
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 February 2025
Sec. Hypertension
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1496534
This article is part of the Research Topic Target Organ Damage in Fabry Disease View all 12 articles
 Rosa Lillo1,2,†
Rosa Lillo1,2,† Alessio Cianci2,†
Alessio Cianci2,† Maria Chiara Meucci1
Maria Chiara Meucci1 Giulia Iannaccone1,2
Giulia Iannaccone1,2 Claudio Di Brango2
Claudio Di Brango2 Filippo Tusa2
Filippo Tusa2 Mario Marsilia2
Mario Marsilia2 Gaetano Antonio Lanza1,2
Gaetano Antonio Lanza1,2 Antonella Lombardo1,2
Antonella Lombardo1,2 Francesco Burzotta1,2,‡
Francesco Burzotta1,2,‡ Francesca Graziani1*‡
Francesca Graziani1*‡
Background: To date, only limited data are available on right atrium (RA) morphofunctional remodeling in Fabry disease (FD).
Purpose: We aimed to investigate RA structural and functional remodeling in patients with FD vs. healthy controls using 2D speckle tracking echocardiography (STE) and to explore whether any differences exist in FD patients with and without left ventricular hypertrophy (LVH).
Methods: We prospectively enrolled patients with FD and controls matched for age, sex, and cardiovascular risk factors. Patients with FD were divided in two groups according to the presence/absence of LVH (LVH+: left ventricular wall thickness >12 mm). All patients underwent standard echocardiography and STE analysis investigating the mechanics of all cardiac chambers, including RA reservoir, contractile and conduit strain.
Results: A total of 64 patients with FD (50% males; mean age 50 ± 17 years; 51.5% LVH+) and 64 control patients were included in the study. Focusing on right chambers, RA and right ventricular (RV) dimensions were similar between FD and controls. No differences were found for tricuspid annular plane systolic excursion (p = 0.073) and RV fractional area change (p = 0.461), while RV systolic Tissue Doppler velocity was reduced in patients with FD (p = 0.041). STE analysis revealed impaired strain values for all cardiac chambers in FD vs controls, specifically: left ventricular global longitudinal strain (LV-GLS, p < 0.001), left atrial (LA) reservoir strain (p = 0.001), conduit strain (p = 0.012), and contractile strain (p < 0.001), RV-GLS and RV free wall strain (p < 0.001). Similarly, all RA strain phases were significantly reduced in patients with FD compared with control patients (RA reservoir 27.4 ± 11.1 vs. 41.9 ± 8.3%, p < 0.001; RA contractile 9.9 ± 5.1 vs. 18.0 ± 4.9%, p < 0.001; RA conduit 19.1 ± 8.1 vs. 24.1 ± 8.1%, p = 0.001). When comparing FD patients without LVH to controls, it was found that RA reservoir and contractile strains were significantly reduced in the former (p < 0.001). In multivariable linear regression analyses, LA reservoir strain (p = 0.010) and LV-GLS (p = 0.044) emerged as independent correlates of RA mechanics after adjustments were made for RA dimensions, RV systolic function parameters and hypertrophy, and LV maximal wall thickness.
Conclusions: In FD impaired RA strain is a common finding. RA reservoir and contractile strains are reduced in FD patients even before LVH ensues, as compared to controls. LA reservoir strain and LV-GLS show an independent correlation with RA reservoir strain.
Anderson–Fabry disease (FD) is a rare X-linked lysosomal storage disorder caused by pathogenic variants of the α-galactosidase A gene, resulting in complete or partial deficiency of α-galactosidase A (α-Gal A) enzyme activity and consequent globotriaosylceramide (Gb3) accumulation (1). Overt cardiac involvement is usually defined by the presence of left ventricular hypertrophy (LVH) (2), but histological studies have proved that all cardiac chambers are affected by Gb3 storage (3, 4).
Two-dimensional speckle tracking echocardiography (2D-STE) is a powerful tool for the assessment of cardiac chamber mechanics, and the evaluation of LV global longitudinal strain (LV-GLS) is widely used in clinical practice. Also right ventricle (RV) and left atrium (LA) strains are emerging as novel markers of dysfunction in cardiomyopathies (5–8), and their impairment is linked to prognosis (9, 10). In the context of Fabry cardiomyopathy, LV (11) and RV strains (5) have already been investigated, as also LA strain (7, 12). Specifically, LA deformation can be impaired even before the occurrence of LVH and overt diastolic dysfunction (13). To date, only limited data are available on right atrium (RA) remodeling in FD. Therefore, our aim in this study is to assess RA structural and functional remodeling in patients with FD compared with sex and age-matched healthy controls (HCs) and to explore whether any differences exist in FD patients with and without LVH.
This is a prospective study performed at the Cardiac Rare Disease Outpatient Clinic, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, between July 2020 and March 2024. All patients with a diagnosis of FD were screened (n = 78). We excluded patients whose speckle tracking analysis was not feasible due to poor image quality (n = 14). FD was diagnosed by measuring plasma and leucocyte alfa-galactosidase A enzyme activity in males and confirmed by sequencing the GLA gene in all patients (14). All patients underwent a comprehensive clinical and echocardiographic evaluation. Patients with FD were divided in two groups according to the maximal LV wall thickness: those with LVH (defined as LV wall thickness >12 mm, LVH+) and those without LVH (LV wall thickness ≤12 mm, LVH−), who were regarded as genetic positive but phenotype negative (6). HCs identified from the students, nurses, and doctors of our center and matched for age, sex, and cardiovascular risk factors were included.
This study complied with the ethical principles of the Declaration of Helsinki, and it was approved by our local ethics committee. Informed written consent was obtained from all patients to participate in the study.
Comprehensive two-dimensional echocardiography was performed in accordance with current guidelines (15), and as previously described (7), by experienced cardiologists (MCM and GI). STE analysis was performed by experienced cardiologists (FG and RL), blinded to patients’ clinical characteristics, using the commercially available software, 2D Cardiac Performance Analysis© by TomTec-Arena TM (TomTec Imaging Systems, Unterschleissheim, Germany). A speckle tracking analysis was performed in all cardiac chambers in accordance with the latest recommendations (16–18). Briefly, to take the measurements of LV-GLS, images from apical four-, two,- and three-chamber views, zoomed on the LV and acquired with frame rates >50 frames/s, were used. The LV endocardial border was traced from an end-systolic frame and automatically tracked throughout the cardiac cycle by the software. The adequacy of tracking was manually verified and the region of interest properly adjusted. LV-GLS was obtained by averaging all segmental strain values and later by averaging the values calculated in each view. For the RV strain analysis, the average values of the longitudinal peak systolic strain from the three segments of the free wall (RV-FWS) and from all six segments of the free wall and septal wall of the RV (RV-GLS) were calculated (16).
LA strains were measured from the apical four-chamber view, and RA strains were measured from the RV-focused apical four-chamber view (16). The three components of atrial strain were identified from the created curves, as follows: reservoir strain was measured as the peak value during the cardiac cycle; contractile strain was assessed during the peak atrial contraction; conduit strain (strain during passive LV filling) was calculated as the difference between reservoir and contractile strains (16). In patients with atrial fibrillation (AF), measurements were obtained by averaging three consecutive cardiac cycles; in this group, atrial strain analysis was limited to the investigation of atrial reservoir and conduit strains, as recommended by guidelines (16) and performed in other studies (7, 19). Strain values are reported as absolute numbers throughout the text. According to current evidence, RA reservoir <25% was considered impaired (20).
Normally distributed continuous variables were presented as mean ± standard deviation and compared between two groups using an unpaired Student's t-test, whereas non-normally distributed data were expressed as median and interquartile range and compared using the Mann–Whitney U test. Categorical data were presented as frequencies and percentages, and a comparison between groups was performed by using χ2 test or Fisher's exact test, as appropriate. One-way analysis of variance with Bonferroni post-hoc tests was used to compare three groups (LVH+, LVH−, and HCs) when continuous variables were normally distributed. Alternatively, the Kruskal–Wallis test was performed when continuous variables were not normally distributed, with Bonferroni post-hoc correction.
Univariable and multivariable linear logistic regression analyses were performed to identify the clinical and echocardiographic determinants of RA reservoir strain in the overall population. Variables with a significant correlation in the univariable analysis (p < 0.05) were further investigated by using a multivariable model. All tests were two-sided, and p-values <0.05 were considered statistically significant. All analyses were performed using SPSS statistical software (SPSS version 23, Inc., Chicago, IL, USA).
A total of 64 patients with FD and 64 HCs were enrolled in the study. The main clinical characteristics of the overall FD population are reported in Table 1, while Supplementary Table S1 reports the differences between two groups of FD patients, LVH+ and LVH−. As shown, 50% of patients were male, and the mean age was 50 ± 17 years. The majority of the patients had a classic phenotype (71.8%) and were on specific therapy (57.8%). Roughly, a quarter of them were hypertensive (28.1%), only 8% had chronic kidney disease, and three patients previously underwent kidney transplantation. All but six patients (9.4%) were in sinus rhythm at the time of evaluation.
Echocardiography showed LVH in 33 patients (LVH+, 51.5%). Table S2 shows a comparison between echocardiographic measurements of the FD population vs. HCs. As expected, several differences emerged among them. Patients with FD had increased LV wall (12 IQR: 9–16 mm vs. 9 IQR: 8–10.9 mm, p < 0.001) and RV wall thickness values (4.9 ± 1.9 mm vs. 3.4 ± 0.5 mm, p < 0.001); 26 patients (40.6%) had right ventricular hypertrophy (RVH), all of whom were in the LVH group. With regard to the atria, patients with FD had increased left atrial volume index (LAVi) values (p < 0.001), while RA dimensions were similar between the two groups defined by RA area (15.3 ± 4 vs. 14.3 ± 2.3 cm2, p = 0.098) or volume index (23.0 ± 8.1 vs. 20.7 ± 5.2 ml/m2, p = 0.056).
The Tissue Doppler analysis revealed lower systodiastolic function indices in patients with FD compared with control patients, while LVEF values were similar in both groups. With regard to RV function, RV systolic velocity was lower in patients with FD (12.3 IQR: 11.0–13.7 cm/s vs. 13 IQR: 12–14 cm/s, p = 0.041), while tricuspid annular plane systolic excursion (TAPSE) values did not significantly differ between the FD population and HCs (p = 0.073), as also right ventricular fractional area change (RVFAC) values (p = 0.461). Pulmonary artery systolic pressure (PASP) was higher in patients with FD (even if mostly within normal range, p = 0.031), while TAPSE/PASP values were lower (p = 0.042). STE analysis revealed impaired values of all chambers strains in patients with FD compared to controls. Specifically with regard to the atria, patients with FD showed lower LA strain (LA reservoir p = 0.001, LA conduit p = 0.012, LA contractile p < 0.001) and RA strain values (RA reservoir: 27.4 ± 11.1% vs. 41.9 ± 8.3%, p < 0.001; RA contractile: 9.9 ± 5.1% vs. 18.0 ± 4.9%, p < 0.001; RA conduit 19.1 ± 8.1% vs. 24.1 ± 8.1%, p = 0.001) when compared with controls (Figure 1); in 27 patients with FD (42.1%), RA reservoir strain was <25%.
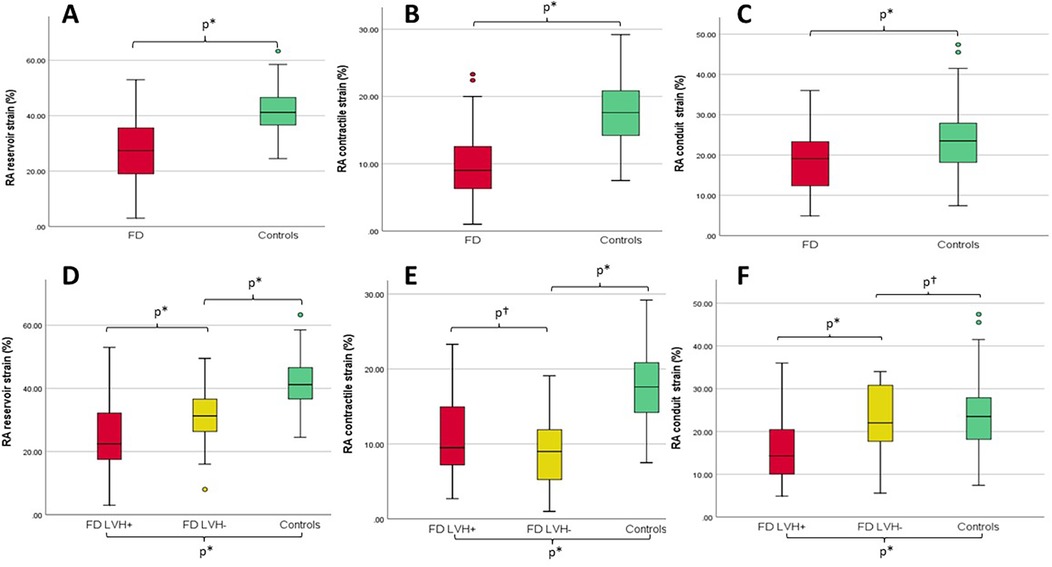
Figure 1. (A–C) Box plots with median and interquartile ranges of RA reservoir, contractile, and conduit strains for patients with FD (overall population, red) compared with controls (green), respectively. (D–F) Box plots with median and interquartile ranges of right RA reservoir, contractile, and conduit strains for FD patients with LVH (LVH+, red) vs. FD patients without LVH (LVH−, yellow) vs. controls (green), respectively. *p ≤ 0.05; †p = not statistically significant; RA, right atrium; LVH, left ventricular hypertrophy.
Even if FD patients with AF were excluded, all chambers strains were significantly reduced in these patients when compared with controls, as reported in Supplementary Table S3.
The main echocardiographic findings in LVH+ vs. LVH− vs. controls are summarized in Table 2.
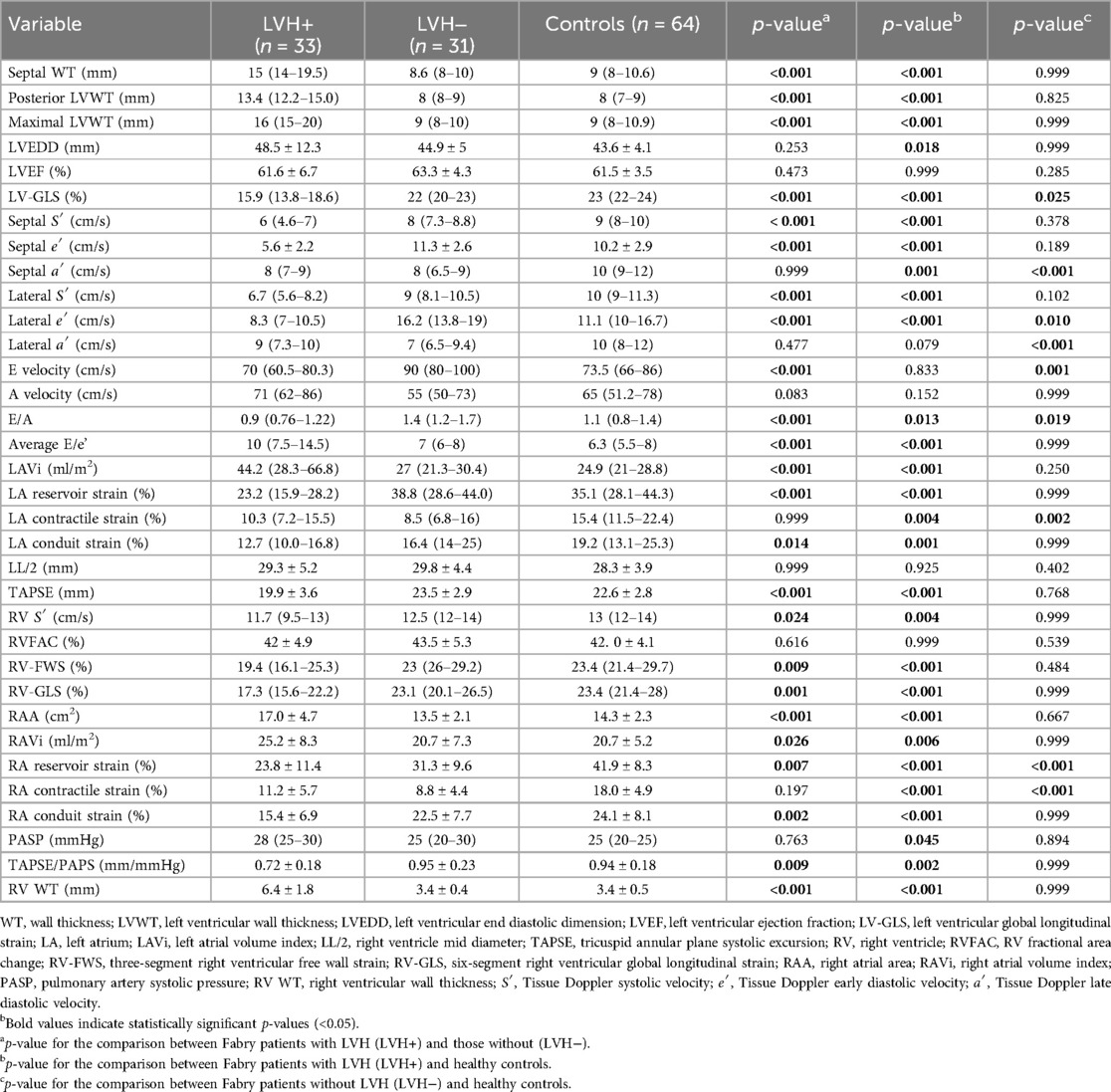
Table 2. Echocardiographic characteristics of Fabry patients with and without left ventricular hypertrophy and controls.
Among the LVH+ group of patients, the median maximal wall thickness was 16 mm (IQR: 15–20) and, as expected, several differences emerged between the three groups (LVH+ vs. LVH− vs. controls), as shown. Of interest, the LVH− patients differed from controls for lower values of LV-GLS (p = 0.025), lateral e′ (p = 0.010), septal and lateral a′ (p < 0.001), RA reservoir (31.3 ± 9.6 vs. 41.9 ± 8.3%, p < 0.001), and contractile (8.8 ± 4.4 vs. 18.0 ± 4.9%, p < 0.001) strains. Figure 2 shows an example of RA strain assessment in a patient with FD LVH+, in a patient with LVH−, and in an HC.
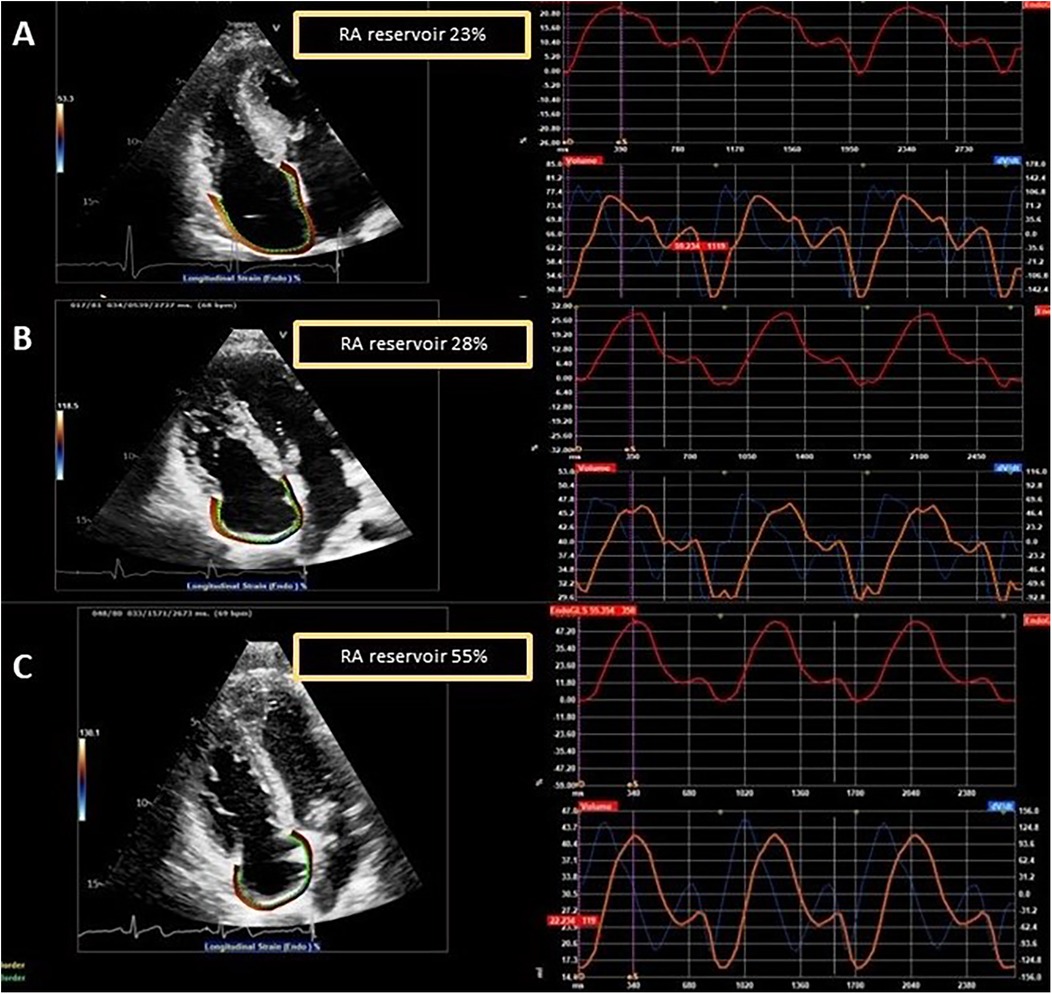
Figure 2. Examples of RA strain assessment in a patient with FD and left ventricular hypertrophy (LVH+, A), in a patient with FD without left ventricular hypertrophy (LVH−, B), and in a healthy control (C).
Supplementary Table S4 reports a univariable linear regression analysis for echocardiographic predictors of RA reservoir strain, while a multivariable analysis is presented in Table 3. LA reservoir strain (p = 0.010) and LV-GLS (p = 0.044) emerged as independent correlates of RA mechanics after adjustments were made for the parameters of RA dimensions, RV systolic function and hypertrophy, and LV maximal wall thickness. No independent predictors of RA reservoir strain have been identified among clinical variables, as reported in Supplementary Table S5.
In this study we investigated RA structural and functional remodeling in patients with FD vs controls. We found that in patients with FD, RA strains were reduced, while RA dimensions were similar between the two groups. Interestingly, RA reservoir and contractile strains were also significantly reduced in patients with FD LVH− when compared with healthy subjects.
Fabry cardiomyopathy is a pan-cardiac disease, as Gb3 accumulates in the lysosomes of all cardiac cellular types (21–23). Indeed, histologic studies have shown that glycosphingolipid deposition also affects atrial cardiomyocytes (24), causing atrial enlargement, impaired function, and a predisposition to supraventricular arrhythmias (25). During the last few decades, studies on patients with FD have mainly focused on left atrial involvement, showing that the mean LA size on echocardiography is greater than in age-matched control subjects (2). STE analysis showed that atrial deformation can be impaired even before the occurrence of LVH and diastolic dysfunction, and in patients with overt cardiomyopathy, LA mechanics impairment correlates with the degree of LVH (13, 18).
However, to date, only limited data are available on RA remodeling in FD. Recently, Mattig et al. (19) retrospectively analyzed the diagnostic accuracy of right heart and LA strain parameters to distinguish cardiac amyloidosis (CA) from FD. The authors found that atrial strain parameters were impaired in both patients with CA and those with FD, with patients with CA demonstrating significantly lower LA and RA strain values. Moreover, the authors demonstrated that a combination of standard and STE imaging, including RA strain (together with age, basal RV diameter, and global RV strain), showed the best diagnostic accuracy to distinguish the two diseases.
To the best of our knowledge, this is the first study specifically comparing RA strain in FD patients with and without LVH and controls. The factors that contribute to the impairment of RA mechanics in FD cannot be deduced by the limited data of our study, but we might speculate that the following may have a role: (1) the intrinsic involvement of the RA myocardium, which may affect RA compliance and contractility; (2) the RV systodiastolic dysfunction, affecting RA pressure and right “atrial afterload”; (3) hemodynamic factors influencing RA pressure reliant on LV and LA involvement (26). With regard to the last-mentioned point, the potential role of “left-sided” cardiomyopathy in RA mechanics is supported by the results of the present work, since LA reservoir strain and LV-GLS emerged as independent correlates of RA mechanics after adjustments for the parameters of RA dimensions, RV systolic function and hypertrophy, LV wall thickness.
The findings of this study add new data for the complex understanding of right-sided cardiac involvement in FD. As we demonstrated previously (27, 28), in FD, RV involvement parallels LV structural changes. Indeed, RVH is a feature of advanced disease (14), as suggested by the fact that it is detected in those with concomitant LVH and is associated with the LV mass index and Mainz Severity Score Index (27). Moreover, in our previous work (5), we found that the conventional parameters of RV systolic function, namely, TAPSE, RVFAC, and tissue Doppler imaging systolic velocity, are usually normal even when RVH is present, while 2D-STE is a more sensitive tool to unveil subtle RV systolic dysfunction, which turns out to be a common finding even when standard parameters are within normal ranges. Interestingly, in the present work, FD patients without LVH had impaired RA reservoir and contractile strain when compared with controls. Thus, we can speculate that RA strain impairement is an early sign of right-sided FD cardiac involvement. These pieces of evidence support the importance of 2D-STE for a comprehensive echocardiographic evaluation in this disease and the importance of assessing all cardiac chambers strains, including RA.
The main limitation of this work is the small sample size, which carries relevant statistical implications; however, this is a common disadvantage for studies on rare diseases. Moreover, the short follow-up interval and the limited number of major cardiac events did not allow us to assess the clinical and prognostic value of RA strain in FD. Previous studies (29) showed that LA strain is a predictor of AF and LA strain can be more useful in patients with normal LA volumes when compared with those with LA dilatation. In this context, future specifically designed studies aiming to assess the clinical value of RA strain in FD patients with and without RA dilatation could be of great interest.
In the present study, we decided to also include patients with AF and those who had undergone pacemaker implantation previously, with the aim to investigate RA mechanics in a real-world population that included patients with arrhythmias. In the AF group, the investigation was limited to atrial reservoir and conduit strains as recommended by guidelines (16) and performed in other studies on cardiomyopathies (7, 19). As expected, cases of AF and previous pacemaker implantation were found only in FD patients with LVH. Both supraventricular arrhythmias and pacing may alter atrial mechanics, and thus, these factors may have a role in influencing our results.
Lastly, cardiac magnetic resonance data obtained within 1 year from an echocardiographic evaluation were available only in a minority of patients, and hence, the correlation between RA mechanics and tissue characterization could not be assessed.
In FD, impaired RA strain is a common finding. The results of this study reveal that RA reservoir and contractile strains are reduced in FD patients even before LVH ensues, as compared to controls. LA reservoir strain and LV-GLS show an independent correlation with RA reservoir strain.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Comitato Etico Fondazione Policlinico universitario “Agostino Gemelli”- Università Cattolica del Sacro Cuore, Largo Gemelli 8 – 00168 Roma. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
RL: Data curation, Investigation, Writing – original draft, Formal Analysis, Methodology. AC: Data curation, Investigation, Writing – original draft, Conceptualization. MM: Conceptualization, Project administration, Writing – original draft. GI: Data curation, Methodology, Writing – original draft. CD: Data curation, Formal Analysis, Visualization, Writing – review & editing. FT: Conceptualization, Data curation, Investigation, Writing – review & editing. MM: Data curation, Investigation, Validation, Writing – review & editing. GL: Resources, Supervision, Validation, Writing – review & editing. AL: Investigation, Methodology, Supervision, Validation, Writing – review & editing. FB: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. FG: Investigation, Methodology, Resources, Software, Supervision, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Italian Ministry of Health Ricerca Corrente 2025, Project ID 2789889.
RL has received advisory board fees from Sanofi Genzyme, Takeda, and Shire; she has also received travel support from Amicus Therapeutics. FG has received research grants and advisory board/speaker fees from Takeda, Shire, Amicus Therapeutics, and Sanofi Genzyme.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1496534/full#supplementary-material
1. Pieroni M, Moon JC, Arbustini E, Barriales-Villa R, Camporeale A, Vujkovac AC, et al. Cardiac involvement in Fabry disease: JACC review topic of the week. J Am Coll Cardiol. (2021) 77(7):922–36. doi: 10.1016/j.jacc.2020.12.024
2. Lillo R, Pieroni M, Camporeale A, Ciabatti M, Lombardo A, Massetti M, et al. Echocardiography in Anderson-Fabry disease. Rev Cardiovasc Med. (2022) 23(6):201. doi: 10.31083/j.rcm2306201
3. Ajmone Marsan N, Graziani F, Meucci MC, Wu HW, Lillo R, Bax JJ, et al. Valvular heart disease and cardiomyopathy: reappraisal of their interplay. Nat Rev Cardiol. (2024) 21(1):37–50. doi: 10.1038/s41569-023-00911-0
4. Sheppard MN, Cane P, Florio R, Kavantzas N, Close L, Shah J, et al. A detailed pathologic examination of heart tissue from three older patients with Anderson-Fabry disease on enzyme replacement therapy. Cardiovasc Pathol. (2010) 19(5):293–301. doi: 10.1016/j.carpath.2009.05.003
5. Lillo R, Graziani F, Panaioli E, Mencarelli E, Pieroni M, Camporeale A, et al. Right ventricular strain in Anderson-Fabry disease. Int J Cardiol. (2021) 330:84–90. doi: 10.1016/j.ijcard.2021.02.038
6. Meucci MC, Lillo R, Lombardo A, Lanza GA, Bootsma M, Butcher SC, et al. Comparative analysis of right ventricular strain in Fabry cardiomyopathy and sarcomeric hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2023) 24(4):542–51. doi: 10.1093/ehjci/jeac151
7. Meucci MC, Lillo R, Mango F, Marsilia M, Iannaccone G, Tusa F, et al. Left atrial structural and functional remodelling in Fabry disease and cardiac amyloidosis: a comparative analysis. Int J Cardiol. (2024) 402:131891. doi: 10.1016/j.ijcard.2024.131891
8. Lillo R, Meucci MC, Malara S, Primiano G, Servidei S, Lombardo A, et al. Early cardiac mechanics abnormalities in patients with mitochondrial diseases. Mitochondrion. (2024) 22:101940. doi: 10.1016/j.mito.2024.101940
9. Huntjens PR, Zhang KW, Soyama Y, Karmpalioti M, Lenihan DJ, Gorcsan J III. Prognostic utility of echocardiographic atrial and ventricular strain imaging in patients with cardiac amyloidosis. JACC Cardiovasc Imaging. (2021) 14(8):1508–19. doi: 10.1016/j.jcmg.2021.01.016
10. Raafs AG, Vos JL, Henkens MTHM, Slurink BO, Verdonschot JAJ, Bossers D, et al. Left atrial strain has superior prognostic value to ventricular function and delayed-enhancement in dilated cardiomyopathy. JACC Cardiovasc Imaging. (2022) 15(6):1015–26. doi: 10.1016/j.jcmg.2022.01.016
11. Krämer J, Niemann M, Liu D, Hu K, Machann W, Beer M, et al. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur Heart J. (2013) 34:1587–96. doi: 10.1093/eurheartj/eht098
12. Bernardini A, Camporeale A, Pieroni M, Pieruzzi F, Figliozzi S, Lusardi P, et al. Atrial dysfunction assessed by cardiac magnetic resonance as an early marker of Fabry cardiomyopathy. JACC Cardiovasc Imaging. (2020) 13(10):2262–4. doi: 10.1016/j.jcmg.2020.05.011
13. Boyd AC, Lo Q, Devine K, Tchan MC, Sillence DO, Sadick N, et al. Left atrial enlargement and reduced atrial compliance occurs early in Fabry cardiomyopathy. J Am Soc Echocardiogr. (2013) 26(12):1415–23. doi: 10.1016/j.echo.2013.08.024
14. Meucci MC, Lillo R, Del Franco A, Monda E, Iannaccone G, Baldassarre R, et al. Prognostic implications of the extent of cardiac damage in patients with Fabry disease. J Am Coll Cardiol. (2023) 82(15):1524–34. doi: 10.1016/j.jacc.2023.07.026
15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39. doi: 10.1016/j.echo.2014.10.003
16. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19(6):591–600. doi: 10.1093/ehjci/jey042
17. Iannaccone G, Graziani F, Del Buono MG, Camilli M, Lillo R, Caffè A, et al. Left atrial strain analysis improves left ventricular filling pressures non-invasive estimation in the acute phase of Takotsubo syndrome. Eur Heart J Cardiovasc Imaging. (2023) 24(6):699–707. doi: 10.1093/ehjci/jead045
18. Pichette M, Serri K, Pagé M, Di LZ, Bichet DG, Poulin F. Impaired left atrial function in Fabry disease: a longitudinal speckle-tracking echocardiography study. J Am Soc Echocardiogr. (2017) 30(2):170–9.e2. doi: 10.1016/j.echo.2016.10.014
19. Mattig I, Steudel T, Klingel K, Barzen G, Frumkin D, Spethmann S, et al. Right heart and left atrial strain to differentiate cardiac amyloidosis and Fabry disease. Sci Rep. (2024) 14(1):2445. doi: 10.1038/s41598-024-52890-y
20. Krittanawong C, Maitra NS, Hassan Virk HU, Farrell A, Hamzeh I, Arya B, et al. Normal ranges of right atrial strain: a systematic review and meta-analysis. JACC Cardiovasc Imaging. (2023) 16(3):282–94. doi: 10.1016/j.jcmg.2022.06.022.21
21. Pieroni M, Ciabatti M, Graziani F, Camporeale A, Saletti E, Lillo R, et al. The heart in Fabry disease: mechanisms beyond storage and forthcoming therapies. Rev Cardiovasc Med. (2022) 23(6):196. doi: 10.31083/j.rcm2306196
22. Graziani F, Leccisotti L, Lillo R, Bruno I, Ingrasciotta G, Leone AM, et al. Coronary microvascular dysfunction is associated with a worse cardiac phenotype in patients with Fabry disease. JACC Cardiovasc Imaging. (2022) 15(8):1518–20. doi: 10.1016/j.jcmg.2022.03.004
23. Lillo R, Ingrasciotta G, Locorotondo G, Lombardo A, Graziani F. An unusual case of mitral valve chordal rupture. Echocardiography. (2021) 38(12):2109–11. doi: 10.1111/echo.15228
24. Linhart A, Elliott PM. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart. (2007) 93(4):528–35. doi: 10.1136/hrt.2005.063818
25. Akhtar MM, Elliott PM. Anderson-Fabry disease in heart failure. Biophys Rev. (2018) 10(4):1107–19. doi: 10.1007/s12551-018-0432-5
26. Olsen FJ, Biering-Sørensen T. Right atrial strain: tapping into a new reservoir of hemodynamic information. Int J Cardiol. (2021) 326:226–8. doi: 10.1016/j.ijcard.2020.11.009
27. Graziani F, Laurito M, Pieroni M, Pennestrì F, Lanza GA, Coluccia V, et al. Right ventricular hypertrophy, systolic function, and disease severity in Anderson-Fabry disease: an echocardiographic study. J Am Soc Echocardiogr. (2017) 30(3):282–91. doi: 10.1016/j.echo.2016.11.014
28. Graziani F, Lillo R, Panaioli E, Pieroni M, Camporeale A, Verrecchia E, et al. Prognostic significance of right ventricular hypertrophy and systolic function in Anderson-Fabry disease. ESC Heart Fail. (2020) 7(4):1605–14. doi: 10.1002/ehf2.12712
Keywords: Anderson–Fabry disease, right atrium, speckle tracking echocardiography, strain, cardiomyopathy
Citation: Lillo R, Cianci A, Meucci MC, Iannaccone G, Di Brango C, Tusa F, Marsilia M, Lanza GA, Lombardo A, Burzotta F and Graziani F (2025) Right atrial strain in Anderson–Fabry disease. Front. Cardiovasc. Med. 12:1496534. doi: 10.3389/fcvm.2025.1496534
Received: 14 September 2024; Accepted: 17 January 2025;
Published: 18 February 2025.
Edited by:
Evgeny Belyavskiy, MVZ of the German Heart Center of the Charité GmbH, GermanyReviewed by:
Luca Arcari, M.G. Ospedale Vannini, ItalyCopyright: © 2025 Lillo, Cianci, Meucci, Iannaccone, Di Brango, Tusa, Marsilia, Lanza, Lombardo, Burzotta and Graziani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Graziani, ZnJhbmNlc2NhLmdyYXppYW5pQHBvbGljbGluaWNvZ2VtZWxsaS5pdA==
†These authors have contributed equally to this work and share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.