
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 06 March 2025
Sec. Heart Valve Disease
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1479200
Background: Previous reports of longer-term outcomes of transcatheter aortic valve implantation (TAVI) focus on higher risk patients and suggest potential temporal changes.
Aims: To evaluate the longer-term and temporal performances of TAVI compared to surgical aortic valve replacement (SAVR).
Methods: Randomized controlled trials reporting outcomes with at least 1-year follow-up. The primary outcome was the composite of all-cause death or disabling stroke.
Results: We included 8 trials with 8,749 patients. TAVI was associated with a higher risk of longer-term (5-year) primary outcome compared to SAVR among higher-risk [odds ratio (OR), 1.25; 95% CI, 1.07–1.47] but not lower-risk participants [1.0 (0.77–1.29)]. However, a significant temporal interaction was detected in both risk profiles. TAVI with balloon-expandable valves was associated with a higher risk of longer-term primary outcome compared to SAVR [1.38 (1.2–1.6)], whereas no statistical difference was found with self-expanding valves [1.03 (0.89–1.19)]. There was a significant interaction between the two valve systems, and a temporal interaction was detected in both systems. Overall landmark analysis revealed a lower risk in TAVI within the initial 30 days [0.76 (0.6, 0.96)], comparable between 30 days to 2 years [1.04 (0.85, 1.28)], and higher beyond 2 years [1.36 (1.15–1.61)]. Analysis for all-cause death generated largely similar results.
Conclusions: TAVI was associated with a higher longer-term risk of primary outcome compared to SAVR in higher-risk patients and with balloon-expandable valves. However, a characteristic temporal interaction was documented in all subgroups. Future studies are warranted to test these findings.
Transcatheter aortic valve implantation (TAVI) has emerged as a popular treatment for patients with severe aortic stenosis, surpassing surgical procedures in some countries (1). We previously indicated a potential higher mortality associated with TAVI compared to surgical aortic valve replacement (SAVR) at 5-year follow-up (2), mainly in high risk patients (3–5). The longer-term performance of TAVI vs. SAVR in patients with lower risk remains uncertain. Additionally, the temporal changes in TAVI performance at different timepoints have yet to be determined. Given the expansion of TAVI to low-risk patients with increased life expectancy, this assessment holds critical clinical importance.
The 5-year follow-up data from nearly all registered comparative randomized controlled trials (RCTs) of TAVI vs. SAVR have recently been published (6–9). We therefore are able to assess the longer-term outcomes of TAVI and conduct a landmark analysis to identify the timepoint at which the performance of TAVI might diverge from SAVR, as indicated in some studies (5). The aim of our study was to evaluate the longer-term and temporal performances of TAVI compared to SAVR, both overall and within important subgroups.
We reported the meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Supplementary Table S1).
PubMed, the Cochrane Central Register of Controlled Trials, EMBASE, and major conference proceedings were systematically searched from inception through October 25, 2023, an update of our previous meta-analysis (2). The computer-based searches combined terms and keywords which included transcatheter aortic valve implantation, transcatheter aortic valve replacement, TAVI, TAVR, and randomized trial (Supplementary Materials). Two investigators independently hand-searched the references of identified studies and relevant reviews to identify any additional relevant trials.
Two reviewers conducted independent screening of titles and abstracts to determine eligibility of the studies. Full-text articles were retrieved for studies that were deemed potentially relevant. In cases where discrepancies arose, a third investigator resolved the discrepancies. Eligible studies had to be RCTs evaluating TAVI vs. SAVR in patients with severe aortic stenosis, and reporting outcomes of interest with at least 1-year follow-up. Nonrandomized observational studies, studies comparing different types of TAVI devices, and studies with less than 1-year follow-up were excluded.
The primary outcome was the composite of all-cause death and disabling stroke. Secondary outcomes included all-cause death, cardiovascular death, myocardial infarction, stroke, transient ischemic attack (TIA), major bleeding, major vascular complications (MVC), permanent pacemaker implantation (PPM), new-onset atrial fibrillation, aortic-valve reintervention, rehospitalization, and moderate or severe paravalvular leak (PVL).
Two investigators independently extracted the data using a pre-specified form. Whenever possible, data from the intention-to-treat analysis were extracted; otherwise, data from the as-treated analysis were extracted. The same investigators also assessed the risk of bias in the included RCTs using the Cochrane Risk of Bias 2.0 tool.
Summary measures were reported as odds ratios (ORs) and pooled using random-effects models (DerSimonian–Laird method). Data were analyzed separately for different time points, including data within 30 days, 1 year, 2 years, and 5 years (one trial reported 4-year outcome and was used), and categorized as early, short-term, midterm, and longer-term outcomes, respectively. Landmark analysis was also conducted for intervals within 1 year, between 1 year and 2 years, and beyond 2 years. Events occurring within 1 year were further divided into events within 30 days and events between 30 days and 1 year to further explore the timing of performance change. For trials in which only one of the arms had no events, the 0.5 continuity correction was applied. Stratified analyses were performed based on surgical risks (higher and lower risks) and TAVI systems [balloon-expandable valves (BEV) and self-expanding valves (SEV)]. The higher-risk group included trials involving patients with extreme, high, and intermediate-to-high surgical risk, while the lower-risk group included trials involving patients with low and low-to-intermediate risk, as determined by the evaluation using the Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score. Between-subgroup differences were assessed using the χ2-test for heterogeneity. Sensitivity analysis was performed for the primary outcomes using Hartung-Knapp-Sidik-Jonkman variance correction, and by removing an individual trial each time. Heterogeneity was evaluated using the Q and I2 statistics. All meta-analyses were performed using Stata software version 16.0, and the Review Manager version 5.3. A 2-tailed p value <0.05 was considered statistically significant.
We included 8 trials and 14 secondary reports that provided eligible data from these trials (3–23), involving a total of 8,749 patients (Supplementary Figure S1). All 8 trials reported outcomes at 30 days and 1 year, 7 reported 2-year outcomes, one reported 4-year outcomes (8), and 6 reported 5-year outcomes (3–6, 9, 20). The mean age was 79.2 years and 57.4% were male. Based on STS-PROM risk score, 4 trials were categorized as lower-risk trials (mean STS PROM 1.9%–3.0%), while the other 4 categorized as higher-risk trials (mean STS PROM 4.5%–11.7%). BEV was used in 3 trials, SEV in 4 trials, and a mixed TAVI system in one trial. Baseline characteristics are presented in Supplementary Tables S2, S3. Blinding of participants and personnel was not feasible in any of the trials (Supplementary Table S4).
TAVI demonstrated a lower rate of primary outcome compared to SAVR at 30 days [odds ratio (OR), 0.76 (95% CI 0.6–0.96)] and 1 year [0.83 (0.72–0.96)]. However, at longer-term follow-up, TAVI was associated with a higher risk [1.17 (1.01–1.36)] (Figure 1). Landmark analysis indicated a significant benefit of TAVI within the first year, comparable events between 1 year and 2 years [1.19 (0.95–1.49)], but a significant disadvantage beyond 2 years [1.36 (1.15–1.61)], with a significant temporal interaction (p for interaction<0.0001) (Figure 2). The most notable benefit of TAVI was observed within the initial 30 days, whereas no significant difference was found between 30 days and 1 year [0.9 (0.74–1.08)] (Supplementary Table S5).
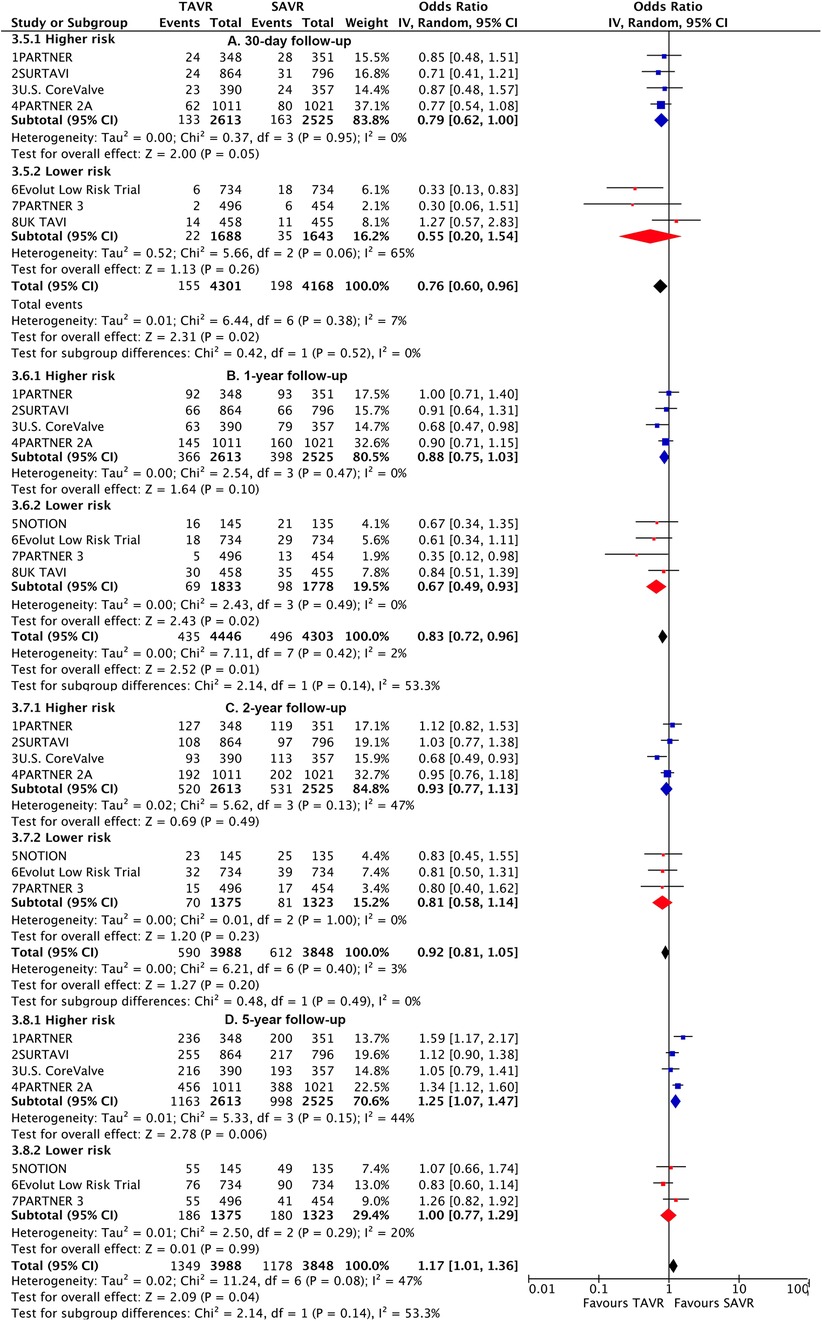
Figure 1. Risk estimates of all-cause death or disabling stroke for TAVI vs SAVR stratified by surgical risks at different lengths of follow-up.
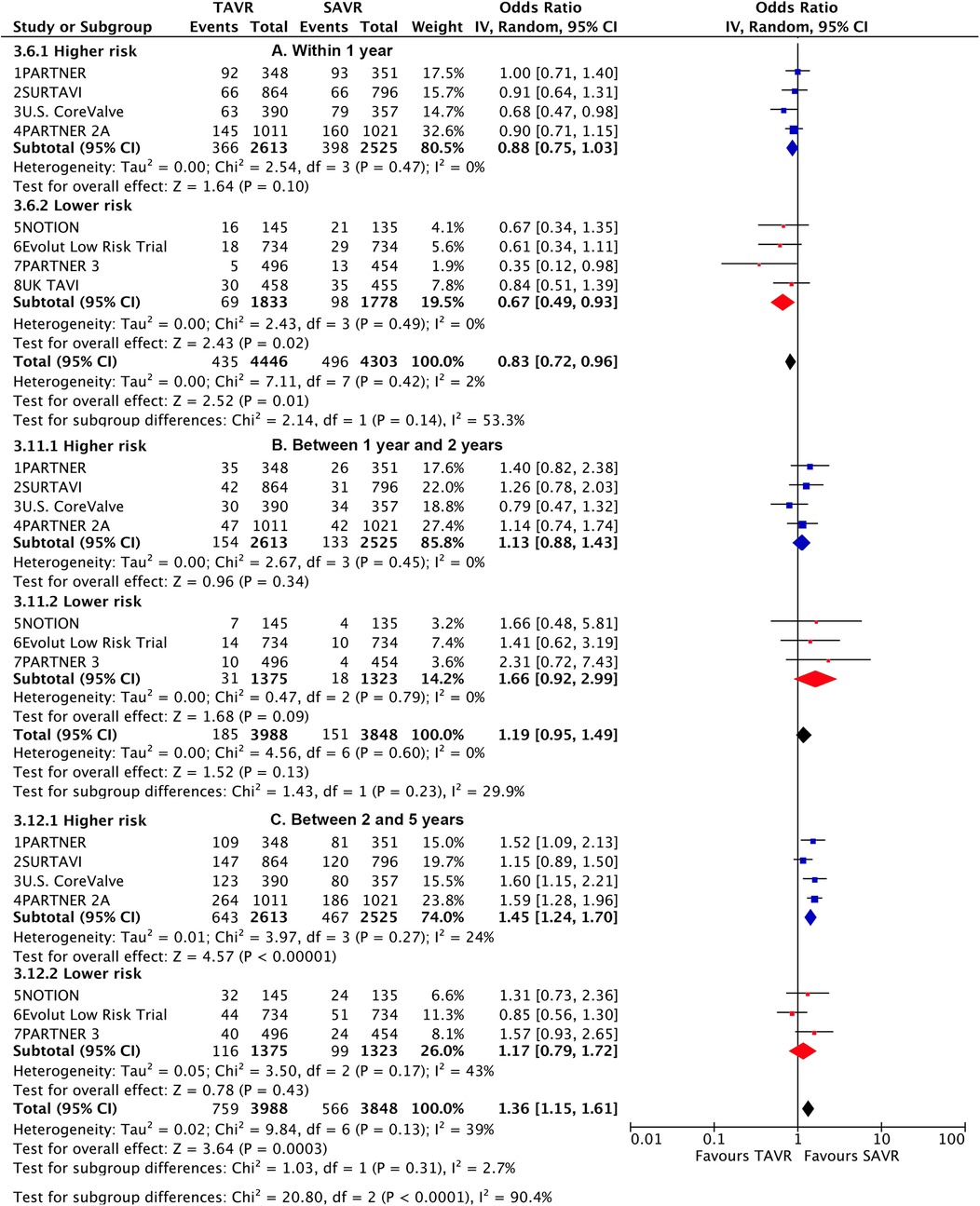
Figure 2. Landmark risk estimates of all-cause death or disabling stroke for TAVI vs SAVR stratified by surgical risks.
Subgroup analysis revealed a higher risk of longer-term primary outcome in TAVI compared to SAVR among participants with higher risk [1.25 (1.07–1.47)], but no statistical difference was found in patients with lower risk [1.00 (0.77–1.29)]. The higher risk of TAVI in higher-risk patients was primarily attributed to events occurring beyond 2 years [1.45 (1.24–1.7)] (p for interaction<0.0001) (Figure 2; Supplementary Table S6). The lower risk of TAVI over SAVR in lower-risk patients within 1 year [0.67 (0.49–0.93)] was not observed at longer-term follow-up, and a significant temporal interaction was detected (p for interaction = 0.01) (Supplementary Table S7).
Subgroup analysis demonstrated a higher risk of longer-term primary outcome in TAVI using BEV compared to SAVR [1.38 (1.2–1.6)], but no statistical difference was found with SEV [1.03 (0.89–1.19)] (Figure 3). A significant interaction was observed between two valve systems (p for interaction = 0.005, Supplementary Table S8). The higher risk of TAVI with BEV was primarily attributed to events occurring beyond 2 years [1.57 (1.32–1.86)] (p for interaction = 0.004) (Figure 4; Supplementary Table S9). The benefit of TAVI with SEV over SAVR within 1 year [0.75 (0.6–0.94)] was not observed at longer-term follow-up [1.21 (0.93–1.56)], and a significant temporal interaction was detected (p for interaction = 0.015) (Supplementary Table S10).
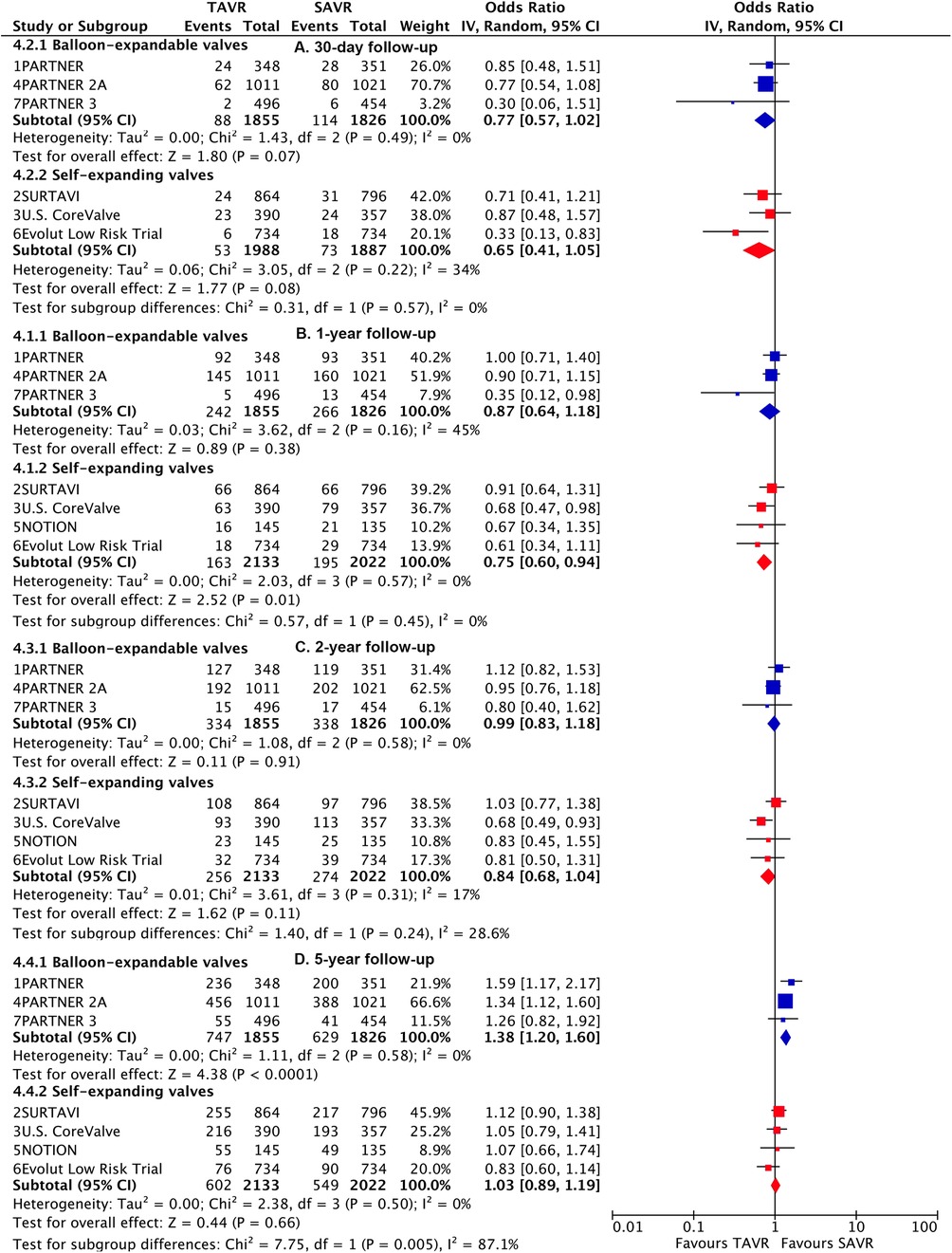
Figure 3. Risk estimates of all-cause death or disabling stroke for TAVI vs SAVR stratified by TAVI valve systems at different lengths of follow-up.
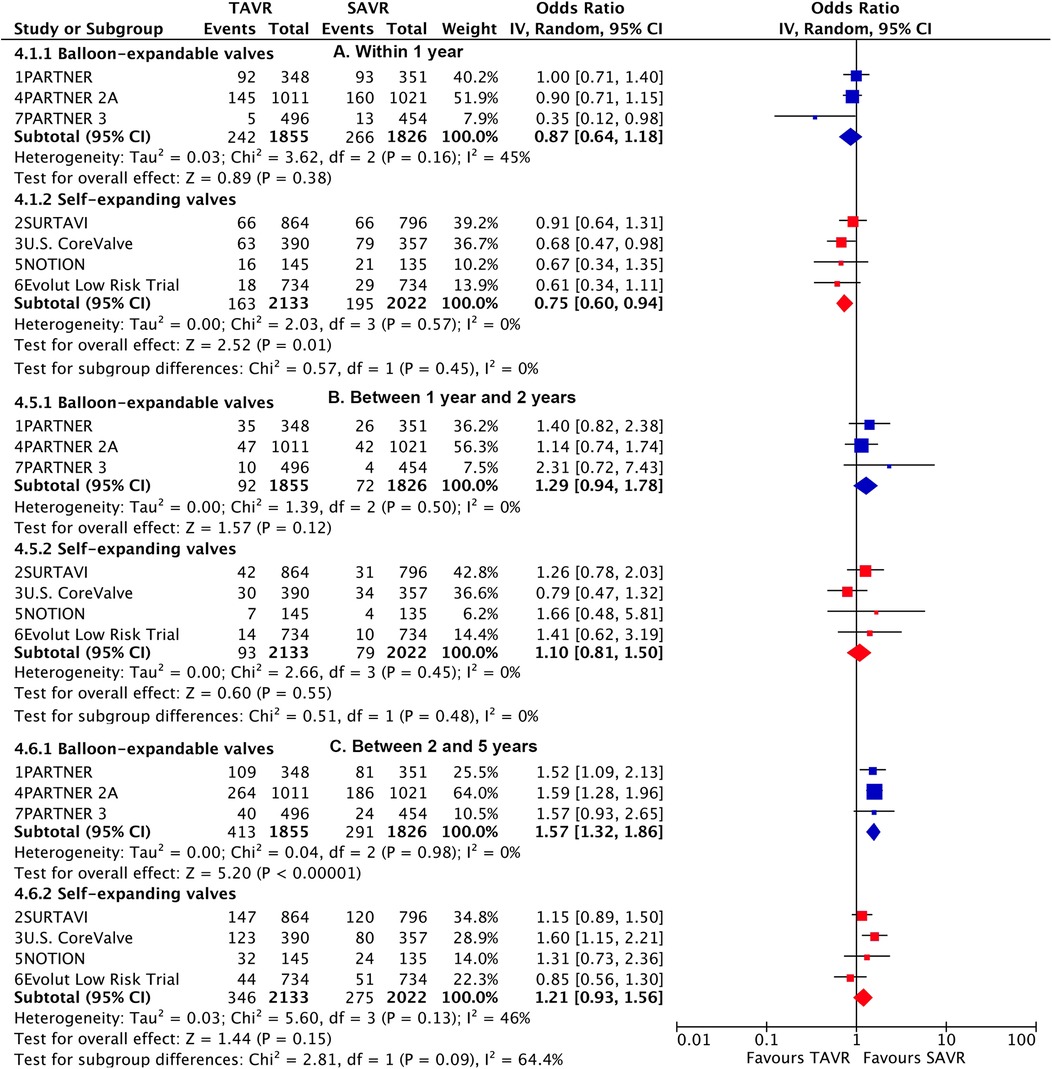
Figure 4. Landmark risk estimates of all-cause death or disabling stroke for TAVI vs SAVR stratified by TAVI valve systems.
Overall and subgroup analysis for all-cause death generated largely similar results with the primary outcome (Supplementary Figures S2, S3). At longer-term follow-up, TAVI was found to have a numerically higher risk of cardiovascular death, a significantly higher risk of TIA, MVC, PPM, reintervention, rehospitalization, and moderate to severe PVL, compared to SAVR. However, TAVI showed a significantly lower risk of major bleeding and new-onset atrial fibrillation, and a comparable risk of stroke and myocardial infarction (Table 1).
The increased risk of TAVI on cardiovascular death was primarily attributed to events occurring beyond 2 years, rehospitalization attributed to events beyond 1 year, while TIA, MVC, and reintervention were primarily associated with events within 1 year. The benefits of TAVI on major bleeding and new-onset atrial fibrillation were mainly attributed to lower events occurring within 1 year. The risk of PPM at longer-term follow-up was primarily attributed to higher events occurring within 1 year in TAVI, with the risk attenuating but still higher in TAVI between 1 year and 2 years and beyond 2 years (Supplementary Table S5).
In subgroup analysis, a statistically higher risk of longer-term reintervention and rehospitalization was observed in TAVI compared to SAVR among participants at higher risk, while no statistical difference was found in patients at lower risk (Supplementary Table S11). Significant interaction was detected between the two risk groups (both p for interaction <0.0001). The lower risk of rehospitalization in TAVI over SAVR in lower-risk patients within the first year was not observed during longer-term follow-up (Supplementary Table S7). Subgroup analysis indicated a statistically higher risk of longer-term PPM in TAVI compared to SAVR, regardless of participants’ higher or lower risk. The SEV showed a higher risk than the BEV, with a significant difference (p for interaction <0.0001) (Supplementary Table S8).
There was minimal heterogeneity observed across trials for both the primary outcomes and all death outcomes across all follow-up durations, as detailed in corresponding figures and tables. Several tests for publication bias were conducted for the primary outcome, and no significant results were found (not shown). However, the assessment of publication bias was limited by the relatively small number of trials, potentially affecting the ability to detect small-study effects. The analysis of primary outcome using the Hartung-Knapp-Sidik-Jonkman variance correction and excluding each trial one time revealed largely similar findings (Supplementary Figures S4–S7).
This present meta-analysis, including comprehensive data from all available trials comparing TAVI with SAVR, with >8,000 patients and longer-term follow-up data from nearly all trials, yields several important conclusions (Central Illustration). First, TAVI was associated with a higher risk of longer-term primary outcome compared to SAVR among participants with higher risk, but not among those with lower risk. However, a significant temporal interaction was detected in both risk profiles. Second, TAVI with BEV was associated with a higher risk of longer-term primary outcome compared to SAVR, whereas no statistical difference was found with SEV. There was a significant interaction between the two valve systems, and a temporal interaction was observed in both TAVI systems. Third, landmark analysis revealed a lower risk of primary outcome in TAVI compared to SAVR within the initial 30 days, comparable between 30 days and 2 years, and a significant higher risk beyond 2 years. Fourth, overall analysis showed that TAVI was associated with a higher longer-term risk of all-cause death, TIA, MVC, PPM, reintervention, rehospitalization, and moderate to severe PVL, a comparable risk of stroke and myocardial infarction, but a lower risk of major bleeding and new-onset atrial fibrillation.
We conducted a comprehensive search on PubMed to identify relevant meta-analyses comparing the longer-term outcomes of TAVI and SAVR. However, these meta-analyses included 3–4 trials with 5-year follow-up data, focusing exclusively on patients with higher risks (2, 24, 25). In contrast, our meta-analysis incorporated a larger dataset, comprising 7 trials with longer-term follow-up data, encompassing both higher- and lower-risk patients. It is important to note that our study utilized longer-term data from nearly all registered large RCTs. One of the identified meta-analyses employed a network meta-analysis approach but considered 1-to-2-year follow-up as long-term (26). Another meta-analysis included only 3 RCTs but supplemented them with 7 propensity-score matching observational studies, which were limited by inadequate adjustment for important confounding (27). We also performed several additional analyses. Firstly, we conducted a landmark analysis to assess the differences in TAVI outcomes within specific time intervals, revealing significant temporal variations in the effect of TAVI. Secondly, we conducted subgroup analyses based on TAVI systems and surgical risks, revealing noteworthy distinctions between subgroups.
None of the trials included were specifically designed to have sufficient statistical power to detect a significant reduction in all-cause death. However, our meta-analysis revealed a significant higher risk of longer-term mortality associated with TAVI. This finding aligns with the temporal trend observed in primary outcome. Further subgroup analysis indicated a significantly higher risk of all-cause death in TAVI among higher-risk patients and with BEV, but no significant difference was observed in lower-risk patients or with SEV. Importantly, the temporal trend was also only evident in the former two subgroups. A separate meta-analysis of 7 propensity matched studies corroborated our findings by showing a significantly higher risk of mortality at 5-year follow-up (27).
TAVI demonstrated initial superiority over SAVR within the first year but lost this advantage thereafter in lower risk patients. Given that lower risk patients typically have good life expectancy, this temporal interaction warrants intensive and close attention. In the PARTNER 3 trial, Kaplan–Meier event curves for the primary outcome crossed around the 2- to 3-year mark, thereafter favoring SAVR, while in the Evolut Low Risk trial, the curves remained parallel, favoring TAVI (8, 9). Although there were some differences, the pooled analysis of longer-term data from these lower-risk trials did not show substantial heterogeneity (I2 = 20%). A large real-world registry including 42,586 patients who underwent isolated SAVR and meeting the inclusion and exclusion criteria for the PARTNER 3 and Evolut Low Risk trials, revealed excellent survival rates in low-risk patients following SAVR, with all-cause mortality of 7.1% at 5 years and 12.4% at 8 years (28). Similar findings were observed in other large registries (29). Determining whether TAVI can achieve such excellent long-term outcomes as SAVR will require robust evidence from follow-up periods exceeding 10 years. The recommendation of TAVI in these patients is pending this evidence.
We showed a higher longer-term risk of primary outcome and all-cause death in TAVI compared to SAVR among higher-risk patients. These observations seem a paradox, i.e., patients with a higher surgical risk actually had better longer-term outcomes when they underwent surgery instead of opting for TAVI. Notably, the short-term risk of all-cause death was not decreased in TAVI in higher risk patients. This observation was similar to several meta-analyses with higher-risk patients (2, 24). Unfortunately, no randomized trials in high-risk patients using newer-generation valves have been conducted thus far. There have been some propensity-matched studies that shed light on this topic. For instance, a study involving 72 pairs of high-risk patients, although utilizing mixed generations of TAVI valves, showed a lower in-hospital mortality rate but a higher risk of all-cause death at 5-year follow-up in the TAVI group (30). Another propensity-matched analysis of 783 pairs of intermediate-risk patients (mean age: 81.7 years, mean STS score: 5.5) using newer-generation SAPIEN 3 valves demonstrated a comparable risk of death or disabling stroke at 5 years compared to SAVR (31). Further studies are warranted to evaluate the performance of TAVI with newer-generation valves compared to SAVR in the context of higher-risk patients.
An interesting finding of our analysis was the significant interaction between BEV and SEV regarding the primary outcome and all-cause death at longer-term follow-up. A temporal interaction was observed in BEV for both the primary outcome and all-cause death, while in SEV, it was observed only for the primary outcome. These temporal trends closely align with those reported in the PARTNER 2A trial (5), which compared early-generation BEV TAVI with SAVR in higher surgical risk patients, and the PARTNER 3 trial (9), which compared newer-generation BEV TAVI with SAVR in lower surgical risk patients. Landmark analyses of clinical events between 2 and 5 years in both trials demonstrated higher rates of all-cause death and the primary outcome in TAVI compared to SAVR. Similarly, in another trial of BEV TAVI, the Kaplan–Meier event curves for all-cause death converged at 2 years (4). In contrast, trials comparing SEV TAVI to SAVR showed Kaplan–Meier event curves for the primary endpoint that remained parallel, favoring TAVI in the Evolut Low Risk trial (8), nearly overlapped in the SURTAVI (6) and NOTION (20) trials, and converged until the 5-year mark in the U.S. CoreValve trial (3). Longer-term data from head-to-head comparisons of BEV with SEV TAVI have been reported in only one RCT (32). In this trial, with 241 high-risk patients randomly assigned to early generation BEV and SEV, all-cause mortality (53.4% vs. 47.6%) and cardiovascular mortality (31.6% vs. 21.5%) at 5 years were numerically higher in the BEV group compared with the SEV group, consistent with our findings. These differences might be attributed to better forward flow hemodynamics and less structural valve deterioration in SEV compared to BEV (32). Several propensity-matched studies showed varied findings, but these conclusions were limited by residual confounders that could not be fully accounted for, such as patients’ anatomical suitability. It is likely that more patients with extensive outflow tract calcifications, low implanted coronary arteries, or complex and small femoral access received SEV (33). We found no significant difference between BEV and SEV at short-term follow-up, which is also consistent with findings from other RCTs (34, 35).
Our analysis had several strengths. Firstly, we incorporated the largest number of RCTs with longer-term follow-up outcomes, ensuring a comprehensive evaluation of the data. Additionally, the trials included in our analysis had nearly identical follow-up durations, enabling landmark analyses and mitigating the potential impact of variations in follow-up durations on the outcomes.
However, it is important to acknowledge some limitations. Firstly, our analysis was based on trial-level rather than patient-level data. Although we performed subgroup analyses based on clinically relevant subgroups, we were unable to conduct more detailed meta-regression analyses to account for potential confounding factors beyond the subgroup variables. Secondly, concomitant procedures were performed in both TAVI and SAVR groups in original trials, which could potentially influence the evaluation of isolated TAVI vs. isolated SAVR. Thirdly, our assessment of publication bias was limited by the relatively small number of trials, potentially affecting the ability to detect small-study effects.
TAVI was associated with a higher longer-term risk of primary outcome compared to SAVR in higher-risk patients and with balloon-expandable valves. However, a characteristic temporal interaction was documented in all subgroups. Long-term follow-up data from low-risk trials and large trials comparing TAVI with balloon-expandable and self-expanding valves are warranted to test these findings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YW: Data curation, Formal analysis, Investigation, Writing – original draft. XiaZ: Conceptualization, Data curation, Methodology, Writing – review & editing. XinZ: Conceptualization, Formal analysis, Methodology, Validation, Writing – review & editing. WX: Conceptualization, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1479200/full#supplementary-material
MVC, major bleeding, major vascular complication; PPM, permanent pacemaker implantation; PVL, paravalvular leak; RCT, randomized controlled trials; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack.
1. Mori M, Gupta A, Wang Y, Vahl T, Nazif T, Kirtane AJ, et al. Trends in transcatheter and surgical aortic valve replacement among older adults in the United States. J Am Coll Cardiol. (2021) 78(22):2161–72. doi: 10.1016/j.jacc.2021.09.855
2. Zhang XL, Zhang XW, Lan RF, Chen Z, Wang L, Xu W, et al. Long-term and temporal outcomes of transcatheter versus surgical aortic-valve replacement in severe aortic stenosis: a meta-analysis. Ann Surg. (2021) 273(3):459–66. doi: 10.1097/SLA.0000000000003906
3. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, et al. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. (2018) 72(22):2687–96. doi: 10.1016/j.jacc.2018.08.2146
4. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. (2015) 385(9986):2477–84. doi: 10.1016/S0140-6736(15)60308-7
5. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. (2020) 382(9):799–809. doi: 10.1056/NEJMoa1910555
6. Van Mieghem NM, Deeb GM, Sondergaard L, Grube E, Windecker S, Gada H, et al. Self-expanding transcatheter vs surgical aortic valve replacement in intermediate-risk patients: 5-year outcomes of the SURTAVI randomized clinical trial. JAMA Cardiol. (2022) 7(10):1000–8. doi: 10.1001/jamacardio.2022.2695
7. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 3-year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. (2023) 81(17):1663–74. doi: 10.1016/j.jacc.2023.02.017
8. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 4-year outcomes of patients with aortic stenosis in the evolut low risk trial. J Am Coll Cardiol. (2023) 82(22):2163–5. doi: 10.1016/j.jacc.2023.09.813
9. Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, et al. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. (2023) 389(21):1949–60. doi: 10.1056/NEJMoa2307447
10. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364(23):2187–98. doi: 10.1056/NEJMoa1103510
11. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. (2012) 366(18):1686–95. doi: 10.1056/NEJMoa1200384
12. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. (2014) 370(19):1790–8. doi: 10.1056/NEJMoa1400590
13. Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. (2015) 66(2):113–21. doi: 10.1016/j.jacc.2015.05.017
14. Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. (2015) 65(20):2184–94. doi: 10.1016/j.jacc.2015.03.014
15. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374(17):1609–20. doi: 10.1056/NEJMoa1514616
16. Sondergaard L, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Two-year outcomes in patients with severe aortic valve stenosis randomized to transcatheter versus surgical aortic valve replacement: the all-comers nordic aortic valve intervention randomized clinical trial. Circ Cardiovasc Interv. (2016) 9(6):e003665. doi: 10.1161/CIRCINTERVENTIONS.115.003665
17. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376(14):1321–31. doi: 10.1056/NEJMoa1700456
18. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380(18):1695–705. doi: 10.1056/NEJMoa1814052
19. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380(18):1706–15. doi: 10.1056/NEJMoa1816885
20. Thyregod HGH, Ihlemann N, Jorgensen TH, Nissen H, Kjeldsen BJ, Petursson P, et al. Five-year clinical and echocardiographic outcomes from the NOTION randomized clinical trial in patients at lower surgical risk. Circulation. (2019) 139(24):2714–23. doi: 10.1161/CIRCULATIONAHA.118.036606
21. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. (2021) 77(9):1149–61. doi: 10.1016/j.jacc.2020.12.052
22. Investigators UTT, Toff WD, Hildick-Smith D, Kovac J, Mullen MJ, Wendler O, et al. Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all-cause mortality in patients with aortic stenosis: a randomized clinical trial. JAMA. (2022) 327(19):1875–87. doi: 10.1001/jama.2022.5776
23. Forrest JK, Deeb GM, Yakubov SJ, Rovin JD, Mumtaz M, Gada H, et al. 2-year outcomes after transcatheter versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol. (2022) 79(9):882–96. doi: 10.1016/j.jacc.2021.11.062
24. Swift SL, Puehler T, Misso K, Lang SH, Forbes C, Kleijnen J, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis: a systematic review and meta-analysis. BMJ Open. (2021) 11(12):e054222. doi: 10.1136/bmjopen-2021-054222
25. Ahmad Y, Howard JP, Arnold AD, Madhavan MV, Cook CM, Alu M, et al. Transcatheter versus surgical aortic valve replacement in lower-risk and higher-risk patients: a meta-analysis of randomized trials. Eur Heart J. (2023) 44(10):836–52. doi: 10.1093/eurheartj/ehac642
26. Ueyama H, Kuno T, Ando T, Hayashida K, Takagi H. Network meta-analysis of surgical aortic valve replacement and different transcatheter heart valve systems for symptomatic severe aortic stenosis. Can J Cardiol. (2021) 37(1):27–36. doi: 10.1016/j.cjca.2020.02.088
27. Takagi H, Hari Y, Nakashima K, Kuno T, Ando T. A meta-analysis of ≥5-year mortality after transcatheter versus surgical aortic valve replacement. J Cardiovasc Surg (Torino). (2020) 61(1):107–16. doi: 10.23736/S0021-9509.19.11030-0
28. Thourani VH, Habib R, Szeto WY, Sabik JF, Romano JC, MacGillivray TE, et al. Survival after surgical aortic valve replacement in low-risk patients: a contemporary trial benchmark. Ann Thorac Surg. (2024) 117(1):106–12. doi: 10.1016/j.athoracsur.2023.10.006
29. Martinsson A, Nielsen SJ, Milojevic M, Redfors B, Omerovic E, Tonnessen T, et al. Life expectancy after surgical aortic valve replacement. J Am Coll Cardiol. (2021) 78(22):2147–57. doi: 10.1016/j.jacc.2021.09.861
30. Mach M, Poschner T, Hasan W, Kerbel T, Szalkiewicz P, Hasimbegovic E, et al. Transcatheter versus isolated surgical aortic valve replacement in young high-risk patients: a propensity score-matched analysis. J Clin Med. (2021) 10(15):3447. doi: 10.3390/jcm10153447
31. Madhavan MV, Kodali SK, Thourani VH, Makkar R, Mack MJ, Kapadia S, et al. Outcomes of SAPIEN 3 transcatheter aortic valve replacement compared with surgical valve replacement in intermediate-risk patients. J Am Coll Cardiol. (2023) 82(2):109–23. doi: 10.1016/j.jacc.2023.04.049
32. Abdel-Wahab M, Landt M, Neumann FJ, Massberg S, Frerker C, Kurz T, et al. 5-year outcomes after TAVR with balloon-expandable versus self-expanding valves: results from the CHOICE randomized clinical trial. JACC Cardiovasc Interv. (2020) 13(9):1071–82. doi: 10.1016/j.jcin.2019.12.026
33. Zhang XL, Wei ZH, Wang HW, Xu W, Wang Y, Xu B. Early and midterm outcomes of transcatheter aortic-valve replacement with balloon-expandable versus self-expanding valves: a meta-analysis. J Cardiol. (2022) 80(3):204–10. doi: 10.1016/j.jjcc.2022.04.011
34. Abdel-Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tolg R, et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. (2014) 311(15):1503–14. doi: 10.1001/jama.2014.3316
Keywords: aortic stenosis, TAVI, SAVR, longer-term, randomized controlled trials (RCT)
Citation: Wang Y, Zhang X, Zhang X and Xu W (2025) Longer-term and landmark analysis of transcatheter vs. surgical aortic-valve implantation in severe aortic stenosis: a meta-analysis. Front. Cardiovasc. Med. 12:1479200. doi: 10.3389/fcvm.2025.1479200
Received: 11 August 2024; Accepted: 19 February 2025;
Published: 6 March 2025.
Edited by:
Bart Meuris, University Hospitals Leuven, BelgiumReviewed by:
Vojko Kanic, Maribor University Medical Centre, SloveniaCopyright: © 2025 Wang, Zhang, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinlin Zhang, eGlubHpoYW5nMDgwN0AxNjMuY29t; Wei Xu, MTMzOTA5MDA4NjhAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.