
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 24 February 2025
Sec. Cardiac Rhythmology
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1501716
This article is part of the Research Topic New Challenges in Arrhythmology View all 11 articles
 Chengming Ma1†
Chengming Ma1† Xianjie Xiao1†
Xianjie Xiao1† Qian Chen2
Qian Chen2 Wenwen Li3
Wenwen Li3 Zhongzhen Wang1
Zhongzhen Wang1 Shiyu Dai1
Shiyu Dai1 Yuanjun Sun1
Yuanjun Sun1 Yunlong Xia1
Yunlong Xia1 Lianjun Gao1
Lianjun Gao1 Xiaomeng Yin1*
Xiaomeng Yin1*
Aims: Whether the intraprocedural anticoagulation regimen and activated clotting time (ACT) in pulsed field ablation (PFA) for atrial fibrillation (AF) are the same as those for radiofrequency catheter ablation (RFCA) is currently unknown.
Methods and results: Our retrospective study included 51 paroxysmal AF patients who underwent PFA (PFA group) and were matched with paroxysmal AF patients who underwent RFCA. Nearest-neighbor propensity score matching was performed at a 1:1 ratio (no tolerance to anticoagulant regimens and a tolerance of 0.02 on the CHA2DS2-VASc score, left atrial diameter, and left ventricular ejection fraction). Compared with the RFCA group, the PFA group had a significantly shorter procedure time but a longer fluoroscopy time. In both groups, an initial heparin dose of 110 U/kg was given. The 30-min ACT in the PFA group (240 ± 95.5 s) was shorter than that in the RFCA group (294.4 ± 82.3 s, P = 0.003). The 60-, 90-, and 120-min ACTs were significantly longer in the PFA group. The percentage of 30 min-ACTs in the therapeutic range in the RFCA group (33.3%) was greater than that in the PFA group (15.7%, P = 0.038). The time to achieve the target ACT was longer in the PFA group. There were no differences in the incidence of periprocedural thromboembolism or bleeding events between the two groups.
Conclusions: Compared with RFCA, PFA was associated with longer intraprocedural ACTs, shorter initial ACTs, fewer initial ACTs in the therapeutic range, and longer times to achieve the target ACT.
Atrial fibrillation (AF) is the most common type of arrhythmia worldwide, and its incidence and prevalence are increasing (1). AF is associated with a 2.4-fold increased risk of stroke and high healthcare costs and expenses for patients, according to previous studies (2). Moreover, the increased risk of heart failure in patients with AF also contributes to the high healthcare burden.
Currently, oral anticoagulants (OACs) and catheter ablation (CA) are the main treatments for patients with AF. OAC is the essential treatment for AF and has been shown to decrease the lifetime risk of stroke (3). Non-vitamin K antagonist oral anticoagulants (NOACs) have become the main drugs used to treat patients with nonvalvular AF. Numerous randomized controlled trials (RCTs) and research involving large registries have revealed that CA is more effective than antiarrhythmic drugs in maintaining sinus rhythm (4, 5). Radiofrequency (RF) has become the main energy source for CA since the pulmonary vein was recognized to play a dominant role in AF in 1998. Cryoablation is an alternative for PVI. Cryoablation and RF ablation are comparable in terms of their effects, degrees of cellular damage, inflammatory response, and thromboembolic risk (6, 7). Notably, patients who undergo CA procedures for AF are at increased risk of stroke and thromboembolism. Periprocedural thromboembolic events (1.0%) and asymptomatic cerebral embolism (5%–15%) are notable complications and are sometimes life-threatening. Therefore, periprocedural anticoagulant management is essential to reduce the risk of thromboembolism, facilitate hemostasis and prevent intra- and postoperative bleeding. Uninterrupted anticoagulation with unfractionated heparin (UFH) to maintain the activated clotting time (ACT) in the safe range during the procedure is the current consensus. Most evidence on UFH and the intraprocedural target ACT (300–350 s) during ablation procedures for AF is derived from studies involving patients undergoing VKA and RFCA procedures (8).
Currently, pulsed field ablation (PFA) is another promising method for PVI in the treatment of AF (9). Unlike RF ablation or cryoablation, PFA is a nonthermal ablation procedure that causes irreversible and selective cardiac electroporation. PFA may not result in collateral damage to noncardiac tissues (10, 11). During PFA, electrical pulses delivered to cardiac cells disrupt the integrity of the cell membrane, causing cell death and replacement fibrosis. Currently, there are few early preclinical and clinical studies supporting the implementation of PFA in clinical practice. Whether PFA is better suited for periprocedural management of CA in patients with AF is unknown because of limited evidence. Whether intraprocedural anticoagulation management and periprocedural thromboembolic risk differs between PFA and RFCA are unknown. Currently, the protocol for intraprocedural anticoagulation management for PFA is the same as that for RFCA. It is uncertain whether the current intraprocedural UFH dosing regimen and target ACT values (300–350 s) are appropriate for PFA. Nevertheless, few studies focusing on the heparin dosage in AF patients who undergo PFA have been reported. We hypothesized that the intraprocedural anticoagulation management and periprocedural thromboembolic risk of PFA would differ from those of RFCA.
We compared the intraprocedural ACTs, time required for the ACT to reach the therapeutic range, actual percentages of measurements at the target ACT, heparin dosage, and incidence of periprocedural thromboembolic and bleeding events between AF patients who underwent PFA and those who underwent RFCA to determine whether the current intraprocedural heparin dosing regimen is appropriate for PFA.
This study was a retrospective, single-center clinical trial involving patients with paroxysmal AF who underwent PFA and RFCA in our center from 1/02/2023–30/06/2024. This trial was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University.
Patients with paroxysmal AF who underwent PFA and RFCA at our center from 1/02/2023 to 30/06/2024 were retrospectively included. Patients with a history of cardiac ablation, cardiac surgery, or cardiovascular implantable electronic devices; severe hepatic/renal insufficiency; cerebrovascular disease within the last 3 months (including stroke and transient ischemic attack); or contraindications to anticoagulants were excluded. Patients with severe cardiac dysfunction [a left ventricular ejection fraction (LVEF) ≤40% or NYHA cardiac function grade III–IV] or a left atrial diameter (LAD) ≥55 mm were also excluded. Patients who refused to participate in this trial and were lost to follow-up were not included (Figure 1).
The patients in the PFA group were collected from the three prospective studies conducted in our center to evaluate the safety and effectiveness of PFA in paroxysmal AF: study A: the AFIRE study (a prospective, multicenter, single arm study with performance goals designed to evaluate the safety and effectiveness of a multielectrode circular IRE catheter and multichannel IRE generator in the treatment of paroxysmal AF, NCT05552963); study B: the PF-Beat-AF Trial [a study of pulse field ablation (PFA) for the treatment of paroxysmal atrial fibrillation, AccuPulse Medical Technology]; and study C: the Comparison of PFA vs. RFA in Patients with Symptomatic Paroxysmal Atrial Fibrillation (NCT06014996, Insight LifeTech). Patients in the RFCA group underwent RFCA at our center at the same time as those in the PFA group underwent PFA. PFA patients were matched 1:1 with RFCA patients via nearest-neighbor propensity score matching with no tolerance of anticoagulant regimens and a tolerance of 0.02 for the CHA2DS2-VASc score, LAD, and LVEF. Aside from the parameters mentioned above, weight, age, hepatic and kidney function, past medical history, date of procedure, and the operator were also considered during the matching process.
All patients were treated with NOACs preoperatively, and atrial thrombosis was excluded by computed tomography or transesophageal echocardiography of left atrial appendage in female patients with CHA2DS2-VASc scores ≥ 3 and male patients with CHA2DS2-VASc scores ≥ 2 within 48 h before the ablation procedure.
The CA procedure was performed under general anesthesia. A bolus of 110 U/kg of heparin was administered immediately after right femoral vein puncture. A decapolar catheter was positioned in the coronary sinus for atrial pacing and signal reference. Transseptal puncture was performed via a modified Brockenbrough technique.
In the PFA group, ablation was performed via the following three specialized PFA generators and PFA catheters: (1) the Multi-Channel Irreversible Electroporation (IRE) generator and Multi-Channel Circular IRE catheter (Biosense Webster, Irvine, USA); (2) the AccuPulse PFA generator and circular-shaped PFA catheter [AccuPulse Medical Technology (Suzhou) Co. Ltd.; Jiangsu, China]; and (3) the PFA generator (Insight Lifetech, Shenzhen, China), a proprietary lotos-shaped PFA catheter (LotosPFA, Insight Lifetech), and a customized steerable sheath to navigate and position the PFA catheter.
In the RFCA group, a PV mapping catheter (Pentaray NAV ECO High Density Mapping Catheter, Biosense Webster, Irvine, USA, or Advisor HD Grid Mapping Catheter, Abbott Laboratories, Chicago, IL) and a saline-irrigated ablation catheter (Thermocool SMART TOUCH SF, Biosense Webster, Irvine, USA., or TactiCath Contact Force Ablation Catheter) were used for mapping and ablation via the CARTO 3 V6 electroanatomic mapping system (Biosense Webster, Irvine, USA) or Ensite Precision Cardiac Mapping System (Abbott Laboratories, Chicago, IL).
The final stage of the CA procedure was complete electrical isolation of the PV, which was confirmed by the absence of PV potentials or PV-left atrium conduction; notably, no tachycardia was induced by the electrophysiologic study (EPS).
The initial dosage of heparin was administered immediately after right femoral vein puncture. The amount of supplemental heparin used was determined by the operator to maintain the ACT between 300 and 350 s based on our previous study (12). An additional dose of heparin was not given if the ACT was ≥350 s. If ACT was 150–300 s, a heparin dose of 800 U was added, if ACT < 150 s, a heparin dose of 1,000 U was added. An additional dose of heparin was not given if the ACT reached the target or was ≥300 s. If severe bleeding complications occurred, heparin was discontinued immediately, and then protamine sulfate was administered.
The ACT was measured every 30 min with an optical coagulation analyzer (OCG-102, Wondfo Biotech, Guangzhou, China). ACT compliance was defined as at least one intraprocedural ACT in the therapeutic range. The intraprocedural ACTs at each 30-min interval (30 min-ACT, 60 min-ACT, 90 min-ACT, 120 min-ACT, and 150 min-ACT), average percentage of measurements at the target ACT, percentage of initial ACTs (i.e., 30 min-ACT) in the therapeutic range, time required for the ACT to reach the therapeutic range, intraprocedural heparin dose, ACT compliance rate, and total amount of heparin administered were collected and analyzed.
The incidence rates of periprocedural bleeding and thromboembolic complications were recorded and analyzed. The periprocedural complications were defined as adverse events that occurred within 30 days after the procedure. The primary endpoints were the percentage of initial ACTs in the therapeutic range, time required for the ACT to reach the therapeutic range, and ACT compliance rate. The secondary endpoints were the percentages of measurements at the target ACT, intraprocedural heparin dosage, and periprocedural bleeding and thromboembolic complications.
SPSS version 27.0 (SPSS Inc., Chicago, USA) was used for all analyses. Continuous variables are expressed as the mean ± standard deviation (SD) if normally distributed; the median and the 25%–75% interquartile range were used for skewed data. An unpaired t test or one-way analysis of variance was performed for measurement data. For non-normally distributed measurement data, the Mann–Whitney U test was performed for comparisons between two groups. The Kruskal‒Wallis H test with Bonferroni correction was used for comparisons between multiple groups. For categorical variables, chi-square tests or Fisher's exact tests were used for comparisons between two groups. The ACTs and actual percentages of measurements at the target ACT were compared between the RFCA group and the PFA group. A 2-tailed P value < 0.05 indicated statistical significance.
A total of 102 AF patients [59.9 ± 9.1 years; 58 (56.9%) males] were included in this study. There were 51 patients in the PFA group and 51 patients in the RFCA group. The mean LAD and LVEF were 36.8 ± 3 mm and 59 ± 1.5%, respectively. The mean CHA2DS2-VASc score was 1.4 (0 in 21 (20.6%) patients, 1 in 38 (37.3%) patients, 2 in 24 (23.5%) patients, 3 in 16 (15.7%) patients, 4 in 2 (2%) patients and 5 in 1 (1%) patient). There was no significant difference between the two groups in terms of age, sex, comorbidities, renal function, hemoglobin, concomitant antiplatelet therapy, CHA2DS2-VASc score, left ventricular diameter, etc. Compared with the RFCA group, the PFA group had significantly shorter procedure times (105.8 ± 33.3 min vs. 155.8 ± 40.2 min, P < 0.001) but longer fluoroscopy times (16.2 ± 7.5 min vs. 6.7 ± 2 min, P = 0.024). The baseline clinical characteristics of the two groups are summarized in Table 1.
During the RFCA procedure, each patient underwent 2.4 ± 1 ACT measurements, and patients in the PFA group underwent 2.3 ± 1 ACT measurements (P = 0.556). The ACTs at 30-min intervals are shown in Figure 2A and Table 2. The 30-min ACT in the PFA group (240 ± 95.5 s) was shorter than that in the RFCA group (294.4 ± 82.3 s, P = 0.003). The 60 min-ACT, 90 min-ACT, and 120 min-ACT were significantly longer in the PFA group than in the RFCA group (Table 2). The average percentage of measurements at the target ACT in the PFA group (33% [0,50%]) was greater than that in the RFCA group (20% [0,50%], P = 0.565), but the difference was not statistically significant.
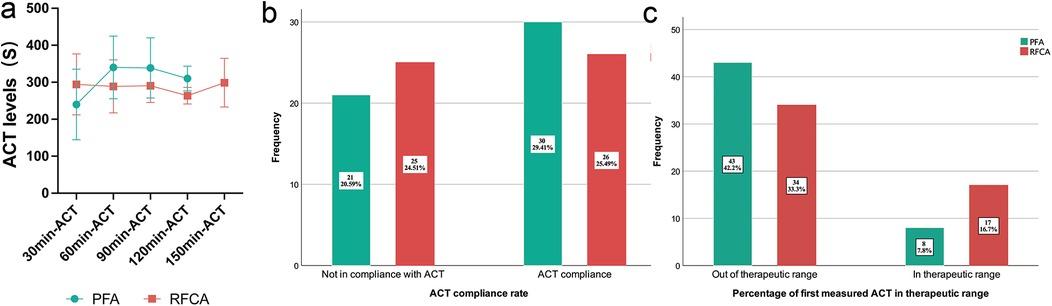
Figure 2. Intraprocedural ACTs of the PFA group and RFCA group. (a) shows the intraprocedural ACTs at each 30-minute interval, (b) shows the ACT compliance rate of the PFA group and the RFCA group and (c) shows the percentage of first measured ACT in the therapeutic range of the PFA group and the RFCA group. The percentages in the bar chart represent the proportion of all patients.
There was no significant difference in the ACT compliance rate between the two groups (58.8% in the PFA group vs. 51% in the RFCA group, P = 0.426; Figure 2B). However, the percentage of initial ACTs (30 min-ACT) in the therapeutic range in the RFCA group (33.3%) was markedly greater than that in the PFA group (15.7%, P = 0.038), indicating that the ACT in the RFCA group reached the target more quickly with the same initial dose of heparin (shown in Figure 2C).
Figure 3A,B show the number of ACT measurements and the percentage of ACTs in or out of the therapeutic range throughout the whole procedure. In the PFA group, 10 patients did not undergo a second ACT measurement, as the procedure was already complete. Thirty-one patients underwent two ACT measurements, representing 64.7% (31/51) of all PFA patients. The patients in the PFA group needed more time to achieve the target ACT than did the patients in the RFCA group (Figure 3C).
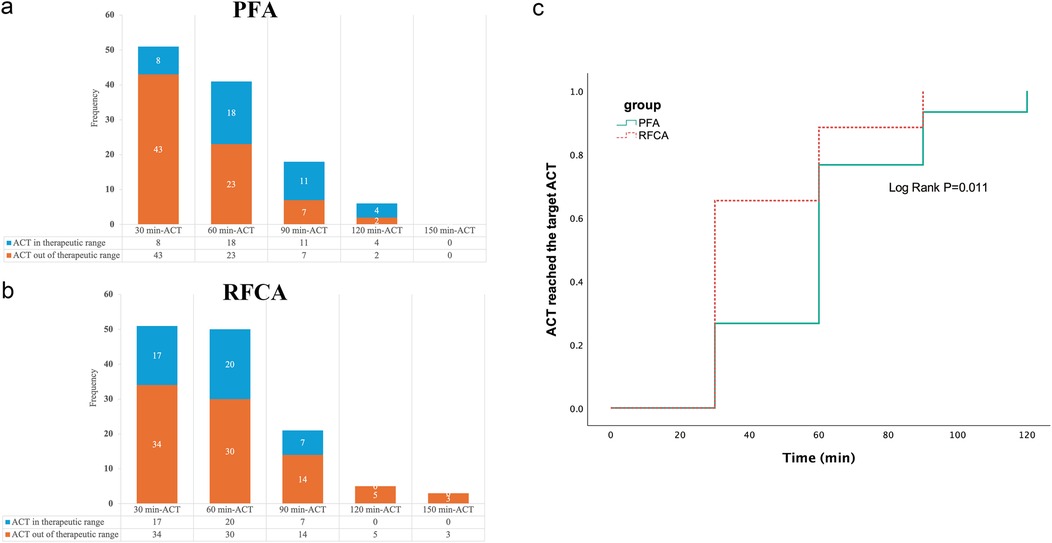
Figure 3. The number of ACT measurements and Kaplan–Meier analysis of the PFA group and RFCA group. (a) shows the number of ACT measurements and the percentage of ACTs in or out of the therapeutic range at each 30-minute interval in the PFA group, (b) shows the number of ACT measurements and the percentage of ACTs in or out of the therapeutic range at each 30-minute interval in the RFCA group and (c) shows that the patients in the PFA group required more time to achieve the target ACT than did the patients in the RFCA group.
In the subgroup analysis of the PFA group, no significant differences in 60 min-ACT, 90 min-ACT or 120 min-ACT were detected among A, B, or C, as shown in Table 3 and Figure 4A. No difference in the average percentage of measurements at the target ACT, ACT compliance, or percentage of initial ACTs in the therapeutic range was detected between the types of PFA (Table 3 and Figure 4B,C).

Figure 4. Intraprocedural ACTs from the subgroup analysis of the PFA group. (a) shows the intraprocedural ACTs of the four groups at each 30-minute interval, (b) shows the ACT compliance rate of the four groups and (c) shows the percentage of first measured ACT in the therapeutic range of the four groups. The percentages in the bar chart represent the proportion of all patients.
The required heparin dosage to achieve the target ACT, including the initial, additional, and total dose of heparin, was similar between the PFA group and the RFCA group (Table 4). The proportion of patients (78.4%) who needed an additional dose of heparin in the RFCA group was greater than that in the PFA group (56.9%, P = 0.02).
There was no significant difference in the incidence of bleeding or thromboembolic complications between the two groups (Table 5). Two inguinal hematomas were observed in the PFA group, and three inguinal hematomas (including one arteriovenous fistula) were observed in the RFCA group. No hematuria/hemoglobinuria was detected in the two groups.
In the present study, we retrospectively collected and analyzed the intraprocedural ACTs, periprocedural bleeding and thromboembolic complication rates, and heparin dosages in paroxysmal AF patients who underwent PFA at our center. The significant findings of our study were as follows: (1) PFA was more effective in terms of ablation and a longer fluoroscopy time, with no significant difference between the types of PFA catheters; (2) compared with RFCA, PFA was associated with longer intraprocedural ACTs, shorter initial ACTs, and fewer initial ACTs in the therapeutic range, independent of the type of PFA catheter; (3) the time to achieve the target ACT was longer in patients who underwent PFA than in those who underwent RFCA; and (4) the additional dose of heparin needed to achieve and maintain the target ACT was similar in patients who underwent PFA to those in patients who underwent RFCA. To our knowledge, this is the first study investigating the differences between PFA and RFCA in terms of intraprocedural ACTs and periprocedural bleeding and thromboembolic complication rates.
PVI has been considered essential in CA for AF since PV potentials have been confirmed to trigger paroxysmal AF and the monitoring of PV potentials has been proven paramount (13). Thermal ablation, predominantly RFCA, has been the main treatment method for CA of AF in the last two decades. However, some thermal-related complications, such as atrioesophageal fistula, esophageal perforation, and adjunctive nerve injury, may be severe or immediately life-threatening and may require emergency management. PFA is a novel and promising procedure (14, 15). Although data for PFA are still limited, the existing evidence indicates that the incidence of adverse extracardiac effects is expected to be significantly lower due to electroporation into cardiomyocytes (16).
Stroke and asymptomatic acute cerebral lesions are serious periprocedural thromboembolic events with an incidence of 0.1%–0.5% and 5%–30%, respectively, that cannot be ignored and have lifelong consequences (17). The incidence of MRI-detected brain lesions after thermal ablation of AF was nearly 30% (18, 19). In contrast to the significant decrease in the incidence of adjunctive tissue damage, the incidence of periprocedural thromboembolic events associated with PFA did not decrease significantly (20). The analysis of the neurological assessment subgroup in the ADVENT trial, which compared the cerebral impact of thermal and PFA ablation in treating PAF, revealed a comparable incidence of silent cerebral events (SCEs) and silent cerebral lesions (SCLs) following PVI ablation between PFA and RFCA (21). Therefore, safe and adequate intraprocedural anticoagulation management is also essential for PFA of AF. However, there is no consensus on the intraprocedural heparin dosing regimen and the target ACT for PFA, and few studies have focused on this issue.
The present intraprocedural anticoagulation protocol for PFA procedures is in accordance with the established guidelines for RFCA (22). The intraprocedural target ACT for PFA maybe clear (23). However, the appropriateness of current intraprocedural heparin regimens for PFA and whether intraprocedural ACTs are dependent on the type of PFA are still unclear. Different energy generates different lesions, consequently leading to diverse formation of microthrombi (24–26). In our study, we found that the PFA was associated with a longer intraprocedural ACT. However, the initial ACT and number of initial ACTs in the therapeutic range in PFA patients were lower than those in RFCA patients, and the time required to achieve the target ACT was longer in PFA than in RFCA. This could be attributed to real-time heparinized saline irrigation shortening the time to reach the target ACT during RFCA. In the later stages of RFCA, the formation of thrombi and microthrombi may have resulted in less pronounced increases in the ACT. The prolonged subsequent intraprocedural ACT observed in the PFA cohort may be attributed to the higher additional heparin bolus administered. Furthermore, the intraprocedural ACTs were found to be correlated with the heparin dosing regimens. Previous studies have indicated that a modified heparin dosing regimen may improve the ACT compliance rate and the required time to reach the target ACT (27–29). The ACT compliance rate in these modified regimens was higher than that observed in the present study, indicating that a novel heparin dosing regimen is a crucial component of the PFA.
In our study, three PFA catheters that were shaped differently were used: two circular shapes (tending to adhere to the thrombus) and one lotos shape, the latter of which was flexible and resulted in better catheter adherence and energy delivery. We also found that the PFA catheter used for saline irrigation in study A (Biosense Webster IRE Catheter) may have prolonged the ACT. PFA catheters are available in a variety of shapes and irrigated configurations, which result in different intraprocedural ACTs. As PFA becomes more widely used in clinical practice and post-market applications, it is essential to consider the impact of this variability on periprocedural anticoagulation management.
In our study, although the total amount of heparin administered was not significantly different between the two groups, the number of additional units of heparin administered to the PFA group was fewer than that administered to the RFCA group. PFA is a short procedure and may have been completed before an additional dose of heparin was administered. The discrepancy in the total heparin dosage was attributable to the necessity of a 30-minute observation period during clinical studies in the PFA cohort, during which additional heparin was administered to mitigate the risk of embolization. Thus, we suggest a higher initial dose of heparin for PFA. Moreover, according to the latest study of 337 patients undergoing PFA for AF, PFA may redefine the blanking period of AF ablation (30). This offer more benefits to AF patients, such as the reduced necessity for post-procedure anticoagulation therapy.
This study has several limitations. This was a single-center retrospective study with a small sample of patients. Due to the low incidence of hemorrhagic event and no thrombotic complication, it was underpowered to detect differences in periprocedural bleeding or thromboembolic complication rates. The incidence of silent cerebral ischemia after ablation was not evaluated. Multicenter prospective RCTs with a larger sample of patients and MRI-detected SCLs are needed to verify this hypothesis. Moreover, few measurements were at the target ACT in this study. This study exclusively examined intraprocedural ACTs for PFA and RFCA, and did not evaluate intraprocedural ACT management and anticoagulation strategies for other emerging technologies, such as cryoablation or laser ablation. This was due to the limited procedural volume and technical constraints. In addition, the study concentrated on the peri-procedural management, and a long-term follow-up was not conducted.
Compared with RFCA, PFA was associated with longer intraprocedural ACTs, shorter initial ACTs, fewer initial ACTs in the therapeutic range, and longer times to achieve the target ACT.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by First Affiliated Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CM: Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. XX: Conceptualization, Investigation, Writing – review & editing. QC: Data curation, Formal analysis, Writing – review & editing. WL: Software, Visualization, Writing – review & editing. ZW: Data curation, Methodology, Writing – review & editing. SD: Investigation, Writing – review & editing. YS: Investigation, Writing – review & editing. YX: Resources, Supervision, Validation, Writing – review & editing. LG: Conceptualization, Supervision, Validation, Writing – review & editing. XY: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2022YFC3601301).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACT, activated clotting time; AF, atrial fibrillation; AFL, atrial flutter; CA, catheter ablation; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NOAC, nonvitamin K antagonist oral anticoagulant; OAC, oral anticoagulants; PV, pulmonary vein; RCT, randomized controlled trials; RFCA, radiofrequency catheter ablation; SD, standard deviation; TIAs, transient ischemic attacks.
1. Du X, Guo L, Xia S, Du J, Anderson C, Arima H, et al. Atrial fibrillation prevalence, awareness and management in a nationwide survey of adults in China. Heart. (2021) 107(7):535–41. doi: 10.1136/heartjnl-2020-317915
2. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. Br Med J. (2016) 354:i4482. doi: 10.1136/bmj.i4482
3. Vinter N, Cordsen P, Johnsen SP, Staerk L, Benjamin EJ, Frost L, et al. Temporal trends in lifetime risks of atrial fibrillation and its complications between 2000 and 2022: Danish, nationwide, population based cohort study. Br Med J. (2024) 385:e077209. doi: 10.1136/bmj-2023-077209
4. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. (2012) 367(17):1587–95. doi: 10.1056/NEJMoa1113566
5. Blomström-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. J Am Med Assoc. (2019) 321(11):1059–68. doi: 10.1001/jama.2019.0335
6. Herrera Siklódy C, Arentz T, Minners J, Jesel L, Stratz C, Valina CM, et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: a randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm. (2012) 9(2):189–96. doi: 10.1016/j.hrthm.2011.09.017
7. Santoro F, Brunetti ND, Rillig A, Reissmann B, Lemeš C, Maurer T, et al. Stroke and left atrial thrombi after cryoballoon ablation of atrial fibrillation: incidence and predictors. Results from a long-term follow-up. J Thromb Thrombolysis. (2021) 51(1):74–80. doi: 10.1007/s11239-020-02148-x
8. Briceno DF, Villablanca PA, Lupercio F, Kargoli F, Jagannath A, Londono A, et al. Clinical impact of heparin kinetics during catheter ablation of atrial fibrillation: meta-analysis and meta-regression. J Cardiovasc Electrophysiol. (2016) 27(6):683–93. doi: 10.1111/jce.12975
9. Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FE, et al. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation. (2023) 147(19):1422–32. doi: 10.1161/CIRCULATIONAHA.123.063988
10. Verma A, Asivatham SJ, Deneke T, Castellvi Q, Neal RE 2nd. Primer on pulsed electrical field ablation: understanding the benefits and limitations. Circ Arrhythm Electrophysiol. (2021) 14(9):e010086. doi: 10.1161/CIRCEP.121.010086
11. Cochet H, Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Nakashima T, et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. (2021) 23(9):1391–9. doi: 10.1093/europace/euab090
12. Zhang RF, Ma CM, Wang N, Yang MH, Li WW, Yin XM, et al. Appropriate intraprocedural initial heparin dosing in patients undergoing catheter ablation for atrial fibrillation receiving uninterrupted non-vitamin-K antagonist oral anticoagulant treatment. BMC Cardiovasc Disord. (2021) 21(1):214. doi: 10.1186/s12872-021-02032-3
13. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339(10):659–66. doi: 10.1056/NEJM199809033391003
14. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. (2021) 7(5):614–27. doi: 10.1016/j.jacep.2021.02.014
15. Schmidt B, Bordignon S, Neven K, Reichlin T, Blaauw Y, Hansen J, et al. European real-world outcomes with pulsed field ablation in patients with symptomatic atrial fibrillation: lessons from the multi-centre EU-PORIA registry. Europace. (2023) 25(7):1–11. doi: 10.1093/europace/euad185
16. Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med. (2023) 389(18):1660–71. doi: 10.1056/NEJMoa2307291
17. Tzeis S, Gerstenfeld EP, Kalman J, Saad E, Shamloo AS, Andrade JG, et al. European heart rhythm association (EHRA)/heart rhythm society (HRS)/Asia pacific heart rhythm society (APHRS)/Latin American heart rhythm society (LAHRS) expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2024) 21:e31–149. doi: 10.1016/j.hrthm.2024.03.017
18. Haeusler KG, Eichner FA, Heuschmann PU, Fiebach JB, Engelhorn T, Callans D, et al. Detection of brain lesions after catheter ablation depends on imaging criteria: insights from AXAFA-AFNET 5 trial. Europace. (2023) 25(12):1–7. doi: 10.1093/europace/euad323
19. Nakamura T, Okishige K, Kanazawa T, Yamashita M, Kawaguchi N, Kato N, et al. Incidence of silent cerebral infarctions after catheter ablation of atrial fibrillation utilizing the second-generation cryoballoon. Europace. (2017) 19(10):1681–8. doi: 10.1093/europace/euw191
20. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner A, et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace. (2022) 24(8):1256–66. doi: 10.1093/europace/euac050
21. Patel C, Gerstenfeld EP, Gupta SK, Winterfield J, Woods C, Natale A, et al. Comparison of cerebral safety following atrial fibrillation using pulsed field and thermal ablation: results of the neurological assessment subgroup in the advent trial. Heart Rhythm. (2024) 21:2103–9. doi: 10.1016/j.hrthm.2024.05.048
22. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2018) 20(1):e1–160. doi: 10.1093/europace/eux274
23. Congxin H, Yangyang B, Kejiang C, Lin C, Minglong C, Shaojie C, et al. Expert consensus on clinical application and operation process of pulsed field ablation for atrial fibrillation. Chin J Card Pacing Electrophysiol. (2024) 38(4):235–48. doi: 10.13333/j.cnki.cjcpe.2024.04.001
24. Neven K, Füting A, Byrd I, Heil RW Jr, Fish JM, Feeney DA, et al. Absence of (sub-)acute cerebral events or lesions after electroporation ablation in the left-sided canine heart. Heart Rhythm. (2021) 18(6):1004–11. doi: 10.1016/j.hrthm.2021.02.015
25. Hsu JC, Gibson D, Banker R, Doshi SK, Gidney B, Gomez T, et al. In vivo porcine characterization of atrial lesion safety and efficacy utilizing a circular pulsed-field ablation catheter including assessment of collateral damage to adjacent tissue in supratherapeutic ablation applications. J Cardiovasc Electrophysiol. (2022) 33(7):1480–8. doi: 10.1111/jce.15522
26. Khairy P, Chauvet P, Lehmann J, Lambert J, Macle L, Tanguay JF, et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. (2003) 107(15):2045–50. doi: 10.1161/01.CIR.0000058706.82623.A1
27. Sairaku A, Morishima N, Matsumura H, Amioka M, Maeda J, Watanabe Y, et al. Intra-procedural anticoagulation and post-procedural hemoglobin fall in atrial fibrillation ablation with minimally interrupted direct oral anticoagulants: comparisons across 4 drugs. J Interv Card Electr. (2021) 61(3):551–7. doi: 10.1007/s10840-020-00851-6
28. Kishima H, Mine T, Fukuhara E, Ashida K, Ishihara M, Masuyama T. A novel protocol for initial heparin administration during catheter ablation for atrial fibrillation in patients taking direct oral anticoagulants. Heart Vessels. (2019) 34(5):832–41. doi: 10.1007/s00380-018-1294-2
29. Bradley CJ, Williamson BD, George J, Haines DE. Protocol driven periprocedural anticoagulation for left atrial ablation. J Cardiovasc Electrophysiol. (2021) 32(3):639–46. doi: 10.1111/jce.14892
Keywords: radiofrequency catheter ablation, pulsed field ablation, activated clotting time, atrial fibrillation, anticoagulant
Citation: Ma C, Xiao X, Chen Q, Li W, Wang Z, Dai S, Sun Y, Xia Y, Gao L and Yin X (2025) Intraprocedural activated clotting time and heparin dosage in pulsed field ablation of paroxysmal atrial fibrillation. Front. Cardiovasc. Med. 12:1501716. doi: 10.3389/fcvm.2025.1501716
Received: 25 September 2024; Accepted: 11 February 2025;
Published: 24 February 2025.
Edited by:
Laura Vitali Serdoz, Klinikum Fuerth, GermanyReviewed by:
Mario Matta, AOU Città della Salute e della Scienza, ItalyCopyright: © 2025 Ma, Xiao, Chen, Li, Wang, Dai, Sun, Xia, Gao and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomeng Yin, ZHIueWlueG1AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.