Abstract
Introduction:
Known risk factors for new-onset atrial fibrillation/flutter (NOAF) include thyrotoxicosis and subclinical hypothyroidism. While prior research has predominantly explored the link between thyrotoxicosis and NOAF, the presence of subclinical hypothyroidism among patients presenting with acute NOAF in the emergency department (ED) remains an underexplored area of inquiry. This study aimed to assess the prevalence of undiagnosed thyrotoxicosis and subclinical hypothyroidism in patients with acute NOAF diagnosed in the ED.
Methods:
This registry-based cohort study was conducted in the ED at Vrinnevi Hospital in Sweden during the years 2018, 2020, and 2022, with a 1-year follow-up period. Patients ≥18 years diagnosed with NOAF in the ED, with no ongoing thyroid hormone substitution or previous documented thyroid abnormality within the past 2 years, were included. The primary outcome was the diagnosis of thyrotoxicosis or subclinical hypothyroidism either in the ED or during a 1-year follow-up period.
Results:
486 patients with NOAF were included in the study (43.6% females). 329 (67.7%) underwent thyroid function testing in the ED or by the end of the 1-year follow-up. In total, 16 (4.9%) patients presented with subclinical hypothyroidism while 4 (1.2%) patients presented with clinical or subclinical thyrotoxicosis.
Discussion:
This study found that subclinical hypothyroidism was more prevalent (4.9%) than thyrotoxicosis (1.2%) among patients presenting with acute NOAF. These findings contrast with previous research that has predominantly linked thyrotoxicosis with acute NOAF, suggesting the need for further studies including both subclinical hypothyroidism and thyrotoxicosis in patients with NOAF.
Introduction
Atrial fibrillation/flutter (AF) is the most frequent cardiac arrhythmia among patients worldwide (1, 2). The true prevalence of AF is unknown, as many patients remain undiagnosed due to asymptomatic disease (3, 4). AF increases the risk of ischemic stroke, heart failure, sudden cardiac death, and cardiovascular (CV) mortality (1, 2). Various risk factors contribute to the development of AF such as genetic predisposition, advanced age, male sex, overweight, increased alcohol consumption, smoking, CV disease (CVD), and thyroid disease (5, 6). In patients with acute AF, spontaneous conversion is observed in up to 70% of cases. This occurrence depends on factors such as atrial size, concomitant heart failure, previous AF episodes, elevated heart rate, and the presence of a reversible thyrotoxic state (7, 8).
Normal thyroid function is particularly important for maintaining normal cardiac function. Thyroid dysfunctions are common and affects approximately 3.8% of the European population, with an annual incidence of 0.23% for hypothyroidism and 0.05% for hyperthyroidism (9). Clinical thyrotoxicosis, subclinical thyrotoxicosis, and subclinical hypothyroidism are established risk factors for new-onset AF (NOAF) and acknowledged in recent guidelines from the European Society of Cardiology (ESC) (10–13). Accordingly, the ESC recommends thyroid hormone testing in cases of NOAF.
Previous studies have primarily focused on the association between thyrotoxicosis and NOAF, reporting a prevalence ranging from 2.7% to 5.5% in the general NOAF population. In comparison, subclinical hypothyroidism—with an approximate prevalence of 5.7% in the general NOAF population—has received considerably less attention in the literature (14–18). Furthermore, there are few studies evaluating the prevalence of thyroid dysfunctions in patients diagnosed with acute NOAF in the ED. In regard of thyrotoxicosis, one study involving a mixed population of AF patients found that its prevalence was as high as 10.8% (19). However, this study relied solely upon thyroid stimulating hormone (TSH) levels to differentiate thyroid dysfunctions. This method could result in diagnostic issues since TSH profiles can overlap across various thyroid conditions that are not pertinent to AF. In regard of subclinical hypothyroidism, there are to the best of our knowledge, no studies investigating its prevalence in patients with acute NOAF diagnosed in the ED.
The aim of this study was to evaluate the prevalence of clinical and subclinical thyrotoxicosis as well as subclinical hypothyroidism in patients with acute NOAF diagnosed in the ED.
Materials and methods
Study design and participants
This was a single-center, registry-based, cohort study including all patients aged 18 years or older diagnosed with acute NOAF [International Statistical Classification of Diseases and Related Health Problems (ICD) −10 I48] visiting the ED during the years 2018, 2020, and 2022 at Vrinnevi Hospital, Norrkoping, Sweden (catchment area of 180,000 inhabitants). A review of medical records was performed to confirm acute NOAF diagnosis by interpretation of electrocardiograms (ECGs) (obtained in the ED or in the ambulance). Patients with prior AF, thyroid dysfunction within two years before the ED visit, or those currently prescribed thyroid hormone replacement therapy were excluded. For patients who had multiple emergency department visits during the study period, data from their initial admission was included, while information from any subsequent re-admissions was excluded, (Figure 1).
Figure 1

Flow chart depicting exclusion of patients during the eligibility process. ED, emergency department; Prev., previous; TFT, thyroid function testing.
The study was approved by the Swedish Ethics Review Authority (2021-05963-01) and adhered to the principles of the Declaration of Helsinki. According to Swedish legislation, patients registered in healthcare quality registers are not required to provide written informed consent for their data to be used in healthcare research or publication (20).
Data sources
Data were obtained by Region Ostergotland decision support and follow-up system (REBUS) and medical records using the unique national identification number assigned to every Swedish resident at birth or at residency. Personal identification numbers given at birth or residency indicate the assigned sex by the penultimate number. Data collected from REBUS included date of visit at the ED, diagnosis (ICD 10), age, sex, date of discharge, and date of death. Data at the time of AF diagnosis regarding: CV risk factors (CVRF) [smoking, obesity (body mass index BMI ≥30 kg/m2)] and comorbidities [heart failure (ICD-10 I50), ischemic heart disease (I20-I25), diabetes mellitus (E10), hypertension (I10)] were recorded. Additionally, heart rhythm (from ECG) and lab results [high sensitivity Troponin T (hsTnT), N-Terminal pro-B-type Natriuretic Peptide (NT-proBNP), hemoglobin, C-Reactive Protein (CRP), and blood glucose] were extracted from the medical records.
Thyroid testing and interpretation
TSH and free thyroxine (fT4) levels were obtained from medical records at the ED visit, as well as those lab results recorded within two years prior to the ED visit. Since the regional guidelines for thyrotoxicosis recommend initial testing with only TSH and fT4 in patients with a suspicion of hyperthyroidism, we did not have information regarding triiodothyronine (fT3) levels. Furthermore, for patients who had not undergone a thyroid function test either previously or in the emergency department, TSH and fT4 levels were evaluated during a 1-year follow-up period through their medical records.
Thyroid dysfunctions were classified according to local reference values (TSH 0.3–4.2 mIE/L and fT4 12–22 pmol/L), (Supplementary Table S1) (21–23). Thyroid function tests were analyzed with electrochemiluminescence immunoassay using Cobas e601 and e602 (Roche, Basel, Switzerland).
Outcome
The primary outcome was the detection of clinical or subclinical thyrotoxicosis as well as subclinical hypothyroidism in patients diagnosed with NOAF in the ED.
Statistical analysis
Baseline data is presented for the entire cohort and then divided into two groups: patients who received thyroid tests and patients who did not. The Kolmogorov-Smirnov test was used on continuous variables to determine whether data followed normal distribution. Continuous data, where normal distribution was not rejected, were presented with mean and standard deviation (SD). Continuous data with skewed distribution were presented with median with interquartile range (IQR). Categorical data were presented as number (n) and percent (%). Between-group differences regarding qualitative data were tested for statistical significance using the chi-squared test. If more than 20% of cells had a frequency <5 Fisher's exact test was used. The two sample t-test was used for comparison of normally distributed variables between two groups of independent samples, and the Mann-Whitney U-test was used on continuous variables that had a skewed distribution.
For statistical analysis, IBM SPSS, Statistics, 28.0 (Armonk, NY, USA) was used. A value of p < 0.05 was set as level of statistical significance.
Results
The study included 1,591 patients with AF during the years of 2018, 2020, and 2022. Of these, 486 patients [median age 73.1 years (IQR 62.6–79.8) and 43.6% female] were eligible for analysis, (Figure 1). Comorbidities were common, including hypertension (63.2%), obesity (34.8%), heart failure (16.9%), ischemic heart disease (18.9%), and type 2 diabetes mellitus (14.7%). Baseline characteristics are presented in Table 1.
Table 1
| n (% of total) | Total 486 |
Thyroid testing in the ED 174 (35.8) |
No thyroid testing in the ED 312 (64.2) |
p-value |
|---|---|---|---|---|
| Female, n (%) | 212 (43.6) | 76 (43.6) | 136 (43.6) | 0.985 |
| Median age, years median (IQR) | 73.1 (62.6–79.8) | 69.3 (56.4–76.8) | 75.0 (66.5–80.9) | <0.001 |
| Age groups, n (%) | <0.001 | |||
| <60 years | 98 (20.2) | 54 (31.0) | 44 (14.1) | |
| 61–70 years | 98 (20.2) | 36 (20.7) | 62 (19.9) | |
| 71–80 years | 175 (36.0) | 57 (32.8) | 118 (37.8) | |
| ≥80 years | 115 (23.7) | 27 (15.5) | 88 (28.2) | |
| Comorbidities and CVRF, n (%) | ||||
| Heart failure | 82 (16.9) | 25 (14.4) | 57 (18.3) | 0.271 |
| Ischemic heart disease | 91 (18.7) | 32 (18.4) | 59 (18.9) | 0.888 |
| Diabetes mellitus type 2 | 72 (14.8) | 19 (10.9) | 53 (17.0) | 0.071 |
| Hypertension | 307 (63.2) | 98 (56.3) | 209 (67.0) | 0.019 |
| Smokinga | 61 (13.9) | 23 (14.2) | 38 (13.7) | 0.889 |
| Obesity (BMI >30 kg/m2)b | 146 (34.8) | 59 (38.8) | 87 (32.6) | 0.198 |
| 1-year mortality, n (%) | 42 (8.6) | 14 (8.0) | 28 (9.0) | 0.727 |
| Heart rate, beats/min mean (SD) | 124 (30) | 127 (29) | 122 (31) | 0.064 |
| ECG rhythm, n (%) | ||||
| Atrial fibrillation | 400 (82.3) | 138 (79.3) | 262 (84.0) | 0.245 |
| Atrial flutter | 84 (17.3) | 36 (20.7) | 48 (15.4) | |
| Pacemaker rhythm | 2 (0.4) | 0 (0) | 2 (0.6) | |
| Laboratory findings | ||||
| hsTnT, ng/L median (IQR)c | 17 (9–28) | 16 (9–25) | 18 (9–28) | 0.257 |
| NT-pro-BNP, ng/L median (IQR)d | 1,825 (658–4,143) | 1,410 (485–3,982) | 2,030 (840–4,235) | 0.168 |
| TSH, mIE/L median (IQR) | – | 1.7 (1.2–2.9) | – | na |
| fT4, pmol/L median (IQR) | – | 16.4 (15.0–18.1) | – | na |
| Hemoglobin, g/L median (IQR)e | 144 (133–154) | 146 (136–154) | 143 (132–154) | 0.078 |
| CRP, mg/L median (IQR)f | 2.5 (2.5–10)* | 2.5 (2.5–14)* | 2.5 (2.5–8)* | 0.023 |
| Creatinine, µmol/L median (IQR)e | 81 (69–97) | 79 (67–95) | 83 (71–99) | 0.056 |
| Blood glucose, mmol/L median (IQR)g | 6.3 (5.4–7.0) | 6.3 (5.5–6.9) | 6.1 (5.3–6.8) | 0.928 |
| Previous thyroid testing, n (%) | 129 (26.5) | 31 (17.8) | 98 (31.4) | 0.001 |
Demographic and clinical characteristics of 486 patients with new-onset atrial fibrillation/flutter in the emergency department at Vrinnevi hospital Norrkoping, Sweden in 2018, 2020, and 2022.
P-values are presented for the groups: thyroid testing in the ED vs. no thyroid testing in the ED. ED, emergency department; ECG, electrocardiography; CVRF, cardiovascular risk factors; BMI, body mass index; hsTnT, high sensitivity Troponin-T; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; TSH, thyroid stimulating hormone; fT4, free thyroxine hormone; CRP, C-reactive protein; n, numbers; SD, standard deviation; IQR, interquartile range; na, not applicable.
Data missing for:
47 patients.
67 patients.
72 patients.
330 patients.
3 patients.
43 patients.
266 patients.
For analytical purposes, CRP levels below the lower level of detection (i.e., values <5) were assigned the value of 2.5.
Out of the 486 patients diagnosed with acute NOAF, 174 (35.8%) underwent thyroid function testing in the ED (43.6% females). During the 1-year follow-up, an additional 155 patients were tested (49.0% females), bringing the total to 329 (67.7%) patients who underwent thyroid testing within the first year of their NOAF diagnosis (46.2% females), (Figure 1). For all the tested patients, the median TSH level was 1.7 (1.2–2.9) mIE/L and fT4 level was 16.4 (15.0–18.1) pmol/L.
Overall, 31 patients (9.4%) with NOAF were found to have previously undiagnosed thyroid hormone abnormalities. Among them, 16 patients (4.9%) had subclinical hypothyroidism, and 4 patients (1.2%) had clinical or subclinical thyrotoxicosis. Regarding subclinical hypothyroidism, 11 patients (3.3%) were diagnosed in the ED, while an additional 5 patients (1.6%) were diagnosed during the 1-year follow-up. Regarding thyrotoxicosis, no cases of clinical or subclinical thyrotoxicosis were diagnosed in the ED, while 1 patient (0.3%) was diagnosed with clinical thyrotoxicosis and 3 patients (0.9%) were diagnosed with subclinical thyrotoxicosis during the 1-year follow-up, (Figure 2). No patients with confirmed thyroid dysfunction received Amiodarone prior to their thyroid function testing, 1 patient diagnosed with subclinical hypothyroidism later received Amiodarone in an outpatient setting. Additionally, 4 patients diagnosed with subclinical hypothyroidism and 1 patient with overt thyrotoxicosis later presented with normalized thyroid function within the follow-up period (re-evaluation occurred within 1–5 months after the initial testing for all but one patient, where it occurred within 9 months). Furthermore, 8 patients diagnosed with subclinical hypothyroidism and 2 patients with subclinical thyrotoxicosis had no re-evaluation within the 1-year follow-up, (Supplementary Table S2).
Figure 2
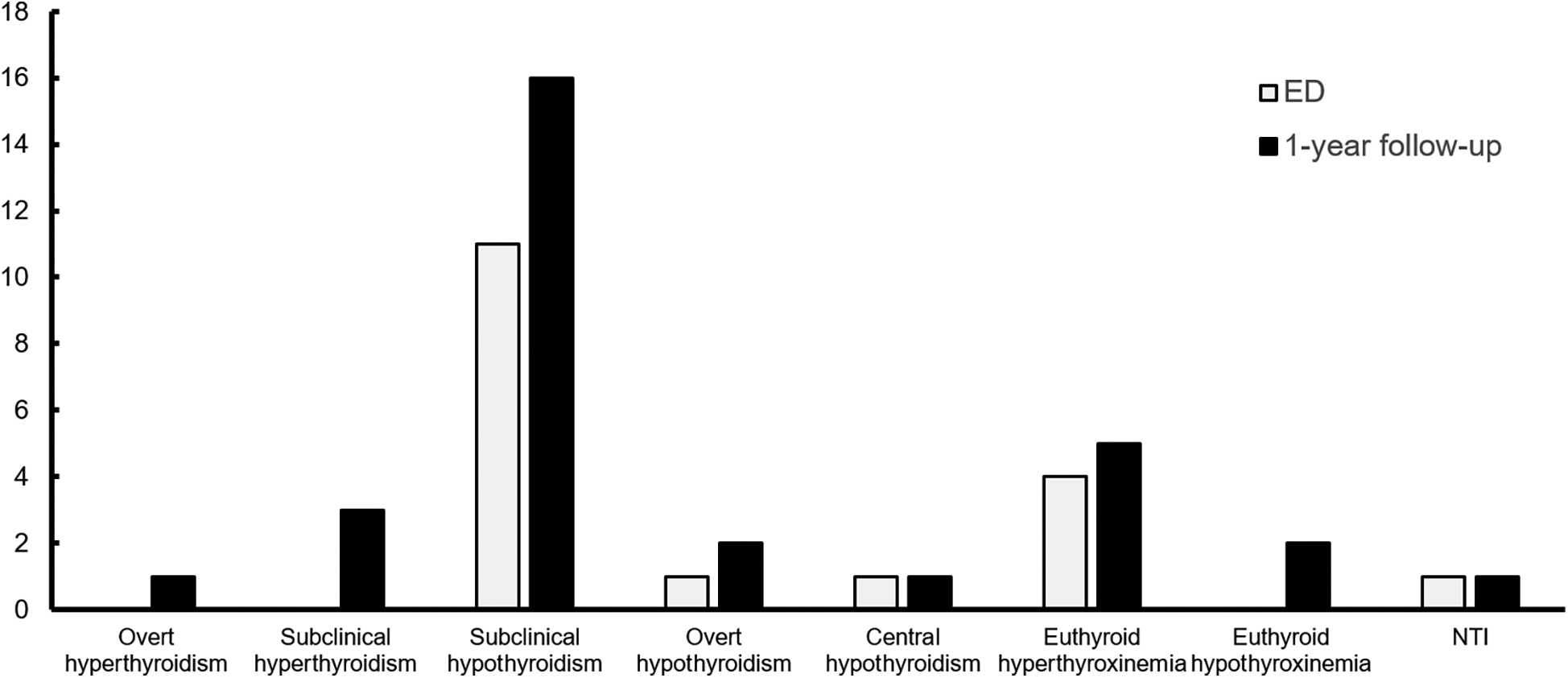
Distribution of thyroid dysfunction in the ED and by the end of the 1-year follow-up. ED, emergency department; NTI, non-thyroid illness.
Patients screened for thyroid dysfunction in the ED were significantly younger (median age 69.3 vs. 75.0 years) than those who were not tested (p < 0.001), had a lower incidence of hypertension (56.3% vs. 67.0%, p = 0.019), and were less likely to have undergone previous thyroid testing (17.8% vs. 31.4%, p = 0.001) compared to those not tested. Differences were also observed in CRP levels between the groups (p = 0.023), although the median CRP level for both groups was the same at 2.5 mg/L, (Table 1). Additionally, patients tested and then diagnosed with clinical thyrotoxicosis, subclinical thyrotoxicosis, or subclinical hypothyroidism did not significantly differ in clinical characteristics when compared to those with normal thyroid function, (Table 2).
Table 2
| n (% of total) | Normal thyroid function 298 (93.7) |
Thyroid dysfunctions* 20 (6.3) |
p-value |
|---|---|---|---|
| Female, n (%) | 133 (44.6) | 12 (60.0) | 0.182 |
| Median age, years (IQR) | 72.0 (61.0–78.0) | 74.5 (58.25–81.0) | 0.468 |
| Comorbidities and CVRF, n (%) | |||
| Heart failure | 51 (17.1) | 4 (20.0) | 0.741 |
| Ischemic heart disease | 55 (18.5) | 3 (15.0) | 0.673 |
| Diabetes mellitus type 2 | 36 (12.1) | 1 (5.0) | 0.488 |
| Hypertension | 191 (64.1) | 15 (75.0) | 0.323 |
| Smokinga | 38 (12.8) | 3 (18.8) | 0.573 |
| Obesity (BMI >30 kg/m2)b | 93 (31.2) | 7 (41.2) | 0.628 |
| 1-year mortality, n (%) | 19 (6.4) | 1 (5.0) | 1.000 |
| Heart rate, beats/min mean (SD) | 125 (30) | 127 (34) | 0.790 |
| ECG rhythm, n (%) | |||
| Atrial fibrillation | 242 (81.2) | 16 (80.0) | 1.000 |
| Atrial flutter | 56 (18.8) | 4 (20.0) | |
| Pacemaker rhythm | 0 (0) | 0 (0) | |
| Laboratory findings | |||
| hsTnT, ng/L median (IQR)c | 16 (10–25) | 24 (16–40) | 0.037 |
| NT-pro-BNP, ng/L median (IQR)d | 1,730 (620–3,828) | 4,520 (390–6,070) | 0.309 |
| Hemoglobin, g/L median (IQR)e | 144 (134–153) | 145 (131–153) | 0.974 |
| CRP, mg/L median (IQR)f | 2.5 (2.5–9.0)** | 6.0 (2.5–32)** | 0.200 |
| Creatinine, µmol/L median (IQR)e | 81 (68–95) | 77 (67–93) | 0.558 |
| Blood glucose, mmol/L median (IQR)g | 6.1 (5.4–6.9) | 7.0 (5.6–7.4) | 0.287 |
| Previous thyroid testing, n (%) | 76 (25.5) | 5 (25.0) | 0.960 |
Demographic and clinical characteristics of patients with normal thyroid function and thyroid dysfunctions associated with new-onset atrial fibrillation/flutter diagnosed in the emergency department at Vrinnevi hospital Norrkoping, Sweden in 2018, 2020, and 2022.
ED, emergency department; ECG, electrocardiography; CVRF, cardiovascular risk factors; BMI, body mass index; hsTnT, high sensitivity Troponin-T; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; TSH, thyroid stimulating hormone; fT4, free thyroxine hormone; CRP, C-reactive protein; n, numbers; SD, standard deviation; IQR, interquartile range; na, not applicable.
Data missing for:
21 patients.
35 patients.
44 patients.
194 patients.
1 patient.
27 patients.
163 patients.
Clinical thyrotoxicosis, subclinical thyrotoxicosis, and subclinical hypothyroidism.
For analytical purposes, CRP levels below the lower level of detection (i.e., values <5 mg/L) were assigned the value of 2.5.
Discussion
Abnormal thyroid hormone levels are an established risk factor for NOAF and recently subclinical hypothyroidism has been highlighted as a clinically relevant disturbance in patients with NOAF (13, 24). The findings in the current study are, to the best of our knowledge, the first to show that subclinical hypothyroidism might be more prevalent than thyrotoxicosis in patients with acute NOAF diagnosed in the ED, with a prevalence of 4.9% compared to 1.2%.
Thyroid dysfunctions are sparsely studied in acute NOAF diagnosed in the ED, and studies have primarily focused on thyrotoxicosis. Buccelletti et al. (19) found by analyzing TSH levels solely that thyrotoxicosis was common, suggesting a prevalence as high as 10.8% vs. 1.2% in the current study. This discrepancy may be attributed to various factors, such as differences in diagnostic thresholds and laboratory assays when diagnosing thyrotoxicosis as well as differences in populations and geographic variations in thyroid disease prevalence. It should be noted that there are differences in the annual incidence of hyperthyroidism in Sweden compared to i.e., northern Italy (0.03% vs. 0.08% respectively), which may account for some of the observed difference (25, 26). Furthermore, when differentiating thyroid disorders, it is essential to understand that TSH levels alone are insufficient for distinguishing between clinical thyrotoxicosis, subclinical thyrotoxicosis, non-thyroidal illness, and central hypothyroidism, as well as between clinical and subclinical hypothyroidism. To improve accuracy and decrease the risk of overestimating non-significant thyroid dysfunctions, additional factors like the clinical context and biomarkers such as fT4 should be included (23).
Conversely, the prevalence of thyroid dysfunctions in the general NOAF population has been extensively studied, but the focus has primarily been on thyrotoxicosis, while subclinical hypothyroidism has often been overlooked. However, a study by Selmer et al. (12) employed a comprehensive diagnostic approach that included both TSH and fT4 levels and presented data on thyrotoxicosis as well as subclinical hypothyroidism, an approach adopted in the current study. Selmer et al. reported a prevalence of 2.7% for thyrotoxicosis and 5.7% for subclinical hypothyroidism, findings that align with the current study. Furthermore, Boriani et al. showed a similar prevalence of thyrotoxicosis (3%) in the general NOAF population. Additionally, Boriani et al. (16) included cases of hypothyroidism but did not differentiate between subclinical and clinical cases. Krahn et al. (15) reported a higher prevalence of thyrotoxicosis (5.4%) in the general NOAF population; however, they did not account for subclinical hypothyroidism and relied on TSH levels for diagnosis. As previously noted, this approach may lead to an overestimation of thyrotoxic cases. The above findings, in synthesis with the findings in the current study, suggest that the prevalence of significant thyroid dysfunctions in acute NOAF diagnosed in the ED is similar to that observed in the general NOAF population.
This study used data from high-quality patient registers with low dropout rates. Nevertheless, studies based on registers and patient medical records invariably encounter issues such as selection bias, confounding variables, measurement inaccuracies, and reporting biases. The hospital houses the only ED in the catchment area, providing a comprehensive real-life population of all NOAF patients diagnosed in the ED within the region. However, even though this study covers the total population of NOAF in the catchment area, the cohort size is limited. While the cohort size is similar to Buccelletti et al. (19), it is smaller than in recent studies of the general NOAF population (16, 17, 19). Within this cohort, only a third underwent thyroid testing in the ED and two thirds totally during the 1-year follow-up which may limit the generalizability of the results. Furthermore, it should be acknowledged that those with newly detected thyroid abnormalities during follow-up may not have had a thyroid dysfunction at the time of admission to the ED, and previous studies have found that NOAF is an independent predictor of subsequent thyrotoxicosis (17, 27). The 1-year follow-up in this study introduces a potential risk of overestimating the prevalence of thyrotoxicosis in the ED; nonetheless, the observed prevalence was notably lower than previously reported. Given that NOAF serves as a risk factor for subsequent thyrotoxicosis and considering the lower prevalence in this study compared to previous studies, the 1-year follow-up might not introduce a significant weaknesses in the study design. Subclinical hypothyroidism has been shown to resolve spontaneously in a majority of cases, suggesting that the follow-up could lead to an underestimation of its prevalence (28). The prevalence of subclinical hypothyroidism tends to increase with age, and the median age of 73 years in the current study may have contributed to the higher prevalence observed compared to thyrotoxicosis. This age-related trend highlights the importance of considering demographic factors when interpreting differences in prevalence between these conditions (29). Additionally, the absence of fT3 levels in the current study is a limitation. However, it does not significantly impact the findings, as the comprehensive use of TSH and fT4 levels still provides a robust assessment of thyroid function.
The clinical implications of these findings are yet to be determined, and the effects of treating subclinical hypothyroidism in patients with NOAF needs further inquiry (24, 30). As recently reviewed, most guidelines recommend treatment for subclinical hypothyroidism only for patients exhibiting symptoms of hypothyroidism, or for those with TSH levels exceeding 10 mIE/L (31). Observational data have suggested a potential CV benefit from active treatment but randomized trials are needed for any firm conclusions to be drawn (32). Whether NOAF should be considered a symptom of subclinical hypothyroidism remains uncertain but cases of AF resolution have been documented following the treatment of subclinical hypothyroidism (33, 34). Larger multicenter studies involving diverse populations seem to be needed to confirm the prevalence rates of subclinical hypothyroidism and thyrotoxicosis in acute NOAF patients. Exploring geographic and demographic variations could identify populations at higher risk and inform targeted interventions to increase adherence to ESC guidelines regarding thyroid hormone testing. Furthermore, younger age and the absence of previous thyroid function testing were factors associated with a higher frequency of thyroid testing in NOAF. However, these factors did not significantly differentiate between those with a diagnosed thyroid disease and those without. This suggests that while certain demographic and clinical factors may influence the decision to conduct thyroid testing, they are not reliable indicators of underlying thyroid pathology in acute NOAF.
Conclusion
In conclusion, subclinical hypothyroidism was more frequently prevalent than thyrotoxicosis (4.9% vs. 1.2%) in patients diagnosed with acute NOAF, suggesting it may play a more significant role than previously recognized. These findings suggest that there is a need for further studies including both thyrotoxicosis as well as subclinical hypothyroidism in acute NOAF and further research into the clinical implications of subclinical hypothyroidism in this population.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Swedish Ethics Review Authority DNR: 2021-05963-01. The studies were conducted in accordance with the Swedish legislation and institutional requirements. Written informed consent for participation was not required from the participants' or their legal guardians/next of kin because the study was registry-based. According to Swedish legislation, patients registered in healthcare quality registers are not obligated to provide written informed consent for their data to be used in healthcare research or for the publication of research findings.
Author contributions
JH: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SC: Methodology, Project administration, Writing – review & editing. LB: Methodology, Project administration, Writing – review & editing. PM: Writing – review & editing. CD: Writing – review & editing. AW: Writing – review & editing. MW: Formal Analysis, Methodology, Writing – review & editing. MS: Formal Analysis, Methodology, Writing – review & editing. LH: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Grants were received from the Medical Research Council of Southeast Sweden (FORSS), and ALF, Region Ostergotland.
Acknowledgments
We would like to thank Sebastian Karlsson, ED, Vrinnevi hospital for his contribution in the data acquisition.
Conflict of interest
MW has served on advisory boards and/or lectured for Astra Zeneca MSD, Lilly, Novo Nordisk and Sanofi, and has organized a professional regional meeting sponsored by Lilly, Rubin Medical, Sanofi, Novartis and Novo Nordisk. LH reports unrelated modest consultation fees from Astellas, Bayer, and Orion Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1518297/full#supplementary-material
References
1.
Chugh SS Havmoeller R Narayanan K Singh D Rienstra M Benjamin EJ et al Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. (2014) 129(8):837–47. 10.1161/CIRCULATIONAHA.113.005119
2.
Odutayo A Wong CX Hsiao AJ Hopewell S Altman DG Emdin CA . Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. Br Med J. (2016) 354:i4482. 10.1136/bmj.i4482
3.
Healey JS Connolly SJ Gold MR Israel CW Van Gelder IC Capucci A et al Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. (2012) 366(2):120–9. 10.1056/NEJMoa1105575
4.
Svennberg E Engdahl J Al-Khalili F Friberg L Frykman V Rosenqvist M . Mass screening for untreated atrial fibrillation the STROKESTOP study. Circulation. (2015) 131(25):2176–84. 10.1161/CIRCULATIONAHA.114.014343
5.
Benjamin EJ Levy D Vaziri SM D’Agostino RB Belanger AJ Wolf PA . Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham heart study. JAMA. (1994) 271(11):840–4. 10.1001/jama.1994.03510350050036
6.
Frost L Vestergaard P Mosekilde L . Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. (2004) 164(15):1675–8. 10.1001/archinte.164.15.1675
7.
Mariani MV Pierucci N Piro A Trivigno S Chimenti C Galardo G et al Incidence and determinants of spontaneous cardioversion of early onset symptomatic atrial fibrillation. Med. (2022) 58(11):1–15. 10.3390/medicina58111513
8.
Wong CL Tam HKV Fok CKV Lam PKE Fung LM . Thyrotoxic atrial fibrillation: factors associated with persistence and risk of ischemic stroke. J Thyroid Res. (2017) 2017. 10.1155/2017/4259183
9.
Taylor PN Albrecht D Scholz A Gutierrez-Buey G Lazarus JH Dayan CM et al Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. (2018) 14(5):301–16. 10.1038/nrendo.2018.18
10.
Huang M Yang S Ge G Zhi H Wang L . Effects of thyroid dysfunction and the thyroid-stimulating hormone levels on the risk of atrial fibrillation: a systematic review and dose-response meta-analysis from cohort studies. Endocr Pract. (2022) 28(8):822–31. 10.1016/j.eprac.2022.05.008
11.
Auer J Scheibner P Mische T Langsteger W Eber O Eber B . Subclinical hyperthyroidism as a risk factor for atrial fibrillation. Am Heart J. (2001) 142(5):838–42. 10.1067/mhj.2001.119370
12.
Selmer C Olesen JB Hansen ML Lindhardsen J Olsen AM Madsen JC et al The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. Br Med J. (2012) (7885):345. 10.1136/BMJ.E7895
13.
Van Gelder IC Rienstra M Bunting KV Casado-Arroyo R Caso V Crijns HJGM et al 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2024) 45:3314–414. 10.1093/eurheartj/ehae176
14.
Kerr CR Boone J Connolly SJ Dorian P Green M Klein G et al The Canadian registry of atrial fibrillation: a noninterventional follow-up of patients after the first diagnosis of atrial fibrillation. Am J Cardiol. (1998) 82(7A):82N–5N. 10.1016/S0002-9149(98)00589-X
15.
Krahn AD Klein GJ Kerr CR Boone J Sheldon R Green M et al How useful is thyroid function testing in patients with recent-onset atrial fibrillation? Arch Intern Med. (1996) 156(19):2221–4. 10.1001/archinte.1996.00440180083010
16.
Boriani G Proietti M Laroche C Fauchier L Marin F Nabauer M et al Contemporary stroke prevention strategies in 11,096 European patients with atrial fibrillation: a report from the EURObservational research programme on atrial fibrillation (EORP-AF) long-term general registry. Europace. (2018) 20(5):747–57. 10.1093/europace/eux301
17.
Selmer C Hansen ML Olesen JB Mérie C Lindhardsen J Olsen AM et al New-onset atrial fibrillation is a predictor of subsequent hyperthyroidism: a nationwide cohort study. PLoS One. (2013) 8(2):e57893. 10.1371/journal.pone.0057893
18.
Forfar JC Miller HC Toft AD . Occult thyrotoxicosis: a correctable cause of “idiopathic” atrial fibrillation. Am J Cardiol. (1979) 44(1):9–12. 10.1016/0002-9149(79)90243-1
19.
Buccelletti F Carroccia A Marsiliani D Gilardi E Silveri NG Franceschi F . Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med. (2011) 29(9):1158–62. 10.1016/j.ajem.2010.06.010
20.
Association WM. World medical association declaration of Helsinki: ethical principles for medical research involving human participants. JAMA. (2024). 10.1001/jama.2024.21972
21.
Levy MJ Koulouri O Gurnell M . How to interpret thyroid function tests. Clin Med (Lond). (2013) 13(3):282–8. 10.7861/clinmedicine.13-3-282
22.
Stockigt JR Barlow JW . The diagnostic challenge of euthyroid hyperthyroxinemia. Aust N Z J Med. (1985) 15(2):277–84. 10.1111/j.1445-5994.1985.tb04036.x
23.
Koulouri O Moran C Halsall D Chatterjee K Gurnell M . Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. (2013) 27(6):745–62. 10.1016/j.beem.2013.10.003
24.
Singh H Shahid MZ Harrison SL Lane DA Lip GYH Logantha SJRJ . Subclinical thyroid dysfunction and the risk of incident atrial fibrillation: a systematic review and meta-analysis. PLoS One. (2024) 19(1):1–16. 10.1371/journal.pone.0296413
25.
Abraham-Nordling M Byström K Törring O Lantz M Berg G Calissendorff J et al Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. (2011) 165(6):899–905. 10.1530/EJE-11-0548
26.
Caputo M Pecere A Sarro A Mele C Ucciero A Pagano L et al Incidence and prevalence of hyperthyroidism: a population-based study in the piedmont region, Italy. Endocrine. (2020) 69(1):107–12. 10.1007/s12020-020-02222-7
27.
Huang PS Cheng JF Chen JJ Wang YC Hwang JJ Wu CK et al Higher risk of incident hyperthyroidism in patients with atrial fibrillation. J Clin Endocrinol Metab. (2024) 109(1):92–9. 10.1210/clinem/dgad448
28.
Van Der Spoel E Van Vliet NA Poortvliet RKE Du Puy RS den Elzen WPJ Quinn TJ et al Incidence and determinants of spontaneous normalization of subclinical hypothyroidism in older adults. J Clin Endocrinol Metab. (2024) 109(3):e1167–74. 10.1210/clinem/dgad623
29.
Bremner AP Feddema P Leedman PJ Brown SJ Beilby JP Lim EM et al Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. (2012) 97(5):1554–62. 10.1210/jc.2011-3020
30.
Pearce SHS Brabant G Duntas LH Monzani F Peeters RP Razvi S et al 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. (2014) 2(4):215–28. 10.1159/000356507
31.
Bekkering GE Agoritsas T Lytvyn L Heen AF Feller M Moutzouri E et al Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. Br Med J. (2019) 365:l2006. 10.1136/bmj.l2006
32.
Sue LY Leung AM . Levothyroxine for the treatment of subclinical hypothyroidism and cardiovascular disease. Front Endocrinol (Lausanne). (2020) 11:1–8. 10.3389/fendo.2020.00001
33.
National Institute for Health and Care Excellence. Thyroid Disease: Assessment and Management. London: National Institute for Health and Care Excellence (2019). https://www.nice.org.uk/guidance/ng145/chapter/Recommendations#managing-and-monitoring-subclinical-hypothyroidism
34.
Kolettis TM Tsatsoulis A . Subclinical hypothyroidism: an overlooked cause of atrial fibrillation?J Atr Fibrillation. (2012) 5(4):6–8. 10.4022/jafib.710
Summary
Keywords
atrial fibrillation, acute new-onset atrial fibrillation, thyroid testing, thyrotoxicosis, subclinical hypothyroidism, hyperthyroidism, emergency department
Citation
Hytting J, Celik S, Bodeström Eriksson L, Mallios P, Digerfeldt C, Waldemar A, Wijkman M, Singull M and Hubbert L (2025) Prevalence of abnormal thyroid hormone levels in acute new-onset atrial fibrillation. Front. Cardiovasc. Med. 11:1518297. doi: 10.3389/fcvm.2024.1518297
Received
28 October 2024
Accepted
23 December 2024
Published
10 January 2025
Volume
11 - 2024
Edited by
Laura Vitali Serdoz, Klinikum Fuerth, Germany
Reviewed by
Vincenzo Mirco La Fazia, Texas Cardiac Arrhythmia Institute, United States
Christian Selmer, University of Copenhagen, Denmark
Updates
Copyright
© 2025 Hytting, Celik, Bodeström Eriksson, Mallios, Digerfeldt, Waldemar, Wijkman, Singull and Hubbert.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jakob Hytting jakob.hytting@liu.se
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.