
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 February 2025
Sec. Cardiac Rhythmology
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1480473
Background: Epicardial adipose tissue (EAT) remodeling is associated with atrial fibrillation (AF). However, there is limited research on the contribution of EAT to the risk of AF recurrence (AFR). The purpose of this research was to assess the relationship between the risk of AFR after radiofrequency catheter ablation and the volume and attenuation of the EAT.
Methods: We included a total of 123 consecutive individuals who received AF ablation, 31 of whom suffered AFR. The volume and mean density of the whole-heart and periatrial EAT were measured on computed tomography images using four attenuation ranges. The clinical, atrial, and EAT characteristics of patients with and without AFR were compared. Logistic regression was used to identify independent risk factors and to build a model to predict recurrence. The relationship between EAT characteristics and recurrence was analyzed for the subtypes of AF.
Results: The AFR group had a larger left atrial anteroposterior diameter (47.4 ± 7.4 vs. 43.7 ± 8.0 mm), left–right diameter (78.6 ± 7.9 vs. 74.7 ± 9.1 mm), and volume (145.9 vs. 127.0 mL) than the non-recurrence group (P = 0.021, 0.037, 0.015, respectively). The total EAT volume in the AFR group was significantly larger than that in the non-recurrence group, for both the overall and persistent AF groups (all P < 0.1). The periatrial EAT volume of the AFR group was significantly larger than that of the non-recurrence group for those with persistent AF (P = 0.047, 0.048, 0.048, 0.031 for four attenuation ranges). The total EAT volume and left atrial anteroposterior diameter were independent risk factors for AFR (P = 0.035, 0.045, respectively).
Conclusion: The EAT volume and left atrial anteroposterior diameter were of great significance in predicting AFR.
Epicardial adipose tissue (EAT) is the visceral fat of cardiac tissue in proximity to cardiac structures and shares blood supply with the cardiac microcirculation. EAT serves as a substantial local reservoir of molecules that can affect the myocardium (1, 2). The growing advancement and accessibility of non-invasive imaging methods such as CT and cardiac magnetic resonance imaging have provided several pieces of evidence on the association between EAT and the risk of atrial fibrillation (AF) (3).
The Framingham Heart Study found that there was a significant correlation between the volume of pericardial fat and the occurrence of AF, even after taking into account the individual's body mass index (4). The growing interest of scientists and clinicians has been sparked by the regional distribution and functions of EAT.
The greatest obstacle to radiofrequency ablation in patients with AF is the unresolved risk of AF recurrence (AFR). After early research identified the pulmonary vein triggers that cause AF, catheter ablation to isolate the pulmonary veins was recognized as an effective therapy for AF (5). Improved approaches such as contact force-sensing catheters, ablation index-guided procedures, high-power short-duration ablation, pulse-field ablation, and pulmonary vein isolation (PVI) are now more effective and durable. However, even with substantial substrate modifications, atrial arrhythmias often recur, particularly in patients with persistent AF (PeAF) (6). Several risk factors for AFR following catheter ablation have been found, however, there is no single indicator that can effectively predict AFR (7).
An increasing number of patients with AF undergo ablation treatments, which often include preprocedural imaging. This enables the investigation of the correlation between EAT and recurrent AF. Several researchers have demonstrated a similar link between increased EAT and postablation AF. Nevertheless, not all the data are consistent (8–10). In previous studies, four attenuation ranges were used to define the heart fat tissue. Thus, the aim of this study was to investigate the effects of total and periatrial EAT CT characteristics based on four attenuation ranges on clinical outcomes in patients with AF undergoing catheter ablation.
This retrospective study was approved by the Institutional Review Board (2023-KY-1286), and the requirement for informed consent was waived.
A total of 362 consecutive adult patients diagnosed with AF who received radiofrequency catheter ablation (RFCA) at the First Affiliated Hospital of Zhengzhou University from January 2020 to January 2021 were screened for inclusion. Ultimately, 123 patients met our inclusion criteria. The inclusion criteria were as follows: initial RFCA was performed in patients with AF, CT imaging was performed before RFCA, and complete follow-up data were collected. Patients who had the following conditions were excluded: rheumatic valvular heart disease, congenital heart disease, pericardial effusion, or infectious endocarditis. Patients for whom the CT images contained artifacts that affected parameter measurements were also excluded.
Epidemiological, laboratory, CT, and echocardiographic data were reviewed. CT image data, including left atrial (LA) characteristics (anteroposterior diameter, left–right diameter, upper-down diameter, and volume) (Figure 1) and EAT characteristics (EAT volume and mean density of the whole heart and LA) were assessed. We used the total area of the EAT to calculate the sum of the EAT volume and then separated it into the whole-heart and periatrial EAT.
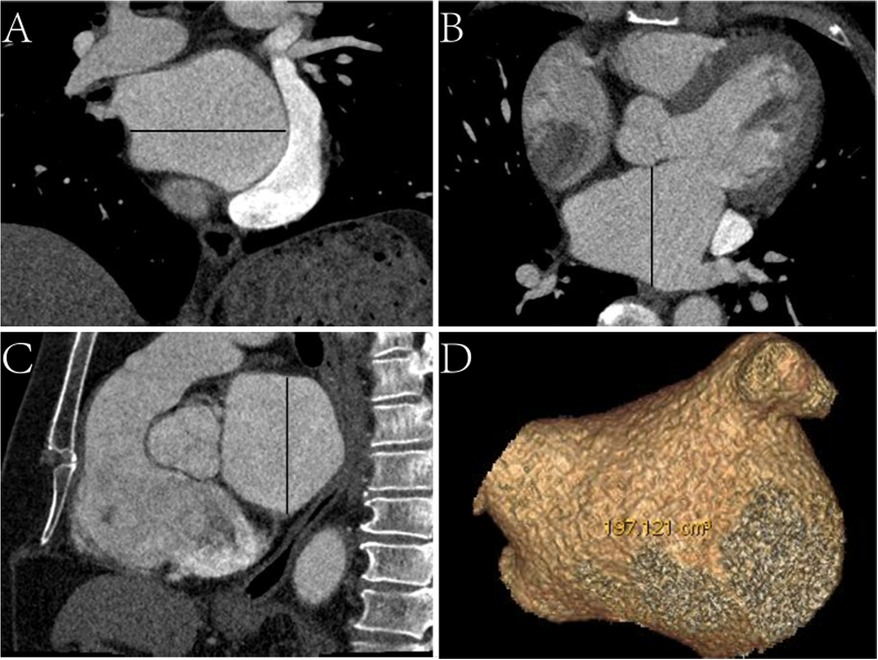
Figure 1. Schematic diagram of measurements of left atrial CT characteristics. (A) Coronal CT image, the black line represents the left–right diameter. (B) Axial CT image, the black line represents the anteroposterior diameter. (C) Sagittal CT image, the black line represents the upper-down diameter. (D) Virtual reality CT image displaying the volume.
All patients underwent isolated LA PVI. The electrophysiologist decided which type of energy to use [radiofrequency (RF) or cryoablation] based on clinical and imaging factors. Wide-area circumferential RF was exclusively performed in patients with PeAF. The endpoint of the procedure was to isolate the entire pulmonary vein from the LA. After the ablation procedure, if the AF continued, electrical cardioversion was conducted. Before each RF ablation, electroanatomical LA bipolar endocardial voltage mapping was performed using either the EnSite NavX System (St. Jude Medical) or the CARTO 3 system (Biosense Webster Inc.). All the voltage maps with sufficient coverage of the anterior and posterior LA walls were included. Low voltage was defined as <0.5 mV.
CT images were acquired using a Revolution CT Scanner (GE Healthcare, Milwaukee, WI, USA) and a SOMATOM Force Scanner (Siemens Medical Solutions, Erlangen, Germany). CT examinations covered the entire heart from 1 cm below the bifurcation of the trachea to the bottom of the heart. The patients were administered a total of 60 mL of ionic contrast material (Ultravist 370, Bayer Schering Pharma) and 40 mL of saline at a rate of 5 mL/s. Two phases of the CT series were obtained 6 s after CT attenuation of the region of interest (ROI) located in the aortic root reached 100 Hounsfield units (HU). The parameters were as follows: tube voltage, 120 kVp; tube current, 400 mA; and reconstructed thickness and increment, 0.75 mm.
EAT volume and density were calculated using postprocessing software on a SyngoVia workstation (Cardiac Risk Assessment and Radiomics, MM Research Frontier SyngoVia, VB2.0, Siemens Healthineers, Forchheim, Germany). In total, 16 EAT characteristics of the whole heart and LA were assessed based on four attenuation ranges (−190 to −30 HU, −195 to −45 HU, −200 to −45 HU, −200 to 0 HU) (Figures 2, 3) (11, 12). The EAT features of the whole heart were automatically recognized, while the features of the LA were semiautomatically recognized by outlining the ROI on every 6-mm axial image from the top of the LA appendage to the bottom of the left atrium. After manually delineating and correcting the ROI in each slice, the regions were fused to obtain the desired features. The EAT data around the whole heart in the two measurements were consistent with the automatic recognition software. The Bland–Altman test was used to evaluate the consistency of EAT data only around the atrium and not around the whole heart. Finally, 30 patients were randomly selected by another experienced radiologist to outline the ROI of the EAT around the LA.
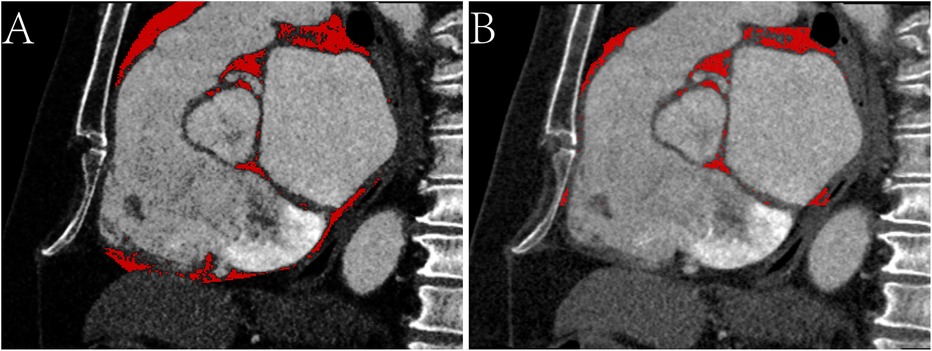
Figure 2. Sagittal CT images of the epicardial adipose tissue on -200 to 0 HU. (A) The red area represents the epicardial adipose tissue (EAT) around the whole heart. (B) The red area represents the EAT around the atrium.
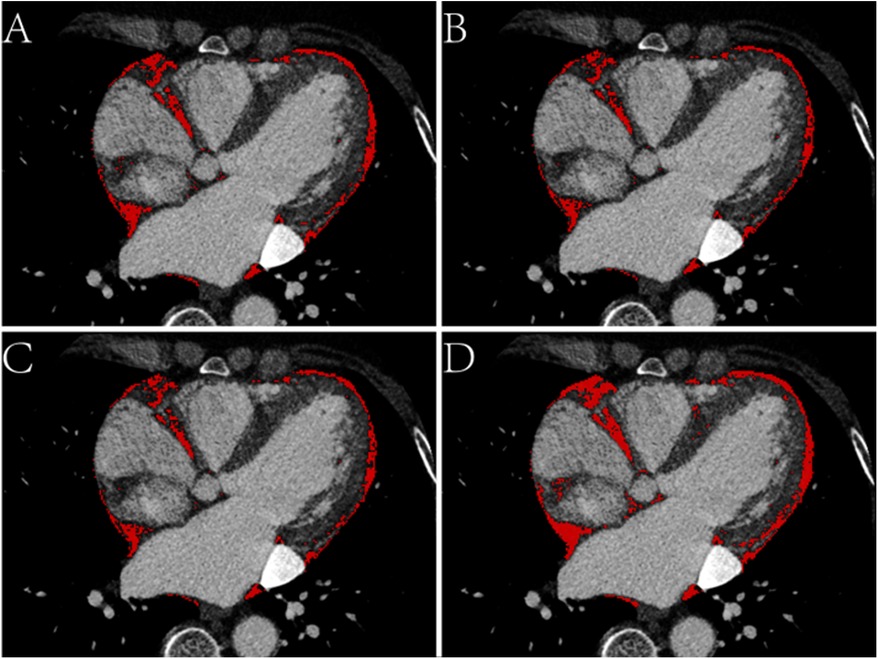
Figure 3. Axial CT images of the epicardial adipose tissue (EAT) based on four attenuation ranges. (A) The red area represents the EAT based on -190 to -30 HU. (B) The red area represents the EAT based on -195 to -45 HU. (C) The red area represents the EAT based on -200 to -45 HU. (D) The red area represents the EAT based on -200 to 0 HU.
A standard 12-lead ECG or systematic 24-h Holter monitor examination was performed at 3 months, 6 months, and 1 year after ablation, or at any time when symptoms were present. AFR was defined as AF, atrial flutter, or atrial tachycardia lasting at least 30 s, recorded 3 months after ablation. Antiarrhythmic drugs were prescribed when AFR was observed 3 months after ablation.
The statistical analyses were performed using MATLAB (version 2017, Natick, MA, USA) and SPSS software (version 22.0, IBM Corp., Armonk, NY, USA). The normality of the data was analyzed and the differences between the two groups were evaluated using the chi-square test or Fisher's exact test, or alternatively, the independent t-test and Mann–Whitney U test. The evaluation of several characteristics in predicting recurrence was conducted by measuring the area under the curve (AUC) of the receiver operating characteristic curve (ROC). A binary logistic regression model (backward Wald) was built to predict recurrence and to screen for independent risk factors. A P-value < 0.1 for the initial screening and a P-value < 0.05 for the final screening were considered significant.
The study included 123 patients who fulfilled the inclusion criteria, of whom 31 (25.2%) had AFR. Tables 1, 2 present an overview of the patients' demographic data and clinical examination characteristics. The recurrence and non-recurrence groups did not vary significantly statistically in terms of sex, smoking history, alcohol intake, history of coronary heart disease, hypertension, hyperlipidemia, hyperuricemia, or stroke. There were no significant differences in brain natriuretic peptide (BNP) and other laboratory indicators between the two groups. The quantitative data of BNP was further analyzed and a P-value close to statistical significance was observed between the two groups [754 (363, 1,092) vs. 460 (160, 889), pg/mL, p = 0.102].
Tables 3, 4 show the correlation between AF phenotype and EAT parameters. The total EAT volume was significantly larger in patients with AFR than in patients without recurrence (P < 0.1). The difference was observed in both the entire AF population and the PeAF subgroup population, but not in the paroxysmal AF (PAF) subgroup. In addition, EAT density was assessed in each member of the AF group. Even in the subgroup analysis, there was no evidence of a difference in the average EAT density between those with and without AFR (P > 0.1).
When the periatrial EAT was examined, patients with PeAF in the recurrence group had a significantly higher periatrial EAT volume than those without such a recurrence (P < 0.05). Nevertheless, neither the whole group nor the patients with paroxysmal AF showed this variance (P > 0.1). There was also no statistically significant difference in the density of atrial epicardial adipose tissue between the groups with AFR and without recurrence, in both the whole population and subgroup analyses.
LA volume, LA anteroposterior diameter, and LA left–right diameter were all greater in the AFR group (P < 0.05). In contrast, neither the diameter nor the volume of the LA varied significantly among the PeAF subgroup (P > 0.1). The LA upper-down diameter did not differ significantly between individuals with and without AFR (P > 0.1) (Table 5).
The two independent readers exhibited a high degree of consistency in the four thresholds around the heart chamber (Figure 4), and the data tendencies were consistent for each of the four different fat thresholds. The AUC of the −200 to 0 HU threshold for whole-heart fat was 0.615. The other three threshold values for predicting AFR were lower with 0.601 for −190 to −30 HU, 0.595 for −195 to −45 HU, and 0.596 for −200 to −45 HU. As shown in Figure 5, the regression analysis accounted for the maximum AUC of the whole-heart EAT (−200 to 0 HU). The most widely used whole-heart EAT criterion (−190 to −30 HU) was also included as a significant variable in the regression model. The regression analysis showed that the whole-heart EAT volume and left atrial anteroposterior diameter were independent risk factors for AFR (P < 0.05) (Table 6).

Figure 4. Bland–Altman scatter plot of four attention range setting methods for two surveyors. (A) epicardial adipose tissue (EAT) as measured by two surveyors based on -190 to -30 HU. (B) EAT as measured by two surveyors based on -195 to -45 HU. (C) EAT as measured by two surveyors based on -200 to -45 HU. (D) EAT as measured by two surveyors based on -200 to 0 HU.
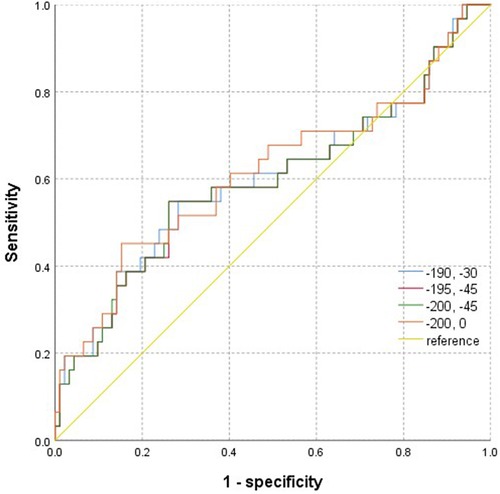
Figure 5. ROC images of epicardial adipose tissue volume measured based on four attenuation ranges for predicting the recurrence of atrial fibrillation after radiofrequency ablation.
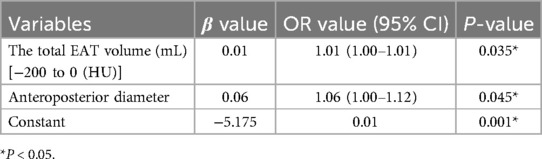
Table 6. Multivariate logistic regression model for predicting atrial fibrillation recurrence after radiofrequency ablation.
Approximately 30% to 40% of individuals have AFR after the first ablation treatment (5). Previous studies have investigated the correlation between CT features of EAT and AFR, however, the results have been inconsistent (8–10, 13). The results of this study indicate that the EAT volume and LA anteroposterior diameter are of great significance in predicting AFR.
Our study assessed that the AFR was 25.2%, which is consistent with a previously documented occurrence rate (5). Patients diagnosed with AFR had a higher probability of having a greater LA diameter and volume compared to those without recurrence. This discovery strongly corroborates the conclusions of previous research that establish a connection between echocardiographic measurements, such as the diameter and volume of the LA and the risk of arrhythmia recurring following catheter ablation (14). Hof et al. discovered that with each 10 mL increase in LA volume, there was a 1.14 adjusted odds ratio (OR) for AFR (15). A meta-analysis of 11 studies found a positive link between an elevated average LA volume and an increased incidence of recurrence (16). This research revealed that the anteroposterior diameter of the LA was a significant factor that influenced AFR. Studies (17) have indicated that BNP might be a biomarker for predicting AFR, but no difference was observed in our study, which may be due to incomplete data.
Inconsistent data exist regarding the function of the EAT and its effect on AFR following ablation. The EAT volume corrected by body surface area was considered an independent factor affecting AFR (18). Al Chekakie et al. found that patients with PeAF had a greater volume of pericardial fat than those with PAF or sinus rhythm (19). EAT volume was associated with the risk of AFR following PVI ablation, according to previous research (8, 20). Other studies have failed to establish a statistically significant correlation between EAT and AFR following ablation (13). In support of the previous conclusion, our research emphasizes the significance of EAT volume in predicting AFR after ablation. In contrast to the PAF group, this distinction was more pronounced in the PeAF group. The difference in the overall volume of EAT was more pronounced compared to the difference in the volume of periatrial EAT. Compared to a previous study (21), four different distinct fat thresholds were analyzed in our study. Although the data trends exhibited consistency, the difference was more pronounced in the −200 to 0 HU range compared to the other thresholds.
The prognosis of patients with recurrent episodes of AF may be enhanced by assessing EAT as a marker of ablation efficiency. Nevertheless, information on the distribution of EAT around the LA and its predictive implications in patients undergoing AF catheter ablation remains limited. Nakanishi et al. first demonstrated that the periatrial EAT volume estimated using multidetector CT accurately predicted the development of new-onset AF in patients with coronary artery disease (22). Tsao et al. discovered that the EAT surrounding the LA had a significant impact on the success of the ablation procedure. A review also emphasized the predictive role of peri-LA EAT in AFR after ablation (21). However, there was no difference in the volume of EAT around the LA between patients with PAF and PeAF (9). In our study, one interesting finding was that the periatrial EAT volume was much greater in patients with PeAF who had recurrence compared to those who did not. Contrary to what was expected, our research did not find a significant difference between those who had a recurrence of PAF and those who did not. This conclusion agrees with that of Huber et al., who discovered that LA-enhancing EAT was independently linked to recurrence after AF ablation (23). Shin and associates focused on the EAT and AF to find inflammatory indicators. Every patient in their experiment had measurements of their periventricular, periatrial, and total EAT volumes. They discovered that periatrial fat in patients with PeAF was thicker than in patients with PAF (24). Our findings emphasize that higher periatrial EAT volume was associated with the recurrence of PeAF after ablation, but the general rather than local effects of EAT play a role in the onset of early recurrence of AF after ablation. Compared to PAF, the pathogenesis of PeAF seems to be more linked with atrial metabolism, although confounding factors may be involved.
The role of EAT density in the early recurrence of AF remains unknown. Situated between the visceral pericardium and the myocardium, EAT is a unique kind of visceral adipose tissue (25). Significant paracrine and vasocrine effects are exerted on nearby cardiomyocytes by the EAT's advantageous position (26). The production of fatty acids and proinflammatory adipokines is one way that the EAT changes from an anti-inflammatory to a pro-inflammatory phenotype in disease situations. EAT radiodensity can reflect the pathological status. Measured by adipose tissue attenuation on CT, periatrial inflammation was associated with AF independent of LA size (27). Inflammation causes higher attenuation of adipose tissue, as assessed by CT in patients with recurrence of AF after catheter ablation (28). It has also been reported that EAT radiodensity is associated with the occurrence, severity, and recurrence of AF (29, 30). Our investigation did not reveal any indication of a difference in average EAT density between patients with and without AFR, which is in contrast to previous results. Similar to previous research, our analysis demonstrated that the attenuation of adipose tissue posterior to the LA was not a predictor when confounding variables were taken into account (30). A possible explanation for this result may be that inflammation may be a factor in the recurrence of AF. However, inflammation alone is not sufficient to determine recurrence. T cells may also be linked to the influence of EAT on AFR (2).
This study suggests that EAT may play a role in risk stratification to identify patients who are more likely to develop progressive and recurrent AF after catheter ablation. The link between EAT and AF might be explained by a number of pathophysiological processes. Cardiovascular disease is associated with the EAT, which is the most metabolically active adipose depot next to the heart (31). As opposed to pericardial fat, EAT has particular properties such as sharing the same blood circulation as the myocardium and exhibiting cell-to-cell contact or infiltration. Furthermore, a few studies have shown a correlation between total adiposity and shorter effective refractory durations in the pulmonary veins in obese individuals with AF (32). This resulted in the hypothesis that individuals with high levels of obesity would be more susceptible to the onset of AF. However, during AF ablation treatments, a previous research study created 3D electroanatomical maps and discovered that regions of fragmented signals were close to the EAT (33). This discovery implies that AF might be triggered by EAT.
This research has some limitations. First, it was constrained by the characteristics of observational research conducted at a single center. However, a multivariate analysis was carried out in order to exclude the influence of important identified factors on the study results. Second, EAT features on echocardiography were not included in the study, mainly because echocardiography is an operation highly dependent on operator proficiency, especially in EAT measurement. The last limitation is a lack of studies on the mechanisms behind the correlation between pericardial or EAT volume and AFR. More research is needed to understand the potential mechanisms behind the association between AFR and the amount of fat in the pericardium or epicardium. Further studies with larger sample sizes and a multicenter methodology should be explored to enhance the applicability of the results.
In conclusion, the EAT was found to play a significant role in AFR, and the effect was more pronounced in patients with PeAF than in those with PAF. The influence of total EAT volume was higher than that of the periatrial EAT volume. The data trends obtained for the four fat thresholds were consistent.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by the Institutional Review Board of The first Affiliated Hospital of Zhengzhou University (2023-KY-1286). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Retrospective study, does not affect treatment strategy.
LF: Data curation, Writing – original draft, Writing – review & editing, Conceptualization, Formal Analysis, Investigation, Methodology, Project administration. LL: Conceptualization, Formal Analysis, Funding acquisition, Software, Writing – review & editing. LB: Data curation, Software, Writing – review & editing, Investigation, Resources. LT: Methodology, Software, Writing – review & editing. YZ: Investigation, Supervision, Validation, Writing – review & editing. XZ: Investigation, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was partly supported by The Henan Association for Science and Technology Youth Talent Support Program (2023HYTP039), Scientific and technological project in Henan Province (242102311026) awarded to LL and LF puts forward ideas, designs experiment and modifies paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vyas V, Hunter RJ, Longhi MP, Finlay MC. Inflammation and adiposity: new frontiers in atrial fibrillation. Europace. (2020) 22:1609–18. doi: 10.1093/europace/euaa214
2. Vyas V, Sandhar B, Keane JM, Wood EG, Blythe H, Jones A, et al. Tissue-resident memory T cells in epicardial adipose tissue comprise transcriptionally distinct subsets that are modulated in atrial fibrillation. Nat Cardiovasc Res. (2024) 3:1067–82. doi: 10.1038/s44161-024-00532-x
3. Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. (2017) 38:1294–302. doi: 10.1093/eurheartj/ehw045
4. Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham heart study. Circ Arrhythm Electrophysiol. (2010) 3:345–50. doi: 10.1161/CIRCEP.109.912055
5. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14:e275–444. doi: 10.1016/j.hrthm.2017.05.012
6. Tzeis S, Gerstenfeld EP, Kalman J, Saad EB, Sepehri Shamloo A, Andrade JG, et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2024) 26:euae043. doi: 10.1093/europace/euae043
7. Vizzardi E, Curnis A, Latini MG, Salghetti F, Rocco E, Lupi L, et al. Risk factors for atrial fibrillation recurrence: a literature review. J Cardiovasc Med (Hagerstown). (2014) 15:235–53. doi: 10.2459/JCM.0b013e328358554b
8. Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. (2011) 57:1745–51. doi: 10.1016/j.jacc.2010.11.045
9. Tsao HM, Hu WC, Wu MH, Tai CT, Lin YJ, Chang SL, et al. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am J Cardiol. (2011) 107:1498–503. doi: 10.1016/j.amjcard.2011.01.027
10. Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, et al. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J. (2011) 75:2559–65. doi: 10.1253/circj.CJ-11-0554
11. Nerlekar N, Thakur U, Lin A, Koh JQS, Potter E, Liu D, et al. The natural history of epicardial adipose tissue volume and attenuation: a long-term prospective cohort follow-up study. Sci Rep. (2020) 10:7109. doi: 10.1038/s41598-020-63135-z
12. Monti CB, Capra D, Zanardo M, Guarnieri G, Schiaffino S, Secchi F, et al. CT-derived epicardial adipose tissue density: systematic review and meta-analysis. Eur J Radiol. (2021) 143:109902. doi: 10.1016/j.ejrad.2021.109902
13. Cruz I, Lopes Fernandes S, Diaz SO, Saraiva F, Barros AS, Primo J, et al. Epicardial adipose tissue volume is not an independent predictor of atrial fibrillation recurrence after catheter ablation. Rev Esp Cardiol (Engl Ed). (2017) 76:539–47. doi: 10.1016/j.rec.2022.11.006
14. Liżewska-Springer A, Dąbrowska-Kugacka A, Lewicka E, Drelich Ł, Królak T, Raczak G. Echocardiographic predictors of atrial fibrillation recurrence after catheter ablation: a literature review. Cardiol J. (2020) 27:848–56. doi: 10.5603/CJ.a2018.0067
15. Hof I, Chilukuri K, Arbab-Zadeh A, Scherr D, Dalal D, Nazarian S, et al. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol. (2009) 20:1005–10. doi: 10.1111/j.1540-8167.2009.01504.x
16. Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace. (2018) 20:33–42. doi: 10.1093/europace/eux013
17. Hammache N, Pegorer-Sfes H, Benali K, Magnin Poull I, Olivier A, Echivard M, et al. Is there an association between epicardial adipose tissue and outcomes after paroxysmal atrial fibrillation catheter ablation? J Clin Med. (2021) 10:3037. doi: 10.3390/jcm10143037
18. Maeda M, Oba K, Yamaguchi S, Arasaki O, Sata M, Masuzaki H, et al. Usefulness of epicardial adipose tissue volume to predict recurrent atrial fibrillation after radiofrequency catheter ablation. Am J Cardiol. (2018) 122:1694–700. doi: 10.1016/j.amjcard.2018.08.005
19. Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, et al. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. (2010) 56:784–8. doi: 10.1016/j.jacc.2010.03.071
20. Kocyigit D, Gurses KM, Yalcin MU, Turk G, Evranos B, Yorgun H, et al. Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon-based pulmonary vein isolation. J Cardiovasc Comput Tomogr. (2015) 9:295–302. doi: 10.1016/j.jcct.2015.03.011
21. Anagnostopoulos I, Kousta M, Kossyvakis C, Paraskevaidis NT, Vrachatis D, Deftereos S, et al. Epicardial adipose tissue and atrial fibrillation recurrence following catheter ablation: a systematic review and meta-analysis. J Clin Med. (2023) 12:6369. doi: 10.3390/jcm12196369
22. Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Sakamoto M, Taguchi H, et al. Peri-atrial epicardial adipose tissue is associated with new-onset nonvalvular atrial fibrillation. Circ J. (2012) 76:2748–54. doi: 10.1253/circj.CJ-12-0637
23. Huber AT, Fankhauser S, Chollet L, Wittmer S, Lam A, Baldinger S, et al. The relationship between enhancing left atrial adipose tissue at CT and recurrent atrial fibrillation. Radiology. (2022) 305:56–65. doi: 10.1148/radiol.212644
24. Shin SY, Yong HS, Lim HE, Na JO, Choi CU, Choi JI, et al. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. (2011) 22:647–55. doi: 10.1111/j.1540-8167.2010.01993.x
25. Wang M, Zhou BG, Zhang Y, Ren XF, Li L, Li B, et al. Association between non-alcoholic fatty liver disease and risk of stroke: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:812030. doi: 10.3389/fcvm.2022.812030
26. Li XX, Tian Y, Shi L, Wang YJ, Zeng LJ, Huang LH, et al. One-stop hybrid procedure combining catheter ablation and left atrial appendage closure increases long-term risk for adverse events in patients with atrial fibrillation. Pacing Clin Electrophysiol. (2020) 43:1358–65. doi: 10.1111/pace.14084
27. Gaibazzi N, Martini C, Benatti G, Palumbo AA, Cacciola G, Tuttolomondo D. Atrial fibrillation and peri-atrial inflammation measured through adipose tissue attenuation on cardiac computed tomography. Diagnostics (Basel). (2021) 11:2087. doi: 10.3390/diagnostics11112087
28. Mahdiui M E, Simon J, Smit JM, Kuneman JH, van Rosendael AR, Steyerberg EW, et al. Posterior left atrial adipose tissue attenuation assessed by computed tomography and recurrence of atrial fibrillation after catheter ablation. Circ Arrhythm Electrophysiol. (2021) 14:e009135. doi: 10.1161/CIRCEP.120.009135
29. Mancio J, Sousa-Nunes F, Martins R, Fragao-Marques M, Conceicao G, Pessoa-Amorim G, et al. Decoding the radiomic and proteomic phenotype of epicardial adipose tissue associated with adverse left atrial remodelling and post-operative atrial fibrillation in aortic stenosis. Eur Heart J Cardiovasc Imaging. (2022) 23:1248–59. doi: 10.1093/ehjci/jeac092
30. Ciuffo L, Nguyen H, Marques MD, Aronis KN, Sivasambu B, de Vasconcelos HD, et al. Periatrial fat quality predicts atrial fibrillation ablation outcome. Circ Cardiovasc Imaging. (2019) 12:e008764. doi: 10.1161/CIRCIMAGING.118.008764
31. Ansaldo AM, Montecucco F, Sahebkar A, Dallegri F, Carbone F. Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol. (2019) 278:254–60. doi: 10.1016/j.ijcard.2018.09.089
32. Munger TM, Dong YX, Masaki M, Oh JK, Mankad SV, Borlaug BA, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol. (2012) 60:851–60. doi: 10.1016/j.jacc.2012.03.042
33. Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune M, et al. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ Arrhythm Electrophysiol. (2012) 5:676–83. doi: 10.1161/CIRCEP.112.971200
Keywords: atrial fibrillation recurrence, epicardial adipose tissue, atrial fibrillation ablation, computed tomography, attenuation ranges
Citation: Feng L, Li L, Bai L, Tang L, Zhao Y and Zhao X (2025) The association between features of epicardial adipose tissue and the risks of early recurrence after catheter ablation in patients with atrial fibrillation. Front. Cardiovasc. Med. 12:1480473. doi: 10.3389/fcvm.2025.1480473
Received: 4 October 2024; Accepted: 14 January 2025;
Published: 18 February 2025.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Maddalena Conte, University of Naples Federico II, ItalyCopyright: © 2025 Feng, Li, Bai, Tang, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Zhao, ZmNjemhhb3h5OEB6enUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.