
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 27 February 2025
Sec. Cardiovascular Epidemiology and Prevention
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1489382
This article is part of the Research TopicNon-Pharmacological Approaches for Cardiovascular Health in Underrepresented PopulationsView all 6 articles
Background: Previous studies have shown that exercise can improve arterial stiffness (AS). However, it remains unclear which type of exercise is most effective for managing AS, particularly in individuals at high risk for cardiovascular diseases (CVD). This review aims to evaluate the effects of various exercises on AS and related variables in individuals at high risk for CVD.
Methods: A comprehensive search strategy was employed to systematically explore MEDLINE (PubMed), Embase, Cochrane Library, EBSCOhost, and Web of Science to identify relevant studies. Inclusion criteria were: (1) randomized controlled trials; (2) participants with known CVD risk factors as per the American College of Sports Medicine guidelines; (3) interventions including interval training (INT), aerobic exercise (AE), resistance exercise, and combined exercise (CT); (4) control groups engaging in no intervention, routine care, or health education; (5) outcome measures of pulse wave velocity (PWV), systolic blood pressure (SBP), and diastolic blood pressure; and (6) studies published in English. Studies were assessed using the Cochrane risk of bias tool and analyzed with a random-effects network meta-analysis.
Results: The review included 2,034 participants from 43 studies. Both CT [standardized mean difference (SMD) = −0.98, p < 0.001, I2 = 84%] and INT (SMD = −0.77, p < 0.001, I2 = 61%) significantly reduced PWV, but both showed considerable heterogeneity. INT (SMD = −0.382, p < 0.001, I2 = 45%) and AE (SMD = −0.369, p < 0.001, I2 = 43%) significantly reduced SBP. Surface under the cumulative ranking curve (SUCRA) showed that CT (SUCRA = 87.2) was the most effective for lowering PWV, while INT (SUCRA = 81.3) was the most effective for lowering SBP.
Conclusion: In high-risk populations for CVD, CT was most effective in improving AS, while INT demonstrated the greatest reduction in SBP. AE showed greater benefits at moderate to low intensities. Due to significant heterogeneity in CT, its results should be interpreted with caution. Further research with larger sample sizes is needed to confirm these findings.
Arterial stiffness (AS) is a critical predictor of cardiovascular disease (CVD) risk, independent of other factors. An increase of 1 m/s in pulse wave velocity (PWV), a primary measure of AS, corresponds to a 12%–14% rise in cardiovascular events and a 13%–15% increase in CVD-related mortality (1). While drug therapy is commonly used to manage AS, its early detection remains challenging. Consequently, preventive strategies, such as dietary changes and exercise, are increasingly important.
Research shows that exercise can improve AS by enhancing arterial remodeling and endothelial function, reducing sympathetic nervous system tone, and lowering inflammatory cytokines (2). However, there is debate about the most effective exercise interventions for different populations, particularly those at high risk for CVD.
Aerobic exercise (AE) is often recommended for improving vascular function due to its effects on vasodilators like calcium (Ca2+), potassium (K+), hydrogen ions (H+), and carbon dioxide (CO2) (3). Interval training (INT) may offer even greater benefits for AS and blood pressure than AE (4). Resistance training (RT) presents mixed results; high-intensity RT might increase blood pressure while reducing AS (5), but it could also induce arteriosclerosis in younger individuals without affecting older adults (3, 6). Combined training (CT), which includes both AE and RT, is considered highly effective for improving AS (7), with the order of exercises affecting results—CT with AE following RT shows better outcomes than the reverse (8).
Several systematic reviews have examined the impact of various exercise interventions on AS. Saz-Lara et al. (3) reviewed AE, RT, INT, CT, physical and mental exercises, and stretching in healthy adults. Montero et al. (9, 10) focused on AE in obese and hypertensive individuals, while Miyachi (6) and Evans et al. (11) reviewed RT in both healthy individuals and those at risk for CVD. Way et al. (12) assessed high-intensity INT and moderate-intensity AE, while Ashor et al. (13) and Zhang et al. (2) reviewed AE, RT, and CT. Ashor et al. focused on healthy and at-risk populations, while Zhang et al. focused on CVD patients. However, both studies lacked detailed analysis of long-term cardiac function and specific subpopulations. The current review addresses these gaps because many existing reviews either focus on narrow groups or lack comprehensive comparisons for high-risk CVD populations.
This study aims to address this gap by referring to the American College of Sports Medicine (ACSM) guidelines to identify high-risk populations, including older adults, those with diabetes, obesity, hypertension, and metabolic syndrome, and postmenopausal women. We conducted a systematic review and network meta-analysis (NMA) of randomized controlled trials (RCTs) to evaluate the effects of INT (14–25), AE (15, 26–35), RT (31, 36–43), and CT (44–55) on AS in these high-risk populations. In addition, we assessed systolic blood pressure (SBP) and diastolic blood pressure (DBP) as secondary outcomes. Our goal is to provide practical recommendations for managing AS in individuals at elevated risk for CVD.
The study protocol was registered with PROSPERO (CRD42023417622) (56). This systematic review and NMA were conducted in accordance with the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA-NMA).
The MEDLINE (PubMed), Embase, Cochrane Library, EBSCO, and Web of Science databases were systematically searched for relevant articles using a comprehensive electronic search strategy, as shown in Supplementary Appendix 2. The search strategy was based on key phrases related to the PICOS tool: (P) Population: “Hypertension” OR “obesity” OR “Type 2 diabetes” OR “T2D” OR “metabolic syndrome” OR “Older persons over 60 years”; (I) Intervention: “physical activity” OR “training” OR “aerobic exercise” OR “moderate intensity continuous training” OR “moderate interval training” OR “high interval training” OR “resistance training” OR “strength training” OR “combined training” or “sprint interval training” or “high intensity interval training”; (C) Comparator: “control group” or “no exercise” or “usual care”; (O) Outcomes: “arterial stiffness” or “pulse wave velocity” or “PWV”; and (S) Study type: “randomized controlled trial”, “randomized”, “placebo”, “RCT”. The search was limited to English-language articles published between the inception of the databases up to February 2024. We included RCTs comparing different types of exercise on AS in high-risk populations of CVD. In addition, we retrieved similar review articles and screened their references to maximize the completeness of the sources.
Duplications were initially eliminated using EndNote X9 software (Clarivate Analytics, Philadelphia, PA, USA). Subsequently, two researchers (R-SW and X-LF) independently screened titles and abstracts to identify all potentially relevant studies. The same two reviewers independently identified and assessed studies that met the inclusion criteria. Disagreements were resolved through discussion or consultation with a third expert (XY), if necessary. The detailed inclusion criteria were as follows: (1) all studies had to be RCTs; (2) subjects had known risk factors associated with CVD according to the American College of Sports Medicine guidelines; (3) interventions included INT, AE, RT, and CT; (4) the comparator received no intervention, usual care, or health education; (5) outcomes included PWV, SBP, and DBP; and (6) the studies had to be written in English.
The exclusion criteria were as follows: (1) the subjects of the study were healthy adults; (2) the type of experiment was animal experiments or randomized crossover experiments; (3) the effect of exercise intervention was acute (<3 weeks); (4) incomplete data or no control group; (5) returned reviews, duplicate publications, letters to the editor, and meta-analysis articles.
In the included randomized controlled trials, exercise interventions comprised INT, AE, RT, and CT. As depicted in Table 1, we operationally defined these four modalities of exercise intervention as follows.
The primary outcome assessed in this study included PWV, with secondary outcomes being SBP and DBP. There are several types of PWV: carotid–femoral pulse wave velocity (cfPWV), brachial-ankle pulse wave velocity (baPWV), central pulse wave velocity (cPWV), central-brachial pulse wave velocity (cbPWV), etc. In general, cfPWV is considered the first gold-standard measure of AS. The cfPWV is defined as the ratio of the distance between the measurement sites to the time difference for the distal pulse wave to reach the measuring sites. The cutoff point for AS was defined as a PWV value exceeding 10 m/s (58).
In this study, PWV primarily refers to the measurement of cfPWV. Since we also included baPWV indicators, we implemented a data transformation method to reconcile the measurement discrepancies between these two metrics. Specifically, we utilized the regression equation to achieve accurate data correction (59). This method effectively addresses the variations in measurements, thereby improving the reliability of our results. The term SBP refers to the maximum arterial pressure during ventricular contraction, while DBP represents the arterial pressure when the heart is in a relaxed state. Pulse pressure (PP) is defined as the difference between SBP and DBP (60). The criteria for Stage I hypertension were defined as SBP of 130–139 mmHg and/or DBP of 80–89 mmHg. These parameters were incorporated into the outcomes and served as the indicators for assessing AS.
The data were extracted independently by two investigators (R-SW and XY), with any disagreements resolved through consensus or the input of a third author (X-LF) if necessary. Information collected included the first author, publication year, country, subject characteristics (sample size, gender, age, PWV, SBP, DBP, and concomitant diseases), intervention details (exercise type, intensity, duration, frequency, and supervision status), and outcome measures reported in each eligible study, as shown in Supplementary Appendix 4. In cases where information was insufficiently provided by studies included in this review article, email communication was initiated to obtain missing values.
The risk of bias (ROB) of the included studies was evaluated by two researchers based on the Cochrane Risk of Bias assessment tool (61), which includes the following seven domains, encompassing seven distinct domains: (a) allocation generation, (b) allocation concealment, (c) blinding of participants and personnel, (d) blinding of outcome assessment, (e) handling of incomplete outcome data, (f) freedom from selective reporting bias, and (g) other forms of bias. These key domains were considered key domains for assessing the risk of bias. However, due to the inherent difficulty in blinding participants to an exercise intervention, this component was not included in the overall risk of bias score. Subsequently, we categorized each study's overall risk of bias into three levels: low risk if none of the above domains were rated as high risk and ≤3 domains were rated as unclear; moderate risk if one study was rated as high risk or no study was rated as high risk but ≥4 studies were rated as unclear; and high risk for all other cases (62).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was utilized to evaluate the certainty of the evidence contributing to the network estimates for both primary and secondary outcomes (63).
The experimental effect was estimated by combining the pre-to-post changes of both the experimental and CON. The standard deviation (SD) of the change value was calculated using the formula provided in the Cochrane Handbook (version 6.3) (64) [the formula is ].
Heterogeneity among studies was assessed using network estimates and pairwise meta-analytic techniques with Review Manager 5.3 (Nordic Cochrane, Denmark). A sensitivity analysis was also conducted to explore this heterogeneity (65). Pooled effect estimates were calculated using a random-effects model, and mean difference (MD) values were determined for PWV, SBP, and DBP. Heterogeneity was quantified using the I2 statistic and Cochran's Q-test, with significant heterogeneity defined as an I2 >50% or a p-value ≤0.10 (66). Publication bias was evaluated with a funnel plot and Begg's test.
Statistical analysis was performed using STATA 16.0 (STATA Corp, College Station, TX, USA) through a frequentist framework and random-effects multivariate NMA (67). Weighted mean differences were reported for continuous variables, with pooled effect estimates accompanied by 95% confidence intervals (CIs) and 95% prediction intervals.
Exercise interventions were compared using network geometry, with lines connecting nodes representing direct relationships. The size of each node and the thickness of connecting lines reflected the number of studies (68).
Inconsistencies were estimated using the loop-specific approach, node-splitting technique, and global inconsistency method to assess ring, local, and global inconsistencies (69). A p-value <0.05 indicated that the inconsistency requirement was met, permitting further NMA analysis (70). The transitivity assumption, which assumes valid indirect comparisons and uniform effect modifiers, was evaluated using a consistency model to ensure random allocation of interventions (70, 71). The network contribution diagram calculated the impact of each direct comparison on both individual and overall NMA results.
The cumulative ranking curve (SUCRA) was used to rank the effects of different exercise modalities (64). SUCRA values range from 0 to 100, with higher values indicating better outcomes (72). Publication bias in the network meta-analysis was assessed using a funnel plot and symmetry criterion.
A total of 1,433 articles were retrieved from the database, while an additional 7 articles were obtained through alternative sources, resulting in a combined pool of 1,440 articles for screening. After eliminating duplicate entries, a total of 845 unique articles remained. Subsequently, after reviewing the titles and abstracts, 568 articles were excluded, leaving us with 277 potential candidates. Further evaluation of the full-text content led to the exclusion of an additional 234 articles, resulting in only 43 remaining articles that met our criteria. In conclusion, 43 eligible articles were ultimately identified as per Figure 1.
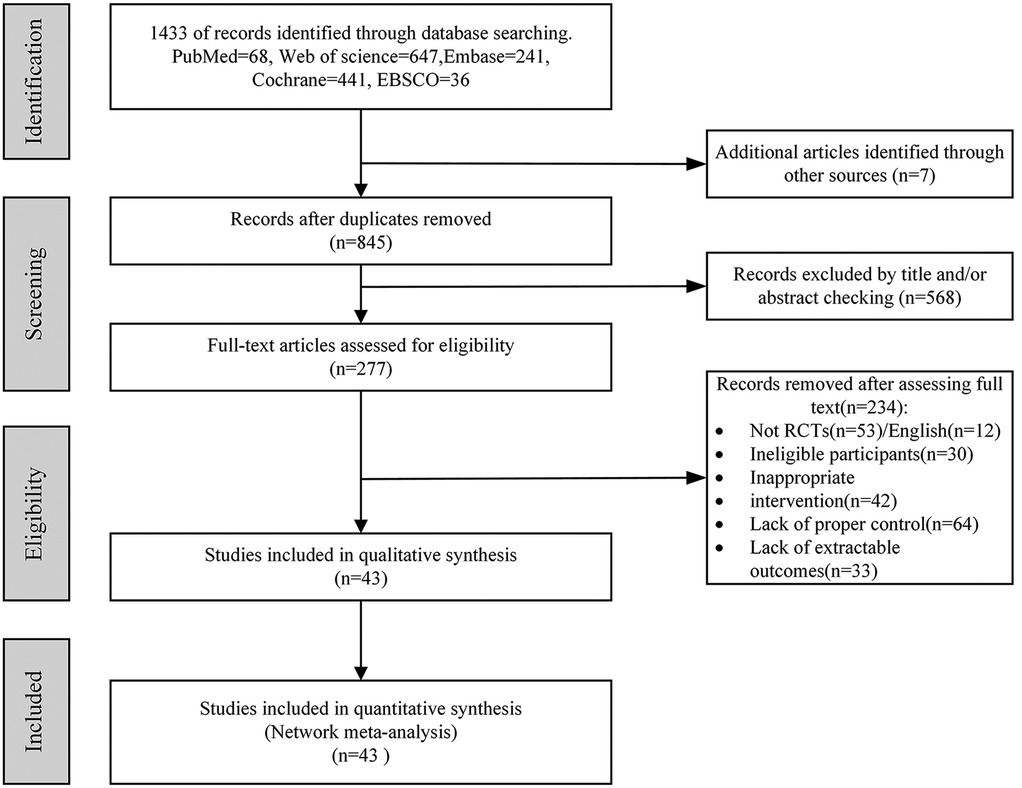
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of each stage of the study selection. RCT, randomized controlled trial.
Supplementary Appendix 4 provides details on the studies included, conducted between 2003 and 2023 across various regions: Asia (12), North America (13), South America (2), Europe (11), Oceania (1), Latin America (2), and Africa (2). The studies involved 2,034 high-risk CVD participants. The experimental group included 1,056 subjects, divided into the following groups: INT (365), AE (327), RT (149), and CT (215). The CON group included 978 subjects.
Participants were about 51% women, aged 10–75 years. Six studies focused solely on men, while 37 studies included both genders. All studies followed the ACSM guidelines for high-risk cardiovascular populations, considering obesity, diabetes, hypertension, and advanced age as risk factors.
Interventions included INT, AE, RT, and CT (AE + RT) at moderate to high intensity. Exercise duration ranged from 8 to 96 weeks, with activities such as running, cycling, rowing, swimming, stepping exercises, and resistance training. Sessions were held three to seven times per week, lasting 30–90 min each. Control groups received usual care or no exercise.
Of the studies, 33 used supervised exercise, 3 combined supervision with home-based interventions, and 7 had no supervision. Carotid–femoral PWV was measured using applanation tonometry (SphygmoCorCPV, ATCor Medical) or SphygmoCor software (version 9.0). Measurements were taken using a high-fidelity micromanometer (Millar Instruments), calculating PWV by dividing the distance between the carotid and femoral recording sites by the time delay between their pulse waves (71).
The ROB assessments for each study are presented in Supplementary Appendix 5. Two studies exhibited a high risk of bias in randomization serialization, while the concealment of allocation methods was reported in 15 studies. Blinding of outcome assessment had a low risk of bias in 30 studies, whereas 7 studies showed a high risk of bias regarding missing outcome values. Selective reporting demonstrated a low risk of bias in 10 studies. In addition, sample sizes smaller than n < 10 or significant errors in the measurement process were considered to have a high risk of other biases, with only 33 studies showing a low risk of bias. In summary, 24 articles were evaluated as having low ROB, while moderate and high ROB were observed in 14 and 5 articles, respectively.
As shown in Figure 2, the forest plots of different exercise intervention effects on PWV report the differences and heterogeneity of intervention effects of the four exercise interventions (INT, AE, RT, and CT) compared to the CON group.
Compared with the CON group, INT [standardized mean difference (SMD) = −0.77, p < 0.0001, 95% CI (−1.17 to −0.36), I2 = 61%], AE [SMD = −0.25, p = 0.000, 95% CI (−0.50 to 0.00), I2 = 80%], and CT [SMD = −0.98, p < 0.00001, 95% CI (−0.73 to −0.35), I2 = 84%] all significantly reduced PWV, with high heterogeneity. RT [SMD = −0.28, p = 0.11, 95% CI (−0.46 to −0.10), I2 = 38%] reduced PWV, with low heterogeneity. The Funnel plot and Begg's test in Supplementary Appendix 6 showed publication bias in the CT group (p < 0.001).
To further verify the sources of heterogeneity, we conducted sensitivity analyses, subgroup analyses, and meta-regression (65). First, in the sensitivity analysis, we sequentially identified the influence of each study on the overall heterogeneity and performed a meta-analysis on the remaining studies (n − 1). By observing changes in the combined results, we evaluated whether the original meta-analysis was significantly influenced by certain studies. The sensitivity analysis identified significant sources of heterogeneity in two intervention methods: INT and AE. Specifically, in AE, heterogeneity (I2 < 50%) was reduced after excluding either Deiseroth et al. (19) or Taha et al. (25), and in INT, heterogeneity was reduced after excluding Kearney et al. (27) (Supplementary Appendix 7.2). A detailed review of these three studies revealed that none of them used cfPWV as the AS measurement index, and all focused on elderly populations. This suggests that the measurement index of AS and the elderly population may be significant factors contributing to heterogeneity.
Second, since the sensitivity analysis did not identify any significant factors contributing to heterogeneity in CT, we conducted subgroup analyses and meta-regression based on PWV assessment site, intervention intensity, duration, content, and participant characteristics (e.g., gender, age) to explore potential sources of heterogeneity (Table 2). However, in these efforts, no significant factors were found. This suggests that a single low-quality study can increase heterogeneity by producing results that significantly differ from other similar studies. Therefore, we speculate that the study quality might have contributed to the heterogeneity (Supplementary Appendix 7.4). Therefore, the heterogeneity in CT is acknowledged as a limitation of this study and warrants further exploration in future research.
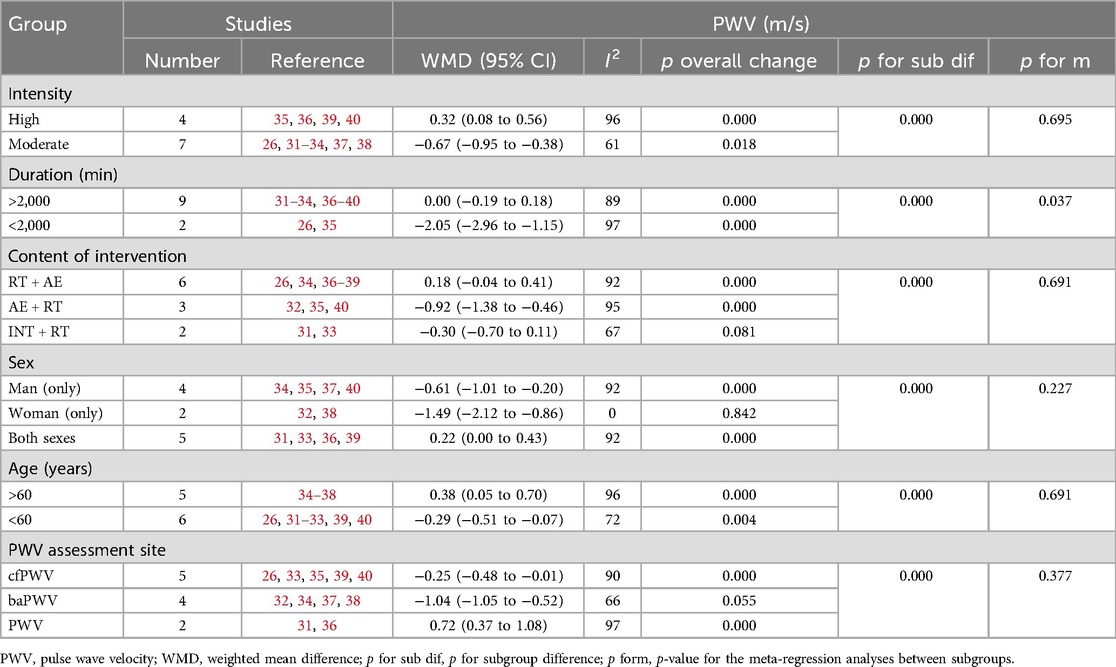
Table 2. Subgroup analyses assessing potential moderating factors for PWV in studies included in the meta-analysis.
The forest plots illustrating the intervention effects of different exercise modalities on secondary indicators (SDP and SBP) are given in Supplementary Appendix 7. Among the four types of exercise interventions (INT, AE, RT, and CT), AE demonstrated a significant reduction in SBP, while CT showed a significant decrease in DBP. The remaining exercise interventions did not have a statistically significant effect on either SBP or DBP. Funnel plot analysis and Begg's test revealed publication bias for RT in both SBP and DBP (p < 0.001), whereas no publication bias was observed in other subgroups (Supplementary Appendix 5).
The primary focus of this study was on PWV (primary index) and blood pressure parameters, specifically SBP and DBP (secondary index), which were subjected to NMA. The supporting materials for PWV, SBP, and DBP primarily consisted of qualified network evidence plots, loop-special approaches, node-splitting techniques, global inconsistency assessments, network forest plots, network contribution plots, funnel plots, and cumulative ranking plots.
The network evidence plot compares the differential impact of various exercise interventions on PWV and secondary outcomes. Figure 3 illustrates the NMA chart depicting PWV, SBP, and DBP. The connecting lines between nodes represent direct relationships between interventions, while the size of each node and the thickness of connecting lines are proportional to the number of studies conducted. As depicted in the figure, AE intervention studies are predominant, whereas RT intervention studies are relatively scarce.
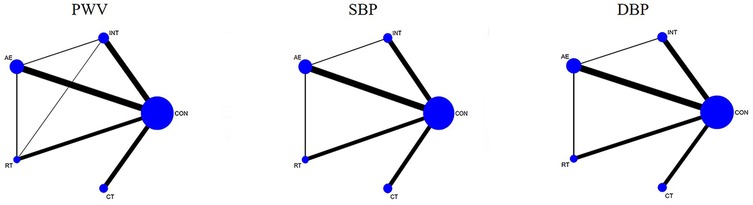
Figure 3. Network meta-analysis of eligible comparisons for pulse wave velocity, systolic blood pressure, and diastolic blood pressure.
The inconsistency test plots include the loop-special approach, node-splitting, and global inconsistency tests, which assess the consistency of PWV, SBP, and DBP at the loop level, local level, and global level, respectively (Supplementary Appendix 9). The results of the loop-special approach indicate that all closed loops involving PWV, SBP, and DBP exhibit good consistency except for INT-AE-RT, which showed inconsistency regarding PWV. Global inconsistency was assessed using an inconsistency model. The results of this model demonstrated that the p-values for PWV, SBP, and DBP were all greater than 0.05, indicating overall good consistency. Furthermore, the node-splitting analysis revealed no significant difference between indirect comparisons (between pairwise comparisons of each item) and direct comparisons (p > 0.05), suggesting reliable results (Supplementary Appendix 9).
The network forest plots illustrate the differences in intervention effects between various exercise types (including the CON) through pairwise comparisons. Supplementary Appendix 10 displays the network forest diagrams for PWV, SBP, and DBP with 95% confidence intervals and 95% prediction intervals.
The contribution of direct and indirect comparisons to NMA is illustrated in the network contribution graph (Supplementary Appendix 8), which also displays the number of studies for each direct comparison.
Funnel plots were used to assess publication bias in the NMA. PWV, SBP, and DBP all showed high symmetry, indicating an absence of publication bias.
SUCRA was employed to rank and compare the intervention effects of different types of exercise on PWV, SBP, and DBP. Supplementary Appendix 12 presents the SUCRA probability results for various exercise interventions.
The comparative effectiveness results of PWV are presented in Table 3. CT [SMD = −0.69, 95% CI (0.21 to 1.17), p < 0.0001] and INT [SMD = −0.50, 95% CI (0.07 to 0.93), p < 0.001] demonstrated significant improvements in PWV compared to the CON group; However, RT [SMD = −0.11, 95% CI (−0.36 to 0.59), p > 0.05] and AE [SMD = −0.33, 95% CI (−0.04 to 0.71), p > 0.05] did not show significant improvements in PWV compared to the CON group.
The SUCRA probability ranking results displayed in Table 3 indicate that CT exhibited the highest efficacy in improving PWV with a SUCRA value of 87.2%, followed by INT with a SUCRA value of 73.1%, and AE with a SUCRA value of 54.2%. Conversely, RT had the lowest improvement effect with a SUCRA value of 9.3%.
The secondary indicators of this study were mainly DBP and SBP. As shown in Table 3, AE [SMD = −3.69, 95% CI (1.36 to 6.02), p < 0.0001] and INT [SMD = −3.82, 95% CI (0.94 to 6.70), p < 0.001] significantly improved PWV compared with the CON group. However, RT [SMD = −1.05, 95% CI (−1.48 to 3.59), p > 0.05] and CT [SMD = −2.22, 95% CI (−0.23 to 4.67), p > 0.05] may not significantly improve SBP compared with the CON group. DBP was not significantly improved by INT, AE, RT, and CT (p > 0). The results of the SUCRA probability ranking in Table 4 show that INT (SUCRA = 81.3) has the best effect on improving SBP among the four exercise interventions: INT, AE, RT, and CT. RT had the least impact on improving SBP (SUCRA = 29.7), CT (SUCRA = 78.8) had the most impact on improving DBP, and RT (SUCRA = 27.4) had the least impact on improving DBP (SUCRA = 29.7).
Because the subjects in this study are persons at risk for cardiovascular disease, which is usually associated with multiple complications such as obesity, hypertension, diabetes, metabolic syndrome, etc., it was challenging to control confounding factors like age and gender within subgroups when analyzing the study results. However, subgroup analysis of exercise intensity was feasible, and we performed subgroup analyses of exercise intensity (Supplementary Appendix 15, 16).
Subgroup analysis of exercise intensity showed that CT [SMD = −0.67, 95% CI (0.07 to 1.28), p < 0.05] significantly improved PWV in the moderate-intensity subgroup (Supplementary Appendix 15.10) and RT [SMD = −0.40, 95% CI (0.12 to 0.68), p < 0.05] and INT [SMD = −0.40, 95% CI (0.16 to 0.64), p < 0.05] can significantly improve PWV in the high-intensity subgroup (Supplementary Appendix 16.10). The results of the SUCRA probability ranking showed that INT (SUCR = 83.3) was the most likely to be the best exercise intervention for PWV in the moderate-exercise-intensity subgroup, while RT (SUCRA = 29.1) was the worst (Supplementary Appendix 15.9). In the high-exercise intensity subgroup, CT (SUCRA = 85.7) may be the most effective exercise intervention for PWV, while AE (SUCRA = 29.1) may be the least effective exercise intervention (Supplementary Appendix 16.9). In conclusion, exercise intensity may be an important factor affecting the effect of exercise intervention in PWV.
Table 4 presents the GRADE evaluation results for PWV, showing a high level of performance with most comparisons achieving medium to high confidence. Supplementary measures, SBP and DBP, were also evaluated using the GRADE framework (Supplementary Appendix 14). This showed lower to moderate confidence for most SBP comparisons and moderate to high confidence for most DBP comparisons. Overall, PWV had the highest confidence in the GRADE assessment, while SBP had relatively low confidence.
This study examined the impact of four exercise interventions (INT, AE, RT, and CT) on PWV, SBP, and DBP in individuals at high risk of CVD. The network meta-analysis, including 43 RCTs with 2,034 participants, found that CT and INT significantly reduced PWV, whereas RT and AE did not. As shown in Figure 4, SUCRA analysis ranked CT as the most effective, followed by INT. AE and RT were less effective. Subgroup analysis showed that moderate-intensity INT was most effective for AS, while high-intensity CT outperformed AE in improving AS.
In studies involving individuals at high risk of CVD, Montero et al. (10) examined the effects of AE on arterial AS in subjects with prehypertension and hypertension. Their results showed that AE, particularly when combined with a reduction in SBP below the median or extended duration, positively affected AS in patients with prehypertension or hypertension.
Montero et al. (9) also studied the impact of AE on AS in obese individuals, finding no significant improvement in AS among middle-aged and older obese adults undergoing AE. However, subgroup and meta-regression analyses suggested that low-intensity AE combined with a reduction in DBP could reduce AS in obese individuals.
Marzolini et al. (7) compared the effects of CT (AE + RT) to AE alone in patients with coronary heart disease. They found that AE + RT led to superior improvements in AS and was more effective than AE alone in enhancing body composition, strength, and certain cardiovascular health indicators.
Evans et al. (11) investigated the effects of RT on AS in high-risk CVD populations. Their study showed that RT did not worsen AS or the blood system, despite increasing sympathetic norepinephrine levels, which may lead to vasoconstriction and higher blood pressure. In fact, RT could be more effective than AE in improving cardiovascular health-related conditions.
Consistent with previous research, this study found that both a CT program (combining AE and RT) (SMD = −0.69, p < 0.001) and an INT program (SMD = −0.50, p < 0.001) effectively reduced PWV in individuals at high risk for CVD. Given that an effect size of 0.5 or greater suggests practical relevance (62), both CT and INT are recommended as effective interventions for improving AS in high-risk CVD populations.
In contrast to previous findings, AE (SMD = −0.33, p > 0.01) did not significantly reduce PWV in high-risk populations despite its known benefits for AS in healthy individuals. AE's positive effects in healthy people are attributed to mechanisms like vascular remodeling, enhanced endothelial function, and reduced oxidative stress (9). However, AE did not significantly lower AS in individuals with hypertension, obesity, or elderly individuals who are at high risk for CVD. Effective AE for high-risk populations typically involves lowering systolic blood pressure below the median, extending the intervention duration, or adjusting exercise intensity. Therefore, AE is not recommended as the primary intervention for improving AS in high-risk CVD populations.
The impact of RT on AS remains debated. Some studies suggest that high-intensity RT may increase blood pressure and reduce AS, while others find no effect (6, 10). Our study also showed that RT (SMD = −0.11, p > 0.01) did not significantly reduce PWV. However, the subgroup analysis revealed that high-intensity RT effectively decreased PWV (SMD = −0.40, p < 0.001), suggesting its potential benefit for improving AS in high-risk individuals. Safety concerns related to high-intensity RT, including potential increases in blood pressure and acute cardiac strain, limit its clinical recommendation for improving AS.
The biological mechanisms behind RT's effects on AS vary by health status. In young, healthy individuals, high-intensity RT can stimulate the sympathetic nervous increased AS (73). In contrast, individuals at high risk for CVD, such as those with metabolic syndrome or diabetes, may experience decreased hormone secretion that mitigates these adverse effects. In addition, RT's impact on arterial structure and load-bearing characteristics can positively influence AS.
Finally, high-intensity CT (SMD = −0.71) was more effective than moderate-intensity CT (SMD = −0.67) in improving AS in high-risk CVD populations. Previous studies have suggested CT is the best intervention for improving AS in general populations. However, the sequence of AE and RT in CT is important; CT with AE following RT but not before RT can reduce AS (8). This study included CT with both AE and RT, but the sequence was not consistently specified. Further research is needed to determine the optimal order of exercises in CT programs.
The secondary findings of this study evaluated the impact of four exercise interventions (INT, AE, RT, and CT) on blood pressure, including SBP and DBP. Analysis of SBP showed that INT (SMD = −0.382, p < 0.001) and AE (SMD = −0.369, p < 0.001) significantly reduced SBP in high-risk CVD populations, while CT (SMD = −2.22, p > 0.01) and RT (SMD = −1.05, p > 0.01) did not. SUCRA ranking indicated that INT had the highest effect (SUCRA = 81.3), followed by AE (SUCRA = 80.4) and CT (SUCRA = 52.5), with RT scoring the lowest (SUCRA = 29.7).
For DBP, none of the interventions showed significant reductions. SUCRA analysis revealed that CT had the best effect (SUCRA = 78.8), followed by AE (SUCRA = 69.2) and INT (SUCRA = 54.9), while RT had the lowest effect (SUCRA = 27.4).
These results align with previous research confirming AE and INT's benefits on SBP. Some studies suggest that high-intensity INT might be more effective than AE for individuals with cardiopulmonary issues or advanced age due to shorter exercise and recovery times. The impact of RT on blood pressure remains debated, with mixed results in prior studies (74). This study found that neither RT nor CT significantly improved blood pressure.
The lack of significant effects on DBP across all interventions may be due to individual variability in high-risk CVD populations. Normal blood pressure values are 130/80 mmHg, and DBP typically should not exceed 90 mmHg (75). Although AE, RT, and CT have been shown to improve various cardiovascular metrics, age-related changes in blood vessels, such as elastin fiber degeneration and increased collagen, may obscure the immediate benefits on DBP for high-risk groups like the elderly, obese, or those with heart disease.
This study examined how exercise intensity affects PWV. The results showed that in the moderate-intensity group, CT significantly improved PWV [SMD = −0.67, 95% CI (0.07 to 1.28), p < 0.05; see Supplementary Appendix 15.10]. In the high-intensity group, both RT [SMD = 0.40, 95% CI (0.12 to 0.68), p < 0.05] and HIIT [SMD = 0.40, 95% CI (0.16 to 0.64), p < 0.05] were effective in improving PWV (see Supplementary Appendix 16.10).
SUCRA rankings showed that for moderate-intensity exercise, INT (SUCRA = 83.3) was the most effective, while RT (SUCRA = 21.6) was the least effective (see Supplementary Appendix 15.9). For high-intensity exercise, CT (SUCRA = 85.7) was the most effective, and AE (SUCRA = 29.5) was the least effective (see Supplementary Appendix 16.9).
These findings underscore the importance of exercise intensity in determining the effectiveness of interventions on PWV. Previous research shows varied effects of INT, AR, RT, and CT based on intensity. For instance, Way et al. (12) found that high-intensity aerobic exercise was more effective for improving AS in healthy adults than moderate-intensity exercise. On the other hand, Montero et al. (9) reported that low-intensity aerobic exercise improved AS in obese individuals, while moderate to high-intensity aerobic exercise did not.
In summary, higher-intensity CT and RT may be more effective for improving PWV, offering valuable guidance for choosing suitable exercise intensities for individuals at high cardiovascular risk.
This study utilized a network meta-analysis, allowing us to compare multiple intervention modalities simultaneously. This approach provides a broader research perspective and significantly enhances the study's value. We focused on high-risk CVD populations, an area that has been underexplored in previous research. Since AS is a common complication of CVD, examining this group is crucial for validating the preventive and therapeutic benefits of exercise on AS.
Our findings are notable and diverge from previous research. We recommend CT and INT as preferred exercise methods for improving arterial stiffness in high-risk CVD populations. In addition, moderate-intensity AE and supervised high-intensity RT are also recommended for these individuals.
However, this study has certain limitations. First, we only included INT, AE, RT, and CT as common interventions, excluding other forms like mental and physical exercises, stretching, and pharmacological treatments, which have limited data. Second, while we focused on PWV, SBP, and DBP as key indicators of AS, other measures such as augmentation index, blood lipids, and blood sugar were not included due to validity concerns. Third, insufficient sample size is also a significant limitation when conducting subgroup analyses on exercise intensity. In addition, despite various analyses, the heterogeneity in CT remains unresolved and is acknowledged as a limitation in this study. Future research with larger sample sizes should address these limitations and explore additional interventions. Potential limitations in future studies may include variability in study populations, differences in study designs, and the influence of unmeasured confounders.
This systematic review and network meta-analysis provides strong evidence that both CT and INT significantly reduce PWV in high-risk CVD populations. In addition, CT and AE effectively lower SBP. SUCRA rankings indicate that CT is the most effective for reducing PWV, while INT is best for lowering SBP. RT is the least effective for reducing PWV, SBP, and DBP. Subgroup analysis by exercise intensity shows that moderate-intensity INT has the greatest impact on AS. Conversely, high-intensity CT contributes to greater improvements in AS compared to AE at a similar intensity. However, these results should be interpreted with caution due to substantial heterogeneity in the CT studies. The variation in CT findings may influence the overall conclusions, and further studies with larger sample sizes and more consistent methodologies are needed to confirm these findings.
Based on the available evidence, individuals at high risk of CVD should consider supervised moderate-intensity AE and high-intensity RT, with INT being the preferred method for effectively improving arterial stiffness.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of the School of Physical Education of Hunan University of Science and Technology (No. ECSPEHUST 2022/0010). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
R-SW: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing, Visualization. YZ: Data curation, Investigation, Writing – review & editing. X-WY: Investigation, Validation, Writing – review & editing. XY: Project administration, Resources, Supervision, Writing – review & editing. X-LF: Resources, Supervision, Validation, Visualization, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1489382/full#supplementary-material
AE, aerobic exercise; AS, arterial stiffness; CON, control group; CT, combined exercise; CVD, cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; HRmax, maximum heart rate; HRR, heart rate reserve; INT, interval training; MIIT, moderate interval training; HIIT, high interval training; cfPWV, carotid–femoral pulse wave velocity; baPWV, brachial arteries pulse wave velocity; RCT, randomized controlled trial; RM, repetition maximum; RT, resistance exercise; VO2max, maximal oxygen uptake.
1. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061
2. Zhang Y, Qi L, Xu L, Sun X, Liu W, Zhou S, et al. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: systematic review and meta-analysis of randomized controlled trials. PLoS One. (2018) 13(7):e0200829. doi: 10.1371/journal.pone.0200829
3. Saz-Lara A, Cavero-Redondo I, Álvarez-Bueno C, Notario-Pacheco B, Reina-Gutiérrez S, Sequí-Domínguez I, et al. What type of physical exercise should be recommended for improving arterial stiffness on adult population? A network meta-analysis. Eur J Cardiovasc Nurs. (2021) 20(7):696–716. doi: 10.1093/eurjcn/zvab022
4. Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. (2015) 45(5):679–92. doi: 10.1007/s40279-015-0321-z
5. Okamoto T, Masuhara M, Ikuta K. Effects of eccentric and concentric resistance training on arterial stiffness. J Hum Hypertens. (2006) 20(5):348–54. doi: 10.1038/sj.jhh.1001979
6. Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med. (2013) 47(6):393–6. doi: 10.1136/bjsports-2012-090488
7. Marzolini S, Oh PI, Brooks D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: a meta-analysis. Eur J Prev Cardiol. (2012) 19(1):81–94. doi: 10.1177/1741826710393197
8. Montero D, Vinet A, Roberts CK. Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol. (2015) 178:69–76. doi: 10.1016/j.ijcard.2014.10.147
9. Montero D, Roberts CK, Vinet A. Effect of aerobic exercise training on arterial stiffness in obese populations. Sports Med. (2014) 44(6):833–43. doi: 10.1007/s40279-014-0165-y
10. Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre-and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. (2014) 173(3):361–8. doi: 10.1016/j.ijcard.2014.03.072
11. Evans W, Willey Q, Hanson ED, Stoner L. Effects of resistance training on arterial stiffness in persons at risk for cardiovascular disease: a meta-analysis. Sports Med. (2018) 48(12):2785–95. doi: 10.1007/s40279-018-1001-6
12. Way KL, Sultana RN, Sabag A, Baker MK, Johnson NA. The effect of high intensity interval training versus moderate intensity continuous training on arterial stiffness and 24 h blood pressure responses: a systematic review and meta-analysis. J Sci Med Sport. (2019) 22(4):385–91. doi: 10.1016/j.jsams.2018.09.228
13. Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. (2014) 9(10):e110034. doi: 10.1371/journal.pone.0110034
14. Guimarães GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. (2010) 33(6):627–32. doi: 10.1038/hr.2010.42
15. Bouaziz W, Lang P-O, Schmitt E, Leprêtre P-M, Lefebvre F, Momas C. Effects of a short-term interval aerobic training program with recovery bouts on vascular function in sedentary aged 70 or over: a randomized controlled trial. Arch Gerontol Geriatr. (2019) 82:217–25. doi: 10.1016/j.archger.2019.02.017
16. Aghaei Bahmanbeglou N, Ebrahim K, Maleki M, Nikpajouh A, Ahmadizad S. Short-duration high-intensity interval exercise training is more effective than long duration for blood pressure and arterial stiffness but not for inflammatory markers and lipid profiles in patients with stage 1 hypertension. J Cardiopulm Rehabil Prev. (2019) 39(1):50–5. doi: 10.1097/HCR.0000000000000377
17. Zhuang X, Xu H, Zheng J. Effect of high intensity interval training on vascular wall elasticity in the elderly. Chin J Phya Med Rehabil. (2023) 45(05):429–31. doi: 10.3760/cma.j.issn.0254-1424.2023.05.009
18. Mora-Rodriguez R, Ramirez-Jimenez M, Fernandez-Elias VE, Guio de Prada MV, Morales-Palomo F, Pallares JG. Effects of aerobic interval training on arterial stiffness and microvascular function in patients with metabolic syndrome. J Clin Hypertens. (2018) 20(1):11–8. doi: 10.1111/jch.13130
19. Deiseroth A, Streese L, Köchli S, Wüst RS, Infanger D, Schmidt-Trucksäss A. Exercise and arterial stiffness in the elderly: a combined cross-sectional and randomized controlled trial (EXAMIN AGE). Front Physiol. (2019) 10:474298. doi: 10.3389/fphys.2019.01119
20. Zhang HM, Xie XF, Xiao Y. Effect of high-intensity interval training and medium-intensity continuous training on vascular health in patients with type 2 diabetes mellitus. J Med Grad Stud. (2022) 35(01):63–8. doi: 10.16571/j.cnki.1008-8199.2022.01.012
21. Kim HK, Hwang CL, Yoo JK, Hwang MH, Handberg EM, Petersen JW. All-extremity exercise training improves arterial stiffness in older adults. Med Sci Sports Exerc. (2017) 49(7):1404–11. doi: 10.1249/MSS.0000000000001229
22. Bellia A, Iellamo F, De Carli E, Andreadi A, Padua E, Lombardo M. Exercise individualized by TRIMPi method reduces arterial stiffness in early onset type 2 diabetic patients: a randomized controlled trial with aerobic interval training. Int J Cardiol. (2017) 248:314–9. doi: 10.1016/j.ijcard.2017.06.065
23. Way KL, Sabag A, Sultana RN, Baker MK, Keating SE, Lanting S. The effect of low-volume high-intensity interval training on cardiovascular health outcomes in type 2 diabetes: a randomised controlled trial. Int J Cardiol. (2020) 320:148–54. doi: 10.1016/j.ijcard.2020.06.019
24. Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer. (2017) 123(20):4057–65. doi: 10.1002/cncr.30859
25. Taha MM, Aneis YM, Hasanin ME, Felaya EE, Aldhahi MI, Abdeen HAA. Effect of high intensity interval training on arterial stiffness in obese hypertensive women: a randomized controlled trial. Eur Rev Med Pharmacol Sci. (2023) 27(9):4069–79. doi: 10.26355/eurrev_202305_32314
26. Millen AME, Norton GR, Avidon I, Woodiwiss AJ. Effects of short-term exercise-training on aortic systolic pressure augmentation in overweight and obese individuals. Eur J Appl Physiol. (2013) 113(7):1793–803. doi: 10.1007/s00421-013-2610-2
27. Kearney TM, Murphy MH, Davison GW, O’Kane MJ, Gallagher AM. Accumulated brisk walking reduces arterial stiffness in overweight adults: evidence from a randomized control trial. J Am Soc Hypertens. (2014) 8(2):117–26. doi: 10.1016/j.jash.2013.10.001
28. Nualnim N, Parkhurst K, Dhindsa M, Tarumi T, Vavrek J, Tanaka H. Effects of swimming training on blood pressure and vascular function in adults >50 years of age. Am J Cardiol. (2012) 109(7):1005–10. doi: 10.1016/j.amjcard.2011.11.029
29. Zhou LY, Wu ZZ, Hong LF. Effect of 12-week aerobic exercise training on brachial-ankle, pulse wave velocity in elderly. Chin J Phya Med Rehabil. (2010) 32(05):356–8. doi: 10.3760/ema.j.issn.0254-1424.2010.05.009
30. Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Aerobic training-induced improvements in arterial stiffness are not sustained in older adults with multiple cardiovascular risk factors. J Hum Hypertens. (2013) 27(5):335–9. doi: 10.1038/jhh.2012.38
31. Horner K, Barinas-Mitchell E, DeGroff C, Kuk JL, Drant S, Lee S. Effect of aerobic versus resistance exercise on pulse wave velocity, intima media thickness and left ventricular mass in obese adolescents. Pediatr Exerc Sci. (2015) 27(4):494–502. doi: 10.1123/pes.2015-0067
32. Huang CY, Pan ML, Zhang L. Effect of maximum fat oxidation intensity exercise on arterial stiffness in overweight/obese young men. Chin J Sport Med. (2018) 37(01):3–9. doi: 10.16038/j.1000-6710.2018.01.001
33. Shin J-H, Lee Y, Kim SG, Choi BY, Lee H-S, Bang S-Y. The beneficial effects of Tai Chi exercise on endothelial function and arterial stiffness in elderly women with rheumatoid arthritis. Arthritis Res Ther. (2015) 17(1):380. doi: 10.1186/s13075-015-0893-x
34. Davis CL, Litwin SE, Pollock NK, Waller JL, Zhu H, Dong Y. Exercise effects on arterial stiffness and heart health in children with excess weight: the SMART RCT. Int J Obes. (2020) 44(5):1152–63. doi: 10.1038/s41366-019-0482-1
35. Chen TT, Li LC, Yin XL. Study on the influence of Taijiquan on PWV, ABL and ADP in middle-aged and elderly people. Chin J Sport. (2018) 35(18):134–5+50. doi: 10.3969/j.issn.1674-151x.2018.18.067
36. Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. (2009) 32(8):1531–5. doi: 10.2337/dc09-0149
37. O’Connor EM, Koufaki P, Mercer TH, Lindup H, Nugent E, Goldsmith D. Long-term pulse wave velocity outcomes with aerobic and resistance training in kidney transplant recipients—a pilot randomised controlled trial. PLoS One. (2017) 12(2):e0171063. doi: 10.1371/journal.pone.0171063
38. Jaime SJ. The effects of twelve weeks of whole-body vibration training and low-intensity resistance exercise training on arterial function, muscle strength, and physical performance in dynapenic postmenopausal women (Doctoral dissertation). The Florida State University, Tallahassee, FL, United States (2017).
39. Croymans DM, Krell SL, Oh CS, Katiraie M, Lam CY, Harris RA. Effects of resistance training on central blood pressure in obese young men. J Hum Hypertens. (2014) 28(3):157–64. doi: 10.1038/jhh.2013.81
40. DeVallance E, Fournier S, Lemaster K, Moore C, Asano S, Bonner D. The effects of resistance exercise training on arterial stiffness in metabolic syndrome. Eur J Appl Physiol. (2016) 116(5):899–910. doi: 10.1007/s00421-016-3348-4
41. Greenwood SA, Koufaki P, Mercer TH, Rush R, O’Connor E, Tuffnell R. Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: a 12-week pilot randomized controlled trial (the exercise in renal transplant trial). Am J Kidney Dis. (2015) 66(4):689–98. doi: 10.1053/j.ajkd.2015.06.016
42. Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. (2013) 26(9):1093–102. doi: 10.1093/ajh/hpt080
43. Fernandez-del-Valle M, Gonzales JU, Kloiber S, Mitra S, Klingensmith J, Larumbe-Zabala E. Effects of resistance training on MRI-derived epicardial fat volume and arterial stiffness in women with obesity: a randomized pilot study. Eur J Appl Physiol. (2018) 118(6):1231–40. doi: 10.1007/s00421-018-3852-9
44. Jaime SJ, Maharaj A, Alvarez-Alvarado S, Figueroa A. Impact of low-intensity resistance and whole-body vibration training on aortic hemodynamics and vascular function in postmenopausal women. Hypertens Res. (2019) 42(12):1979–88. doi: 10.1038/s41440-019-0328-1
45. Ramírez-Vélez R, Castro-Astudillo K, Correa-Bautista JE, González-Ruíz K, Izquierdo M, García-Hermoso A, et al. The effect of 12 weeks of different exercise training modalities or nutritional guidance on cardiometabolic risk factors, vascular parameters, and physical fitness in overweight adults: cardiometabolic high-intensity interval training-resistance training randomized controlled study. J Strength Cond Res. (2020) 34(8):2178–88. doi: 10.1519/JSC.0000000000003533
46. Hetherington-Rauth M, Magalhães JP, Júdice PB, Melo X, Sardinha LB. Vascular improvements in individuals with type 2 diabetes following a 1 year randomised controlled exercise intervention, irrespective of changes in cardiorespiratory fitness. Diabetologia. (2020) 63(4):722–32. doi: 10.1007/s00125-020-05089-5
47. Wong A, Sanchez-Gonzalez MA, Son W-M, Kwak Y-S, Park S-Y. The effects of a 12-week combined exercise training program on arterial stiffness, vasoactive substances, inflammatory markers, metabolic profile, and body composition in obese adolescent girls. Pediatr Exerc Sci. (2018) 30(4):480–6. doi: 10.1123/pes.2017-0198
48. Magalhães JP, Melo X, Correia IR, Ribeiro RT, Raposo J, Dores H. Effects of combined training with different intensities on vascular health in patients with type 2 diabetes: a 1-year randomized controlled trial. Cardiovasc Diabetol. (2019) 18(1):34. doi: 10.1186/s12933-019-0840-2
49. Lee YH, Park SH, Yoon ES, Lee C-D, Wee SO, Fernhall B, et al. Effects of combined aerobic and resistance exercise on central arterial stiffness and gait velocity in patients with chronic poststroke hemiparesis. Am J Phys Med Rehabil. (2015) 94(9):687–95. doi: 10.1097/PHM.000000000000000233
50. Shiotsu Y, Watanabe Y, Tujii S, Yanagita M. Effect of exercise order of combined aerobic and resistance training on arterial stiffness in older men. Exp Gerontol. (2018) 111:27–34. doi: 10.1016/j.exger.2018.06.020
51. Stewart KJ, Bacher AC, Turner KL, Fleg JL, Hees PS, Shapiro EP. Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med. (2005) 165(7):756–62. doi: 10.1001/archinte.165.7.756
52. Park W, Jung W-S, Hong K, Kim Y-Y, Kim S-W, Park H-Y. Effects of moderate combined resistance- and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int J Environ Res Public Health. (2020) 17(19):7233. doi: 10.3390/ijerph17197233
53. Son W-M, Sung K-D, Cho J-M, Park S-Y. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause. (2017) 24(3):262–8. doi: 10.1097/GME.0000000000000765
54. Dobrosielski DA, Gibbs BB, Ouyang P, Bonekamp S, Clark JM, Wang N-Y. Effect of exercise on blood pressure in type 2 diabetes: a randomized controlled trial. J Gen Intern Med. (2012) 27(11):1453–9. doi: 10.1007/s11606-012-2103-8
55. Loimaala A, Groundstroem K, Rinne M, Nenonen A, Huhtala H, Parkkari J. Effect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitus. Am J Cardiol. (2009) 103(7):972–7. doi: 10.1016/j.amjcard.2008.12.026
56. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162(11):777–84. doi: 10.7326/M14-2385
57. O’Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev. (2021) 22(2):e13137. doi: 10.1111/obr.13137
58. Ferrari R. 2013 ESH/ESC guidelines for the management of arterial hypertension. J Hypertens. (2013) 31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc
59. Bramwell JC, Hill AV. Velocity of transmission of the pulse-wave: and elasticity of arteries. Lancet. (1922) 199(5149):891–2. doi: 10.1016/S0140-6736(00)95580-6
60. Flack JM, Calhoun D, Schiffrin EL. The new ACC/AHA hypertension guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults. Am J Hypertens. (2018) 31(2):133–5. doi: 10.1093/ajh/hpx207
61. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
62. Chen X, He H, Xie K, Zhang L, Cao C. Effects of various exercise types on visceral adipose tissue in individuals with overweight and obesity: a systematic review and network meta-analysis of 84 randomized controlled trials. Obes Rev. (2024) 25(3):e13666. doi: 10.1111/obr.13666
63. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS One. (2014) 9(7):e99682. doi: 10.1371/journal.pone.0099682
64. Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016
65. Jackson D, Riley R, White IR. Multivariate meta-analysis: potential and promise. Stat Med. (2011) 30(20):2481–98. doi: 10.1002/sim.4172
66. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
67. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Syn Meth. (2012) 3(2):80–97. doi: 10.1002/jrsm.1037
68. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. (2015) 15:1–9. doi: 10.1186/s12874-015-0060-8
69. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. (2013) 8(10):e76654. doi: 10.1371/journal.pone.0076654
70. White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. (2012) 3(2):111–25. doi: 10.1002/jrsm.1045
71. Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. (2013) 11:1–8. doi: 10.1186/1741-7015-11-159
72. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. (2017) 6:1–5. doi: 10.1186/s13643-016-0385-3
73. Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac A-M, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement: validation and clinical application studies. Hypertension. (1995) 26(3):485–90. doi: 10.1161/01.HYP.26.3.485
74. Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. (2011) 58(5):950–8. doi: 10.1161/HYPERTENSIONAHA.111.177071
Keywords: arterial stiffness, blood pressure, high-risk population for cardiovascular disease, exercise intervention, network meta-analysis
Citation: Wu R-S, Zhang Y, Yuan X-W, Yan X and Fu X-L (2025) Comparative effectiveness of exercise interventions on arterial stiffness in individuals at risk for cardiovascular disease: a systematic review and network meta-analysis. Front. Cardiovasc. Med. 12:1489382. doi: 10.3389/fcvm.2025.1489382
Received: 1 September 2024; Accepted: 20 January 2025;
Published: 27 February 2025.
Edited by:
Yao Jie Xie, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Audrey Adji, Victor Chang Cardiac Research Institute, AustraliaCopyright: © 2025 Wu, Zhang, Yuan, Yan and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Yan, MTMyNzIxMzEzNjFAMTYzLmNvbQ==; Xiao-Lei Fu, MTMzMjczMzMyNDlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.