
Step 4: Documentation and Record Keeping
A record of all decisions made during the study selection process was maintained. This included documenting reasons for excluding studies at the full-text review stage, which was critical for transparency and to allow for a clear understanding of the review process.
Step 5: Final Compilation
The final list of selected studies was compiled after completing the full-text review and resolution of discrepancies. This list represented the studies that were deemed most relevant and suitable for inclusion in the scoping review.
By employing this structured and detailed study selection process, the review aimed to ensure that the final compilation of studies was representative, unbiased, and adhered strictly to the predetermined inclusion and exclusion criteria. This approach was fundamental to the integrity and reliability of the findings presented in the scoping review.
2.6 Data extraction and synthesis method
The data extraction and synthesis process for this scoping review was planned and executed to ensure accurate and comprehensive analysis of the selected studies. A standardized data extraction form was developed to gather key information from each study. This form was designed to capture essential details such as study author, year of publication, study design, participant demographics, intervention specifics (including dosage and duration of MitoQ supplementation), and key findings related to CMD. Two reviewers (MP, SW) independently extracted data from each study to minimize the risk of errors and bias. Any discrepancies in the extracted data between reviewers were resolved a third reviewer (JC). The data extraction process was particularly focused on outcomes pertinent to metabolic health (mitochondrial function, oxidative stress, obesity, and diabetes), cardiovascular health (endothelial function, blood pressure measurements, exercise tolerance, and other relevant cardiovascular biomarkers) and liver health.
Given the diversity of study designs and outcomes, a narrative synthesis approach was adopted. This method allowed for a comprehensive and contextual interpretation of the findings, acknowledging the variability and complexity inherent in clinical research. The synthesis involved summarizing the key findings of each study and discussing them in relation to the review's objectives. This included a critical assessment of the strengths and limitations of the studies, as well as an exploration of the implications of the findings for clinical practice and future research. Particular attention was given to variations in study results, seeking to understand and explain discrepancies or consistencies in the context of study methodologies, populations, and MitoQ dosages used.
While the primary focus was on human-based trials, in vivo and in vitro studies were included as supplementary material. These studies were used to provide additional context and support for the mechanistic understanding of MitoQ's effects on CMD. This supplementary analysis helped in drawing connections between clinical outcomes and underlying biological processes, thereby enriching the review's overall narrative.
The final synthesis was compiled into a coherent narrative, structured to provide a clear and comprehensive overview of the current state of research on the effect of MitoQ on CMD. This synthesis was reported in a manner that emphasizes clarity and accessibility, ensuring that the findings were understandable to both clinical practitioners and researchers in the field. By following this detailed methodology for data extraction and synthesis, the scoping review aimed to provide a thorough and balanced overview of the existing research, highlighting key findings and identifying areas for further investigation in the field of CMD and MitoQ.
3 Results
3.1 MitoQ and metabolic health
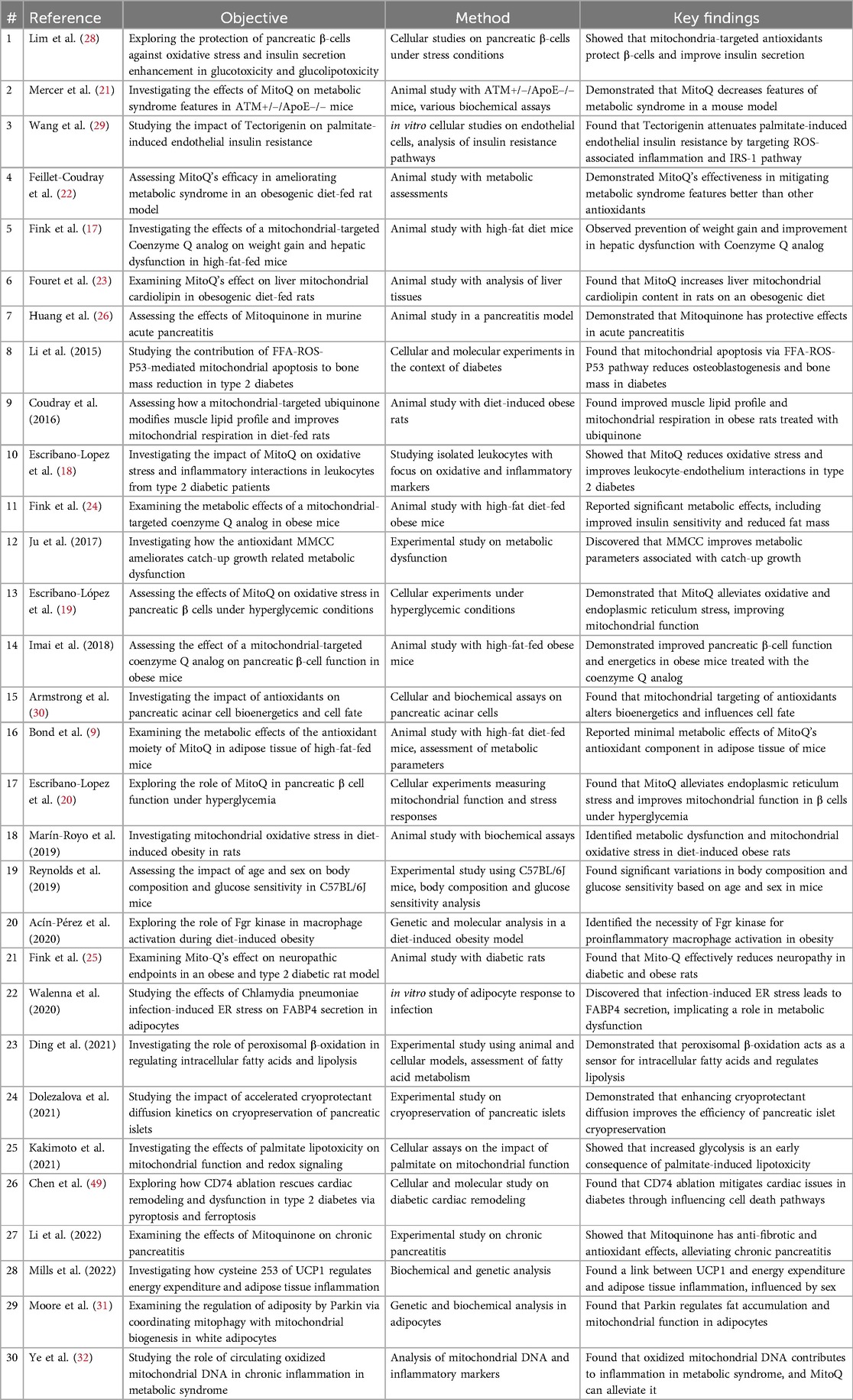
Focusing on metabolic health, a total of thirty studies were analyzed and reviewed, each contributing insights into the role of mitochondrial-targeted antioxidants in metabolic health and cellular function. These studies spanned a diverse range of objectives, from examining the protective effects of antioxidants on pancreatic β-cells to explore the genetic regulation of adiposity and mitochondrial function. The reviewed literature provided a robust foundation for understanding the multifaceted impact of mitochondrial dysfunction and its therapeutic targeting on various aspects of metabolic syndrome and related diseases. A summary table of reviewed studies was shown in Table 1 Summary Table of Studies on MitoQ's Metabolic Health Impacts.
3.1.1 Mitochondrial-Targeted antioxidants and metabolic health
A significant portion of the studies within this review focused on how mitochondrial dysfunction influenced metabolic health and the role of mitochondrial-targeted antioxidants, particularly MitoQ, in addressing CMD. Escribano-Lopez et al.'s research on white blood cell function in type 2 diabetes individuals demonstrated the efficacy of MitoQ in reducing oxidative stress, protecting against endoplasmic reticulum stress, and improving mitochondrial function under hyperglycemic conditions. The authors reported MitoQ's amelioration of endoplasmic reticulum stress and NFκB activation, as well as increased insulin secretion and preventing the enhancement of reactive oxygen species (ROS) production and O₂ consumption and decrease in glutathione (GSH) levels that were characteristic under hyperglycemic conditions (18–20). These findings highlighted the potential of MitoQ as a therapeutic agent for metabolic disorders characterized by high blood sugar levels. Similarly, Mercer et al. (21) showed that MitoQ could decrease features (increased adiposity, hypercholesterolemia, and hypertriglyceridemia) of metabolic syndrome in a mouse model, while Feillet-Coudray et al. noted its superiority for xanthine oxidase and NADPH oxidase-dependent ROS production in mitigating metabolic syndrome features in an obesogenic diet-fed rat model (22). This suggested a promising role for MitoQ in managing complex metabolic disorders.
Fouret et al. and Fink et al. presented MitoQ as an intervention that not only improved metabolic syndrome features but also prevented weight gain and hepatic dysfunction, markedly reduced hepatic lipid hydroperoxides and reduced circulating alanine aminotransferase, as well as decreased pathogenic alterations to cardiolipin content and profiles in rodents (17, 23–25). These studies emphasized MitoQ's regulatory capabilities in metabolic pathways, suggesting its utility in managing diet-induced obesity and associated hepatic issues, especially in preventing weight gain. In contrast, Bond et al. reported minimal metabolic effects of MitoQ's antioxidant component in adipose tissue of high fat fed mice, using excised fresh subcutaneous adipose tissue samples from treated mice and measuring their respiratory capacity, indicating that the efficacy of such interventions might be context-dependent and warrant further investigation (9).
3.1.2 Impact on cellular function and disease conditions
The effect of mitochondrial-targeted antioxidants extended beyond metabolic health, influencing various cellular functions in healthy and disease states. For instance, Huang et al. and Li et al. explored the protective effects of MitoQ in acute and chronic pancreatitis models, respectively (26, 27). Moreover, Lim et al. established that mitochondrial-targeted antioxidants could shield pancreatic β-cells from oxidative stress, thereby enhancing insulin secretion and offering a novel approach to managing diabetes-induced β-cell dysfunction (28).
Wang et al. investigated the role of tectorigenin, a mitochondrially active flavonoid, in attenuating palmitate-induced endothelial insulin resistance, shedding light on potential strategies for managing diabetes-associated vascular complications (29). Armstrong et al. found that mitochondrial targeting of antioxidants alters pancreatic acinar cell bioenergetics and influences cell fate, markedly reduced mitochondrial ATP turnover capacity and cellular ATP concentration, suggesting implications for diseases involving acinar cells, like acute pancreatitis (30).
3.1.3 Genetic regulation of metabolic processes
A new frontier in understanding metabolic health was being uncovered by studies investigating genetic regulation. Moore et al. provided a mechanistic insight, showing that Parkin regulated adiposity by coordinating mitophagy with mitochondrial biogenesis in white adipocytes (31). This study underscored the importance of mitochondrial dynamics in adipose tissue function and the pathophysiology of obesity.
Additionally, Ye et al. delved into the role of circulating oxidized mitochondrial DNA in chronic inflammation in metabolic syndrome (32). Their discovery that MitoQ could alleviate DNA-induced inflammation opens avenues for addressing systemic inflammation in CMD. This finding pointed to the potential of targeting mitochondrial integrity and function to manage systemic inflammatory responses that were central to metabolic syndrome.
These studies collectively underscored the promising roles of mitochondrial-targeted interventions in metabolic health. They highlighted the therapeutic promise of mitochondrial antioxidants in ameliorating various aspects of metabolic dysfunction, from improving insulin secretion and reducing weight gain to manage systemic inflammation. However, some contradictory findings also indicated the need for further research to elucidate the differential effects in various metabolic contexts and to optimize therapeutic strategies for clinical use. The results affirmed the importance of a nuanced approach to treatment, considering individual genetic and metabolic profiles to maximize therapeutic efficacy.
3.2 Mitoq and CVDs
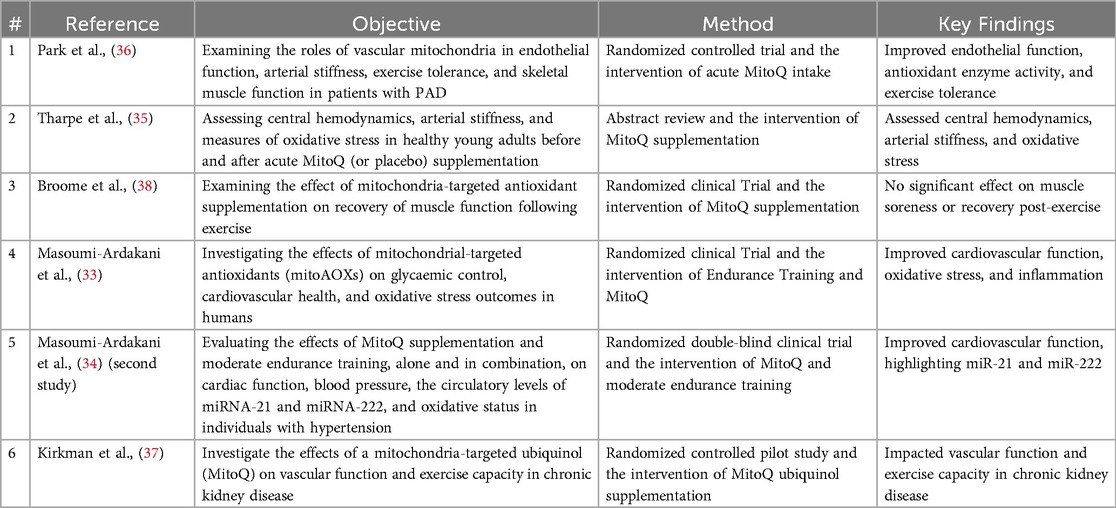
Focusing on CVDs, the scoping review analyzed six primary studies, each offering insights into the cardiovascular impacts of MitoQ supplementation. These studies provided a broad perspective on how MitoQ might benefit various aspects of cardiovascular health across different populations. in vivo studies showed that MitoQ had demonstrated multiple positive effects on cardiovascular health (33, 34), anti-oxidative stress (34, 35) and exercise tolerance (33, 34, 36, 37), especially in patients with chronic conditions. Different studies had produced different conclusions about the effects of muscle recovery, with some not finding significant benefits (38). A summary table of reviewed studies was illustrated in Table 2 Studies on MitoQ's Cardiovascular Health Impacts.
The comparative analysis of the reviewed studies revealed several key observations and implications regarding MitoQ's role in CVDs. Firstly, the diversity in the study populations was noteworthy, ranging from patients with specific conditions, such as individuals with hypertension (33), peripheral artery disease (36) and chronic kidney disease (37), to healthy young adults (35). The wide-ranging demographic representation provided valuable insights into the effects of MitoQ across various groups, suggesting its potential relevance to a broad spectrum of individuals. Secondly, there was notable variation in the interventions across these studies, particularly concerning the dosage and duration of MitoQ supplementation, some studies, such as Masoumi-Ardakani et al., combined MitoQ with endurance training, revealing enhanced cardiovascular outcomes and reductions in oxidative stress and inflammation (33, 34), while others focused on standalone supplementation, which didn't observe significant effects on post-exercise recovery (38).
While majority of the studies reported positive effects of MitoQ on cardiovascular parameters, such as improved endothelial function (36), reduced oxidative stress and better blood pressure regulation (33, 34), a few studies, for example, Broome et al. didn't observe significant effects of MitoQ on physiological outcomes such as inflammatory recovery post-exercise (38), highlighting the complexity of MitoQ's impact on different health aspects.
3.3 MitoQ and liver health
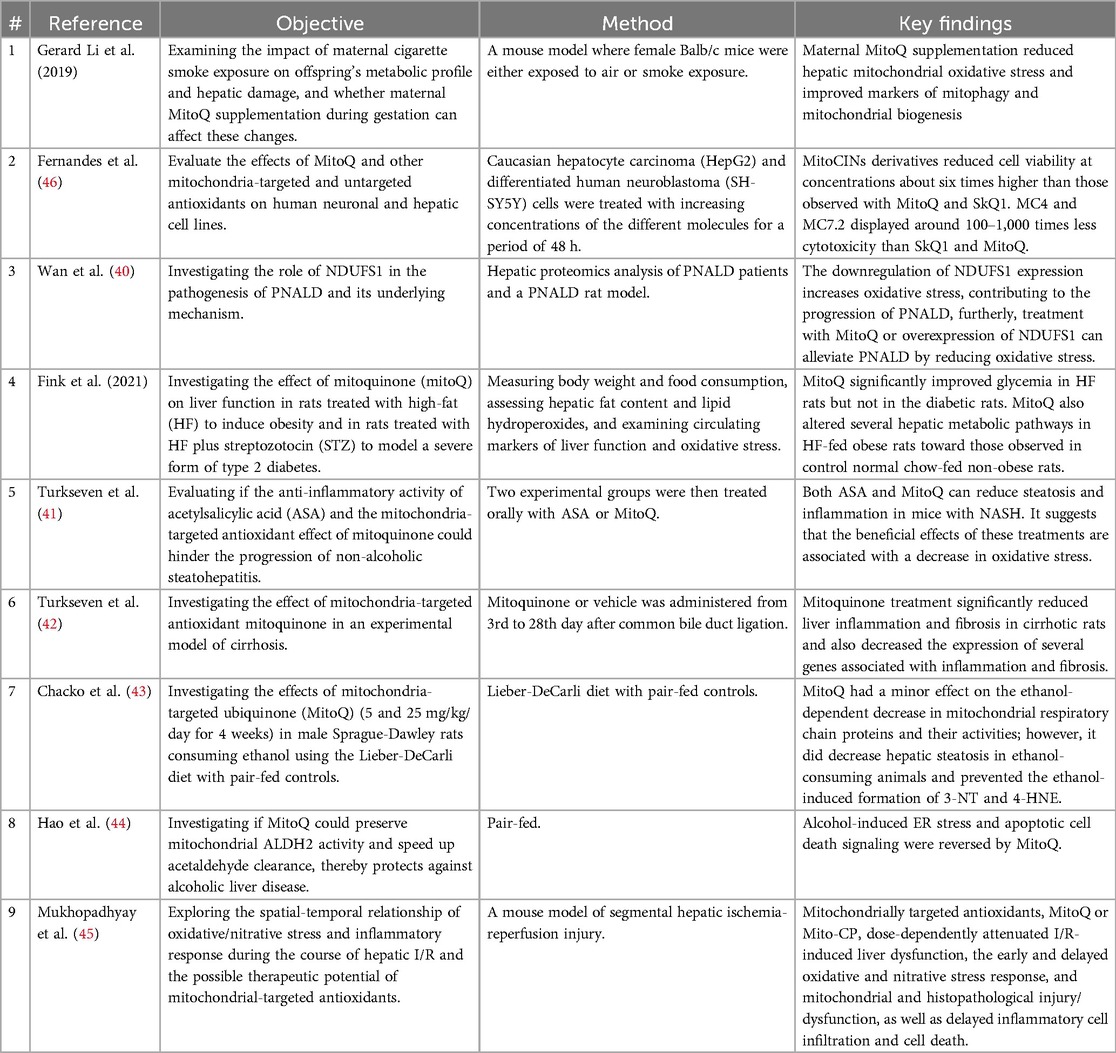
Focusing on liver health, a total of 9 articles were reviewed in this study, these studies contributed insights into the potential benefits of MitoQ in liver health management. in vivo studies showed that in animal models (e.g., mice or rats) the role of MitoQ in specific diseases or pathological states was analysed, mainly related to liver function, steatosis, fibrosis, and inflammation (16, 39–45). The only in vitro study, using hepatocytes and neuronal cell lines, revealed the cytotoxicity of MitoQ at high concentrations while comparing the toxicity and efficacy of different mitochondria-targeted antioxidants (46). A summary table of the reviewed articles on MitoQ's impacts on liver health was presented in Table 3 Studies on MitoQ's Liver Health Impacts.
Multiple studies had shown that MitoQ reduced oxidative stress in the liver and improved liver cell survival by reducing mitochondrial oxidative stress levels (45). Studies also had found that MitoQ could help improve mitochondrial function, including promoting mitochondrial autophagy and mitochondrial biosynthesis, leading to improved liver health (39, 44). Furtherly, MitoQ had potential therapeutic effects on specific liver diseases, such as non-alcoholic fatty liver disease, liver fibrosis, and hepatitis, by reducing the severity of the disease and improving the associated pathophysiologic processes (42–44).
4 Discussion
This scoping review had synthesized the findings from 45 studies that collectively contributed to the understanding of mitochondrial-targeted antioxidants and their role in CMD. The evidence suggested promising therapeutic potential for compounds such as MitoQ in mitigating various metabolic and cellular dysfunctions.
The discussion of mitochondrial function in CMD was complex, the study by Moore et al. presented Parkin as a regulator of adiposity, indicating a key role for mitochondrial quality control in adipocyte metabolism (31). However, the intricacies of mitophagy and its regulation by Parkin in human adipocytes remained to be fully elucidated.
Recent research had explored the potential role of MitoQ in managing CMD. Mitochondrial dysfunction and oxidative stress were implicated in the pathogenesis of conditions such as CVDs, diabetes, liver diseases and metabolic syndrome. MitoQ, by selectively targeting mitochondria and reducing reactive oxygen species production, had shown promise in preclinical studies for its ability to improve mitochondrial function and attenuate oxidative stress. For instance, studies had demonstrated that MitoQ supplementation can enhance endothelial function (47), and reduce oxidative damage to lipids and proteins (48). These studies underscored the importance of mitochondrial health and oxidative balance in the pathophysiology of CMD. Moreover, studies like those by Escribano-Lopez et al. and Chen et al. highlighted the role of mitochondrial-targeted antioxidants in reducing oxidative stress and inflammation (18–20, 49). However, the complexity of redox biology in human physiology suggested that the balance between pro-oxidant and antioxidant mechanisms might be more delicate than currently understood. Oversimplification of these dynamics could introduce bias in interpreting the efficacy of antioxidant therapies (36, 38).
In CVDs, the effect of MitoQ mainly existed in serving as cardiovascular health protectors and chronic disease management tools, as well as enhancing healthy individual function. MitoQ enhanced endothelial function by improving vascular mitochondrial function (36), while significantly reducing arterial stiffness, improving hemodynamic parameters, and attenuating atherosclerosis (35). Furtherly, in patients with chronic kidney disease, MitoQ helped to enhance vascular elasticity and exercise tolerance (37), another study also showed MitoQ increased endothelial function in non-exercisers with lower cardiorespiratory fitness (50). Some studies had illustrated that MitoQ didn't significantly improve recovery of muscle function or reduce muscle soreness after exercise (38), while other study clarified that training-induced increased in peak power were enhanced following MitoQ supplementation (51).Furthermore, when combined with endurance training, it was more effective in improving cardiovascular and antioxidant function (33, 34). Although MitoQ had a limited effect on post-exercise recovery, it had potential benefits in optimizing vascular function and antioxidant status, as well as reducing cardiac hypertrophy (47, 52).
For liver health, MitoQ targeted mitochondria and significantly reduced oxidative stress, alleviated liver inflammation and fat accumulation, inhibited liver fibrosis, and improved metabolic abnormalities, it demonstrated protective effects in various liver disease models (16, 39–46). MitoQ could protect the liver from some pathways; some studies focused on chronic alcohol-induced liver disease study, illustrated the pathway from reducing alcohol-induced hepatic fat deposition, preventing the formation of ethanol-induced oxidation products (43), as well as ameliorating endoplasmic reticulum stress and apoptotic signalling induced by alcohol (44), furtherly, some studies focused on other types liver disease illustrated that it could alleviate liver fibrosis (42, 53) as well as relieve arsenic-induced acute liver injury and immune imbalance (54, 55).
However, some studies, such as the one conducted by Broome et al. (38), did not find significant effects of MitoQ in muscle recovery, which contrasted with the positive outcomes reported in other research. However, they did report decreased inflammation via F2-isoprostanes after exercise. This discrepancy highlighted the need for caution in interpreting the results and suggested that further research was necessary to understand the conditions under which MitoQ was most effective.
This scoping review provided valuable insights into the therapeutic potential of MitoQ for the prevention and management of CMD, however, several limitations should be acknowledged. Firstly, the variability in study designs and methodologies across the included, differences in study populations ranged from healthy individuals to patients with various conditions, as well as inconsistencies in dosage and duration of MitoQ supplementation, make it challenging to generalize findings and draw definitive conclusions. Secondly, most of the evidence reviewed originates from preclinical studies and small-scale clinical trials, lack of the robust, large-scale clinical trials to validate the efficacy and safety of MitoQ in diverse patient populations. Thirdly, there was a lack of consistency regarding the dosage and duration of MitoQ supplementation in the reviewed studies, this variability complicated the interpretation of findings, given the limited clinical trials, caution should be exercised when considering the use of this drug.
For metabolic diseases, the findings from this review underscored the potential of mitochondrial-targeted therapies in treating metabolic disorders, such as improved insulin sensitivity and reduced inflammation. For CVDs, MitoQ's ability to enhance endothelial function and mitigate oxidative damage could play a significant role in the prevention and management of CVDs. For liver health, recent studies had noted the potential of MitoQ in promoting liver health. Evidence suggested that MitoQ could effectively reduce oxidative damage and prevent hepatic fat accumulation, thereby mitigating liver dysfunction, particularly in cases induced by high-fat diets.
5 Conclusion
The exploration of mitochondrial-targeted antioxidants represented a burgeoning field of research with significant implications for CMD. The studies reviewed in this scoping analysis collectively highlighted the therapeutic potential of compounds such as MitoQ in modulating various aspects of CMD, including CVDs, liver health and metabolic health. The synthesis of these findings illustrated a broad spectrum of benefits ranging from enhanced insulin secretion to improved lipid profiles and mitochondrial function.
The potential of mitochondrial antioxidants to serve as a novel therapeutic approach for CMD was clear, yet the path to clinical application was paved with the need for further investigation. Future research need not only focus on confirming these findings in human populations but also on understanding the precise mechanisms by which these compounds exert their effects. This would entail detailed studies into mitochondrial dynamics, the interplay between different cellular stress pathways, and the impact of genetic and environmental factors on individual responses to treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TS: Conceptualization, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. JC: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from MitoQ Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The research team greatly appreciated the funding support. The authors were deeply grateful to MitoQ research team for their valuable insights and consultations, which greatly enhanced the quality of this study.
Conflict of interest
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. The University of Edinburgh. Cardiometabolic diseases. (2024). Available online at: https://www.ed.ac.uk/medicine-vet-medicine/our-research/research-themes/cardiometabolic-diseases (Accessed September 10, 2024).
2. Ungvari Z, Kunutsor SK. Coffee consumption and cardiometabolic health: a comprehensive review of the evidence. Geroscience. (2024) 46(6):6473–510. doi: 10.1007/s11357-024-01262-5
3. Li Y, Liu X, Lv W, Wang X, Du Z, Liu X, et al. Metformin use correlated with lower risk of cardiometabolic diseases and related mortality among US cancer survivors: evidence from a nationally representative cohort study. BMC Med. (2024) 22(1):269. doi: 10.1186/s12916-024-03484-y
4. Chen W, Zhao H, Li Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct Target Ther. (2023) 8(1):333. doi: 10.1038/s41392-023-01547-9
5. Yang J, Suo H, Song J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Crit Rev Food Sci Nutr. (2021) 61(22):3857–75. doi: 10.1080/10408398.2020.1809344
6. Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension. (2018) 71(6):1056–63. doi: 10.1161/HYPERTENSIONAHA.117.10787
7. Sukjamnong S, Chan YL, Zakarya R, Nguyen LT, Anwer AG, Zaky AA, et al. Mitoq supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci Rep. (2018) 8(1):6631. doi: 10.1038/s41598-018-24949-0
8. Emont MP, Jacobs C, Essene AL, Pant D, Tenen D, Colleluori G, et al. A single-cell atlas of human and mouse white adipose tissue. Nature. (2022) 603(7903):926–33. doi: 10.1038/s41586-022-04518-2
9. Bond ST, Kim J, Calkin AC, Drew BG. The antioxidant moiety of MitoQ imparts minimal metabolic effects in adipose tissue of high fat fed mice. Front Physiol. (2019) 10:543. doi: 10.3389/fphys.2019.00543
10. Kim SR, Song JH, Ahn JH, Jeong MS, Yang YM, Cho J, et al. Obesity exacerbates coxsackievirus infection via lipid-induced mitochondrial reactive oxygen Species generation. Immune Netw. (2022) 22(2):e19. doi: 10.4110/in.2022.22.e19
11. World Health Organization. Cardiovascular diseases (CVDs). (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (Accessed September 15, 2024).
12. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592(7855):524–33. doi: 10.1038/s41586-021-03392-8
13. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139(10):e56–e528. doi: 10.1161/CIR.0000000000000659
14. Liu X, Wang H, Liang X, Roberts MS. Chapter 30 - hepatic metabolism in liver health and disease. In: Muriel P, editor. Liver Pathophysiology. Boston: Academic Press (2017). p. 391–400.
15. Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. (2023) 79(2):516–37. doi: 10.1016/j.jhep.2023.03.017
16. Fink BD, Yu L, Coppey L, Obrosov A, Shevalye H, Kerns RJ, et al. Effect of mitoquinone on liver metabolism and steatosis in obese and diabetic rats. Pharmacol Res Perspect. (2021) 9(1):e00701. doi: 10.1002/prp2.701
17. Fink BD, Herlein JA, Guo DF, Kulkarni C, Weidemann BJ, Yu L, et al. A mitochondrial-targeted coenzyme q analog prevents weight gain and ameliorates hepatic dysfunction in high-fat-fed mice. J Pharmacol Exp Ther. (2014) 351(3):699–708. doi: 10.1124/jpet.114.219329
18. Escribano-Lopez I, Diaz-Morales N, Rovira-Llopis S, de Maranon AM, Orden S, Alvarez A, et al. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. (2016) 10:200–5. doi: 10.1016/j.redox.2016.10.017
19. Escribano-López I, Dàz-Morales N, de Marañón AM, Iannantuoni F, López-Domenech S, Abad-Jimenez Z, et al. P-116 - the mitochondrial antioxidant MitoQ alleviates oxidative stress, endoplasmic reticulum stress and mitochondrial function in pancreatic β cells under hyperglycaemic conditions. Free Radic Biol Med. (2018) 120:S79–80. doi: 10.1016/j.freeradbiomed.2018.04.263
20. Escribano-Lopez I, Banuls C, Diaz-Morales N, Iannantuoni F, Rovira-Llopis S, Gomis R, et al. The mitochondria-targeted antioxidant MitoQ modulates mitochondrial function and endoplasmic Reticulum stress in pancreatic beta cells exposed to hyperglycaemia. Cell Physiol Biochem. (2019) 52(2):186–97. doi: 10.33594/000000013
21. Mercer JR, Yu E, Figg N, Cheng KK, Prime TA, Griffin JL, et al. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/-/ApoE-/- mice. Free Radic Biol Med. (2012) 52(5):841–9. doi: 10.1016/j.freeradbiomed.2011.11.026
22. Feillet-Coudray C, Fouret G, Ebabe Elle R, Rieusset J, Bonafos B, Chabi B, et al. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than apocynin or allopurinol. Free Radic Res. (2014) 48(10):1232–46. doi: 10.3109/10715762.2014.945079
23. Fouret G, Tolika E, Lecomte J, Bonafos B, Aoun M, Murphy MP, et al. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Biochim Biophys Acta. (2015) 1847(10):1025–35. doi: 10.1016/j.bbabio.2015.05.019
24. Fink BD, Guo DF, Kulkarni CA, Rahmouni K, Kerns RJ, Sivitz WI. Metabolic effects of a mitochondrial-targeted coenzyme Q analog in high fat fed obese mice. Pharmacol Res Perspect. (2017) 5(2):e00301. doi: 10.1002/prp2.301
25. Fink B, Coppey L, Davidson E, Shevalye H, Obrosov A, Chheda PR, et al. Effect of mitoquinone (mito-Q) on neuropathic endpoints in an obese and type 2 diabetic rat model. Free Radic Res. (2020) 54(5):311–8. doi: 10.1080/10715762.2020.1754409
26. Huang W, Cash N, Wen L, Szatmary P, Mukherjee R, Armstrong J, et al. Effects of the mitochondria-targeted antioxidant mitoquinone in murine acute pancreatitis. Mediators Inflamm. (2015) 2015:901780. doi: 10.1155/2015/901780
27. Li M, Yuan Y, Han X, Liu X, Zhang W, Hao J. Antioxidant mitoquinone alleviates chronic pancreatitis via anti-fibrotic and antioxidant effects. J Inflamm Res. (2022) 15:4409–20. doi: 10.2147/JIR.S357394
28. Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, et al. Mitochondria-targeted antioxidants protect pancreatic beta-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cell Physiol Biochem. (2011) 28(5):873–86. doi: 10.1159/000335802
29. Wang Q, Cheng XL, Zhang DY, Gao XJ, Zhou L, Qin XY, et al. Tectorigenin attenuates palmitate-induced endothelial insulin resistance via targeting ROS-associated inflammation and IRS-1 pathway. PLoS One. (2013) 8(6):e66417. doi: 10.1371/journal.pone.0066417
30. Armstrong JA, Cash NJ, Morton JC, Tepikin AV, Sutton R, Criddle DN. Mitochondrial targeting of antioxidants alters pancreatic acinar cell bioenergetics and determines cell fate. Int J Mol Sci. (2019) 20(7):1700. doi: 10.3390/ijms20071700
31. Moore TM, Cheng L, Wolf DM, Ngo J, Segawa M, Zhu X, et al. Parkin regulates adiposity by coordinating mitophagy with mitochondrial biogenesis in white adipocytes. Nat Commun. (2022) 13(1):6661. doi: 10.1038/s41467-022-34468-2
32. Ye W, Wen C, Zeng A, Hu X. Increased levels of circulating oxidized mitochondrial DNA contribute to chronic inflammation in metabolic syndrome, and MitoQ-based antioxidant therapy alleviates this DNA-induced inflammation. Mol Cell Endocrinol. (2023) 560:111812. doi: 10.1016/j.mce.2022.111812
33. Masoumi-Ardakani Y, Najafipour H, Nasri HR, Aminizadeh S, Jafari S, Safi Z. Moderate endurance training and MitoQ improve cardiovascular function, oxidative stress, and inflammation in hypertensive individuals: the role of miR-21 and miR-222: a randomized, double-blind, clinical trial. Cell J. (2022) 24(10):577–85. doi: 10.22074/cellj.2022.8089
34. Masoumi-Ardakani Y, Najafipour H, Nasri HR, Aminizadeh S, Jafari S, Moflehi D. Effect of combined endurance training and MitoQ on cardiac function and Serum level of antioxidants, NO, miR-126, and miR-27a in hypertensive individuals. Biomed Res Int. (2022) 2022:8720661. doi: 10.1155/2022/8720661
35. Tharpe M, Barnett A, Hutchison Z, Linder B, Kavazis A, Brown M, et al. Effects of MitoQ on central hemodynamics, arterial stiffness, and oxidative stress in healthy, young adults. FASEB J. (2021) 35(S1). doi: 10.1096/fasebj.2021.35.S1.05402
36. Park SY, Pekas EJ, Headid RJ 3rd, Son WM, Wooden TK, Song J, et al. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am J Physiol Heart Circ Physiol. (2020) 319(2):H456–H67. doi: 10.1152/ajpheart.00235.2020
37. Kirkman DL, Stock JM, Shenouda N, Bohmke NJ, Kim Y, Kidd J, et al. Effects of a mitochondrial-targeted ubiquinol on vascular function and exercise capacity in chronic kidney disease: a randomized controlled pilot study. Am J Physiol Renal Physiol. (2023) 325(4):F448–F56. doi: 10.1152/ajprenal.00067.2023
38. Broome SC, Atiola RD, Braakhuis AJ, Mitchell CJ, Merry TL. Mitochondria-targeted antioxidant supplementation does not affect muscle soreness or recovery of maximal voluntary isometric contraction force following muscle-damaging exercise in untrained men: a randomized clinical trial. Appl Physiol Nutr Metab. (2022) 47(7):762–74. doi: 10.1139/apnm-2021-0767
39. Li G, Chan YL, Sukjamnong S, Anwer AG, Vindin H, Padula M, et al. A mitochondrial specific antioxidant reverses metabolic dysfunction and fatty liver induced by maternal cigarette smoke in mice. Nutrients. (2019) 11(7):1669. doi: 10.3390/nu11071669
40. Wan S, Maitiabula G, Wang P, Zhang Y, Gao X, Zhang L, et al. Down regulation of NDUFS1 is involved in the progression of parenteral-nutrition-associated liver disease by increasing oxidative stress. J Nutr Biochem. (2023) 112:109221. doi: 10.1016/j.jnutbio.2022.109221
41. Turkseven S, Turato C, Villano G, Ruvoletto M, Guido M, Bolognesi M, et al. Low-Dose acetylsalicylic acid and mitochondria-targeted antioxidant mitoquinone attenuate non-alcoholic steatohepatitis in mice. Antioxidants (Basel). (2023) 12(4):971. doi: 10.3390/antiox12040971
42. Turkseven S, Bolognesi M, Brocca A, Pesce P, Angeli P, Di Pascoli M. Mitochondria-targeted antioxidant mitoquinone attenuates liver inflammation and fibrosis in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. (2020) 318(2):G298–304. doi: 10.1152/ajpgi.00135.2019
43. Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, et al. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. (2011) 54(1):153–63. doi: 10.1002/hep.24377
44. Hao L, Sun Q, Zhong W, Zhang W, Sun X, Zhou Z. Mitochondria-targeted ubiquinone (MitoQ) enhances acetaldehyde clearance by reversing alcohol-induced posttranslational modification of aldehyde dehydrogenase 2: a molecular mechanism of protection against alcoholic liver disease. Redox Biol. (2018) 14:626–36. doi: 10.1016/j.redox.2017.11.005
45. Mukhopadhyay P, Horváth B, Zsengellėr Z, Bátkai S, Cao Z, Kechrid M, et al. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radical Biol Med. (2012) 53(5):1123–38. doi: 10.1016/j.freeradbiomed.2012.05.036
46. Fernandes C, Videira AJC, Veloso CD, Benfeito S, Soares P, Martins JD, et al. Cytotoxicity and mitochondrial effects of phenolic and quinone-based mitochondria-targeted and untargeted antioxidants on human neuronal and hepatic cell lines: a comparative analysis. Biomolecules. (2021) 11(11):1605. doi: 10.3390/biom11111605
47. Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RAJ, Cochemé HM, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension (Dallas, Tex: 1979). (2009) 54(2):322–8. doi: 10.1161/HYPERTENSIONAHA.109.130351
48. Gonzalo-Skok O, Casuso RA. Effects of mitoquinone (MitoQ) supplementation on aerobic exercise performance and oxidative damage: a systematic review and meta-analysis. Sports Med Open. (2024) 10(1):77. doi: 10.1186/s40798-024-00741-5
49. Chen L, Yin Z, Qin X, Zhu X, Chen X, Ding G, et al. CD74 ablation rescues type 2 diabetes mellitus-induced cardiac remodeling and contractile dysfunction through pyroptosis-evoked regulation of ferroptosis. Pharmacol Res. (2022) 176:106086. doi: 10.1016/j.phrs.2022.106086
50. Carlini NA, Harber MP, Fleenor BS. Acute effects of MitoQ on vascular endothelial function are influenced by cardiorespiratory fitness and baseline FMD in middle-aged and older adults. J Physiol. (2024) 602(9):1923–37. doi: 10.1113/JP285636
51. Broome SC, Pham T, Braakhuis AJ, Narang R, Wang HW, Hickey AJR, et al. Mitoq supplementation augments acute exercise-induced increases in muscle PGC1α mRNA and improves training-induced increases in peak power independent of mitochondrial content and function in untrained middle-aged men. Redox Biol. (2022) 53:102341. doi: 10.1016/j.redox.2022.102341
52. Milagros Rocha M, Victor VM. Targeting antioxidants to mitochondria and cardiovascular diseases: the effects of mitoquinone. Med Sci Monit. (2007) 13(7):RA132–RA45.17599037
53. Vilaseca M, García-Calderó H, Lafoz E, Ruart M, López-Sanjurjo CI, Murphy MP, et al. Mitochondria-targeted antioxidant mitoquinone deactivates human and rat hepatic stellate cells and reduces portal hypertension in cirrhotic rats. Liver Int. (2017) 37(7):1002–12. doi: 10.1111/liv.13436
54. Li H, Guo Y, Su W, Zhang H, Wei X, Ma X, et al. The mitochondria-targeted antioxidant MitoQ ameliorates inorganic arsenic-induced DCs/Th1/Th2/Th17/treg differentiation partially by activating PINK1-mediated mitophagy in murine liver. Ecotoxicol Environ Saf. (2024) 277:116350. doi: 10.1016/j.ecoenv.2024.116350
Keywords: MitoQ, cardiometabolic diseases, metabolic health, cardiovascular diseases, liver health
Citation: Pang M, Wang S, Shi T and Chen J (2025) Overview of MitoQ on prevention and management of cardiometabolic diseases: a scoping review. Front. Cardiovasc. Med. 12:1506460. doi: 10.3389/fcvm.2025.1506460
Received: 5 October 2024; Accepted: 24 February 2025;
Published: 11 March 2025.
Edited by:
Takuro Miyazaki, Showa University, JapanReviewed by:
Yike Yang, Peking University Third Hospital, ChinaDi Zhu, Air Force Medical University, China
Copyright: © 2025 Pang, Wang, Shi and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsong Chen, MDYyM2Q2MkB6anUuZWR1LmNu; amluc29uZy5jaGVuQGF1Y2tsYW5kLmFjLm56
†These authors have contributed equally to this work and share first authorship
 Mingli Pang
Mingli Pang Shidi Wang3,†
Shidi Wang3,†