- 1School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China
- 2School of Stomatology, Changsha Medical University, Changsha, China
- 3Hunan key Laboratory of the Research and Development of Novel Pharmaceutical Preparations, School of Pharmaceutical Science, Changsha Medical University, Changsha, China
Cardiometabolic diseases (CMD) are leading causes of death and disability worldwide, with complex pathophysiological mechanisms in which inflammation plays a crucial role. This review aims to elucidate the molecular and cellular mechanisms within the inflammatory microenvironment of atherosclerosis, hypertension and diabetic cardiomyopathy. In atherosclerosis, oxidized low-density lipoprotein (ox-LDL) and pro-inflammatory cytokines such as Interleukin-6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-α) activate immune cells contributing to foam cell formation and arterial wall thickening. Hypertension involves the activation of the renin-angiotensin system (RAS) alongside oxidative stress-induced endothelial dysfunction and local inflammation mediated by T cells. In diabetic cardiomyopathy, a high-glucose environment leads to the accumulation of advanced glycation end products (AGEs), activating the Receptor for Advanced Glycation Endproducts (RAGE) and triggering inflammatory responses that further damage cardiac and microvascular function. In summary, the inflammatory mechanisms in different types of metabolic cardiovascular diseases are complex and diverse; understanding these mechanisms deeply will aid in developing more effective individualized treatment strategies.
Introduction
Cardiometabolic diseases (CMD) represent a complex cluster of disorders, primarily encompassing atherosclerosis, coronary artery disease, and hypertension, all of which are intricately linked to metabolic dysfunction (1–3). These diseases are frequently associated with metabolic syndrome (MetS), a condition characterized by a constellation of symptoms including central obesity, insulin resistance, hyperglycemia, dyslipidemia, and hypertension (4–7). As societies continue to urbanize and modernize, the adverse lifestyle practices have become increasingly prevalent, leading to a rising incidence of cardiometabolic diseases globally (8–11). According to a large number of relevant literature findings, CMD have become one of the leading causes of mortality worldwide, exerting a considerable burden on individuals, families, and public health systems (12, 13). Given that these diseases often progress insidiously, with many patients remaining asymptomatic until severe cardiovascular events such as myocardial infarction, heart failure, or stroke occur, there is an urgent need to unravel the underlying mechanisms of CMD to develop effective preventive and therapeutic strategies that can improve patient outcomes and quality of life (14, 15).
The inflammatory microenvironment plays a critical role in the onset and progression of CMD (16–18). A growing body of research indicates that chronic low-grade inflammation in the cardiovascular system is a pivotal driver of the onset and progression of these diseases (19, 20). Inflammation impacts cardiovascular health through its regulation of processes such as lipid uptake, glucose metabolism, endothelial function, and vascular remodeling (21–24). During the development of atherosclerosis, inflammatory cells such as macrophages, T cells, and mast cells, along with various inflammatory mediators they secrete—including cytokines and chemokines—contribute to the formation and progression of arterial plaques (25, 26). These cells and molecules form a complex inflammatory network that affects multiple aspects of the cardiovascular system, not only causing direct damage to blood vessel walls but also modulating metabolic functions and activating immune responses (17, 26). Despite substantial evidence supporting the critical role of inflammation in CMD, the precise mechanisms by which it is initiated and sustained, and how it interacts with metabolic processes to accelerate disease progression, remain inadequately understood and warrant further investigation.
To address these challenges, this review aims to systematically explore the molecular mechanisms and cellular foundations underpinning the development and maintenance of the inflammatory microenvironment in cardiometabolic diseases. By synthesizing current research advancements, the article seeks to elucidate the specific roles of various inflammatory mediators in CMD, and how their interplay with metabolic abnormalities influences disease progression and outcomes. As our understanding of these pathological mechanisms deepens, we hope to lay a robust scientific foundation for future therapeutic innovations designed to improve the prognosis of patients with cardiometabolic diseases and alleviate the global health burden they pose. By enhancing our understanding of the pathological mechanisms at play, we aim to pave the way for the development of innovative interventions that improve patient outcomes and reduce the global burden of cardiometabolic diseases.
Metabolic factors are involved in the occurrence and development of cardiovascular diseases
Cardiovascular disease (CVD) is the leading cause of death worldwide and is characterized by atherosclerosis, endothelial dysfunction, inflammation, and oxidative stress (27–30). Metabolic factors play a crucial role in the onset and progression of CVD, primarily including hyperlipidemia, hyperglycemia, and insulin resistance (31). Mendelian randomization (MR) is an analytical method that employs genetic variants as instrumental variables and can be widely used to study the causal associations between exposures and outcomes (32–35). In a MR study, MetS exhibits significant causal relationships with various CVD (36). In clinical trials, patients with MetS have a significantly higher risk of developing CVD compared to those without MetS (37). In patients with hypopituitarism, the high prevalence of MetS is primarily linked to abdominal fat deposition, dyslipidemia, and insulin resistance, with growth hormone replacement therapy showing benefits for lipid profiles and body fat but potentially transiently worsening glucose tolerance (38).
Recent molecular and cellular mechanisms linking inflammation and metabolism
The interplay between inflammation and metabolism is crucial for physiological balance and disease pathogenesis, including type 2 diabetes (T2D), cardiovascular diseases, obesity, and autoimmune disorders (39). Silent Information Regulator 2 Ortholog 1 (SIRT1) regulates inflammation through various pathways, including affecting metabolic pathways, inflammatory cells and mediators, as well as key signaling pathways, thus playing a significant role in metabolic and immune diseases and serving as a potential therapeutic target (40). Research indicates that small dense low-density lipoprotein-cholesterol (sdLDL-C) and remnant-like particle cholesterol (RLP-C) induce inflammation by activating immune cells, while hyperlipidemia exacerbates inflammatory responses, leading to dysregulated lipid metabolism and alterations in lipoprotein profiles (41). These insights provide new directions for developing targeted therapies for chronic inflammatory and metabolic disorders, potentially leading to more precise and effective treatments.
By targeting these key biological processes, it is possible to develop more precise and effective interventions to improve patient health in the future. Therefore, an in-depth understanding of the mechanisms underlying the formation of the inflammatory microenvironment is essential for disease prevention and treatment. This review will focus on the formation mechanism of the inflammatory microenvironment of atherosclerosis, hypertension and diabetic cardiomyopathy from the perspective of molecular and cellular levels, focusing on the specific factors affecting the inflammatory response.
Inflammatory mechanisms in atherosclerosis
Initial events and the role of oxidized Low-density lipoprotein
Atherosclerosis is a chronic inflammatory disease characterized by the formation of lipid deposits, immune cell infiltration, and fibrosis in the arterial wall, eventually leading to atherosclerotic plaque formation (42, 43). One of the initial events in atherosclerosis is the accumulation of LDL in the subendothelial space of arteries (44, 45). In these regions, LDL becomes susceptible to oxidation by ROS, forming ox-LDL (46). ox-LDL not only exhibits pro-inflammatory properties but also activates various immune responses (46). ox-LDL binds to scavenger receptors on endothelial cell (EC), macrophages, and smooth muscle cells, triggering intracellular signaling pathways that initiate inflammatory responses (47). For instance, ox-LDL activates the Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, promoting the expression of various pro-inflammatory cytokines and chemokines like IL-6 and TNF-α (47, 48). Ox-LDL has different effects on vascular smooth muscle cells (VSMCs) and ECs (49, 50). In ECs, ox-LDL activates the NF-κB pathway through Toll-like receptors (TLRs) and other receptors, increasing the expression of pro-inflammatory cytokines such as IL-6, Interleukin-8 (IL-8), and Monocyte Chemoattractant Protein-1 (MCP-1), which attract monocytes to the vessel wall and promote plaque formation (51). In VSMCs, ox-LDL also activates the NF-κB pathway but primarily promotes cell proliferation and migration, influencing plaque stability or instability (52). Additionally, the phenotypic switch of VSMCs from a contractile to a synthetic phenotype is associated with NF-κB activation (52).
Inflammation amplification and immune cell recruitment
The presence of ox-LDL and lipid accumulation in the arterial wall further induces the overproduction of pro-inflammatory cytokines (such as IL-6 and TNF-α) and chemokines, such as Chemokine (C-C motif) ligand 2 (CCL2) and Chemokine (C-X-C motif) ligand 10 (CXCL10) (53, 54). These molecules bind to their respective receptors on EC and immune cells, further amplifying the inflammatory response. IL-6 and TNF-α activate the Janus Kinase—Signal Transducer and Activator of Transcription (JAK-STAT) and NF-κB signaling pathways, promoting inflammatory responses and stimulating the proliferation and migration of vascular wall cells (55, 56). Chemokines (like CCL2) recruit monocytes to the arterial wall, enhancing the local immune response (57). CCL2 binds to the Chemokine (C-C motif) receptor 2 (CCR2) on monocytes, facilitating their adhesion to the endothelium and subsequent infiltration into the vascular wall (57). As atherosclerosis progresses, the accumulation of cholesterol and its metabolites triggers additional inflammatory responses by activating the NLR family, pyrin domain containing 3 (NLRP3) inflammasome, a key intracellular inflammatory signaling complex (58). Activation of the NLRP3 inflammasome leads to Cysteine-aspartic protease 1 (caspase-1) activation, which promotes the conversion of Pro-Interleukin-1β (pro-IL-1β) and Pro-Interleukin-18 (pro-IL-18) into their mature forms, Interleukin-1β (IL-1β) and Interleukin-18 (IL-18) (59, 60). These cytokines further amplify local inflammation and induce apoptosis and necrosis of vascular wall cells (60). Activation of the inflammasome is also associated with plaque instability, potentially leading to plaque rupture and thrombosis, which can trigger acute cardiovascular events such as myocardial infarction and stroke (61).
Endothelial cell activation and foam cell formation
In the early stages of atherosclerosis, EC become activated due to various risk factors such as ox-LDL, hyperglycemia, and hypertension (62). Activated EC express various adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and Endothelial Selectin (E-selectin) (63). The upregulation of ICAM-1 and VCAM-1 is a hallmark of endothelial cell activation (64). These molecules bind to integrins on monocytes, promoting firm adhesion of monocytes to the endothelium and their subsequent infiltration into the subendothelial space (65, 66). Endothelial cell activation also involves the release of pro-inflammatory cytokines like IL-1β and TNF-α, which further enhance endothelial adhesion and permeability, promoting monocyte migration (67). Monocytes that adhere to activated EC, guided by chemokines, cross the endothelial layer and infiltrate the intima (68). Once in the intima, monocytes differentiate into macrophages, which then phagocytose large amounts of ox-LDL through scavenger receptors (47). These macrophages, laden with excessive lipid, transform into foam cells (69). The formation of foam cells is a hallmark of atherosclerotic plaques and involves the release of various inflammatory mediators, further promoting the development of local inflammation (69, 70). The apoptosis and death of foam cells contribute to plaque instability by releasing large amounts of lipids and cellular debris, increasing the risk of plaque rupture and thrombosis (71).
Smooth muscle cell phenotypic switching and changes in plaque stability
In the progression of atherosclerosis, smooth muscle cells undergo proliferation and a shift towards a synthetic phenotype under the influence of inflammatory mediators (72). This process involves a phenotypic change that enables smooth muscle cells to produce extracellular matrix components such as collagen, forming fibrous caps and thickening the arterial intima (73). Smooth muscle cell proliferation and synthetic phenotype transformation play a critical role in stabilizing plaques (74). However, as the disease progresses, the apoptosis and necrosis of smooth muscle cells can lead to the rupture of fibrous caps, increasing plaque instability and the risk of cardiovascular events (75). In summary, the inflammatory mechanisms of atherosclerosis involve complex interactions among multiple cells and molecules. In Figure 1, the molecules and cells involved in the inflammatory microenvironment of atherosclerosis can be seen. The oxidation of LDL, the release of pro-inflammatory cytokines, the role of cholesterol metabolites, and the dynamic changes in EC, monocytes, and smooth muscle cells all contribute significantly to the progression of this disease.
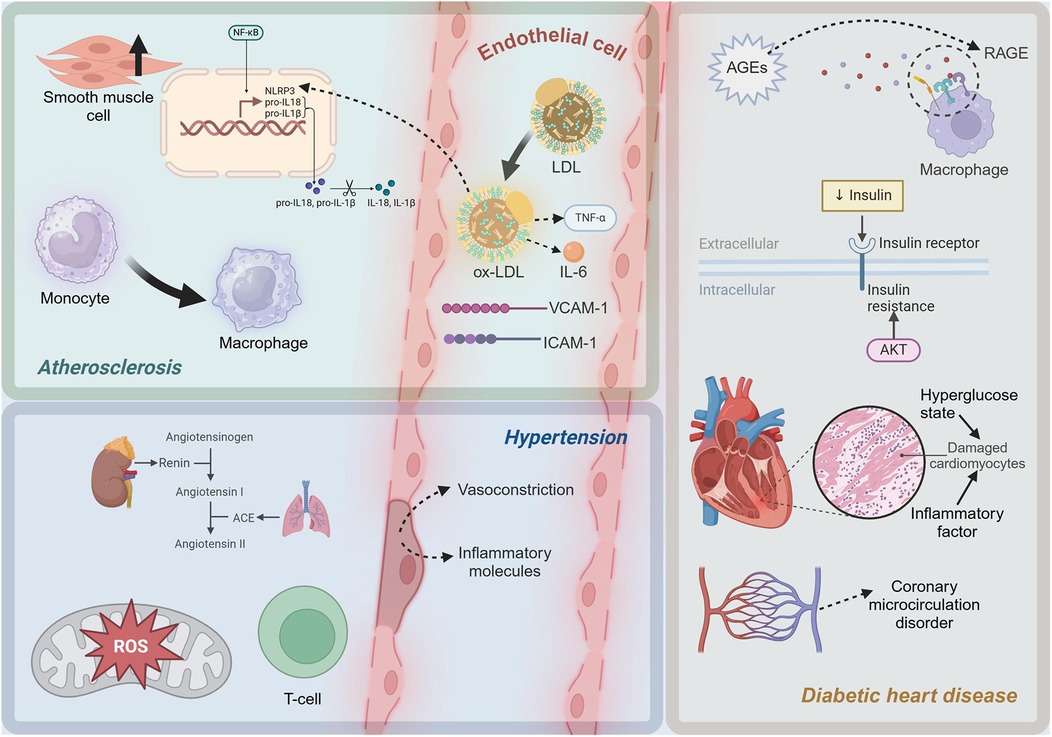
Figure 1. Diagram of inflammatory mechanisms in cardiometabolic diseases. LDL, low-density lipoprotein; ox-LDL, oxidized low-density lipoprotein; TNF-α, Tumor Necrosis Factor-alpha; IL-6, Interleukin-6; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; NF-κB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family, pyrin domain containing 3; pro-IL-1β, Pro-Interleukin-1β; pro-IL-18, Pro-Interleukin-18; IL-1β, Interleukin-1β; IL-18, Interleukin-18; ROS, reactive oxygen species; ACE, Angiotensin-Converting Enzyme; AGEs, advanced glycation end products; RAGE, Receptor for Advanced Glycation Endproducts. This figure was created with BioRender.com.
Inflammatory mechanisms in hypertension
Role of RAS in hypertension-related inflammation
Hypertension is a complex chronic disease with pathophysiological mechanisms involving multiple aspects, with inflammatory mechanisms receiving increasing attention in recent years (76, 77). Inflammation not only serves as a consequence of hypertension but also plays a crucial role in its progression and maintenance. The Renin-Angiotensin System (RAS) plays a pivotal role in the pathogenesis and progression of hypertension, with Angiotensin II (Ang II) being the key effector molecule (78). Ang II, by binding to the Angiotensin II type 1 receptor (AT1R), activates multiple signaling pathways, leading to vasoconstriction, sodium and water retention, and increased blood pressure (79). These inflammatory mediators further attract and activate immune cells, leading to vascular wall inflammation. ROS, including superoxide, hydrogen peroxide, and hydroxyl radicals, play significant roles in the inflammatory mechanisms of hypertension (80). Ang II increases ROS production via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system, which can directly damage vascular EC and activate inflammatory signaling pathways (81). In hypertensive patients, the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx) often decreases, leading to weakened ROS clearance and further exacerbating oxidative stress and inflammation (82).
Oxidative stress and endothelial damage
Endothelial dysfunction is a critical aspect of inflammatory mechanisms in hypertension (83). Upon exposure to Ang II, ROS, and other stimuli, EC release molecules like endothelin-1 (ET-1) and VCAM-1, which promote vasoconstriction and increase leukocyte adhesion and infiltration, leading to vascular wall inflammation (84, 85). Under normal conditions, EC produce nitric oxide (NO) through endothelial nitric oxide synthase (eNOS), which has vasodilatory and anti-inflammatory effects (86, 87). However, in hypertension, eNOS activity declines, reducing NO production, resulting in vasoconstriction and heightened inflammation (88). The immune system plays a crucial role in the inflammatory mechanisms of hypertension, with lymphocytes, particularly T cells, being significantly involved (89). In hypertensive patients and animal models, there is significant infiltration of T cells around blood vessels and in renal tissues (90). These T cells release cytokines such as interferon-γ (IFN-γ) and interleukin-17 (IL-17), further promoting local inflammatory responses (91). T Helper 1 (Th1) cells primarily secrete IFN-γ, which activates macrophages and promotes inflammation; T Helper 17 (Th17) cells mainly secrete IL-17, which is involved in neutrophil recruitment and activation (91, 92). These cytokines work together to cause chronic inflammation in blood vessels and kidneys, further maintaining and exacerbating hypertension.
The inflammatory mechanisms in hypertension are a complex process involving multiple interactions at both molecular and cellular levels. Figure 1 shows the molecules and cells involved in the inflammatory microenvironment of hypertension. At the molecular level, activation of the RAS, particularly the pro-inflammatory effects of Ang II, and exacerbation of oxidative stress, are crucial drivers of inflammation. At the cellular level, endothelial dysfunction and the involvement of lymphocytes, particularly T cells, further promote local and systemic inflammatory responses. A deeper understanding of these mechanisms not only elucidates the pathophysiological basis of hypertension but also provides a theoretical foundation for developing new anti-inflammatory therapeutic strategies.
Inflammatory mechanisms in diabetic heart disease
Advanced glycation end products and inflammation in diabetic cardiomyopathy
Diabetes is classified into three primary types: type 1, type 2, and gestational diabetes (93–96). Diabetes is a long-term condition associated with various complications (97–105). Among these, diabetic cardiomyopathy (DCM) is a common and severe complication, characterized by myocardial dysfunction, cardiac remodeling, and heart failure (106). Inflammation plays a crucial role in the initiation and progression of DCM (107). In a hyperglycemic state, excess glucose can undergo non-enzymatic reactions with proteins, lipids, or nucleic acids to form advanced glycation end products (AGEs) (108). The formation of AGEs is a significant trigger of chronic inflammation in diabetic patients (109). In high glucose conditions, glucose reacts with proteins and lipids through the Maillard reaction to form Schiff bases, which then undergo Amadori rearrangement to produce stable AGEs (110). AGEs bind to their specific receptor, the receptor for advanced glycation end products (RAGE), and activate various pro-inflammatory signaling pathways (111). RAGE, a member of the immunoglobulin superfamily, is widely expressed in cardiomyocytes, EC, and macrophages (112). The binding of AGEs to RAGE can initiate multiple pro-inflammatory signaling cascades (113). Upon AGE-RAGE binding, the IκB kinase (IKK) complex is activated, leading to the phosphorylation and degradation of the inhibitory protein Inhibitor of κB alpha (IκBα) (114). This releases NF-κB, enabling its translocation to the nucleus and initiating the transcription of pro-inflammatory genes such as TNF-α and IL-6 (114). The AGE-RAGE complex can also activate the mitogen-activated protein kinase (MAPK) pathway (115). These kinases promote the expression of inflammatory factors and contribute to apoptosis (115).
Insulin resistance and oxidative stress in diabetic cardiomyopathy
Insulin resistance, a hallmark of T2D, is closely associated with inflammatory responses (116). Insulin resistance induces oxidative stress, leading to increased production of ROS, which in turn activates the expression of pro-inflammatory factors (117). In insulin-resistant states, the activity of NADPH oxidase and the mitochondrial electron transport chain increases, leading to elevated ROS production (118). ROS can activate multiple signaling pathways, including NF-κB and MAPK, promoting the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which further exacerbate myocardial inflammation and damage (119). Myocardial cells are severely damaged under the dual impact of high glucose toxicity and inflammatory factors (120). A high glucose environment directly causes metabolic disturbances in cardiomyocytes, including imbalances in glycolysis and fatty acid oxidation, leading to energy metabolism disorders and cell damage (121). Pro-inflammatory cytokines such as TNF-α and IL-6 activate apoptotic signaling pathways, including the Caspase family and B-cell Lymphoma 2 (Bcl-2) family, leading to myocardial cell apoptosis (122). High glucose and AGEs can activate EC through the NF-κB pathway (123). The cardiac microvascular system in diabetic patients is severely affected by inflammation, leading to coronary microcirculatory disorders, which further exacerbate myocardial ischemia and injury (124). As depicted in Figure 1, in diabetic cardiomyopathy, a high-glucose environment induces the formation of AGEs, which activate pro-inflammatory pathways through the RAGE. Additionally, insulin resistance increases pro-inflammatory factors related to oxidative stress. Myocardial cells are damaged, and microvascular inflammation leads to coronary microcirculatory dysfunction, exacerbating heart injury.
The role of gut microbiota in obesity-related cardiovascular metabolic disorders
Obesity-related CMD are closely associated with chronic low-grade inflammation (125). Key mechanisms include inflammation in adipose tissue, insulin resistance, and cardiac inflammation (126). Recent research has highlighted the role of gut microbiota in the inflammatory microenvironment of CMD (127). Impaired gut barrier function, reduced production of short-chain fatty acids (SCFAs), and increased generation of microbial metabolites like trimethylamine N-oxide (TMAO) are all linked to the development and progression of CMD (128, 129). Future research needs to further explore individual variations in gut microbiota, interactions with the host immune system, and potential interventions based on gut microbiota.
Systems biology integration of multi-omics data to elucidate mechanisms of CMD inflammatory microenvironment
From a systems biology perspective, integrating multi-omics data can provide a comprehensive theoretical framework and data support for elucidating the mechanisms underlying the formation of the inflammatory microenvironment in CMD. Firstly, genomics analysis can reveal genetic variations associated with CMD inflammation and identify potential risk genes (130). Secondly, proteomics data can elucidate the functions and interaction networks of key proteins in the inflammatory microenvironment, revealing the roles of proteins in signal transduction and functional regulation (131). Simultaneously, metabolomics research can capture the dynamic changes in metabolites during the inflammatory process, revealing the critical roles of metabolic pathways in inflammation regulation (132). This integrated analysis not only helps to uncover the complex regulatory network of the CMD inflammatory microenvironment but also provides a theoretical foundation and experimental basis for developing precision intervention strategies based on specific targets, thereby promoting the advancement of CMD-related research to a deeper level.
Conclusion
This review has explored the complex inflammatory mechanisms in CMD diseases, including atherosclerosis, hypertension, and DCM. These conditions involve intricate interactions among various inflammatory factors, cell types, and signaling pathways. In atherosclerosis, ox-LDL and cytokines like IL-6 and TNF-α drive foam cell formation and arterial thickening. Hypertension involves RAS activation, oxidative stress, and T cell-mediated inflammation. DCM results from AGEs accumulation, RAGE activation, and cardiac inflammation induced by high glucose levels. By understanding these mechanisms, we can develop targeted treatments that manage and potentially reverse disease progression. Personalized strategies based on specific inflammatory pathways are crucial for improving patient outcomes, and precision medicine offers promising avenues to enhance the quality of life and prognosis for individuals with these conditions.
Author contributions
ML: Writing – original draft. RC: Writing – original draft. ZZ: Writing – original draft. SX: Writing – original draft. CH: Writing – original draft. YD: Writing – original draft. MZ: Writing – original draft. MB: Writing – review & editing. BH: Writing – review & editing. SL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant No. 81973698 and 81703942), the BUCM Precision Cultivation Program (Grant No. JZPY-202205), the BUCM Research Development Fund (Grant No. 2024-JYB-900203-009), and the Key Research Projects of Hunan Provincial Department of Education (Grant No. 23A0662).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Silveira Rossi JL, Barbalho SM, Reverete de Araujo R, Bechara MD, Sloan KP, Sloan LA. Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors. Diabetes Metab Res Rev. (2022) 38(3):e3502. doi: 10.1002/dmrr.3502
2. Qiao Q, Gao W, Zhang L, Nyamdorj R, Tuomilehto J. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem. (2007) 44(3):232–63. doi: 10.1258/000456307780480963
3. Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. (2006) 38(1):64–80. doi: 10.1080/07853890500401234
4. Han TS, Lean ME. Metabolic syndrome. Medicine. (2015) 43(2):80–7. doi: 10.1016/j.mpmed.2014.11.006
5. Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. (2008) 29(7):777–822. doi: 10.1210/er.2008-0024
6. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. (2009) 2(5-6):231–7. doi: 10.1242/dmm.001180
7. Li JM, Li X, Chan LWC, Hu R, Zheng T, Li H, et al. Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. (2023) 66(12):2368–86. doi: 10.1007/s00125-023-05992-7
8. O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. (2015) 16(1):1–12. doi: 10.1111/obr.12229
9. Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. (1998) 97(6):596–601. doi: 10.1161/01.CIR.97.6.596
10. Wang H, Liu J, Feng Y, Ma A, Wang T. The burden of cardiovascular diseases attributable to metabolic risk factors and its change from 1990 to 2019: a systematic analysis and prediction. Front Epidemiol. (2023) 3:1048515. doi: 10.3389/fepid.2023.1048515
11. Wang K, Ma J, Li Y, Han Q, Yin Z, Zhou M, et al. Effects of essential oil extracted from Artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front Nutr. (2022) 9:1024722. doi: 10.3389/fnut.2022.1024722
12. Ferreira S, Chiavegatto Filho A, Lebrão M, Duarte Y, Laurenti R. Cardiometabolic diseases. Rev Bras Epidemiol. (2019) 21(Suppl 02):e180008.
13. Rao G. Cardiometabolic diseases: a global perspective. J Cardiol Cardiovasc Ther. (2018) 12(2):555834.
14. Flora GD, Nayak MK. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr Pharm Des. (2019) 25(38):4063–84. doi: 10.2174/1381612825666190925163827
15. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longevity. (2019) 2019(1):7092151.
16. Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. (2011) 75(12):2739–48. doi: 10.1253/circj.CJ-11-1184
17. Tsoupras A, Lordan R, Zabetakis I. Inflammation and cardiovascular diseases. In: Zabetakis I, Lordan R, Tsoupras A, editors. The Impact of Nutrition and Statins on Cardiovascular Diseases. Amsterdam: Elsevier (2019). p. 53–117.
18. Hamjane N, Benyahya F, Nourouti NG, Mechita MB, Barakat A. Cardiovascular diseases and metabolic abnormalities associated with obesity: what is the role of inflammatory responses? A systematic review. Microvasc Res. (2020) 131:104023. doi: 10.1016/j.mvr.2020.104023
19. Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. (2017) 113(4):389–98. doi: 10.1093/cvr/cvx012
20. Lasselin J, Capuron L. Chronic low-grade inflammation in metabolic disorders: relevance for behavioral symptoms. Neuroimmunomodulation. (2014) 21(2-3):95–101. doi: 10.1159/000356535
21. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus–atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. (2020) 21(5):1835. doi: 10.3390/ijms21051835
22. Lamb R, Goldstein B. Modulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int J Clin Pract. (2008) 62(7):1087–95. doi: 10.1111/j.1742-1241.2008.01789.x
23. Bao MH, Li JM, Zhou QL, Li GY, Zeng J, Zhao J, et al. Effects of miR-590 on oxLDL-induced endothelial cell apoptosis: roles of p53 and NF-κB. Mol Med Rep. (2016) 13(1):867–73. doi: 10.3892/mmr.2015.4606
24. Yi J, Li L, Yin ZJ, Quan YY, Tan RR, Chen SL, et al. Polypeptide from Moschus suppresses lipopolysaccharide-induced inflammation by inhibiting NF-κ B-ROS/NLRP3 pathway. Chin J Integr Med. (2023) 29(10):895–904. doi: 10.1007/s11655-023-3598-z
25. Kritikou E, Kuiper J, Kovanen PT, Bot I. The impact of mast cells on cardiovascular diseases. Eur J Pharmacol. (2016) 778:103–15. doi: 10.1016/j.ejphar.2015.04.050
26. Amin MN, Siddiqui SA, Ibrahim M, Hakim ML, Ahammed MS, Kabir A, et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. (2020) 8:2050312120965752. doi: 10.1177/2050312120965752
27. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc Pharmacol. (2018) 100:1–19. doi: 10.1016/j.vph.2017.05.005
28. Donia T, Khamis A. Management of oxidative stress and inflammation in cardiovascular diseases: mechanisms and challenges. Environ Sci Pollut Res. (2021) 28(26):34121–53. doi: 10.1007/s11356-021-14109-9
29. Victor VM, Rocha M, Sola E, Banuls C, Garcia-Malpartida K, Hernandez-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. (2009) 15(26):2988–3002. doi: 10.2174/138161209789058093
30. Fu Q, Chen R, Ding Y, Xu S, Huang C, He B, et al. Sodium intake and the risk of various types of cardiovascular diseases: a Mendelian randomization study. Front Nutr. (2023) 10:1250509. doi: 10.3389/fnut.2023.1250509
31. Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. (2009) 42(13–14):1331–46. doi: 10.1016/j.clinbiochem.2009.05.018
32. Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian Randomization. Nat Rev Methods Primers. (2022) 2:1–21.
33. Jiang Y, Chen R, Xu S, Ding Y, Zhang M, Bao M, et al. Assessing causal associations of hyperparathyroidism with blood counts and biochemical indicators: a Mendelian randomization study. Front Endocrinol. (2023) 14:1295040. doi: 10.3389/fendo.2023.1295040
34. Huang C, Xu S, Chen R, Ding Y, Fu Q, He B, et al. Assessing causal associations of bile acids with obesity indicators: a Mendelian randomization study. Medicine. (2024) 103(25):e38610. doi: 10.1097/MD.0000000000038610
35. Han L, Xu S, Zhou D, Chen R, Ding Y, Zhang M, et al. Unveiling the causal link between metabolic factors and ovarian cancer risk using Mendelian randomization analysis. Front Endocrinol. (2024) 15:1401648. doi: 10.3389/fendo.2024.1401648
36. Wu Z, Luo S, Cai D, Lin W, Hu X, Zhou T, et al. The causal relationship between metabolic syndrome and its components and cardiovascular disease: a Mendelian randomization study. Diabetes Res Clin Pract. (2024) 211:111679. doi: 10.1016/j.diabres.2024.111679
37. Ueno K, Kaneko H, Suzuki Y, Okada A, Matsuoka S, Fujiu K, et al. Metabolic syndrome and cardiovascular disease in cancer survivors. J Cachexia Sarcopenia Muscle. (2024) 15(3):1062–71. doi: 10.1002/jcsm.13443
38. Laway BA, Baba MS. Sheehan syndrome: cardiovascular and metabolic comorbidities. Front Endocrinol. (2023) 14:1086731. doi: 10.3389/fendo.2023.1086731
39. Varra F-N, Varras M, Varra V-K, Theodosis-Nobelos P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options. Mol Med Rep. (2024) 29(6):95. doi: 10.3892/mmr.2024.13219
40. Li X, Li Y, Hao Q, Jin J, Wang Y. Metabolic mechanisms orchestrated by Sirtuin family to modulate inflammatory responses. Front Immunol. (2024) 15:1448535. doi: 10.3389/fimmu.2024.1448535
41. Xiong P, Zhang F, Liu F, Zhao J, Huang X, Luo D, et al. Metaflammation in glucolipid metabolic disorders: pathogenesis and treatment. Biomed Pharmacother. (2023) 161:114545. doi: 10.1016/j.biopha.2023.114545
42. Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des. (2012) 18(28):4266–88. doi: 10.2174/138161212802481237
43. Bao MH, Luo HQ, Chen LH, Tang L, Ma KF, Xiang J, et al. Impact of high fat diet on long non-coding RNAs and messenger RNAs expression in the aortas of ApoE(−/−) mice. Sci Rep. (2016) 6:34161. doi: 10.1038/srep34161
44. Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. (2007) 116(16):1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890
45. Jiang Y, Chen R, Xu S, Ding Y, Zhang M, Bao M, et al. Endocrine and metabolic factors and the risk of idiopathic pulmonary fibrosis: a Mendelian randomization study. Front Endocrinol. (2023) 14:1321576. doi: 10.3389/fendo.2023.1321576
46. Vekic J, Zeljkovic A, Cicero AF, Janez A, Stoian AP, Sonmez A, et al. Atherosclerosis development and progression: the role of atherogenic small, dense LDL. Medicina (B Aires). (2022) 58(2):299. doi: 10.3390/medicina58020299
47. Malekmohammad K, Bezsonov EE, Rafieian-Kopaei M. Role of lipid accumulation and inflammation in atherosclerosis: focus on molecular and cellular mechanisms. Front Cardiovasc Med. (2021) 8:707529. doi: 10.3389/fcvm.2021.707529
48. Li W, Zhi W, Zhao J, Yao Q, Liu F, Niu X. Cinnamaldehyde protects VSMCs against ox-LDL-induced proliferation and migration through S arrest and inhibition of p38, JNK/MAPKs and NF-κB. Vasc Pharmacol. (2018) 108:57–66. doi: 10.1016/j.vph.2018.05.005
49. Guo J, Du L. An update on ox-LDL-inducing vascular smooth muscle cell-derived foam cells in atherosclerosis. Front Cell Dev Biol. (2024) 12:1481505. doi: 10.3389/fcell.2024.1481505
50. Li T, Wang B, Ding H, Chen S, Cheng W, Li Y, et al. Effect of extracellular vesicles from multiple cells on vascular smooth muscle cells in atherosclerosis. Front Pharmacol. (2022) 13:857331. doi: 10.3389/fphar.2022.857331
51. Yan B, Yu X, Cai X, Huang X, Xie B, Lian D, et al. A review: the significance of toll-like receptors 2 and 4, and NF-κB signaling in endothelial cells during atherosclerosis. Front Biosci. (2024) 29(4):161. doi: 10.31083/j.fbl2904161
52. Yu Y, Cai Y, Yang F, Yang Y, Cui Z, Shi D, et al. Vascular smooth muscle cell phenotypic switching in atherosclerosis. Heliyon. (2024) 10(18):e37727.39309965
53. Gibson MS, Domingues N, Vieira OV. Lipid and non-lipid factors affecting macrophage dysfunction and inflammation in atherosclerosis. Front Physiol. (2018) 9:654. doi: 10.3389/fphys.2018.00654
54. Han Z, Liu Q, Li H, Zhang M, You L, Lin Y, et al. The role of monocytes in thrombotic diseases: a review. Front Cardiovasc Med. (2023) 10:1113827. doi: 10.3389/fcvm.2023.1113827
55. Feng Y, Ye D, Wang Z, Pan H, Lu X, Wang M, et al. The role of interleukin-6 family members in cardiovascular diseases. Front Cardiovasc Med. (2022) 9:818890. doi: 10.3389/fcvm.2022.818890
56. Su J-H, Luo M-Y, Liang N-, Gong S-X, Chen W, Huang W-Q, et al. Interleukin-6: a novel target for cardio-cerebrovascular diseases. Front Pharmacol. (2021) 12:745061. doi: 10.3389/fphar.2021.745061
57. Gencer S, Evans BR, van der Vorst EP, Döring Y, Weber C. Inflammatory chemokines in atherosclerosis. Cells. (2021) 10(2):226. doi: 10.3390/cells10020226
58. Duez H, Pourcet B. Nuclear receptors in the control of the NLRP3 inflammasome pathway. Front Endocrinol. (2021) 12:630536. doi: 10.3389/fendo.2021.630536
59. Van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. (2011) 32(3):110–6. doi: 10.1016/j.it.2011.01.003
60. Barker BR, Taxman DJ, Ting JP. Cross-regulation between the IL-1β/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol. (2011) 23(5):591–7. doi: 10.1016/j.coi.2011.07.005
61. An N, Gao Y, Si Z, Zhang H, Wang L, Tian C, et al. Regulatory mechanisms of the NLRP3 inflammasome, a novel immune-inflammatory marker in cardiovascular diseases. Front Immunol. (2019) 10:1592. doi: 10.3389/fimmu.2019.01592
62. Jiang H, Zhou Y, Nabavi SM, Sahebkar A, Little PJ, Xu S, et al. Mechanisms of oxidized LDL-mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front Cardiovasc Med. (2022) 9:925923. doi: 10.3389/fcvm.2022.925923
63. Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immun. (1996) 156(7):2558–65. doi: 10.4049/jimmunol.156.7.2558
64. Singh V, Kaur R, Kumari P, Pasricha C, Singh R. ICAM-1 and VCAM-1: gatekeepers in various inflammatory and cardiovascular disorders. Clin Chim Acta. (2023) 548:117487. doi: 10.1016/j.cca.2023.117487
65. Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. (1994) 84(7):2068–101. doi: 10.1182/blood.V84.7.2068.2068
66. Van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. (2009) 85(2):195–204. doi: 10.1189/jlb.0708400
67. Medrano-Bosch M, Simón-Codina B, Jiménez W, Edelman ER, Melgar-Lesmes P. Monocyte-endothelial cell interactions in vascular and tissue remodeling. Front Immunol. (2023) 14:1196033. doi: 10.3389/fimmu.2023.1196033
68. Čejková S, Králová-Lesná I, Poledne R. Monocyte adhesion to the endothelium is an initial stage of atherosclerosis development. Cor Vasa. (2016) 58(4):e419–25. doi: 10.1016/j.crvasa.2015.08.002
69. Lee-Rueckert M, Lappalainen J, Kovanen PT, Escola-Gil JC. Lipid-laden macrophages and inflammation in atherosclerosi s and cancer: an integrative view. Front Cardiovasc Med. (2022) 9:777822. doi: 10.3389/fcvm.2022.777822
70. Farahi L, Sinha SK, Lusis AJ. Roles of macrophages in atherogenesis. Front Pharmacol. (2021) 12:785220. doi: 10.3389/fphar.2021.785220
71. da Luz PL, Chagas ACP, Dourado PMM, Laurindo FR. Endothelium in atherosclerosis: plaque formation and its complications. In: Da Luz PL, Libby P, Chagas ACP, Laurindo FRM, editors. Endothelium and Cardiovascular Diseases. Amsterdam: Elsevier (2018). p. 493–512.
72. Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. (2010) 47(2):168–80. doi: 10.1159/000250095
73. Jaminon A, Reesink K, Kroon A, Schurgers L. The role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. (2019) 20(22):5694. doi: 10.3390/ijms20225694
74. Harman JL, Jørgensen HF. The role of smooth muscle cells in plaque stability: therapeutic targeting potential. Br J Pharmacol. (2019) 176(19):3741–53. doi: 10.1111/bph.14779
75. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. (2014) 276(6):618–32. doi: 10.1111/joim.12296
76. Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. (2016) 39(8):567–73. doi: 10.1038/hr.2016.35
77. Totoń-Żurańska J, Mikolajczyk TP, Saju B, Guzik TJ. Vascular remodelling in cardiovascular diseases: hypertension, oxidation, and inflammation. Clin Sci. (2024) 138(13):817–50. doi: 10.1042/CS20220797
78. Su C, Xue J, Ye C, Chen A. Role of the central renin-angiotensin system in hypertension. Int J Mol Med. (2021) 47(6):1–16.
79. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. (2018) 98(3):1627–738. doi: 10.1152/physrev.00038.2017
81. Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signaling. (2013) 19(10):1085–94. doi: 10.1089/ars.2012.4604
82. Wang W, Kang PM. Oxidative stress and antioxidant treatments in cardiovascular diseases. Antioxidants. (2020) 9(12):1292. doi: 10.3390/antiox9121292
83. Brandes RP. Endothelial dysfunction and hypertension. Hypertension. (2014) 64(5):924–8. doi: 10.1161/HYPERTENSIONAHA.114.03575
84. Goncharov N, Popova P, Avdonin P, Kudryavtsev I, Serebryakova M, Korf E, et al. Markers of endothelial cells in normal and pathological conditions. Biochem (Mosc) Suppl A Membr Cell Biol. (2020) 14:167–83. doi: 10.1134/S1990747819030140
85. Xiao L, Liu Y, Wang N. New paradigms in inflammatory signaling in vascular endothelial cells. Am J Physiol Heart Circ Physiol. (2014) 306(3):H317–25. doi: 10.1152/ajpheart.00182.2013
86. Tran N, Garcia T, Aniqa M, Ali S, Ally A, Nauli SM. Endothelial nitric oxide synthase (eNOS) and the cardiovascular system: in physiology and in disease states. Am J Biomed Sci Res. (2022) 15(2):153.35072089
87. Yang YY, Shi LX, Li JH, Yao LY, Xiang DX. Piperazine ferulate ameliorates the development of diabetic nephropathy by regulating endothelial nitric oxide synthase. Mol Med Rep. (2019) 19(3):2245–53.30664213
88. Li Q, Youn J-Y, Cai H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J Hypertens. (2015) 33(6):1128–36. doi: 10.1097/HJH.0000000000000587
89. Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: new insights and potential therapeutic targets. Front Immunol. (2023) 13:1098725. doi: 10.3389/fimmu.2022.1098725
90. Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. (2016) 68(1):123–32. doi: 10.1161/HYPERTENSIONAHA.116.07237
91. Eid RE, Rao DA, Zhou J, Lo S-fL, Ranjbaran H, Gallo A, et al. Interleukin-17 and interferon-γ are produced concomitantly by human coronary artery–infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. (2009) 119(10):1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618
92. Mills KH. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. (2023) 23(1):38–54. doi: 10.1038/s41577-022-00746-9
93. Liang D, Cai X, Guan Q, Ou Y, Zheng X, Lin X. Burden of type 1 and type 2 diabetes and high fasting plasma glucose in Europe, 1990–2019: a comprehensive analysis from the global burden of disease study 2019. Front Endocrinol. (2023) 14:1307432. doi: 10.3389/fendo.2023.1307432
94. Zuberi Z, Sauli E, Cun L, Deng J, Li WJ, He XL, et al. Insulin-delivery methods for children and adolescents with type 1 diabetes. Ther Adv Endocrinol Metab. (2020) 11:2042018820906016. doi: 10.1177/2042018820906016
95. Zhou Y, Chai X, Yang G, Sun X, Xing Z. Changes in body mass index and waist circumference and heart failure in type 2 diabetes mellitus. Front Endocrinol. (2023) 14:1305839. doi: 10.3389/fendo.2023.1305839
96. Fu Q, Chen R, Xu S, Ding Y, Huang C, He B, et al. Assessment of potential risk factors associated with gestational diabetes mellitus: evidence from a Mendelian randomization study. Front Endocrinol. (2023) 14:1276836. doi: 10.3389/fendo.2023.1276836
97. He K, Chen R, Xu S, Ding Y, Wu Z, Bao M, et al. Environmental endocrine disruptor-induced mitochondrial dysfunction: a potential mechanism underlying diabetes and its complications. Front Endocrinol. (2024) 15:1422752. doi: 10.3389/fendo.2024.1422752
98. Chen R, Xu S, Ding Y, Li L, Huang C, Bao M, et al. Dissecting causal associations of type 2 diabetes with 111 types of ocular conditions: a Mendelian randomization study. Front Endocrinol. (2023) 14:1307468. doi: 10.3389/fendo.2023.1307468
99. Chen J, Li X, Liu H, Zhong D, Yin K, Li Y, et al. Bone marrow stromal cell-derived exosomal circular RNA improves diabetic foot ulcer wound healing by activating the nuclear factor erythroid 2-related factor 2 pathway and inhibiting ferroptosis. Diabet Med. (2023) 40(7):e15031. doi: 10.1111/dme.15031
100. Su M, Hu R, Tang T, Tang W, Huang C. Review of the correlation between Chinese medicine and intestinal microbiota on the efficacy of diabetes mellitus. Front Endocrinol. (2022) 13:1085092. doi: 10.3389/fendo.2022.1085092
101. Xu Z, Zhang P, Chen Y, Jiang J, Zhou Z, Zhu H. Comparing SARC-CalF with SARC-F for screening sarcopenia in adults with type 2 diabetes Mellitus. Front Nutr. (2022) 9:803924. doi: 10.3389/fnut.2022.803924
102. Yu T, Xu B, Bao M, Gao Y, Zhang Q, Zhang X, et al. Identification of potential biomarkers and pathways associated with carotid atherosclerotic plaques in type 2 diabetes mellitus: a transcriptomics study. Front Endocrinol. (2022) 13:981100. doi: 10.3389/fendo.2022.981100
103. Luo M, Cao Q, Wang D, Tan R, Shi Y, Chen J, et al. The impact of diabetes on postoperative outcomes following spine surgery: a meta-analysis of 40 cohort studies with 2.9 million participants. Int J Surg. (2022) 104:106789. doi: 10.1016/j.ijsu.2022.106789
104. Mi W, Xia Y, Bian Y. Meta-analysis of the association between aldose reductase gene (CA)n microsatellite variants and risk of diabetic retinopathy. Exp Ther Med. (2019) 18(6):4499–509.31777552
105. Wei Y, Xu S, Wu Z, Zhang M, Bao M, He B. Exploring the causal relationships between type 2 diabetes and neurological disorders using a Mendelian randomization strategy. Medicine. (2024) 103(46):e40412. doi: 10.1097/MD.0000000000040412
106. Salvatore T, Pafundi PC, Galiero R, Albanese G, Di Martino A, Caturano A, et al. The diabetic cardiomyopathy: the contributing pathophysiological mechanisms. Front Med. (2021) 8:695792. doi: 10.3389/fmed.2021.695792
107. Luo B, Huang F, Liu Y, Liang Y, Wei Z, Ke H, et al. NLRP3 Inflammasome as a molecular marker in diabetic cardiomyopathy. Front Physiol. (2017) 8:519. doi: 10.3389/fphys.2017.00519
108. Mengstie MA, Chekol Abebe E, Behaile Teklemariam A, Tilahun Mulu A, Agidew MM, Teshome Azezew M, et al. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front Mol Biosci. (2022) 9:1002710. doi: 10.3389/fmolb.2022.1002710
109. Hu H, Jiang H, Ren H, Hu X, Wang X, Han C. AGEs and chronic subclinical inflammation in diabetes: disorders of immune system. Diabetes Metab Res Rev. (2015) 31(2):127–37. doi: 10.1002/dmrr.2560
110. Peng H, Gao Y, Zeng C, Hua R, Guo Y, Wang Y, et al. Effects of Maillard reaction and its product AGEs on aging and age-related diseases. Food Sci Hum Wellness. (2024) 13(3):1118–34. doi: 10.26599/FSHW.2022.9250094
111. Teissier T, Boulanger É. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology. (2019) 20(3):279–301. doi: 10.1007/s10522-019-09808-3
112. Dong H, Zhang Y, Huang Y, Deng H. Pathophysiology of RAGE in inflammatory diseases. Front Immunol. (2022) 13:931473. doi: 10.3389/fimmu.2022.931473
113. Bhattacharya R, Alam MR, Kamal MA, Seo KJ, Singh LR. AGE-RAGE axis culminates into multiple pathogenic processes: a central road to neurodegeneration. Front Mol Neurosci. (2023) 16:1155175. doi: 10.3389/fnmol.2023.1155175
114. Gąsiorowski K, Brokos B, Echeverria V, Barreto GE, Leszek J. RAGE-TLR crosstalk sustains chronic inflammation in neurodegeneration. Mol Neurobiol. (2018) 55:1463–76. doi: 10.1007/s12035-017-0419-4
115. Waghela BN, Vaidya FU, Ranjan K, Chhipa AS, Tiwari BS, Pathak C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol Cell Biochem. (2021) 476:585–98. doi: 10.1007/s11010-020-03928-y
116. Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. (2016) 23:1–18. doi: 10.1186/s12929-016-0303-y
117. Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. (2008) 294(3):R673–80. doi: 10.1152/ajpregu.00561.2007
118. Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol. (2017) 233(1):R15–42. doi: 10.1530/JOE-16-0598
119. Zhang H, Dhalla NS. The role of pro-inflammatory cytokines in the pathogenesis of cardiovascular disease. Int J Mol Sci. (2024) 25(2):1082. doi: 10.3390/ijms25021082
120. Palomer X, Salvadó L, Barroso E, Vázquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol. (2013) 168(4):3160–72. doi: 10.1016/j.ijcard.2013.07.150
121. Chen Z, Jin Z-X, Cai J, Li R, Deng K-Q, Ji Y-X, et al. Energy substrate metabolism and oxidative stress in metabolic cardiomyopathy. J Mol Med. (2022) 100(12):1721–39. doi: 10.1007/s00109-022-02269-1
122. Li J, Yang Y, Wang H, Ma D, Wang H, Chu L, et al. Baicalein ameliorates myocardial ischemia through reduction of oxidative stress, inflammation and apoptosis via TLR4/MyD88/MAPKS/NF-κB pathway and regulation of Ca2 + homeostasis by L-type Ca2 + channels. Front Pharmacol. (2022) 13:842723. doi: 10.3389/fphar.2022.842723
123. Shu M, Cheng W, Jia X, Bai X, Zhao Y, Lu Y, et al. AGEs promote atherosclerosis by increasing LDL transcytosis across endothelial cells via RAGE/NF-κB/caveolin-1 pathway. Mol Med. (2023) 29(1):113. doi: 10.1186/s10020-023-00715-5
124. Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, et al. Coronary microvascular dysfunction in diabetes mellitus: pathogenetic mechanisms and potential therapeutic options. Biomedicines. (2022) 10(9):2274. doi: 10.3390/biomedicines10092274
125. Liu S, Gu Y. INFLA Score: a novel inflammatory marker for assessing cardiometabolic disease risk in obese individuals. Diabetol Metab Syndr. (2024) 16(1):151. doi: 10.1186/s13098-024-01396-8
126. Butcko AJ, Putman AK, Mottillo EP. The intersection of genetic factors, aberrant nutrient metabolism and oxidative stress in the progression of cardiometabolic disease. Antioxidants. (2024) 13(1):87. doi: 10.3390/antiox13010087
127. Guiducci L, Nicolini G, Forini F. Dietary patterns, gut microbiota remodeling, and cardiometabolic disease. Metabolites. (2023) 13(6):760. doi: 10.3390/metabo13060760
128. Hijová E. Benefits of biotics for cardiovascular diseases. Int J Mol Sci. (2023) 24(7):6292. doi: 10.3390/ijms24076292
129. Wang A, Guan B, Zhang H, Xu H. Danger-associated metabolites trigger metaflammation: a crowbar in cardiometabolic diseases. Pharmacol Res. (2023) 198:106983.37931790
130. Zheng S, Tsao PS, Pan C. Abdominal aortic aneurysm and cardiometabolic traits share strong genetic susceptibility to lipid metabolism and inflammation. Nat Commun. (2024) 15(1):5652. doi: 10.1038/s41467-024-49921-7
131. Börschel CS, Ortega-Alonso A, Havulinna AS, Jousilahti P, Salmi M, Jalkanen S, et al. Inflammatory proteomics profiling for prediction of incident atrial fibrillation. Heart. (2023) 109(13):1000–6. doi: 10.1136/heartjnl-2022-321959
132. Rath S, Hawsawi YM, Alzahrani F, Khan MI. Epigenetic regulation of inflammation: the metabolomics connection. Semin Cell Dev Biol. (2024) 154(Pt C):355–63. doi: 10.1016/j.semcdb.2022.09.008
Glossary
CMD Cardiometabolic diseases
ox-LD Loxidized low-density lipoprotein
IL-6 Interleukin-6
TNF-α Tumor Necrosis Factor-alpha
RAS renin-angiotensin system
AGE sadvanced glycation end products
RAGE Receptor for Advanced Glycation Endproducts
MetS metabolic syndrome
CVD Cardiovascular disease
MR Mendelian randomization
T2D type 2 diabetes
SIRT1 Silent Information Regulator 2 Ortholog 1
sdLDL-C small dense LDL cholesterol
RLP-C remnant-like particle cholesterol
VSMCs vascular smooth muscle cells
EC endothelial cells
TLRs Toll-like receptors
IL-8 Interleukin-8
MCP-1 Monocyte Chemoattractant Protein-1
LDL-C low-density lipoprotein cholesterol
ROS reactive oxygen species
DNA Deoxyribonucleic Acid
LDL low-density lipoprotein
FFA free fatty acids
NF-κB Nuclear Factor kappa-light-chain-enhancer of activated B cells
CCL2 Chemokine (C-C motif) ligand 2
CXCL10 Chemokine (C-X-C motif) ligand 10
JAK-STAT Janus Kinase—Signal Transducer and Activator of Transcription
CCR2 Chemokine (C-C motif) receptor 2
NLRP3 NLR family, pyrin domain containing 3
caspase-1 Cysteine-aspartic protease 1
pro-IL-1β Pro-Interleukin-1β
pro-IL-18 Pro-Interleukin-18
IL-1β Interleukin-1β
IL-18 Interleukin-18
ICAM-1 intercellular adhesion molecule-1
VCAM-1 vascular cell adhesion molecule-1
E-selectin Endothelial Selectin
Ang II Angiotensin II
AT1R Angiotensin II type 1 receptor
NADPH nicotinamide adenine dinucleotide phosphate
SOD superoxide dismutase
GPx glutathione peroxidase
ET-1 endothelin-1
NO nitric oxide
eNOS nitric oxide synthase
IFN-γ interferon-γ
IL-17 interleukin-17
Th1 T Helper 1
Th17 T Helper 17
DCM Diabetic cardiomyopathy
IKKI κB kinase
IκBα inhibitory protein Inhibitor of κB alpha
MAPK mitogen-activated protein kinase
Bcl-2 B-cell Lymphoma 2
SCFAs short-chain fatty acids
TMAO trimethylamine N-oxide
Keywords: cardiometabolic diseases, inflammatory microenvironment, atherosclerosis, hypertension, diabetic cardiomyopathy
Citation: Liu M, Chen R, Zheng Z, Xu S, Hou C, Ding Y, Zhang M, Bao M, He B and Li S (2025) Mechanisms of inflammatory microenvironment formation in cardiometabolic diseases: molecular and cellular perspectives. Front. Cardiovasc. Med. 11:1529903. doi: 10.3389/fcvm.2024.1529903
Received: 18 November 2024; Accepted: 26 December 2024;
Published: 14 January 2025.
Edited by:
Lei Wang, Guangdong Provincial Hospital of Chinese Medicine, ChinaReviewed by:
Deng Bo, Guangzhou Medical University, ChinaBin Liu, Guangzhou Medical University, China
Copyright: © 2025 Liu, Chen, Zheng, Xu, Hou, Ding, Zhang, Bao, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meihua Bao, bWhiYW83OEAxNjMuY29t; Binsheng He, aGJzY3NtdUAxNjMuY29t; Sen Li, c2VubGlAYnVjbS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Menghua Liu
Menghua Liu Rumeng Chen
Rumeng Chen Zhiwei Zheng1,†
Zhiwei Zheng1,† Yining Ding
Yining Ding Meihua Bao
Meihua Bao Binsheng He
Binsheng He Sen Li
Sen Li