- 1Hangzhou Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Department of Cardiology, Hangzhou Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Objective: This meta-analysis aims to evaluate the safety and efficacy of indobufen in the treatment of cardiovascular diseases, cerebrovascular diseases, and thromboembolic disorders. The primary focus is on the incidence of major adverse cardiovascular events (MACE), thrombosis, bleeding events, and adverse reactions. The results are intended to provide a reference for the clinical application of indobufen and suggest directions for further large-scale, multi-center, prospective studies.
Methods: This review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A comprehensive search was conducted in PubMed, Embase, Web of Science, and the Cochrane Library databases to identify all relevant studies on indobufen. Twelve trials, all randomized controlled trials (RCTs), met the inclusion criteria. The results were presented as risk ratios (RR) with 95% confidence intervals (CI), and meta-analysis was performed using RevMan 5.3 and Stata 18.0 software.
Results: The meta-analysis included 12 randomized controlled trials. Regarding safety, indobufen showed superior clinical outcomes compared to other antiplatelet agents regarding bleeding events, gastrointestinal adverse reactions, and overall adverse reactions, with these differences being more pronounced in cardiovascular diseases. However, the effects of both treatments on efficacy outcomes, including MACE, myocardial infarction, angina, mortality, and thrombotic events, were similar. For stroke events, particularly in patients with cerebrovascular diseases, the use of indobufen was associated with some risk.
Conclusion: Indobufen is associated with a lower risk of adverse reactions and bleeding, making it a viable option for patients at risk of bleeding or adverse effects, particularly in those with cardiovascular diseases. However, compared to anticoagulants such as aspirin and clopidogrel, indobufen has a shorter history of use, and its evidence base is relatively limited, highlighting the need for further research. Currently, indobufen is widely used in secondary cardiovascular and cerebrovascular prevention and provides some guidance for antiplatelet therapy in patients with gastrointestinal discomfort or bleeding risk. However, due to the potential risks in MACE, stroke, and other events, further clinical trials are needed to assess the clinical applicability of indobufen.
Systematic Review Registration: https://www.crd.york.ac.uk/, identifier (CRD42024587938).
1 Introduction
Indobufen, a non-steroidal anti-inflammatory drug (NSAID), has demonstrated significant antiplatelet aggregation effects, making it widely used to prevent and treat cardiovascular diseases. Cardiovascular diseases, one of the leading causes of death and disability worldwide, are steadily increasing in prevalence. According to statistics from the World Health Organization (WHO), ischemic heart disease and stroke rank first among the top ten causes of death worldwide. Cardiovascular and cerebrovascular diseases cause approximately 14 million deaths annually, accounting for 23% of the total global mortality (1). With the aging population and lifestyle changes, the incidence of cardiovascular diseases is on the rise, highlighting the critical need for effective antithrombotic and antiplatelet therapies.
The formation of intravascular thrombosis is the primary cause of cardiovascular and cerebrovascular events (2). Arterial thrombosis primarily comprises platelets and forms at sites of atherosclerotic vascular injury under increased shear stress and disturbed blood flow (3). Antiplatelet drugs, which reduce thrombosis by inhibiting platelet aggregation, play a key role in significantly reducing the incidence of cardiovascular events and are an essential therapeutic approach for treating cardiovascular diseases. Indobufen exerts its antiplatelet effects by inhibiting cyclooxygenase activity (COX-1 and COX-2), thereby reducing the synthesis of platelet aggregation-promoting substances (4). In recent years, there have been an increasing number of studies assessing the effectiveness and safety of indobufen in treating cardiovascular conditions. However, results from various studies have been inconsistent; some demonstrate significant efficacy in preventing cardiovascular events (5), while others fail to confirm its superiority (6). Hence, a systematic evaluation of indobufen's role in cardiovascular disease treatment is crucial.
This study aims to assess the efficacy and safety of indobufen in treating cardiovascular diseases through a systematic review and meta-analysis systematic review of existing literature, thus exploring its potential clinical value. This provides a basis for further clinical research and offers valuable guidance for treating patients with cardiovascular diseases.
2 Methods
According to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), the protocol for this meta-analysis was registered on PROSPERO (Registration No: CRD42024587938) (7).
2.1 Inclusion criteria
Study Population: Adult patients with cardiovascular or thromboembolic diseases. Intervention: With no dosage or treatment duration restrictions, Indobufen can be used as a single treatment or in combination with other standard treatments. Control: A placebo or another positive control drug, alone or in combination with other standard treatments, without restrictions on dosage or duration. Outcome Measures: Efficacy outcomes including MACE (major adverse cardiovascular events), stroke, myocardial infarction, thrombosis, and mortality, and safety outcomes such as adverse reactions and bleeding. Study Design: Randomized controlled trials (RCTs).
2.2 Exclusion criteria
Studies such as conference abstracts, reviews, animal experiments, or republished research; studies where the treatment group does not use indobufen; and studies lacking a control group will be excluded.
2.3 Search strategy
One reviewer conducted a computer-based search of the PubMed, Embase, The Cochrane Library, and Web of Science databases using the keywords “indobufen”, “cardiovascular diseases” ,“cerebrovascular diseases”, “thromboembolic diseases”, and “randomized controlled trials”. The search strategy combined both MeSH terms and free-text terms. The search covered the period from establishing each database until September 2, 2024 (See the Supplementary Material for details).
2.4 Study selection
Two reviewers independently screened the literature based on the inclusion and exclusion criteria. The full texts were downloaded and thoroughly reviewed for studies meeting the criteria, followed by a second screening and cross-checking. Data were then independently extracted by the two reviewers according to a pre-designed data extraction form, including the essential characteristics of the included studies and their results. Based on the Cochrane Risk of Bias tool, the two reviewers independently assessed the methodological quality of the included studies.
2.5 Assessment of risk of bias and quality of evidence
Based on Cochrane risk-of-bias criteria, two reviewers (XL and CL) independently assessed the quality of all included trials. Stata was used to calculate the risk of bias.
2.6 Data analysis
Analyses were conducted using Review Manager 5.3 and Stata 18.0 software. A heterogeneity test was performed on the data before the meta-analysis. If the studies had no significant heterogeneity (P > 0.05 and I2 < 50%), a fixed-effect model was used to weigh and combine the effect sizes. If heterogeneity was present (P < 0.05 and I2 > 50%), studies with significant heterogeneity were excluded from meeting the heterogeneity threshold, after which a fixed-effect model was used to weigh and combine the effect sizes. The results were expressed as relative risk (RR) with 95% confidence intervals (CI). Subgroup analysis was conducted based on the disease type, study country or region, study type, and drug type. Publication bias was assessed using Egger's test, Begg's test, and funnel plot analysis (P > 0.05 indicates no publication bias; confidence level: Egger's test > Begg's test > funnel plot). A P-value of less than 0.05 was considered statistically significant. Additionally, sensitivity analysis was performed using the “metaninf” tool in Stata software.
3 Results
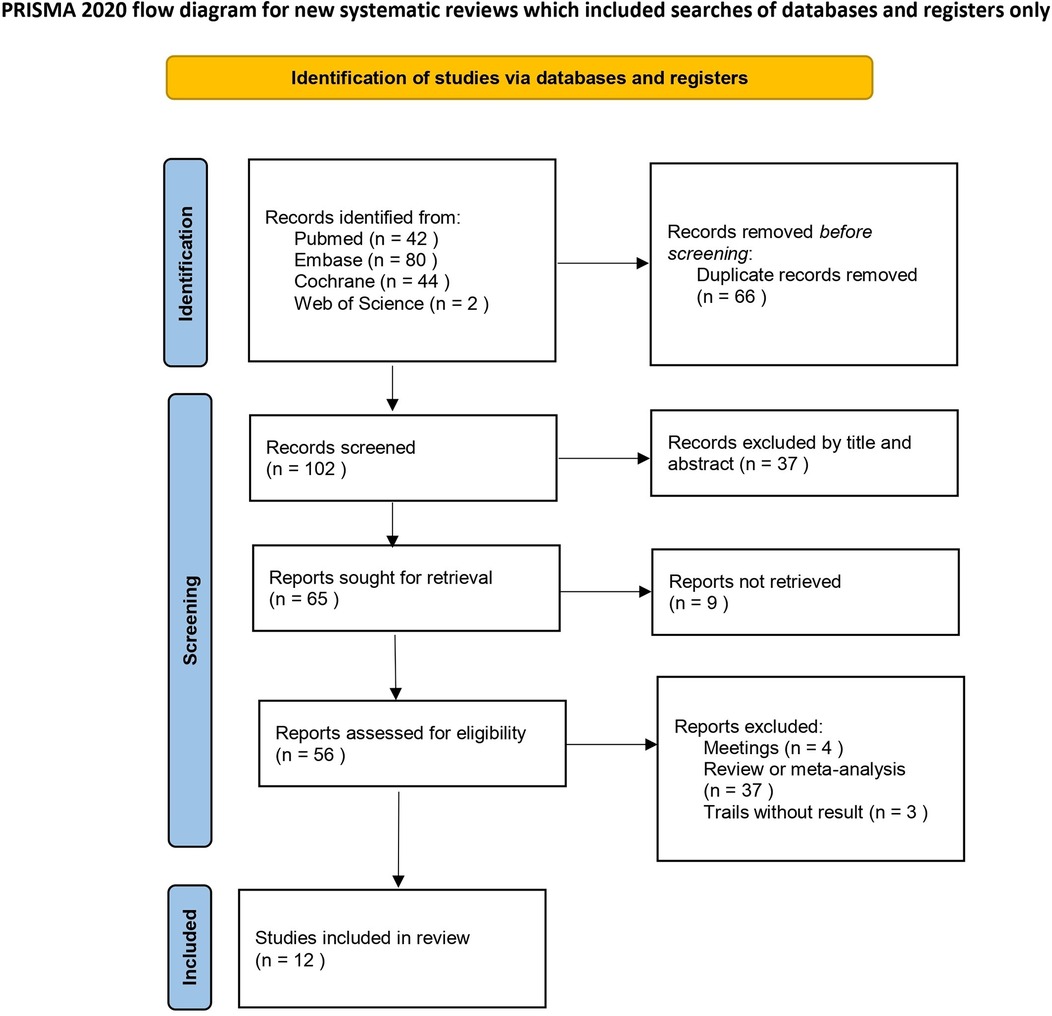
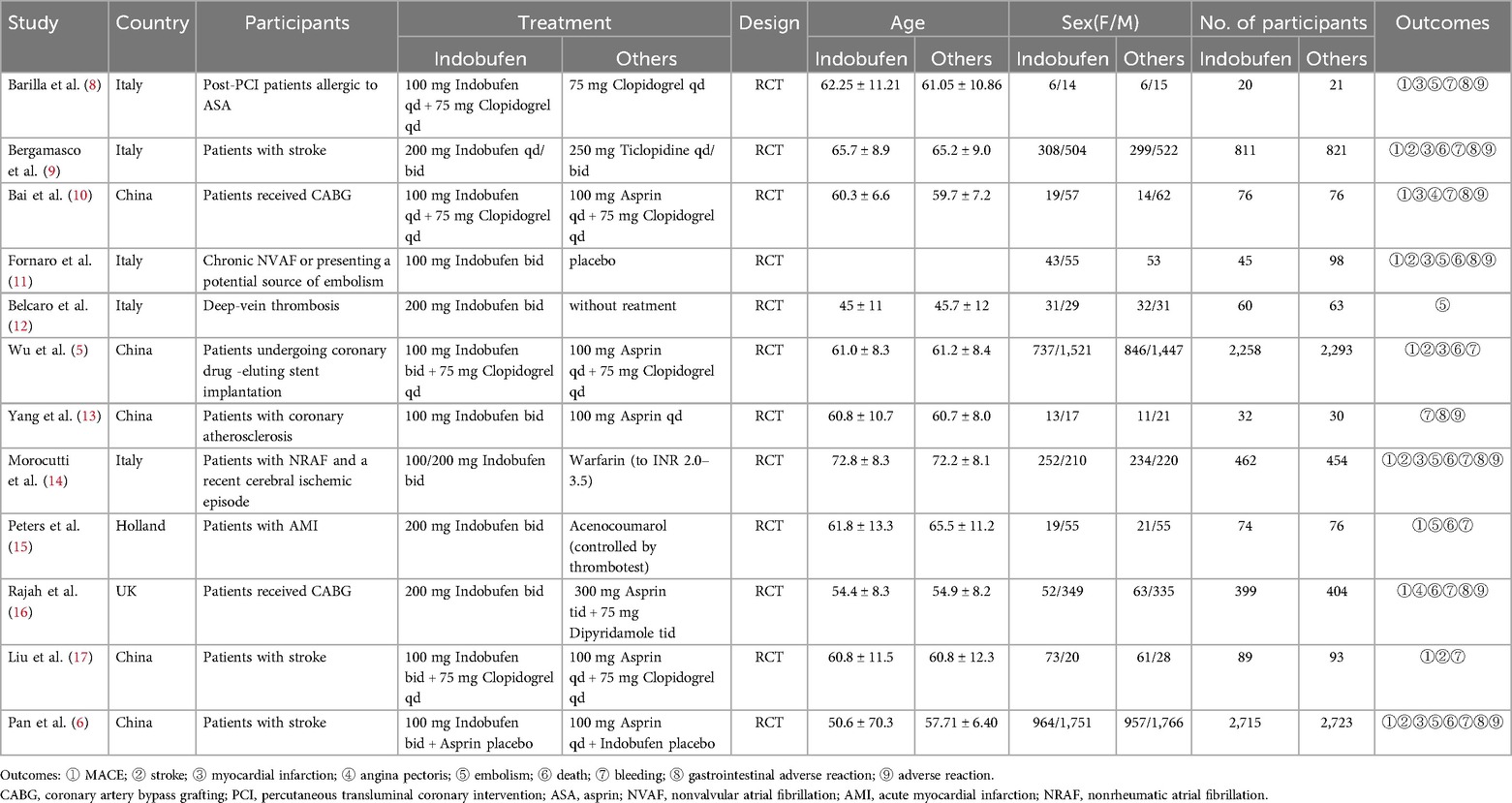
After screening the databases, 168 studies were initially identified. After 66 duplicate studies were removed, 37 irrelevant studies were excluded based on their titles and abstracts. Additionally, 37 articles were excluded because they were reviews or meta-analyses. The full texts of 28 articles were further reviewed, resulting in the exclusion of 16 articles. Among these, 3 trials lacked results, 4 were conference abstracts, and 9 studies had unavailable full texts, making data extraction impossible. Ultimately, 12 randomized controlled trials (RCTs) were included in this meta-analysis. A brief description of the literature screening process is shown in Figure 1, while a summary of the key characteristics of the included studies is provided in Table 1.
3.1 Risk of bias assessment
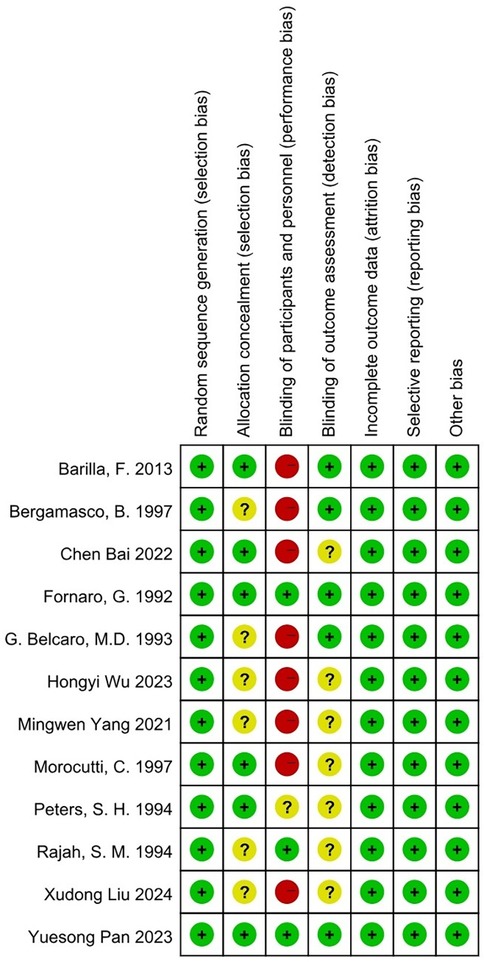
An assessment of bias was conducted using Cochrane Collaboration's tool for evaluating randomized controlled trials (RCTs) (18; Figure 2).
3.2 MACE (major adverse cardiovascular events)
Ten studies reported Major Adverse Cardiovascular Events (MACE) as the primary outcome (5, 6, 8–11, 14–17), which primarily included fatal cardiovascular diseases, myocardial infarction, non-fatal stroke, and recurrent angina. The combined results showed no significant difference in MACE incidence between the indobufen and other treatment groups (RR: 1.05, 95% CI: 0.93–1.20, I2 = 44%, P = 0.41; Figure 3A).
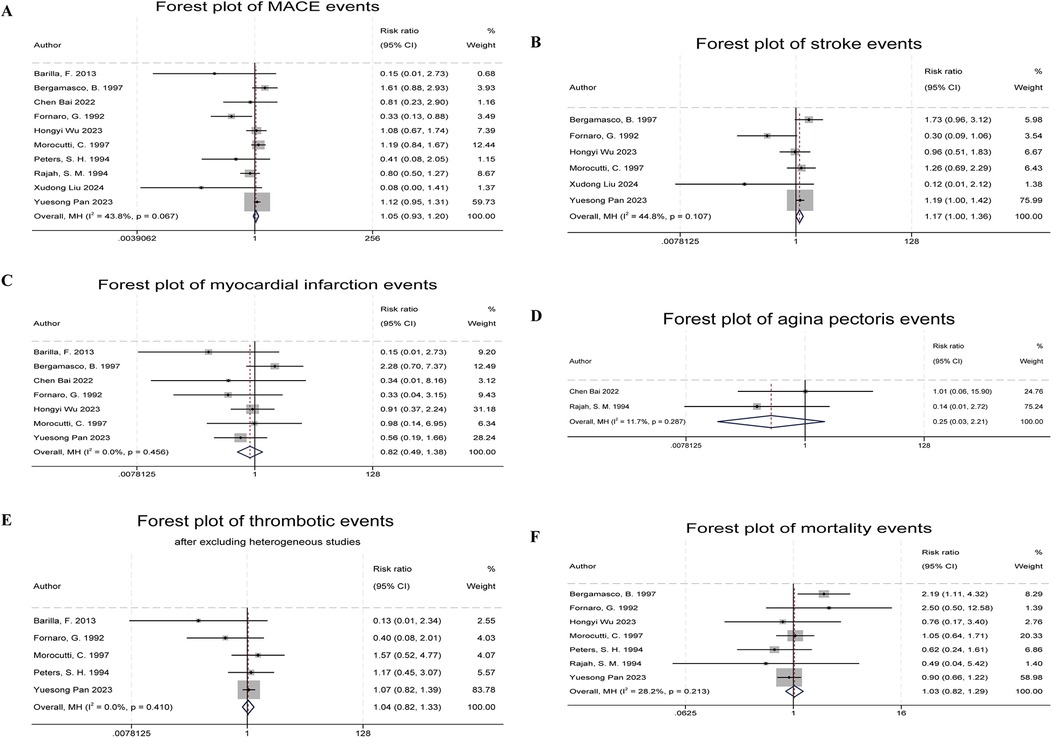
Figure 3. Forest plots of indobufen treatment efficacy evaluations. (A) Forest plot of MACE events. (B) Forest plot of stroke events. (C) Forest plot of myocardial infarction events. (D) Forest plot of angina pectoris events. (E) Forest plot of thrombotic events. (F) Forest plot of mortality events.
3.3 Stroke
Six studies reported stroke events (5, 6, 9, 11, 14, 17). The combined results showed a significant difference in stroke event rates between the indobufen and other treatment groups (RR: 1.17, 95% CI: 1.00–1.36, I2 = 45%, P = 0.049; Figure 3B).
3.4 Myocardial infarction
Seven studies reported myocardial infarction events (5, 6, 8–11, 14). The combined results showed no significant difference in the incidence of myocardial infarction between the indobufen group and other treatment groups (RR: 0.82, 95% CI: 0.49–1.38, I2 = 0%, P = 0.45; Figure 3C).
3.5 Recurrence of angina pectoris
Two studies reported recurrent angina events (10, 16). The combined results showed no significant difference in the incidence of recurrent angina between the indobufen group and other treatment groups (RR: 0.25, 95% CI: 0.03–2.21, I2 = 12%, P = 0.21; Figure 3D).
3.6 Thrombotic events
Six studies reported thrombotic events (8, 11, 12, 14, 15, 17). After heterogeneity testing (I2 = 58.5%, Q test P = 0.03), the results indicated statistical heterogeneity between the included studies. After excluding the study by Belcaro et al. (12), which had a significant impact on heterogeneity, the combined results showed no significant difference in the incidence of thrombotic events between the indobufen group and other treatment groups (RR: 1.04, 95% CI: 0.82–1.33, I2 = 0%, P = 0.74; Figure 3E).
3.7 Death
Seven studies reported mortality events (5, 6, 9, 11, 14–16). The combined results showed no significant difference in the incidence of mortality between the indobufen group and other treatment groups (RR: 1.03, 95% CI: 0.82–1.29, I2 = 28%, P = 0.80; Figure 3F).
3.8 Bleeding events
Ten studies reported bleeding events (5, 6, 8–10, 13–17). One study was excluded due to a lack of event occurrence (8). Heterogeneity testing showed that the study by Morocutti et al. (14) had a significant impact on heterogeneity (I2 = 62%, Q test P = 0.008). After excluding this study, the combined results showed a significant difference in bleeding event rates between the indobufen group and other treatment groups (RR: 0.77, 95% CI: 0.63–0.94, I2 = 39%, P = 0.01; Figure 4A).
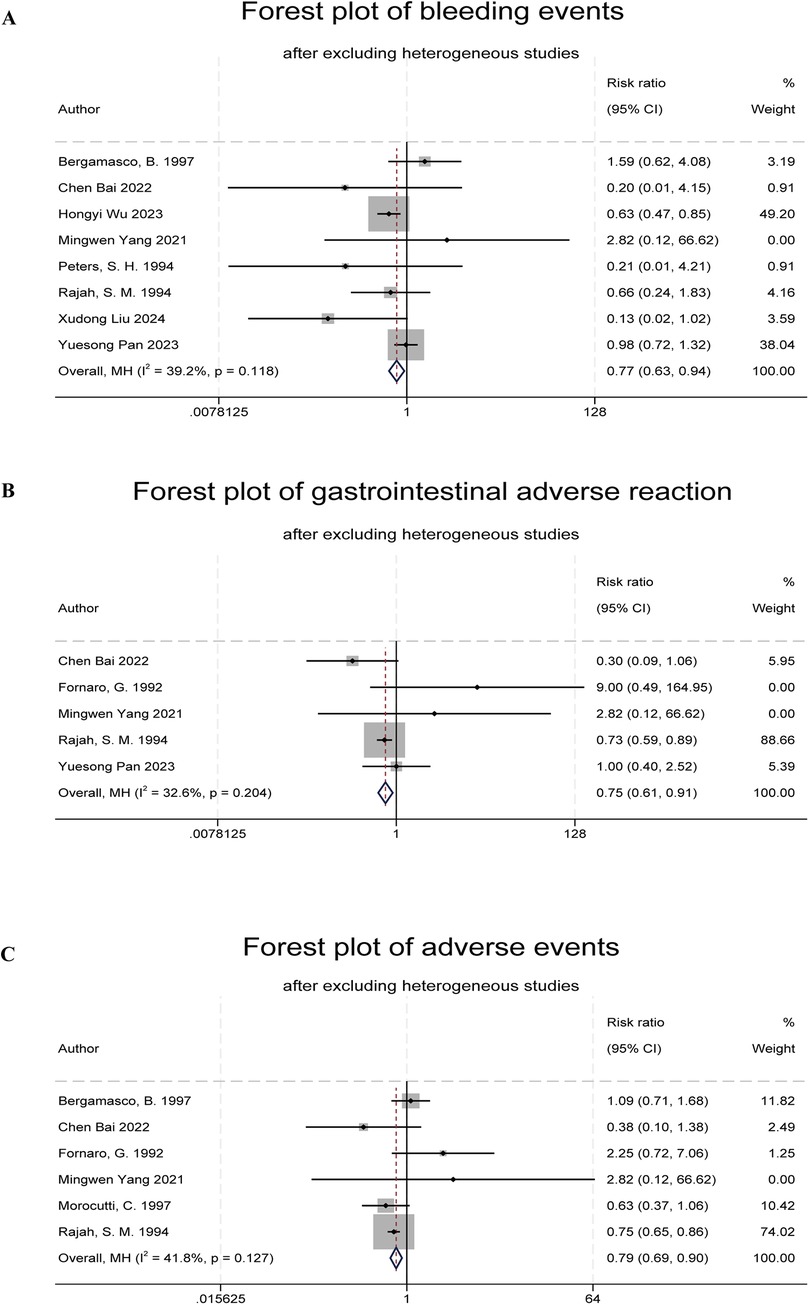
Figure 4. Forest plots of indobufen treatment safety evaluations. (A) Forest plot of bleeding events. (B) Forest plot of gastrointestinal adverse reaction. (C) Forest plot of adverse events.
3.9 Gastrointestinal adverse reactions
Six studies reported gastrointestinal adverse reactions (6, 9–11, 13, 14, 16). One study was excluded due to a lack of event occurrence (12). Heterogeneity testing (I2 = 55.6%, Q test P = 0.05) indicated statistical heterogeneity. After excluding the study by Bergamasco et al. (9), the combined results showed a significant difference in the overall adverse reaction rates between the indobufen group and other treatment groups (RR: 0.75, 95% CI: 0.61–0.91, I2 = 33%, P = 0.004; Figure 4B).
3.10 Total adverse reaction events
Eight studies reported total adverse reaction events (6, 8–11, 13, 14, 16), which included gastrointestinal adverse reactions, dizziness, rash, allergic reactions, and anemia. One study was excluded due to a lack of event occurrence (7). Heterogeneity testing (I2 = 67%, Q test P = 0.006) indicated statistical heterogeneity. After excluding the study by Yuesong Pan et al. (6), the combined results showed a significant difference in the total adverse reaction rates between the indobufen group and other treatment groups (RR: 0.79, 95% CI: 0.69–0.90, I2 = 42%, P = 0.0004; Figure 4C).
3.11 Subgroup analysis
3.11.1 Subgroup analysis of efficacy evaluation
The subgroup analysis for cerebrovascular diseases indicated a significant difference in stroke event rates between the indobufen and other treatment groups (RR: 1.22, 95% CI: 1.04–1.43, I2 = 23%, P = 0.02). No meaningful results were found in the other subgroup analyses (Supplementary Tables S1–S4).
3.11.2 Subgroup analysis of safety evaluation
In the subgroup analysis of cardiovascular diseases, significant differences were found in the bleeding events (RR: 0.62, 95% CI: 0.47–0.83, I2 = 0%, P = 0.001), gastrointestinal adverse reactions (RR: 0.73, 95% CI: 0.60–0.90, I2 = 45%, P = 0.002), and total adverse reaction events (RR: 0.76, 95% CI: 0.67–0.88, I2 = 43%, P = 0.0001) between the indobufen group and other treatment groups. Additionally, in the subgroup analysis based on the treatment drug, indobufen showed a more favorable effect compared to aspirin and clopidogrel for bleeding events and gastrointestinal adverse reactions (RR: 0.75, 95% CI: 0.61–0.92, I2 = 41%, P = 0.0006; RR: 0.72, 95% CI: 0.59–0.88, I2 = 1%, P = 0.001). No meaningful results were found in the other subgroup analyses (Supplementary Tables S1–S4).
3.12 Sensitivity analysis
Heterogeneity tests were conducted when synthesizing effect sizes for each group. If the heterogeneity test indicated statistical significance, studies that contributed substantially to the heterogeneity were excluded. In the final pooled results, heterogeneity was within acceptable limits. Sensitivity analyses were also performed, and the results remained stable (Supplementary Figures S1–S9).
3.13 Publication bias
Publication bias was tested for each outcome by performing Egger's test, Begg's test, and funnel plot analysis (Supplementary Figures S10–S18). Both Egger's and Begg's tests showed P > 0.05, indicating no significant publication bias for the studies included in the analysis of each outcome.
4 Discussion
Based on meta-analysis, this study systematically evaluated the efficacy and safety of indobufen in treating cardiovascular diseases, cerebrovascular diseases, and thromboembolic diseases. Through this meta-analysis, we confirmed that indobufen demonstrates better clinical outcomes than other antiplatelet drugs in safety endpoints, including bleeding, gastrointestinal, and overall adverse reactions. Specifically, subgroup analyses of monotherapy or combination therapy with aspirin or clopidogrel, as well as cardiovascular disease subgroup analysis, revealed that indobufen had a significant advantage in bleeding events and gastrointestinal adverse reactions, which may be attributed to the reversible antiplatelet effect of indobufen. Regarding efficacy, no significant clinical superiority was observed for indobufen in MACE, myocardial infarction, angina, thrombosis, and mortality events compared to other antiplatelet therapies. However, in the case of stroke events, especially among cerebrovascular disease patients, indobufen demonstrated some risks.
Additionally, heterogeneity issues were of considerable concern in this study. Although studies with significant heterogeneity were excluded in the combined analysis, factors such as study design differences, participants' baseline characteristics, and drug dosages may still influence the results. The subgroup analyses in this study showed that heterogeneity in thrombotic events, gastrointestinal adverse reactions, and total adverse reaction outcomes were primarily caused by inconsistent disease types among the included studies. No significant heterogeneity was found in the outcomes mentioned above after performing subgroup analyses based on different disease types (Supplementary Figures S19–S22). For bleeding events, heterogeneity caused by the study by Morocutti et al. (14) and publication bias visible in the funnel plot were further explored. We speculated that the close monitoring of patients in this study during follow-up may have kept the relevant indicators within optimal ranges, which could explain why the original data from this study may not reflect the most realistic research situation compared to other studies.
Although no other meaningful results could be obtained in this study apart from the findings mentioned above, several interesting issues were observed. In cerebrovascular diseases, indobufen treatment may lead to higher risks of thrombosis and gastrointestinal adverse reactions. Further analysis of the included studies suggests that the high risk could be attributed to the few included studies (only two), which may have led to biased results and cannot be generalized. Furthermore, in subgroup analyses of cerebrovascular diseases and Asian patients, indobufen exhibited a higher risk in MACE events and stroke events, which is consistent with the conclusions from the study by Morocutti et al. (14). However, no statistical differences were found between the treatment groups after adjusting for prognostic factors. Multivariable analysis revealed that these factors were female sex, history of stroke, and myocardial infarction at admission (14). It is also well-known that antiplatelet drugs may show low responsiveness in certain diseases or populations due to metabolic pathways or genetic effects, such as the CYP2C19 enzyme for clopidogrel metabolism and the COX-1 gene and P2Y12 gene polymorphisms for aspirin metabolism (19–23). Due to variations in drug dosages, potential drug synergistic effects, the polymorphisms of the genes mentioned above, and the larger weight of findings from studies with large sample sizes, the higher risk associated with indobufen may have been influenced.
Our meta-analysis confirmed that indobufen has certain advantages for patients with gastrointestinal dysfunction or aspirin intolerance in cardiovascular diseases, consistent with expert consensus in clinical guidelines (19). Previous clinical studies, such as OPTION (indobufen or aspirin with clopidogrel for coronary artery drug-eluting stent implantation) and INSURE (indobufen vs. aspirin in acute ischemic stroke), large multi-center clinical trials comparing indobufen and aspirin, reported similar results to our meta-analysis. Indobufen demonstrated advantages in bleeding outcomes compared to other antiplatelet drugs, but for preventing recurrence in cerebrovascular diseases, especially in stroke patients, indobufen was not superior to aspirin. Moreover, a series of previous systematic reviews evaluating the efficacy and safety of indobufen have also pointed out that its effectiveness is significantly lower than other antiplatelet therapies in patients with gastrointestinal discomfort or those at risk of bleeding (24–26).
This meta-analysis comprehensively analyzed the efficacy and safety of indobufen in cardiovascular and cerebrovascular diseases, as well as thromboembolic diseases, and conducted subgroup analyses with possible explanations for significant results. However, due to differences in study designs, such as varied inclusion and exclusion criteria, baseline characteristics of patient populations, and differences in the diseases studied [e.g., coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), and non-rheumatic atrial fibrillation (AF)], as well as time spans between studies (from 1993 to 2024). Inconsistent definitions of outcome assessment, these factors could have influenced the final results of the meta-analysis. Nevertheless, it cannot be denied that indobufen shows advantages compared to other antiplatelet drugs in safety outcomes.
5 Limitation
Several limitations remain in this study. Firstly, the open-label design of some randomized trials may potentially influence the outcomes and introduce bias into the results. Additionally, Different studies used various metrics to assess treatment efficacy, and the statistical forms of these metrics were inconsistent across studies, such as the thromboxane B2 (TXB2) levels in plasma and urine, making it difficult to compare the outcomes of these studies. Therefore, the research focuses only on safety indicators, such as bleeding events and adverse reactions, as well as efficacy indicators, such as cardiovascular events and stroke.
Third, the included studies had inconsistent dosing regimens and insufficient studies with the same dosing protocols. This inconsistency in dosing regimens limits the interpretation of differences between the two groups in the final pooled analysis and introduces bias. In particular, the limited number of studies related to thromboembolic diseases included in this research made it difficult to evaluate the efficacy and safety of indobufen in treating thromboembolic diseases. Fourth, the studies from Asian countries included in this research were limited to China. While these studies provide valuable references for indobufen use in China and other Asian countries, future research should involve more countries and ethnic groups to obtain better results.
6 Conclusion
There are several antiplatelet drugs currently available in clinical practice, including aspirin, clopidogrel, and warfarin. Indobufen, as a secondary medication for preventing cardiovascular and cerebrovascular diseases, exhibits high selectivity and reversible binding to its receptor (27), offering certain advantages over other anticoagulants. However, compared to other anticoagulants, indobufen is used for a shorter duration, and there are fewer large-scale clinical studies, which limits the strength of the available evidence. Additionally, the increased risk of MACE and stroke observed in the indobufen group in this study suggests that, in specific conditions such as cerebrovascular diseases, these risks may be more pronounced. In conclusion, indobufen may be a suitable choice for certain populations. Its low association with bleeding events and adverse reactions provides some guidance for clinical antiplatelet therapy in patients at potential risk of bleeding or adverse reactions. Nevertheless, compared to widely recognized antiplatelet drugs like aspirin and clopidogrel, indobufen still shows certain limitations in clinical efficacy. Therefore, using indobufen should be based on carefully assessing its effectiveness and safety with individualized treatment decisions. Further research focusing on different disease types and populations will be crucial for better defining the indications and scope of use for indobufen in future clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XL: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. CL: Investigation, Software, Validation, Visualization, Writing – original draft. TC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1509010/full#supplementary-material
References
1. World Health Organization. E. coli. (2024). The top 10 causes of death (who.int). (accessed August 7, 2024).
2. Schafer AI. Antiplatelet therapy. Am J Med. (1996) 101(2):199–209. doi: 10.1016/S0002-9343(96)80077-5
3. Bhana N, McClellan KJ. Indobufen: an updated review of its use in the management of atherothrombosis. Drugs Aging. (2001) 18(5):369–88. doi: 10.2165/00002512-200118050-00007
4. Lee JY, Sung KC, Choi HI. Comparison of aspirin and indobufen in healthy volunteers. Platelets. (2024) 27(2):105–9. doi: 10.3109/09537104.2015
5. Wu H, Xu L, Zhao X, Zhang H, Cheng K, Wang X, et al. Indobufen or aspirin on top of clopidogrel after coronary drug-eluting stent implantation (OPTION): a randomized, open-label, end point-blinded, noninferiority trial. Circulation. (2023) 147(3):212–22. doi: 10.1161/CIRCULATIONAHA.122.062762
6. Pan Y, Meng X, Yuan B, Johnston SC, Li H, Bath PM, et al. Indobufen versus aspirin in patients with acute ischaemic stroke in China (INSURE): a randomised, double-blind, double-dummy, active control, non-inferiority trial. Circulation. (2023) 22(6):485–93. doi: 10.1016/S1474-4422(23)00113-8
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
8. Barillà F, Pulcinelli FM, Mangieri E, Torromeo C, Tanzilli G, Dominici T, et al. Clopidogrel plus indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets. (2013) 24(3):183–8. doi: 10.3109/09537104.2012.686072
9. Bergamasco B, Benna P, Carolei A, Rasura M, Rudelli G, Fieschi C. A randomized trial comparing ticlopidine hydrochloride with indobufen for the prevention of stroke in high-risk patients (tiss study). Funct Neurol. (1997) 12(1):33–43.9127122
10. Bai C, Li JX, Yu Y, Liu R, Gao MX, Zhang F, et al. A randomized controlled trial of indobufen versus aspirin in the prevention of bridging restenosis after coronary artery bypass grafting. Zhonghua Xin Xue Guan Bing Za zhi. (2022) 50(5):466–70. doi: 10.3760/cma.j.cn112148-20210701-00560
11. Fornaro G, Rossi P, Mantica PG, Caccia ME, Aralda D, Lavezzari M, et al. Indobufen in the prevention of thromboembolic complications in patients with heart disease. A randomized, placebo-controlled, double-blind study. Circulation. (1993) 87(1):162–4. doi: 10.1161/01.cir.87.1.162
12. Belcaro G, Errichi BM, De Simone P. Prevention of recurrent deep venous thrombosis with indobufen. A 3-year follow-up study using color duplex scanning. Angiology. (1993) 44(4):328–31. doi: 10.1177/000331979304400410
13. Yang M, Ye Z, Mei L, Ullah I, Tan C, Wang G, et al. Pharmacodynamic effects of indobufen compared with aspirin in patients with coronary atherosclerosis. Eur J Clin Pharmacol. (2021) 77(12):1815–23. doi: 10.1007/s00228-021-03177-y
14. Morocutti C, Amabile G, Fattapposta F, Nicolosi A, Matteoli S, Trappolini M, et al. Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (studio Italiano fibrillazione atriale) investigators. Stroke. (1997) 28(5):1015–21. doi: 10.1161/01.str.28.5.1015
15. Peters SHA, Jonker JJC, De Boer AC, Den Ottolander GJH. The incidence of deep venous thrombosis in patients with an acute myocardial infarction treated with acenocoumarol or indobufen. Thromb Haemostasis. (1982) 48(2):222–5. doi: 10.1055/s-0038-1657261
16. Rajah SM, Nair U, Rees M, Saunders N, Walker D, Williams G, et al. Effects of antiplatelet therapy with indobufen or aspirin-dipyridamole on graft patency one year after coronary artery bypass grafting. J Thorac Cardiovasc Surg. (1994) 107(4):1146–53. doi: 10.1016/S0022-5223(94)70392-2
17. Liu X, Lv X, Peng Y, Wang J, Lei J, Tang C, et al. Clopidogrel with indobufen or aspirin in minor ischemic stroke or high-risk transient ischemic attack: a randomized controlled clinical study. BMC Neurol. (2024) 24(1):81. doi: 10.1186/s12883-024-03585-4
18. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
19. Yong Huo YW, Gu Y, Huang K, Xu A, Zheng Y, Ge J. Expert consensus on the diagnosis and treatment of patients with intolerance and low response to commonly used oral antiplatelet drugs. Chin J Intervent Cardiol. (2021) 29(05):240–9. doi: 10.3760/cma.j.cn116031.2021.1000076
20. Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. (2003) 107(23):2908–13. doi: 10.1161/01.CIR.0000072771.11429.83
21. Lev EI, Patel RT, Maresh KJ, Guthikonda S, Granada J, DeLao T, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol. (2006) 47(1):27–33. doi: 10.1016/j.jacc.2005.08.058
22. Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. (2010) 38(1):92–9. doi: 10.1124/dmd.109.029132
23. Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta analysis. JAMA. (2010) 304(16):1821–30. doi: 10.1001/jama.2010.1543
24. Yang Xia LW, Ken C, Shujie D, Suodi Z. Efficacy and safety of indobuprofen tablets for prevention and treatment of ischemic cardio-cerebrovascular disease: a meta-analysis. Chin J Clin Pharmacol. (2017) 33(04):359–62. doi: 10.13699/j.cnki.1001-6821.2017.04.019
25. Xu Rongbin YJ, Shen H, Feng S, Jun M. Effectiveness and efficacy of indobufen in preventing cardiovascular and cerebrovascular events: a meta-analysis. Chin J Evid-Based Cardiovasc Med. (2017) 9(05):532–8.
26. Zhang X, Yan Q, Jiang J, Luo H, Ren Y. Safety and efficacy of aspirin and indobufen in the treatment of coronary heart disease: a systematic review and meta-analysis. Front Cardiovasc Med. (2024) 11:1412944. doi: 10.3389/fcvm.2024.1412944
Keywords: indobufen, cardiovascular, cerebrovascular, efficacy, meta-analysis
Citation: Luo X, Lai C and Chen T (2025) The efficacy and safety of indobufen in patients with ischemic cardiovascular or cerebrovascular diseases: systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1509010. doi: 10.3389/fcvm.2024.1509010
Received: 10 October 2024; Accepted: 24 December 2024;
Published: 9 January 2025.
Edited by:
Nicola Mumoli, ASST Ovest Milanese, ItalyReviewed by:
Han Naung Tun, University of Vermont, United StatesYi-Ming Huang, Capital Medical University, China
Copyright: © 2025 Luo, Lai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tielong Chen, Y3Rsa3R6QDE2My5jb20=
 Xiaolu Luo
Xiaolu Luo Chenglu Lai1
Chenglu Lai1