
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 13 February 2025
Sec. Cardiovascular Surgery
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1513149
This article is part of the Research Topic Surgical Revascularization of the Ischemic Myocardium in the third millennium View all 3 articles
Objective: To investigate the short-term prognosis and risk factors associated with in-hospital mortality in patients undergoing coronary artery bypass grafting (CABG) with low ejection fraction.
Methods: Clinical data were collected from 765 patients who underwent CABG with an ejection fraction of less than 40% between 2019 and 2023 at Anhui Chest Hospital and Beijing Anzhen Hospital, Capital Medical University. The patients were categorized into a in-hospital mortality group (n = 38) and a in-hospital survival group (n = 727), based on whether they died within 30 days post-operation. Univariate and multivariate logistic regression analyses were employed to identify risk factors for in-hospital mortality. The relationship between these risk factors and the likelihood of in-hospital mortality was assessed using restricted cubic splines (RCS). Additionally, predictive values were evaluated by plotting receiver operating characteristic curves (ROC).
Results: In-hospital mortality occurred in 38 out of the 765 patients, resulting in an incidence rate of 4.97%. Compared to the survival group, those in the mortality group exhibited significantly higher rates of exploratory thoracotomy, intra-aortic balloon pump usage, extracorporeal membrane oxygenation application, gastrointestinal bleeding incidents, and acute renal failure occurrences. Independent risk factors for in-hospital mortality included preoperative age, left ventricular ejection fraction (LVEF), fasting glucose levels (Glu), and glomerular filtration rate (eGFR). Conversely, standardized preoperative administration of oral nitrates and aspirin as well as intraoperative utilization of internal mammary arteries emerged as protective factors against in-hospital mortality. ROC analysis revealed predictive efficiencies for age at 68.5%, LVEF at 76.6%, Glu at 60.5%, while eGFR demonstrated a predictive efficiency of 78.1%.
Conclusion: The incidence of in-hospital mortality in patients undergoing coronary artery bypass grafting with low ejection fraction is correlated with several factors, including advanced age, LVEF, Glu, eGFR, and the standardized preoperative administration of oral nitrates and aspirin. These findings serve as a guide for enhancing the in-hospital prognosis for this patient population in clinical practice.
Low LVEF in the context of coronary artery disease (CAD) is defined as CAD accompanied by an LVEF of less than 40%, with the reduced LVEF attributed to ischemic cardiomyopathy. Although surgical interventions such as CABG have significantly improved the prognosis for these patients (1, 2), in-hospital mortality rates and complication incidences remain alarmingly high, resulting in relatively poor outcomes (3–6).
As the prevalence of CAD continues to rise, so too does the number of patients presenting with low LVEF (7). For this patient population, it is crucial to identify risk factors early on, as they play a significant role in clinical decision-making and ensuring appropriate medical support for critically ill individuals. Therefore, this study aims to investigate the short-term prognosis and risk factors associated with in-hospital mortality in patients undergoing CABG with low LVEF.
The study consecutively enrolled 765 patients undergoing CABG surgery from January 2019 to December 2023 at both Anhui Chest Hospital and Beijing Anzhen Hospital. Based on the occurrence of in-hospital mortality, patients were categorized into two groups: the in-hospital death group (N = 38) and the in-hospital survival group (N = 727). In-hospital mortality was defined as the incidence of death among patients during their hospitalization.
Inclusion Criteria:
1. Patients who have undergone CABG surgery,
2. Patients aged over 18 years,
3. Patients with transesophageal echocardiography (TEE) or transthoracic echocardiography (TTE) assessment indicating a LVEF of less than 40%.
Exclusion Criteria:
1. Patients diagnosed with hypothyroid heart disease,
2. Patients experiencing acute decompensated heart failure,
3. Patients requiring emergency surgical intervention,
4. Patients with a history of cardiac arrest or cardiopulmonary resuscitation and those exhibiting bradycardia defined as a heart rate of fewer than 50 beats per minute.
This study conducted a retrospective collection and analysis of patients' baseline data, encompassing factors such as sex, age, body mass index (BMI), medical history (including hypertension, diabetes, hyperglycemia, stroke, and myocardial infarction), NYHA classification, echocardiographic parameters (LVEF and E/A ratios), prior medications, preoperative laboratory assessments, and procedural details. Additionally, information regarding patients' complications and the utilization of mechanical support devices was also gathered.
This study was conducted in accordance with the Declaration of Helsink. The studies involving human participants were reviewed and approved by Anhui Chest Hospital and Beijing Anzhen Hospital of ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Statistical analyses were performed using R version 4.3. Numerical variables are presented as mean ± standard deviation, while categorical variables are expressed as frequency (percentage). For inter-group comparisons, either the chi-square test or Fisher's exact test was employed. Data exhibiting a skewed distribution are reported as median (inter-quartile range), with the Mann-Whitney U test utilized for inter-group comparison. Logistic regression analysis was conducted to identify factors influencing in-hospital mortality, incorporating gender, age, BMI, history of hypertension, history of diabetes, and troponin levels as covariates in subsequent multivariate logistic regression models. ROCwere generated for illustrative purposes. Additionally, RCS were applied to examine the linear relationship between risk factors and in-hospital death. A P-value of less than 0.05 was considered statistically significant.
As presented in Table 1, among the 765 patients studied, there were 553 males (72.29%), with a mean age of 62 years (ranging from 55 to 68 years). A total of 38 cases (4.97%) resulted in in-hospital mortality. In comparison to surviving patients, those who died during hospitalization were older, with increased prevalence of stroke history, elevated plasma glucose levels, preoperative troponin levels, and preoperative CK-MB levels. Additionally, a greater proportion underwent on-pump CABG surgery (all P < 0.05). Patients who experienced in-hospital death demonstrated lower LVEF, reduced usage of ACE inhibitors (ACEI), angiotensin receptor blockers (ARB), statins, nitrate ester medications, as well as diminished estimated glomerular filtration rate (eGFR) levels and lower utilization of left internal mammary artery grafts (LIMA) compared to survivors (all P < 0.05).
As illustrated in Table 2, patients who suffered in-hospital mortality had significantly higher rates of exploratory thoracotomy, intra-aortic balloon pump placement, extracorporeal membrane oxygenation use, gastrointestinal bleeding incidents, and acute renal failure occurrences when compared to surviving patients (all P < 0.05).
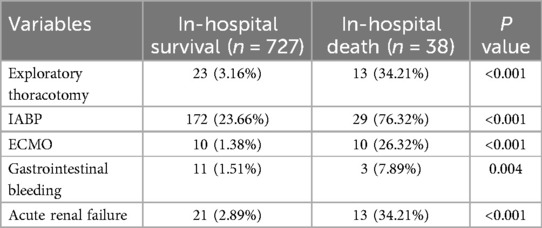
Table 2. Comparison of short-term outcomes between patients in in-hospital death group and patients in in-hospital survival group.
According to Table 3 findings, patients undergoing on-pump CABG exhibited a higher rate of in-hospital mortality than those receiving off-pump CABG procedures (P < 0.05). No significant differences were noted regarding exploratory thoracotomy rates or the incidence of intra-aortic balloon pump use; extracorporeal membrane oxygenation application; gastrointestinal bleeding events; or acute renal failure occurrences between the two groups (all P > 0.05).
As presented in Table 4, several risk or protective factors were screened via uni-variate logistic regression analysis. High age (OR = 1.10, 95% CI: 1.04–1.16, P = 0.002), high in-hospital Glu levels (OR = 1.14, 95% CI: 1.06–1.24, P < 0.001), high in-hospital CK-MB levels (OR = 1.02, 95% CI: 1.00–1.03, P = 0.010), low LVEF (OR = 0.84, 95% CI: 0.79–0.90, P < 0.001) and low eGFR (OR = 0.97, 95% CI: 0.95–0.98, P < 0.001) were screened as risk factors. Male gender (OR = 0.35, 95% CI: 0.15–0.81, P = 0.014), prior administration of nitrates (OR = 0.12, 95% CI: 0.05–0.28, P < 0.001), aspirin therapy (OR = 0.41, 95% CI: 0.18–0.91, P = 0.029), and the use of LIMA (OR = 0.28, 95% CI: 0.12–0.65, P = 0.003) were screened as protective factors.
Further multi-variate logstics analysis identified several significant independent risk factors for in-hospital mortality: high age (OR: 1.10, 95% CI: 1.05–1.16, P < 0.001), high in-hospital Glu levels (OR: 1.10, 95% CI: 1.01–1.21, P = 0.030), low LVEF (OR: 0.83, 95% CI: 0.77–0.88, P < 0.001), and low eGFR (OR: 0.97, 95% CI: 0.95–0.99, P < 0.001). The use of LIMA (OR: 0.39, 95% CI: 0.17–0.90, P = .028), prior administration of nitrate esters [OR: .15; [CI]: .06–.38; [P] < .001], and aspirin therapy [OR: .43; [CI]: .20–.93; [P] = .032] were found to be significantly protective factors against in-hospital mortality.
As illustrated in Figure 1, the RCS analysis indicated that age, LVEF, eGFR, and GLU are non-linearly associated with in-hospital mortality. The identified cut-off values include age >63 years, eGFR <82 ml/min, GLU >6.7 mmol/l, and LVEF <38%.
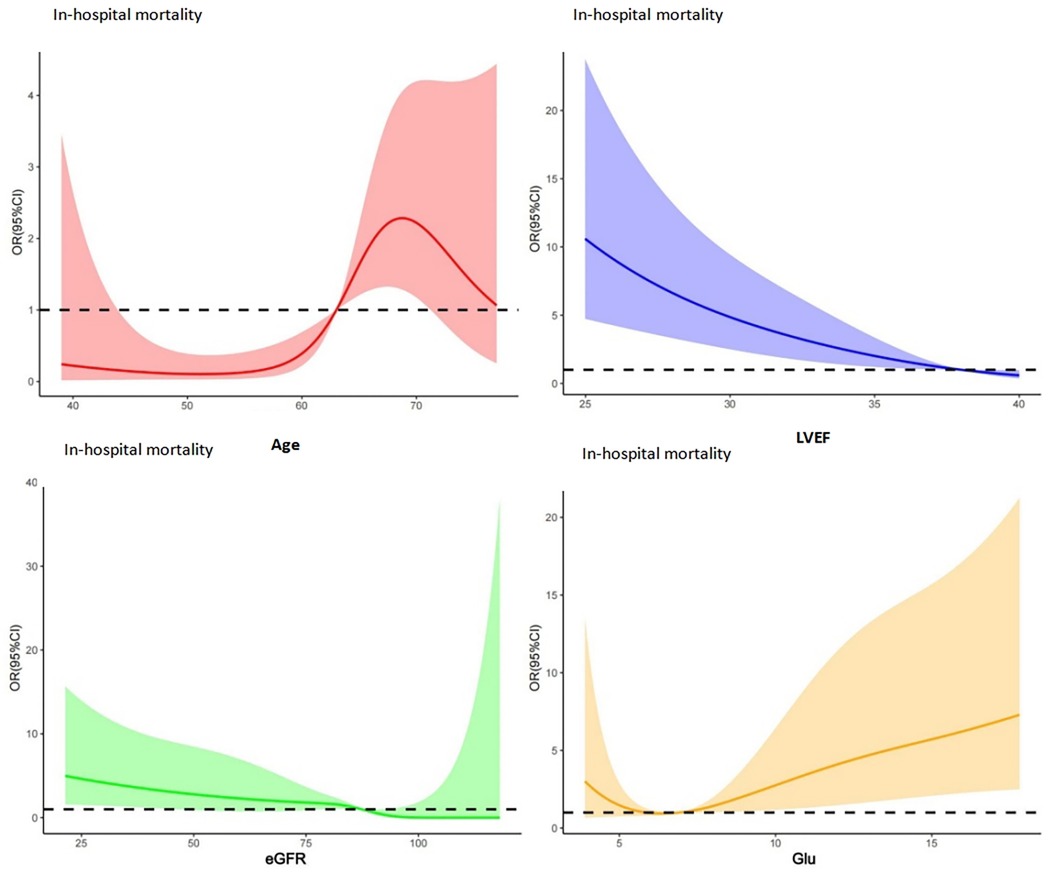
Figure 1. RCS analysis results for patients' in-hospital mortality: red: RCS for age; blue: RCS for LVEF; green: RCS for eGFR; yellow: RCS for Glu, and the identified cut-off values include age >63 years, eGFR <82 ml/min, GLU >6.7 mmol/l, and LVEF <38%.
As illustrated in Figure 2, the ROC curve analysis indicated that the area under the ROC curve for age, LVEF, eGFR, and Glu were 68.5%, 76.6%, 78.1%, and 60.1%, respectively, which indicated that a moderate diagnositic value of LVEF and eGFR and relative low diagnositic value of age and Glu. In addition, the Hosmer-Lemeshow goodness-of-fit statistic P-value was 0.944, which valided the fit of the model.
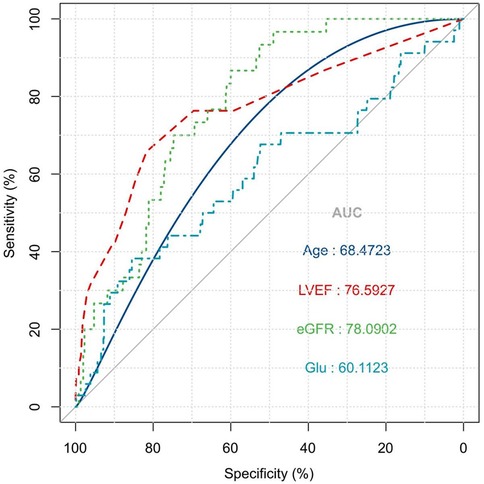
Figure 2. ROC curve for risk factor of patients' in-hospital mortality: the ROC curve analysis indicated that the area under the ROC curve for age, LVEF, eGFR, and GLU were 68.5%, 76.6%, 78.1%, and 60.1%, respectively.
This retrospective study analyzes the outcomes and risk factors associated with in-hospital mortality among patients undergoing CABG with low LVEF. The results indicate that patients with low LVEF exhibit a poorer prognosis compared to those with normal LVEF. In our cohort, the in-hospital mortality rate was 4.97%, which aligns closely with other reported cohorts worldwide (8–10), and is significantly higher than that of patients with normal LVEF who underwent CABG surgery (11). This disparity can be attributed to coronary stenosis leading to acute or chronic ischemic myocardial damage and severe remodeling of the left ventricle. Consequently, these patients often present with compromised cardiac functional reserve, categorizing them as a high-risk population for CABG surgery. Given this context, it is crucial to conduct comprehensive preoperative risk stratification for these patients beyond merely focusing on their LVEF levels. Therefore, this study aims to provide valuable insights for clinical practice concerning CABG candidates with low LVEF.
To date, guidelines recommend CABG as the preferred revascularization strategy for patients presenting with low LVEF combined with multivessel disease (1, 2). Numerous large clinical cohorts have demonstrated that compared to those receiving pharmacological therapy and percutaneous coronary intervention (PCI), CABG offers greater benefits (11–13). However, due to an increased prevalence of myocardial hibernation and diminished myocardial activity post-procedure, these patients remain at elevated risk following CABG revascularization. Our findings corroborate this notion through a higher incidence of in-hospital gastrointestinal bleeding (1.8%) and acute kidney injury (4.4%) observed in our study (14–16). Additionally, we noted a notably high utilization rate of intra-aortic balloon pump (IABP) support at 26.2%, which substantially exceeds the average IABP usage rate of approximately 5% seen in other populations (17).
Previous studies have indicated that advanced age is a significant factor influencing recovery in patients with low LVEF post-CABG (18–20). Older patients typically exhibit lower cardiac reserve and often present with more preoperative comorbidities. After controlling for other confounding variables, our analysis revealed that age remains an independent predictor of in-hospital mortality. The utilization of the LIMA has been shown to significantly enhance long-term survival rates among CABG patients (21). Our findings suggest that LIMA usage plays a protective role against in-hospital mortality, potentially due to its ability to self-regulate blood flow and improve ischemic myocardial perfusion.
The advantages of utilizing the left internal mammary artery (LIMA) in coronary artery bypass grafting (CABG) are as follows: 1. High long-term patency rate; 2. Enhancement of myocardial perfusion and cardiac function; 3. Decrease in mortality rates and the incidence of major adverse cardiovascular events (MACE); 4. Improvement in patients' quality of life. These benefits have been substantiated by numerous studies (22, 23), including our own research, which has arrived at a similar conclusion: for CABG patients with low ejection fraction, LIMA serves an independent protective role for these individuals. From the aspect of cardiac function, it helps to increase LVEF. Studies that followed up patients after CABG found that the recovery of cardiac function in patients who used LIMA was better than that in patients who did not use LIAM. This is because the stable blood flow provided by theLIMA can allow hibernating or stunned myocardium to recover, enhance myocardial contractility, and thereby improve the overall pumping function of the heart (24, 25).
Because patients with CAD and low LVEF exhibit characteristics such as intraoperative hemodynamic instability and often present with secondary mitral valve disease, some individuals may require CABG under cardiopulmonary bypass. Our findings indicate that cardiopulmonary bypass is associated with increased in-hospital mortality in patients with low LVEF, while its impact on other outcomes appears to be less pronounced. Several prior studies have demonstrated that off-pump CABG can enhance short-term outcomes for patients with low LVEF (26, 27). Although CABG may offer short-term advantages in off-pump scenarios, we recommend that surgeons customize their surgical approach based on the severity of each patient's condition and preoperative status.
In conclusion, the in-hospital risk for patients undergoing CABG with low LVEF is significantly elevated compared to routine CABG surgery performed on patients with normal LVEF. This study identified age, LVEF, Glu, and eGFR as independent risk factors predictive of in-hospital mortality. Conversely, regular administration of nitrate esters, aspirin use, and utilization of LIMA were found to be independent protective factors against in-hospital mortality. Further prospective studies involving larger sample sizes are warranted to validate our findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YZ: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. XY: Formal Analysis, Project administration, Validation, Writing – review & editing. XM: Data curation, Methodology, Project administration, Supervision, Writing – original draft. LZ: Data curation, Funding acquisition, Methodology, Resources, Supervision, Visualization, Writing – review & editing. ZW: Data curation, Formal analysis, Validation, Resources, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization [published correction appears in Eur Heart J. 2019 Oct 1;40(37):3096]. Eur Heart J. (2019) 40(2):87–165. doi: 10.1093/eurheartj/ehy394
2. Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. (2023) 148(9):e9–e119. doi: 10.1161/CIR.0000000000001168
3. Darwazah AK, Abu Sham’a RA, Hussein E, Hawari MH, Ismail H. Myocardial revascularization in patients with low ejection fraction < or =35%: effect of pump technique on early morbidity and mortality. J Card Surg. (2006) 21(1):22–7. doi: 10.1111/j.1540-8191.2006.00163.x
4. Alderman EL, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, et al. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation. (1983) 68(4):785–95. doi: 10.1161/01.CIR.68.4.785
5. Shapira OM, Hunter CT, Anter E, Bao Y, DeAndrade K, Lazar HL, et al. Coronary artery bypass grafting in patients with severe left ventricular dysfunction–early and mid-term outcomes. J Card Surg. (2006) 21(3):225–32. doi: 10.1111/j.1540-8191.2006.00221.x
6. Topkara VK, Cheema FH, Kesavaramanujam S, Mercando ML, Cheema AF, Namerow PB, et al. Coronary artery bypass grafting in patients with low ejection fraction. Circulation. (2005) 112(9 Suppl):I344–50. doi: 10.1161/CIRCULATIONAHA.104.526277
7. The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on cardiovascular health and diseases in China 2019: an updated summary. Chinese Circulation Journal,. (2020) 35(9):833–54. doi: 10.3969/j.issn.1000-3614.2020.09.001
8. Hillis GS, Zehr KJ, Williams AW, Schaff HV, Orzulak TA, Daly RC, et al. Outcome of patients with low ejection fraction undergoing coronary artery bypass grafting: renal function and mortality after 3.8 years. Circulation. (2006) 114(1 Suppl):I414–9. doi: 10.1161/CIRCULATIONAHA.105.000661
9. Ascione R, Narayan P, Rogers CA, Lim KH, Capoun R, Angelini GD. Early and midterm clinical outcome in patients with severe left ventricular dysfunction undergoing coronary artery surgery. Ann Thorac Surg. (2003) 76(3):793–9. doi: 10.1016/S0003-4975(03)00664-7
10. Yuan X, Zhang H, Zheng Z, Rao C, Zhao Y, Wang Y, et al. Trends in mortality and major complications for patients undergoing coronary artery bypass grafting among Urban Teaching Hospitals in China: 2004 to 2013. Eur Heart J Qual Care Clin Outcomes. (2017) 3(4):312–8. doi: 10.1093/ehjqcco/qcx021
11. Velazquez EJ, Bonow RO. Revascularization in severe left ventricular dysfunction. J Am Coll Cardiol. (2015) 65(6):615–24. doi: 10.1016/j.jacc.2014.10.070
12. O’Connor CM, Velazquez EJ, Gardner LH, Smith PK, Newman MF, Landolfo KP, et al. Comparison of coronary artery bypass grafting versus medical therapy on long-term outcome in patients with ischemic cardiomyopathy (a 25-year experience from the Duke Cardiovascular Disease Databank). Am J Cardiol. (2002) 90(2):101–7. doi: 10.1016/S0002-9149(02)02429-3
13. Sun LY, Gaudino M, Chen RJ, Bader Eddeen A, Ruel M. Long-term outcomes in patients with severely reduced left ventricular ejection fraction undergoing percutaneous coronary intervention vs coronary artery bypass grafting [published correction appears in JAMA Cardiol. 2020 Jun 1;5(6):732]. JAMA Cardiol. (2020) 5(6):631–41. doi: 10.1001/jamacardio.2020.0239
14. Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S. Adverse events in coronary artery bypass graft (CABG) trials: a systematic review and analysis. Heart. (2003) 89(7):767–72. doi: 10.1136/heart.89.7.767
15. Ailawadi G, Chang HL, O’Gara PT, O’Sullivan K, Woo YJ, DeRose JJ, et al. Pneumonia after cardiac surgery: experience of the national institutes of health/Canadian institutes of health research cardiothoracic surgical trials network. J Thorac Cardiovasc Surg. (2017) 153(6):1384–1391.e3. doi: 10.1016/j.jtcvs.2016.12.055
16. Gaudino M, Rahouma M, Di Mauro M, Yanagawa B, Abouarab A, Demetres M, et al. Early versus delayed stroke after cardiac surgery: a systematic review and meta-analysis. J Am Heart Assoc. (2019) 8(13):e012447. doi: 10.1161/JAHA.119.012447
17. Zaky SS, Hanna AH, Sakr Esa WA, Xu M, Lober C, Sessler DI, et al. An 11-year, single-institution analysis of intra-aortic balloon pump use in cardiac surgery. J Cardiothorac Vasc Anesth. (2009) 23(4):479–83. doi: 10.1053/j.jvca.2008.12.027
18. Bouchart F, Tabley A, Litzler PY, Haas-Hubscher C, Bessou JP, Soyer R. Myocardial revascularization in patients with severe ischemic left ventricular dysfunction. Long term follow-up in 141 patients. Eur J Cardiothorac Surg. (2001) 20(6):1157–62. doi: 10.1016/S1010-7940(01)00982-4
19. Youn YN, Chang BC, Hong YS, Kwak YL, Yoo KJ. Early and mid-term impacts of cardiopulmonary bypass on coronary artery bypass grafting in patients with poor left ventricular dysfunction: a propensity score analysis. Circ J. (2007) 71(9):1387–94. doi: 10.1253/circj.71.1387
20. Wrobel K, Stevens SR, Jones RH, Selzman CH, Lamy A, Beaver TM, et al. Influence of baseline characteristics, operative conduct, and postoperative course on 30-day outcomes of coronary artery bypass grafting among patients with left ventricular dysfunction: results from the surgical treatment for ischemic heart failure (STICH) trial. Circulation. (2015) 132(8):720–30. doi: 10.1161/CIRCULATIONAHA.114.014932
21. Zhao Q, Yang Y, Zhu Y. The state of the art for arterial coronary artery bypass grafting. Chin Surg J. (2020) 58(5):337–40. doi: 10.3760/cma.j.cn112139-20200131-00050
22. Sá MP, Ferraz PE, Escobar RR, Nunes EO, Lustosa P, Vasconcelos FP, et al. Patency of skeletonized versus pedicled internal thoracic artery in coronary bypass graft surgery: a systematic review, meta-analysis and meta-regression. Int J Surg. (2014) 12(7):666–72. doi: 10.1016/j.ijsu.2014.05.071
23. Shadrin IY, Holmes DR, Behfar A. Left internal mammary artery as an endocrine organ: insights into graft biology and long-term impact following coronary artery bypass grafting. Mayo Clin Proc. (2023) 98(1):150–62. doi: 10.1016/j.mayocp.2022.10.003
24. Soylemez N, Balli M, Köksal F, Yilmaz M, Erturk Sag Ft, Erturk Tekin E, et al. A new current to the armamentarium: is the CHA2DS2-vasc-HS score predictive of low left internal mammary artery (LIMA) flow in patients underwent coronary bypass surgery? Heart Surg Forum. (2021) 24(4):E631–6. doi: 10.1532/hsf.3837
25. Chaudhri MS, Shah MU, Asghar MI, Siddiqi R, Janjua AM, Iqbal A. Skeletonization of left internal mammary artery in coronary artery bypass grafting. J Coll Physicians Surg Pak. (2016) 26(9):736–9.27671175
26. Keeling WB, Williams ML, Slaughter MS, Zhao Y, Puskas JD. Off-pump and on-pump coronary revascularization in patients with low ejection fraction: a report from the society of thoracic surgeons national database. Ann Thorac Surg. (2013) 96(1):83–8; discussion 88–9. doi: 10.1016/j.athoracsur.2013.03.098
Keywords: coronary artery bypass grafting, cardiac insufficiency, in-hospital mortality, risk factors, LVEF
Citation: Zhao Y, Yu X, Ma X, Zhang L and Wang Z (2025) Outcomes and risk factors associated with in-hospital mortality in patients undergoing coronary artery bypass grafting with low ejection fraction. Front. Cardiovasc. Med. 11:1513149. doi: 10.3389/fcvm.2024.1513149
Received: 17 October 2024; Accepted: 30 December 2024;
Published: 13 February 2025.
Edited by:
Antonio Maria Calafiore, Henry Dunant Hospital, GreeceReviewed by:
Michele Di Mauro, Maastricht University Medical Centre, NetherlandsCopyright: © 2025 Zhao, Yu, Ma, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Zhang, 326065404@qq.com; Zehui Wang, doctor_wang912@aliyun.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.