- 1Department of Cardiology, Campus Kerckhoff of the Justus-Liebig-University Giessen, Bad Nauheim, Germany
- 2German Centre for Cardiovascular Research (DZHK), Partner Site RheinMain, Frankfurt am Main, Germany
- 3Department of Cardiology and Angiology, Justus-Liebig-University Giessen, Giessen, Germany
- 4Department of Cardiology and Vascular Medicine, GRN Hospital Weinheim, Weinheim, Germany
- 5Cardiac Imaging Center Weinheim, Hector Foundation, Weinheim, Germany
Coronary artery bypass grafting (CABG) is a common and effective treatment for patients with complex coronary artery disease. This case report discusses a 75-year-old male patient who presented with angina and shortness of breath due to thrombus formation in a venous graft 20 years after CABG. Initial diagnostics indicated non-ST-elevation myocardial infarction, leading to immediate intervention. Cardiac catheterization revealed thrombus in the vein graft to the large first diagonal branch, necessitating percutaneous coronary intervention. Despite initial efforts, thrombus aspiration and further catheter advancement were unsuccessful. A combination of balloon angioplasty, stent implantation, and intra-arterial thrombolysis with recombinant tissue plasminogen activator (rt-PA) was employed, resulting in significant thrombus reduction and improved coronary flow. Follow-up coronary CT angiography (CCTA) confirmed complete thrombus resolution and patent graft. The patient was discharged with dual antiplatelet therapy and showed favorable outcomes. This case emphasizes the challenges of managing thrombotic complications in venous bypass grafts and highlights the effectiveness of a multifaceted interventional approach combined with CCTA for non-invasive patient follow-up and assessment of treatment success. Furthermore, a review of the current literature on the role of local thrombolysis for occluded coronary artery bypass grafts is provided.
Introduction
Coronary artery bypass grafting (CABG) is the most frequently performed procedure in cardiac surgery (1), being the foremost preferred strategy of myocardial revascularization in patients with extensive, multi-vessel coronary artery disease (CAD). Beyond improvements in cardiovascular outcomes (2), patients undergoing CABG were reported to exhibit low clinical event rates and improved quality of life in recent prospective cohort studies (3).
Long-term patient outcomes after CABG are primarily dependent on the patency of grafts used for revascularization (1, 4). The incidence of graft failure is reported to be in the range of 10%–50% within approximately 10 years, with the highest failure rates found for venous graft material (1, 4, 5). Graft failure can occur within 1 month of surgery or later with different underlying causes. Most often, neointimal hyperplasia leads to obstructing atherosclerotic plaque formation being the primary cause of late vein graft failure, which may be associated with superimposed thrombus (5). Treatment of failed venous grafts—whether surgical or interventional—is feasible, but carries a high risk of peri-procedural complications. Furthermore, it is unclear whether in case of venous graft failure, the treatment of the native recipient vessel could be safer and more effective compared to the treatment of the occluded graft (6, 7).
Herein, we present the case of a 75-year-old man with recurrent ischemia due to thrombus formation in a venous bypass graft.
Case description
A 75-year-old male patient presented to our emergency department with suspected acute coronary syndrome (ACS) due to new onset angina and shortness of breath (case flowchart in Figure 1). He had undergone CABG 20 years ago and percutaneous coronary intervention (PCI) with placement of one drug-eluting stent (DES) into the vein graft to his right coronary artery (RCA) 7 years ago at our department. Beyond a history of hypertension, hyperlipidemia, and type 2 diabetes mellitus, no other clinical health conditions were known.
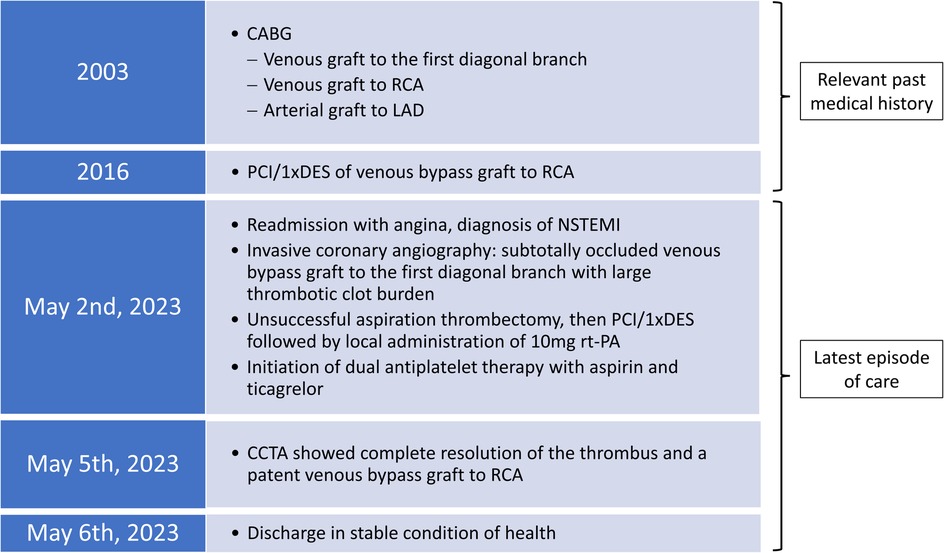
Figure 1. Chronological presentation of relevant data from the patient's cardiac medical history and the latest episode of care.
The physical examination exhibited an elevated heart rate (100 bpm), which electrocardiogram confirmed to be sinus tachycardia without ischemic signs. Echocardiography revealed mildly reduced systolic left ventricular function without regional wall motion abnormalities. Because laboratory chemical diagnostics revealed an elevated and increasing high-sensitive cardiac troponin T (84.8–165.7 ng/L at 2 h), the diagnosis of non-ST-elevation myocardial infarction (NSTEMI) was established. In addition, chronic renal failure was present with a creatinine value of 1.86 mg/dl. Subsequently, the patient received aspirin i.v., the loading dose of ticagrelor p.o., and fondaparinux s.c. and was transferred to the intensive care unit.
Cardiac catheterization was performed urgently on the same day using the right common femoral artery and showed occlusion of all three native coronaries (syntax score of 62). Calcification was non-severe in all three coronary arteries and no collaterals were noted. Selective angiography of the left coronary artery revealed retrograde filling of the saphenous vein graft (SVG) through the first diagonal branch. The SVG showed a very tight stenosis at the distal anastomosis with thrombus being responsible for TIMI flow grade 2 (Figures 2A,B, Supplementary Videos 1A,B,E,F). The left internal mammary artery graft to the left anterior descending (LAD) coronary artery was found patent (Supplementary Video 1C), whereas a good long-term result after PCI and stent placement 7 years ago was observed in the vein graft to the RCA (Supplementary Video 1D). Using a 6 French (6F) Amplatz left curve (AL) guiding catheter and a SION blue guidewire (ASAHI) for lesion crossing and thrombus, aspiration was subsequently attempted using a 6F catheter (Eliminate™, Terumo); however, it was not possible to advance the thrombectomy catheter through the high-grade stenosis. An intravascular ultrasound (IVUS) catheter could also not be advanced through the lesion. In addition, since the lesion and the thrombus were located at the distal part of the bypass graft, the placement of an embolic filter protection device was not deemed technically feasible. Activated clotted time (ACT) was controlled, being at 325 s at this time point. After lesion predilatation using a 2.0×15 mm semi-compliant balloon, two DESs (3.5 mm × 16 mm and 4.0 mm × 12 mm) were deployed with short overlap. However, the remaining thrombus was noticed distally to the implanted stents (Figure 2D, Supplementary Video 1G). The implantation of another DES was deferred in the absence of residual vascular plaque and due to the risk of distal embolization with bypass graft occlusion. Instead, an intra-arterial injection of 10 mg Actilyse (recombinant tissue plasminogen activator, rt-PA; Boehringer Ingelheim, Ingelheim, Germany) was performed through the guideliner directly to the thrombus, which resulted in substantial thrombus reduction within 15 min (Figure 2E, Supplementary Video 1H).
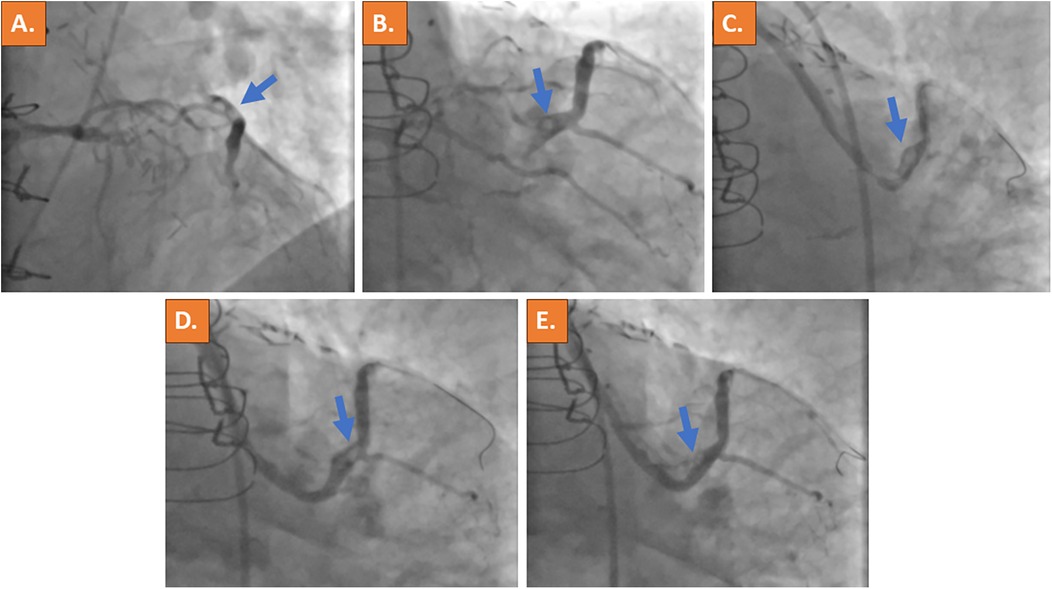
Figure 2. Acute invasive coronary angiography with selective contrast material injection into the left coronary artery ostium revealed retrograde filling of the vein bypass graft to the large first diagonal branch with suspected thrombus formation at the distal site of the bypass anastomosis (blue arrows in A,B). A high-grade stenosis of the vein graft was present (C). After PCI, a remaining thrombus was noticed distally to the implanted DESs (D). Because thrombus material remained, intra-arterial application of thrombolysis (rt-PA) was performed and significantly reduced the clot burden (E). The thrombotic material is indicated by the blue arrow.
After completion of the procedure, vascular access hemostasis was achieved by Starclose deployment. The patient was transferred to our intermediate care unit. No bleeding complications occurred. After coronary reperfusion, the patient stopped complaining of symptoms. He was put on dual platelet inhibition with aspirin once per day and ticagrelor twice per day for 12 months and additionally received fondaparinux for the next 3 days.
Coronary computed tomography angiography (CCTA), which was performed after 3 days, showed complete resolution of the thrombus and a patent vein graft (Figures 3A,B). The patient was discharged on the following day and remained on treatment with dual platelet inhibition for 12 months.
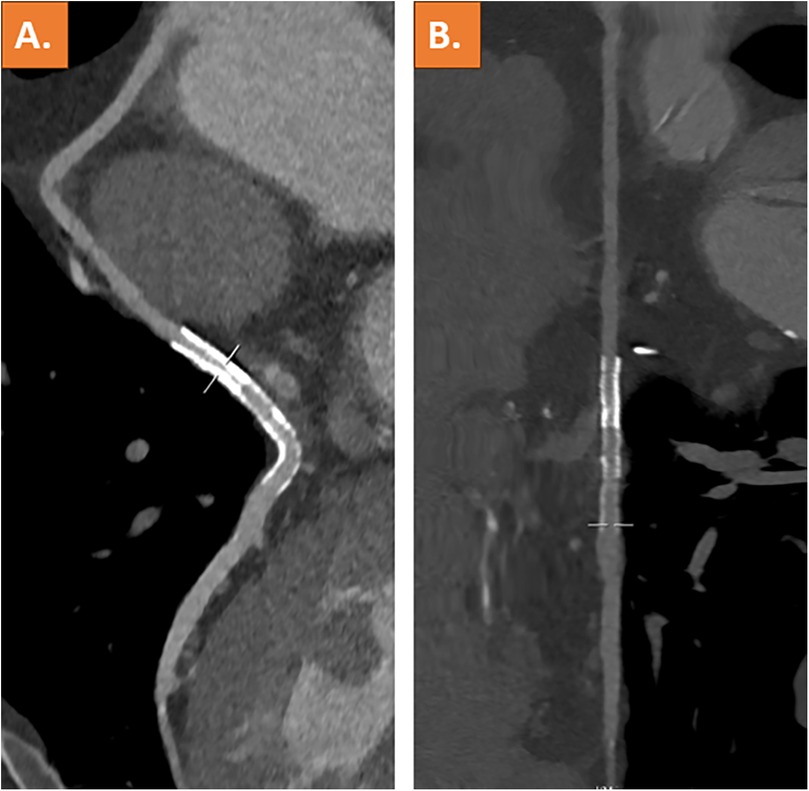
Figure 3. Coronary computed tomography angiography with curved multiplanar reformation (A) and stretched vessel view (B) showing a patent venous coronary artery bypass graft including stent lumen and complete resolution of the thrombus.
Discussion
After CABG, patients can present with challenging lesions in the setting of an acute coronary syndrome. Degenerated SVGs typically show lesions with high thrombus burden. Techniques including thrombectomy in combination with local delivery of glycoprotein-IIb/IIIa-inhibitors have been reported for these venous graft lesions (8, 9). Because thrombectomy was unsuccessful and the thrombotic clot burden remained high despite PCI with stent placement at the proximal part of the lesion, intra-arterial rt-PA was used as a bail-out strategy in the present case. Although data on the use of catheter-directed thrombolysis is present with thrombosed peripheral bypass grafts, data on the effectiveness of local lysis in coronary bypass grafts are scarce (10). Indeed, findings of the most relevant trial data on intracoronary thrombolysis in ST-elevation myocardial infarction (STEMI) were subsumed within a recent meta-analysis by Rehan et al. (11) suggesting that it could improve major adverse cardiac events in patients undergoing primary PCI, without significantly increasing the rate of major bleeding. While in the presented case local application of rt-PA was performed at the interventionalist's discretion with the rationale that it may be more efficient than glycoprotein-IIb/IIIa (GP-IIb/IIIa) inhibitors, one can controversially discuss the comparative value of both treatment strategies. The only prospective randomized data within this context stem from a small single-center pilot study, that could not prove a reduction of infarct size by intracoronary application of tenecteplase vs. the meanwhile withdrawn GP-IIb/IIIa-inhibitor abciximab in patients with anterior STEMI undergoing primary PCI (12). Of course, these findings warrant further investigation in sufficiently powered contemporary randomized controlled trials. This evidence is necessary to determine the future role of intracoronary thrombolysis beyond a case-by-case decision. Nevertheless, local thrombolysis can be a viable option in case of no-reflow or slow-flow, which often occurs in case of SVG PCI (13). A table with principal reports on pharmacological treatment in addition to PCI of thrombotic SVG failure is provided (Table 1) (8, 9, 14–16).
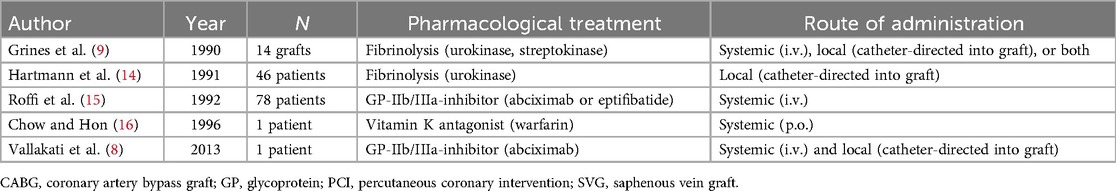
Table 1. Principal reports on pharmacological treatment in addition to percutaneous coronary intervention of thrombotic saphenous bypass graft failure.
In the management of recurrent ischemia due to thrombus formation in a venous bypass graft, alternative treatment options need to be considered carefully (13). One option is PCI or even recanalization of native coronary arteries. This approach is technically challenging and may not always be immediately successful due to the complexity of the procedure and the condition of the native arteries after long-term occlusion. An additional alternative is redo-CABG, which generally is not advisable in elderly patients with a history of previous cardiac surgery.
In this case, aspiration thrombectomy was unsuccessful. Nevertheless, thrombectomy can be used in case of ectatic vessels and large thrombotic burden. The underlying concept is to remove thrombotic material from the site of the ruptured plaque to effectively restore antegrade blood flow, alleviate distal thrombus embolization during PCI, to preserve downstream microcirculation, and to avoid a no-reflow phenomenon. However, the relevance of unselective thrombectomy for patient outcomes remains controversial. Since large randomized controlled trials, such as Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) by Fröbert et al. (17) and ThrOmbecTomy with PCI vs. PCI ALone in Patients with STEMI (TOTAL) by Jolly et al. (18), failed to improve outcomes, current guidelines do not recommend the routine use of thrombectomy (4, 19). However, a meta-analysis showed reduced cardiovascular mortality in the subgroup of patients with large thrombus burden (20), thus emphasizing the role of patient selection. Recently, dedicated mechanical thrombectomy devices were reported to overcome the limitations of manual thrombus aspiration for coronary artery use because of a larger diameter of the catheter lumen and continuous aspirating force during the procedure (21, 22). Although a novel dedicated mechanical thrombectomy device was not available in the present case, this constitutes a viable first-treatment approach. Thrombolysis could then serve as an escalation strategy to restore sufficient flow in the affected vessel with superimposed thrombus. PCI of a culprit lesion should be performed, whenever this is found to be an adequate target. Modern DESs are the gold standard for this purpose. While early generation bioresorbable vascular scaffolds have been associated with target lesion failure and scaffold thrombosis, the concept of these devices may be suited for PCI in venous grafts (23). Nevertheless, recent device improvements, such as reduced strut thickness, need to be proven effective in large randomized controlled trials before wide clinical adoption.
While utilization of embolic protection was not deemed technically feasible here, these devices could be particularly helpful when treating the culprit lesion in venous grafts due to the high risk of distal embolization of thrombotic material. Although the available study data do not support their routine use (24), embolic protection devices in conjunction with advanced interventional techniques and intravascular imaging techniques could mitigate the risk of restenosis and target vessel failure in selective clinical scenarios of vein graft PCI.
The standard of care after complex coronary intervention involves an invasive coronary angiography as a second look. However, due to thrombolysis, another invasive coronary angiography would have posed further risks and potential complications for our patient. Based on a thorough risk-benefit analysis, CCTA was chosen as a non-invasive alternative for the re-evaluation of the thrombotic burden of the venous graft. CCTA represents a particularly well-suited imaging method for assessing coronary artery bypass graft patency with excellent spatial and temporal resolution (25, 26). In addition, CCTA vs. invasive coronary angiography was recently shown to reduce procedural time and contrast-induced nephropathy (27, 28). In line with current recommendations (28, 29), CCTA may be an alternative to second-look invasive coronary angiography for the assessment of stent patency in patients with stent diameters of 3 mm and above. With the advent of CT systems offering ultra-high resolution, the future role of CCTA in these clinical scenarios could be expanded to inner stent diameters below 3 mm (30).
In addition, the role of intravascular imaging needs to be mentioned. Thus, IVUS and optical coherence tomography (OCT) are well-established methods for the characterization of lesion extent and morphology. In our case, the advancement of an IVUS catheter was unfortunately not technically feasible due to vessel tortuosity. Despite these limitations, the present case report highlights challenges of acute and recurrent ischemic events in patients with a history of extensive revascularization. In this context, the potential of a multifaceted management approach involving advanced interventional treatment in combination with local drug therapy for optimizing patient outcomes is underscored. Finally, CCTA proved to be an effective non-invasive complement as a second-look strategy for judging the effectiveness of the invasive pharmaco-chemical treatment.
Patient perspective
The patient, who gave written informed consent for publication of this case report, was reserved toward a repeated invasive coronary angiography procedure after pharmaco-mechanical treatment of his subtotally occluded vein bypass. Indeed, after discussing the alternatives, he expressed a clear preference for a non-invasive follow-up assessment of the treatment result graft by CCTA. Our patient’s preference seems supportive of the finding of Jones et al. that the use of CCTA versus invasive coronary angiography for the assessment of CABG patency leads to improved patient satisfaction (30).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MR: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SS: Supervision, Writing – original draft, Writing – review & editing. CS: Writing – original draft, Writing – review & editing. GK: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
MR received proctor fees from Boston Scientific and Medtronic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1471462/full#supplementary-material
Abbreviations
ACS, acute coronary syndrome; ACT, activated clotting time; CCTA, coronary computed tomography angiography; CABG, coronary artery bypass grafting; DES, drug-eluting stent; i.v., intravenous; IVUS, intravascular ultrasound; LAD, left anterior descending; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; rt-PA, recombinant tissue plasminogen activator; s.c., subcutaneous; SVG, saphenous vein graft.
References
1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
2. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. (2016) 374:1511–20. doi: 10.1056/NEJMoa1602001
3. Sandner S, Misfeld M, Caliskan E, Böning A, Aramendi J, Salzberg SP, et al. Clinical outcomes and quality of life after contemporary isolated coronary bypass grafting: a prospective cohort study. Int J Surg. (2023) 109:707–15. doi: 10.1097/JS9.0000000000000259
4. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e21–e129. doi: 10.1016/j.jacc.2021.09.006
5. Harik L, Perezgrovas-Olaria R, Soletti G Jr, Dimagli A, Alzghari T, An KR, et al. Graft thrombosis after coronary artery bypass surgery and current practice for prevention. Front Cardiovasc Med. (2023) 10:1125126. doi: 10.3389/fcvm.2023.1125126
6. Galassi AR, Vadalà G, Werner GS, Cosyns B, Sianos G, Hill J, et al. Evaluation and management of patients with coronary chronic total occlusions considered for revascularisation. A clinical consensus statement of the European association of percutaneous cardiovascular interventions (EAPCI) of the ESC, the European association of cardiovascular imaging (EACVI) of the ESC, and the ESC working group on cardiovascular surgery. EuroIntervention. (2024) 20:e174–84. doi: 10.4244/EIJ-D-23-00749
7. Guo L, Lv H, Yin X. Chronic total occlusion percutaneous coronary intervention in patients with prior coronary artery bypass graft: current evidence and future perspectives. Front Cardiovasc Med. (2022) 9:753250. doi: 10.3389/fcvm.2022.753250
8. Vallakati A, Mastrine L, Ayzenberg S. Rheolytic therapy combined with intragraft Abciximab for treatment in acute myocardial infarction. J Invasive Cardiol. (2013) 25:E33–5. doi: 10.3389/fcvm.2022.753250
9. Grines CL, Booth DC, Nissen SE, Gurley JC, Bennett KA, O'Connor WN, et al. Mechanism of acute myocardial infarction in patients with prior coronary artery bypass grafting and therapeutic implications. Am J Cardiol. (1990) 65:1292–6. doi: 10.1016/0002-9149(90)91315-W
10. Korosoglou G, Torsello G, Saratzis A, Isernia G, Kontopodis N, González TM, et al. Editor’s choice—endovascular versus surgical treatment for all comer patients with prosthetic bypass graft occlusion: the multicentre ENSUPRO study. Eur J Vasc Endovasc Surg. (2024) 67:786–96. doi: 10.1016/j.ejvs.2023.07.054
11. Rehan R, Virk S, Wong CCY, Passam F, Layland J, Keech A, et al. Intracoronary thrombolysis in ST-elevation myocardial infarction: a systematic review and meta-analysis. Heart. (2024) 110:988–96. doi: 10.1136/heartjnl-2024-324078
12. Morales-Ponce FJ, Lozano-Cid FJ, Martinez-Romero P, Gonzalez-Perez P, Sanchez-Brotons JA, Diaz-Torres I, et al. Intracoronary tenecteplase versus Abciximab as adjunctive treatment during primary percutaneous coronary intervention in patients with anterior myocardial infarction. EuroIntervention. (2019) 14:1668–75. doi: 10.4244/EIJ-D-18-00885
13. Vadalà G, Galassi AR, Werner GS, Sianos G, Boudou N, Garbo R, et al. Contemporary outcomes of chronic total occlusion percutaneous coronary intervention in Europe: the ERCTO registry. EuroIntervention. (2024) 20:e185–97. doi: 10.4244/EIJ-D-23-00490
14. Hartmann JR, McKeever LS, Stamato NJ, Bufalino VJ, Marek JC, Brown AS, et al. Recanalization of chronically occluded aortocoronary saphenous vein bypass grafts by extended infusion of urokinase: initial results and short-term clinical follow-up. J Am Coll Cardiol. (1991) 18:1517–23. doi: 10.1016/0735-1097(91)90684-2
15. Roffi M, Mukherjee D, Chew DP, Bhatt DL, Cho L, Robbins MA, et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation. (2002) 106:3063–7. doi: 10.1161/01.CIR.0000041250.89627.A9
16. Chow WH, Hon PH. Successful stenting after Coumadin therapy and thrombus resolution in a stenotic saphenous vein graft. Cathet Cardiovasc Diagn. (1996) 39:438–9. doi: 10.1002/(SICI)1097-0304(199612)39:4%3C438::AID-CCD26%3E3.0.CO;2-7
17. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. (2013) 369:1587–97. doi: 10.1056/NEJMoa1308789
18. Jolly SS, Cairns JA, Yusuf S, Rokoss MJ, Gao P, Meeks B, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. (2016) 387:127–35. doi: 10.1016/697.S0140-6736(15)00448-1
19. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44:3720–3826. doi: 10.1093/eurheartj/ehad191
20. Peng S, Rempakos A, Mastrodemos OC, Rangan BV, Alexandrou M, Allana S, et al. Use of the indigo CAT RX aspiration system during percutaneous coronary intervention. Catheter Cardiovasc Interv. (2024) 103:695–702. doi: 10.1002/ccd.30994
21. Mathews SJ, Parikh SA, Wu W, Metzger DC, Chambers JW, Ghali MGH, et al. Sustained mechanical aspiration thrombectomy for high thrombus burden coronary vessel occlusion: the multicenter CHEETAH study. Circ Cardiovasc Interv. (2023) 16:e012433. doi: 10.1161/CIRCINTERVENTIONS.122.012433
22. Stone GW, Biensock SW, Neumann FJ. Bioresorbable coronary scaffolds are ready for a comeback: pros and cons. EuroIntervention. (2023) 19:199–202. doi: 10.4244/EIJ-E-23-00015
23. Xenogiannis I, Zenati M, Bhatt DL, Rao SV, Rodés-Cabau J, Goldman S, et al. Saphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation. (2021) 144(9):728–45. doi: 10.1161/CIRCULATIONAHA.120.052163
24. Paul TK, Bhatheja S, Panchal HB, Zheng S, Banerjee S, Rao SV, et al. Outcomes of saphenous vein graft intervention with and without embolic protection device: a comprehensive review and meta-analysis. Circ Cardiovasc Interv. (2017) 10:e005538. doi: 10.1161/CIRCINTERVENTIONS.117.005538
25. Malagutti P, Nieman K, Meijboom WB, van Mieghem CA, Pugliese F, Cademartiri F, et al. Use of 64-slice CT in symptomatic patients after coronary bypass surgery: evaluation of grafts and coronary arteries. Eur Heart J. (2007) 28:1879–85. doi: 10.1093/eurheartj/ehl155
26. Barbero U, Iannaccone M, d'Ascenzo F, Barbero C, Mohamed A, Annone U, et al. 64 slice-coronary computed tomography sensitivity and specificity in the evaluation of coronary artery bypass graft stenosis: a meta-analysis. Int J Cardiol. (2016) 216:52–7. doi: 10.1016/j.ijcard.2016.04.156
27. Jones DA, Beirne AM, Kelham M, Rathod KS, Andiapen M, Wynne L, et al. Computed tomography cardiac angiography before invasive coronary angiography in patients with previous bypass surgery: the BYPASS-CTCA trial. Circulation. (2023) 148:1371–80. doi: 10.1161/CIRCULATIONAHA.123.064465
28. Narula J, Chandrashekhar Y, Ahmadi A, Abbara S, Berman DS, Blankstein R, et al. SCCT 2021 Expert consensus document on coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. (2021) 15:192–217. doi: 10.1016/j.jcct.2020.11.001
29. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 144:e368–454. doi: 10.1016/j.jacc.2021.07.052
30. Stein T, von Zur Muhlen C, Verloh N, Schürmann T, Krauss T, Soschynski M, et al. Evaluating small coronary stents with dual-source photon-counting computed tomography: effect of different scan modes on image quality and performance in a phantom. Diagn Interv Radiol. (2025) 31:29–38. doi: 10.4274/dir.2024.242893
Keywords: acute coronary syndrome, computed tomography angiography, coronary artery bypass graft, percutaneous coronary intervention, thrombectomy, local thrombolysis
Citation: Renker M, Sossalla S, Schoefthaler C and Korosoglou G (2025) Successful pharmaco-mechanical treatment of a subtotally occluded venous bypass graft in a patient presenting with acute coronary syndrome: a case report and review of the current literature on the role of local thrombolysis. Front. Cardiovasc. Med. 12:1471462. doi: 10.3389/fcvm.2025.1471462
Received: 27 July 2024; Accepted: 20 February 2025;
Published: 17 March 2025.
Edited by:
Michail Papafaklis, University of Patras, GreeceReviewed by:
Jiang Ming Fam, National Heart Centre Singapore, SingaporeGiuseppe Vadalà, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, Italy
Copyright: © 2025 Renker, Sossalla, Schoefthaler and Korosoglou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Renker, bS5yZW5rZXJAa2VyY2tob2ZmLWtsaW5pay5kZQ==
 Matthias Renker
Matthias Renker Samuel Sossalla1,2,3
Samuel Sossalla1,2,3 Grigorios Korosoglou
Grigorios Korosoglou