- 1Department of Cardiopulmonary Function, Zhengzhou University People's Hospital (Henan Provincial People's Hospital), Zhengzhou, Henan, China
- 2Department of Cardiopulmonary Function, Fuwai Central China Cardiovascular Hospital, Zhengzhou, Henan, China
- 3Department of Cardiology, Fuwai Central China Cardiovascular Hospital, Zhengzhou, Henan, China
- 4Department of Cardiology, Zhengzhou University People's Hospital (Henan Provincial People's Hospital), Zhengzhou, Henan, China
Background: Deceleration capacity (DC) and acceleration capacity (AC) are used to characterize autonomic regulation. The purpose of this study was to evaluate the autonomic nervous function in patients with vasovagal syncope (VVS) and to evaluate the diagnostic value of DC and AC for VVS.
Methods: A total of 94 consecutive patients with VVS [51.0 (38.0–60.0) years; 48 males] and 76 healthy subjects [53.0 (44.3–62.8) years; 46 males] were recruited as controls. The study compared DC, AC, and heart rate variability (HRV) in 24-h ECG, echocardiogram, and biochemical examinations between the two groups.
Results: DC was significantly higher (9.3 ± 2.1 vs. 7.4 ± 1.4 ms, p < .001) and AC was lower (−9.3 ± 2.1 vs. −7.3 ± 1.3 ms, p < .001) in the syncope group compared to the control group. HRV indicators were higher in the syncope group. In multivariable analyses, DC [odds ratio = 1.746 (95% CI, 1.389–2.195); p < .001], AC [odds ratio = 0.553 (95% CI, 0.435–0.702); p < .001] were independently associated with syncope. Mean HR was associated with syncope only in patients <60 years of age. Receiver operating characteristics (ROC) curves showed areas under curve (AUC) of DC/AC for predicting syncope are 0.755/0.765 with sensitivity of 56.4%/60.6% and specificity of 93.4%/88.2%.
Conclusion: Patients with VVS exhibit higher DC and lower AC. Both DC and AC are independently correlated with syncope. A DC value >9.0 ms and an AC value −9.0 ms could potentially be valuable indicators for monitoring cardiac autonomic nervous dysfunction.
1 Introduction
Syncope is defined as a brief episode of unconsciousness and Vasovagal syncope (VVS) is the most prevalent type of syncope (1, 2). VVS is characterized by sudden onset and spontaneous recovery, usually with a benign course. However, frequent episodes can significantly impact quality of life and increase the risk of complications (3). Studies have shown that patients with VVS tend to have a lower quality of life compared to control populations, with factors such as gender, age, and frequency of syncopes correlating with this decline (4). Additionally, VVS is associated with an elevated risk of physical injury, particularly concerning for individuals in high-risk occupational settings (5). The exact pathophysiological mechanisms of VVS are still not fully understood (6).
Cardiovascular autonomic dysfunction plays a key role in the pathophysiology of VVS. VVS is thought to be closely linked to the dysregulation of autonomic nerves, leading to an imbalance between sympathetic and vagal nerves (3, 7). While a diagnosis of VVS can often be based on the patient's medical history in the presence of typical triggers, it may not always be feasible to rely solely on historical information (1).
The head-up tilt-table test (HUT) is crucial for identifying the underlying causes of unexplained syncope. However, some patients may find the tilt testing uncomfortable and challenging to maintain in an upright position (8, 9).
Traditional measurement of heart rate variability (HRV) has been used to analyze the function of the cardiac autonomic nervous system. Previous studies have presented conflicting results regarding VVS. HRV analysis is a well-established method for evaluating beat-to-beat neural heart rate modulation and its changes in various diseases. It can be challenging to distinguish the effects of vagal and sympathetic modulators on the heart for analysis (10, 11).
Deceleration capacity (DC) and acceleration capacity (AC) of heart rate (HR) have been developed as quantitative measures to evaluate cardiac vagal and sympathetic function (12, 13). DC has been validated as a predictor of mortality in patients following a myocardial infarction (12), while AC has been identified as a predictor for exacerbation of heart failure in patients with dilated cardiomyopathy (14).
A decrease in cardiac DC is indicative of reduced vagal tone in cardiac autonomic function. Previous studies have indicated that individuals with VVS exhibit higher DC values compared to control groups. A DC value greater than 7.5 ms could potentially be a useful tool for monitoring cardiac vagal activity and distinguishing VVS (5). However, existing research has mainly focused on young patients, with a gap in literature exploring the relationship between AC and VVS. Therefore, our study aims to investigate the impact of autonomic nervous system (ANS) function, as assessed by DC and AC, on both VVS and control groups, irrespective of age.
2 Methods
2.1 Ethical approval
The study was approved by the Ethics Committee of Henan Provincial People's Hospital (2018 Ethics Review No. 24) prior to conducting performance and clinical investigations, adhering to the principles outlined in the Declaration of Helsinki.
2.2 Study population
We included 94 consecutive patients [51.0 (38.0–60.0) years; 48 males] who were admitted to the Henan Provincial People's Hospital with suspected VVS. All patients underwent a tilt table test (TTT) as part of their diagnostic assessment. VVS was diagnosed based on clinical characteristics suggestive of a reflex mechanism and after excluding other differential diagnoses (5, 15). Exclusion criteria comprised individuals with a history of overt heart failure, myocardial infarction, left ventricular ejection fraction less than 50%, significant valvular heart disease, dilated cardiomyopathy, hypertrophic cardiomyopathy, sinus node dysfunction, arrhythmogenic right ventricular cardiomyopathy, Brugada syndrome, Long QT syndrome, and symptomatic orthostatic hypotension. Seventy-six healthy subjects [53.0 (44.3–62.8) years; 46 males] were enrolled as controls. They had undergone normal routine physical examinations and had never reported syncopal episodes in the past. Additionally, all subjects had normal 24-h Holter monitoring and echocardiograms.
2.3 Holter recording
A Holter monitor test was performed on each patient using a portable electrocardiogram device (CONTEC Medical System LTD, Qinhuangdao, China). The test measured various indices included average, fastest and slowest HR, deceleration capacity, acceleration capacity and HRV. HRV included standard deviation of normal-to-normal (NN) intervals (SDNN), standard deviation average of NN intervals (SDANN), root mean square successive difference of normal R-R intervals (RMSSD), and the percent of the number of times that the difference between adjacent normal RR intervals >50 ms in the total number of NN intervals (PNN50). The SDNN and SDANN were considered as measured of vagal and sympathetic influences, while RMSSD and PNN50 were regarded as indicators of parasympathetic nerve activity (16).
2.4 Deceleration capacity and acceleration capacity
The heart rate deceleration and acceleration capacities were measured by the Holter system (5). Calculation methods as previous described: Firstly, heart beat intervals were selected as decelerating anchors when >1.00 but ≤1.05 of the preceding heartbeat interval; heart beat intervals were selected as accelerating anchors when <1.00 but ≥0.95 of the preceding heartbeat interval. Secondly, the segments of heartbeat intervals around decelerating and accelerating anchors were collected. Thirdly, the above segments were aligned at the decelerating and accelerating and the signals of segments were averaged to obtain the phase-rectified signal averaging signal X(i).
In the end, the following formula was used to quantify deceleration capacity (DC) and acceleration capacity (AC): .
The values of DC are over 0, quantifying vagal nerve activity and the values of AC are less than 0, quantifying sympathetic nerve activity (17).
2.5 Echocardiographic evaluation
In our study, all patients underwent transthoracic echocardiography (TTE) using a Sonos 5500 Ultrasound machine (Philips). The following parameters measured by the M-mode technique: right atrial diameter (RAd) and left atrial diameter (Lad), left ventricular end-diastolic diameter (LVEDd), and left ventricular end-systolic diameter (LVESd). Simpson's biplane method was used to measure the left ventricular ejection fraction (LVEF).
2.6 Statistical analysis
Continuous data are presented as mean ± SD or median (25th–75th percentile). Continuous variables were compared using a Student t-test for normally distributed data or a Wilcoxon ranksum test otherwise. Categorical variables were presented as sample size (percentage) and were compared using a Pearson χ2 test. multivariate logistic regression were employed to assess associations among various variables of interest with syncope. The receiver operator characteristics curve was performed to test the best cutoff value and the area under curve (AUC) of DC and AC to differentiate VVS and controls. All statistical analyses were two-sided, and a P-value < 0.05 was considered statistically significant.
3 Results
3.1 Comparison between syncopal patients and controls
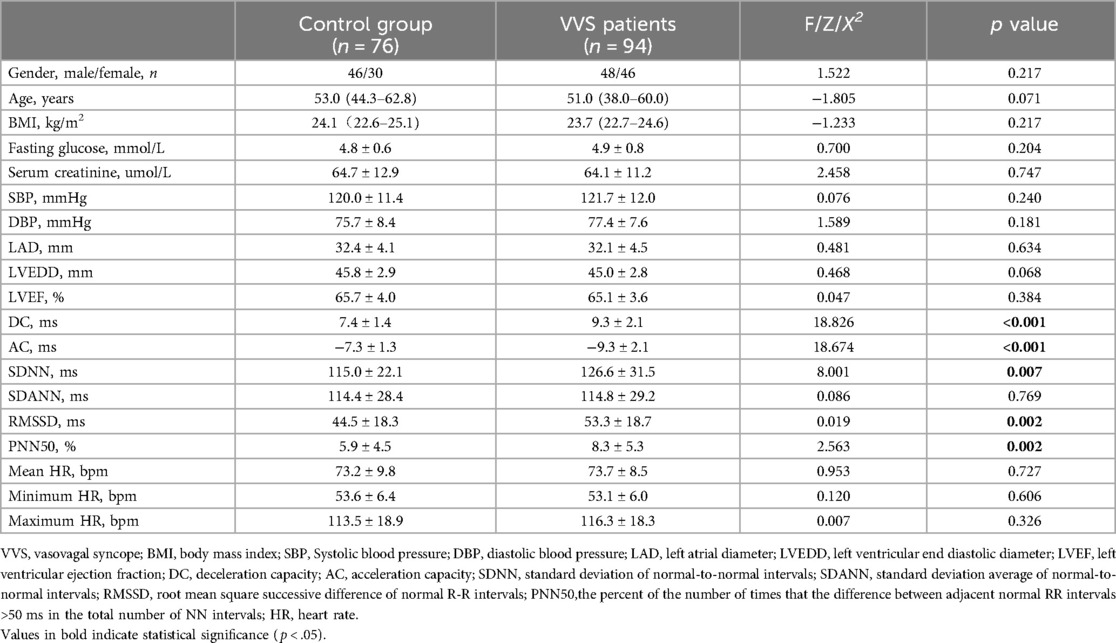
There were no significant differences in gender, body mass index (BMI), echocardiogram, biochemical examinations and HR (all p > .05). DC was significantly higher (9.3 ± 2.1 vs. 7.4 ± 1.4 ms, p < .001) and AC was significantly lower (−9.3 ± 2.1 vs. −7.3 ± 1.3 ms, p < .001) in patients with VVS compared with controls. The HRV parameters including SDNN, RMSSD and PNN50 were increased in VVS group compared with controls (p < .05). Clinical characteristics of both groups are summarized in Table 1.
3.2 Comparison of DC and HRV parameters between VVS patients and controls in different age groups
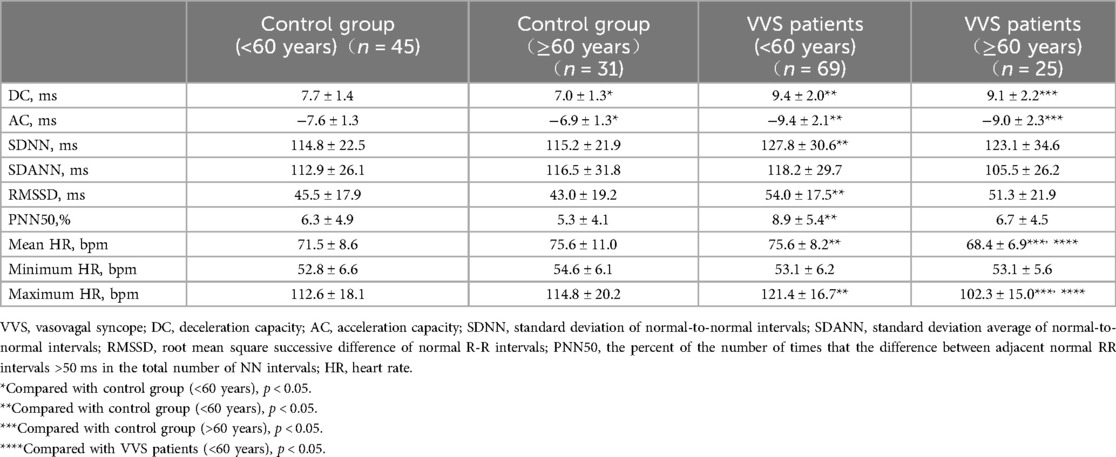
There are 114 participants <60 years of age (45 in the control group and 69 in the VVS group) and 56 participants ≥60 years of age (31 in the control group and 25 in the VVS group). Table 2 shows that in patients with VVS, the DC and absolute value of AC were significantly higher compared to controls, regardless of age.
However, SDNN, RMSSD, and PNN50 differed between VVS and control groups only in participants <60 years of age. The mean HR and maximum HR were higher in the VVS group compared to controls in participants <60 years of age, lower in participants ≥60 years of age. Interestingly, the DC and absolute value of AC were lower in control groups across different age groups, while there was no significant difference in DC and absolute value of AC in VVS groups across different age groups
3.3 Prediction of syncope in different age groups
The univariate regression analysis (Supplementary Table 1) showed that DC, AC, SDNN, RMSSD, and PNN50 were associated with VVS. However, the results of the multivariable regression analyses indicated that only DC [odds ratio = 1.746 (95% CI, 1.389–2.195); p < .001] and AC [odds ratio = 0.553 (95% CI, 0.435–0.702); p < .001] were significantly correlated with syncope (Table 3).
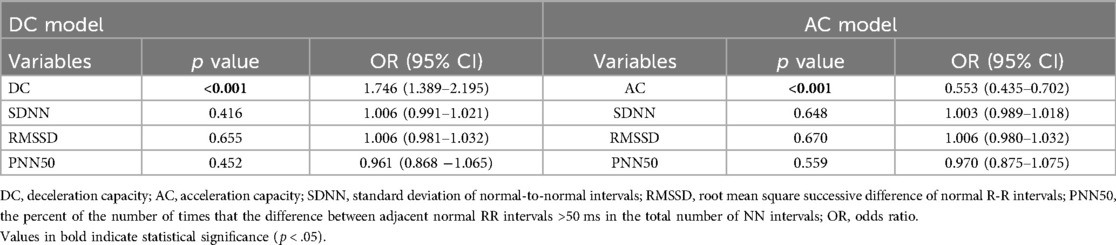
Table 3. The relationships between VVS and deceleration capacity, acceleration capacity in the entire cohort.
In participants under 60 years of age, the univariate regression analysis indicated that DC, AC, SDNN, RMSSD, PNN50, mean HR, and maximum HR were correlated with VVS (Supplementary Table 2). Furthermore, the multivariable regression analyses demonstrated that not only DC and AC, but also mean HR (odds ratio = 1.111, 95% CI 1.018–1.213; p = .000), were significantly linked to syncope (Table 4).
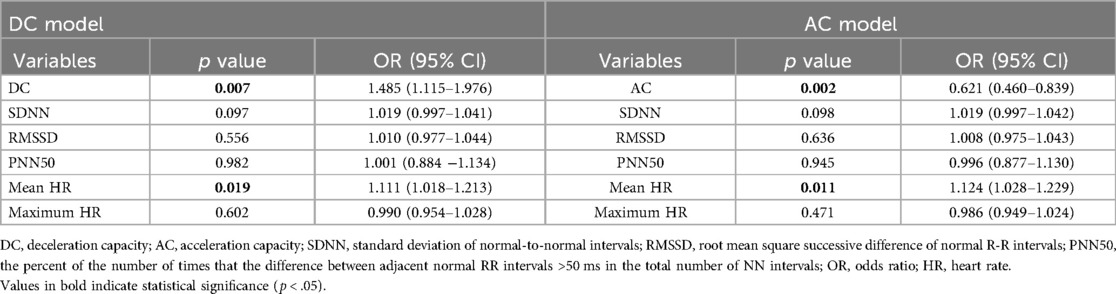
Table 4. The relationship between VVS and deceleration capacity, acceleration capacity in <60 years of age.
In participants aged 60 years and above, the univariate regression analysis showed correlations between VVS and DC, AC, mean HR, and maximum HR (Supplementary Table 3). Further multivariable regression analyses revealed that only DC and AC were significantly associated with syncope (Table 5).
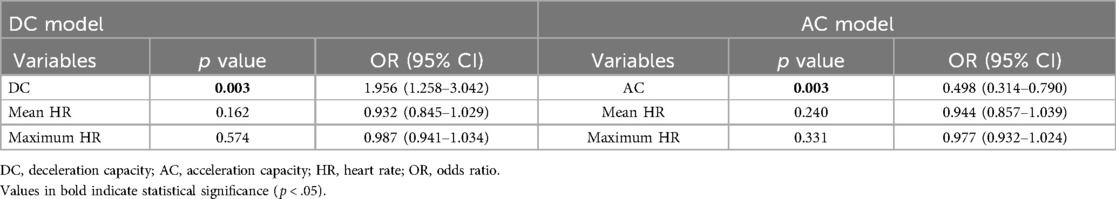
Table 5. The relationship between VVS and deceleration capacity, acceleration capacity in ≥60 years of age.
3.4 Receiver operator characteristic curve for prediction of VVS with DC and AC
ROC analysis was utilized to assess the predictive value of DC and AC in determining VVS. The AUC values for DC/AC in predicting syncope were 0.755/0.765, with sensitivities and specificities of 56.4%/60.6% and 93.4%/88.2% respectively at a cutoff of 9.4 ms/−9.0 ms.
In participants under 60 years of age, the area under the curve (AUC) for DC/AC predicting syncope was 0.743/0.758, with a sensitivity and specificity of 66.7%/50.7% and 82.2%/97.8% at the cutoff of 8.8 ms/−9.8 ms, respectively. For participants aged 60 years or above, the AUC for DC/AC predicting syncope was 0.759/0.767, with a sensitivity and specificity of 52.0%/52.0% and 100%/96.8% at the cutoff of 9.5 ms/−9.0 ms (Figures 1, 2). The AUC was similar for DC, AC, DC combined with HRV index, and AC combined with HRV index (Supplementary Table 4). In Supplementary Figure 1, the ROC curves almost completely overlap.
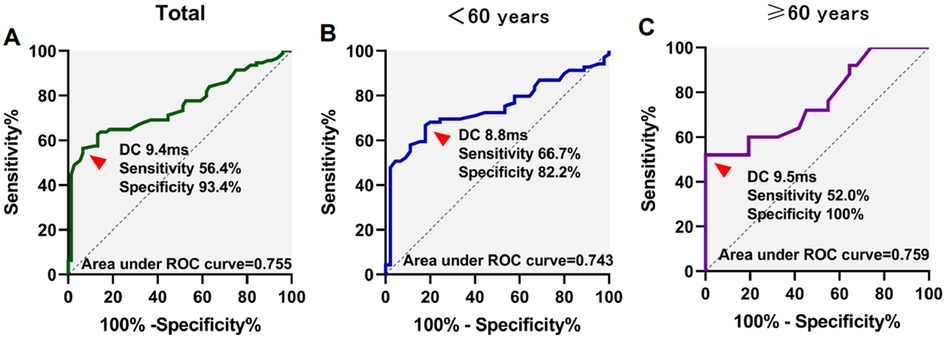
Figure 1. Receiver operating characteristics (ROC) curves for deceleration capacity (DC) for differentiation of syncope. ROC curves in total patients (A), <60 years of age (B), and ≥60 years of age (C) patients were showed.
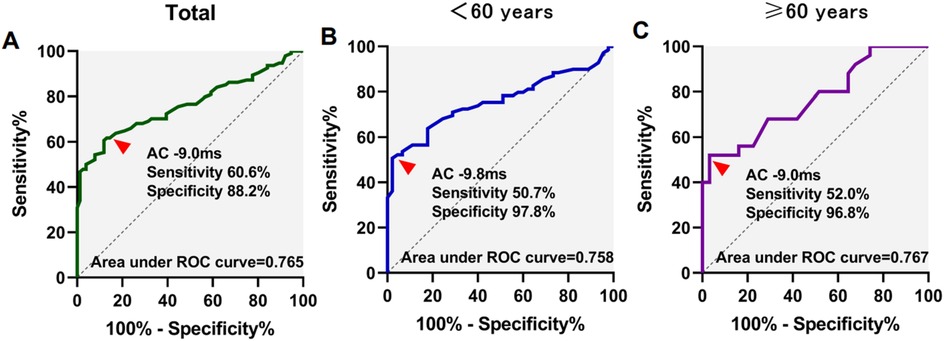
Figure 2. Receiver operating characteristics (ROC) curves for acceleration capacity (AC) for differentiation of syncope. ROC curves in total patients (A), <60 years of age (B), and ≥60 years of age (C) patients were showed.
4 Discussion
The mechanisms involved in VVS have not been fully elucidated. In the research of VVS, focus has been on the role of autonomic nerves. It is believed that VVS is directly associated with altered regulatory functions of autonomic nerves, leading to an imbalance between sympathetic and vagal nerves (3, 7).
Sympathetic nervous system activity serves as a rapid regulatory system that enables the cardiovascular system to adapt to postural changes. Previous research has indicated that the vasodilation observed during vasovagal syncope (VVS) is due to a decrease in sympathetic tone, with sympathetic control of total peripheral resistance being the primary mechanism of VVS (18, 19).
Hypotension and/or bradycardia were observed during VVS, with bradycardia primarily mediated by parasympathetic means through the vagal nerve. Histological studies of the human heart have shown a higher presence of parasympathetic nerves compared to sympathetic nerves in the atrium (20). Recent research suggests that selective vagal denervation in the atrial subendocardium through catheter ablation could potentially prevent VVS recurrence (21, 22). Therefore, heightened vagal activity plays a significant role in the pathophysiology of VVS.
Tilt table testing has been recognized as a valuable diagnostic tool for VVS, although it does have certain limitations. The sensitivity and specificity of TTT are not optimal, with the test yielding positive results in only 78%–92% of patients meeting the clinical diagnostic criteria for VVS. Additionally, some individuals may experience discomfort, particularly during a positive TTT result (8, 9, 23, 24).
The effects of vagal and sympathetic modulators on the heart can be challenging to differentiate for analysis. Various studies have examined whether patients with VVS exhibit differences in baseline autonomic tone compared to healthy controls, with conflicting results. Some studies indicate that patients with VVS have heightened vagal autonomic tone, while others propose the opposite (10, 25). HRV reflects the integrated changes in autonomic functions controlled by both sympathetic and vagal regulation, without isolating the vagal component. Additionally, HRV is influenced by various factors (10).
DC and AC are innovative indicators of the autonomic nervous system. They utilize a signal processing algorithm to distinguish between deceleration and acceleration of heart rate, serving as a metric for cardiac autonomic nervous modulation. DC and AC offer advantages over traditional techniques like TTT and HRV. Firstly, they enable a quantitative assessment of autonomic activity in patients with VVS. Secondly, DC and AC values, calculated through phased-rectified signal averaging, are less susceptible to noise interference and demonstrate superior sensitivity, specificity, and stability compared to HRV (5, 12).
Our study revealed abnormally increased vagal tone, as assessed by DC, and decreased sympathetic activation, as assessed by AC, in patients with VVS compared to healthy controls. The findings suggest that the heightened baseline vagal regulation, along with sympathetic nervous system modulation during upright posture, may contribute to these patients being more susceptible to bradycardia, hypotension, and ultimately syncope.
Aging is known to affect autonomic function and responses to head-up tilt (HUT) in patients with syncope. Studies have shown changes in serum catecholamine levels during HUT testing, with younger fainters (<40 years) exhibiting higher Epi/NE ratios. Variations in clinical characteristics and response patterns to head-up tilt have been observed between young (≤35 years) and older (≥65 years) patients, suggesting potential differences in the underlying pathophysiological mechanisms (26). The frequency of cardioinhibitory response decreases with age, possibly due to increased vagal activity in younger patients compared to older individuals (27). Previous studies have found that DC was increased in VVS, but this was only applicable to young patients (<60 years) (5).
In our study, we found that DC was lower and AC was higher in VVS patients. This indicates vagal tone withdrawal and increased sympathetic activity in VVS patients, irrespective of age.
5 Conclusion
The absolute values of AC and DC were found to be higher in patients with VVS compared to the control group. Both AC and DC were identified as independent correlation factors for VVS. These findings suggest that DC and AC have diagnostic significance not only for younger VVS patients, but also for older individuals with VVS.
5.1 Study limitations
This study has several limitations. Firstly, there were only 94 patients with VVS included in our study, and more prospective studies are needed to investigate the association between deceleration and acceleration capacities with VVS patients. Secondly, we did not specifically exclude conditions such as diabetes, hypertension, obesity, and chronic lung disease, which might have an impact on autonomic activity. For instance, cardiovascular autonomic neuropathy (CAN) is an under-recognized yet highly prevalent microvascular complication of diabetes, affecting approximately 20% of those with the condition. However, the enrolled patients were relatively healthy, and few demonstrated the above conditions. Third, further detailed, larger sample, multicenter and longitudinal studies may be required in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Henan Provincial People's Hospital (2018 Ethics Review No. 24). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JW: Data curation, Writing – original draft, Writing – review & editing. JX: Data curation, Writing – original draft. YQ: Methodology, Writing – review & editing. RY: Formal Analysis, Writing – review & editing. WW: Validation, Writing – review & editing. CG: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National key research and development plan “digital medical equipment research and development” pilot project (2018YFC0114502). The funding was not involved in data processing or analysis and writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1495129/full#supplementary-material
Abbreviations
DC, deceleration capacity; AC, acceleration capacity; VVS, vasovagal syncope; HR, heart rate; HRV, HR variability; SDNN, standard deviation of normal-to-normal (NN) intervals; SDANN, standard deviation average of NN intervals; RMSSD, root mean square successive difference of normal R-R intervals; PNN50, the percent of the number of times that the difference between adjacent normal RR intervals >50 ms in the total number of NN intervals; ANS, autonomic nervous system; TTT, tilt-table test; RAd, right atrial diameter; LAd, left atrial diameter; LVEDd, left ventricular end-diastolic diameter; LVESd, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; BMI, body mass index; ROC, receiver operator characteristic; AUC, area under curve.
References
1. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. (2018) 39:1883–948. doi: 10.1093/eurheartj/ehy037
2. Torabi P, Rivasi G, Hamrefors V, Ungar A, Sutton R, Brignole M, et al. Early and late-onset syncope: insight into mechanisms. Eur Heart J. (2022) 43:2116–23. doi: 10.1093/eurheartj/ehac017
3. Longo S, Legramante JM, Rizza S, Federici M. Vasovagal syncope: an overview of pathophysiological mechanisms. Eur J Intern Med. (2023) 112:6–14. doi: 10.1016/j.ejim.2023.03.025
4. Barón-Esquivias G, Cayuela A, Gómez S, Aguilera A, Campos A, Fernández M, et al. [Quality of life in patients with vasovagal syncope. Clinical parameters influence]. Med Clin (Barc). (2003) 121:245–9. doi: 10.1016/S0025-7753(03)75188-4
5. Zheng L, Sun W, Liu S, Liang E, Du Z, Guo J, et al. The diagnostic value of cardiac deceleration capacity in vasovagal syncope. Circ Arrhythm Electrophysiol. (2020) 13:e008659. doi: 10.1161/CIRCEP.120.008659
6. Rose MS, Koshman ML, Spreng S, Sheldon R. The relationship between health-related quality of life and frequency of spells in patients with syncope. J Clin Epidemiol. (2000) 53:1209–16. doi: 10.1016/S0895-4356(00)00257-2
7. Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU, et al. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. (1997) 96:2509–13. doi: 10.1161/01.CIR.96.8.2509
8. Forleo C, Guida P, Iacoviello M, Resta M, Monitillo F, Sorrentino S, et al. Head-up tilt testing for diagnosing vasovagal syncope: a meta-analysis. Int J Cardiol. (2013) 168:27–35. doi: 10.1016/j.ijcard.2012.09.023
9. Sutton R, Fedorowski A, Olshansky B, van Dijk JG, Abe H, Brignole M, et al. Tilt testing remains a valuable asset. Eur Heart J. (2021) 42:1654–60. doi: 10.1093/eurheartj/ehab084
10. Onishi Y, Minoura Y, Chiba Y, Onuki T, Ito H, Adachi T, et al. Daily dysfunction of autonomic regulation based on ambulatory blood pressure monitoring in patients with neurally mediated reflex syncope. Pacing Clin Electrophysiol. (2015) 38:997–1004. doi: 10.1111/pace.12661
11. Huang F, Xu CF, Deng XY, Zuo P, Lin F, Fan JJ, et al. Deceleration capacity-a novel measure for autonomic nervous system in patients with vasovagal syncope on tilt-table testing. J Huazhong Univ Sci Technolog Med Sci. (2017) 37:326–31. doi: 10.1007/s11596-017-1735-7
12. Bauer A, Kantelhardt JW, Barthel P, Schneider R, Mäkikallio T, Ulm K, et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet. (2006) 367:1674–81. doi: 10.1016/S0140-6736(06)68735-7
13. Liu X, Xiang L, Tong G. Predictive values of heart rate variability, deceleration and acceleration capacity of heart rate in post-infarction patients with LVEF ≥35. Ann Noninvasive Electrocardiol. (2020) 25:e12771. doi: 10.1111/anec.12771
14. Zou C, Dong H, Wang F, Gao M, Huang X, Jin J, et al. Heart acceleration and deceleration capacities associated with dilated cardiomyopathy. Eur J Clin Invest. (2016) 46:312–20. doi: 10.1111/eci.12594
15. Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 Heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. (2015) 12:e41–63. doi: 10.1016/j.hrthm.2015.03.029
16. Grégoire JM, Gilon C, Carlier S, Bersini H. Autonomic nervous system assessment using heart rate variability. Acta Cardiol. (2023) 78:648–62. doi: 10.1080/00015385.2023.2177371
17. Yan L, Jin J, Zhao X, Huang X, Zhu W, Jiang S, et al. Heart rate acceleration and deceleration capacities associated with circadian blood pressure variation. Ann Noninvasive Electrocardiol. (2020) 25:e12748. doi: 10.1111/anec.12748
18. Lambert E, Lambert GW. Sympathetic dysfunction in vasovagal syncope and the postural orthostatic tachycardia syndrome. Front Physiol. (2014) 5:280. doi: 10.3389/fphys.2014.00280
19. Márquez MF, Gómez-Flores JR, González-Hermosillo JA, Ruíz-Siller TJ, Cárdenas M. Role of the sympathetic nervous system in vasovagal syncope and rationale for beta-blockers and norepinephrine transporter inhibitors. Medwave. (2016) 16:e6824. doi: 10.5867/medwave.2016.6824
20. Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. (2003) 18:32–9. doi: 10.1007/s003800300005
21. Cai S, Zheng L, Yao Y. Cardioneuroablation for vasovagal syncope alters head-up tilt test response and reduces cardiac deceleration capacity. HeartRhythm Case Rep. (2023) 9:773–8. doi: 10.1016/j.hrcr.2023.08.001
22. Traykov V, Shalganov T. Cardioneuroablation for the treatment of vasovagal syncope: current status and impact on quality of life. Curr Cardiol Rep. (2023) 25:1839–49. doi: 10.1007/s11886-023-01997-1
23. Sutton R. The value of tilt testing and autonomic nervous system assessment. Cardiol Clin. (2015) 33:357–60. doi: 10.1016/j.ccl.2015.04.003
24. Aponte-Becerra L, Novak P. Tilt test: a review. J Clin Neurophysiol. (2021) 38:279–86. doi: 10.1097/WNP.0000000000000625
25. Erdem A, Uenishi M, Küçükdurmaz Z, Matsumoto K, Kato R, Hara M, et al. Cardiac autonomic function measured by heart rate variability and turbulence in pre-hypertensive subjects. Clin Exp Hypertens. (2013) 35:102–7. doi: 10.3109/10641963.2012.690475
26. Kochiadakis GE, Papadimitriou EA, Marketou ME, Chrysostomakis SI, Simantirakis EN, Vardas PE. Autonomic nervous system changes in vasovagal syncope: is there any difference between young and older patients. Pacing Clin Electrophysiol. (2004) 27:1371–7. doi: 10.1111/j.1540-8159.2004.00641.x
Keywords: deceleration capacity, acceleration capacity, vasovagal syncope, autonomic nervous function, syncope
Citation: Wang J, Xu J, Qiu Y, Yang R, Wang W and Gao C (2024) Cardiac deceleration capacity and acceleration capacity have diagnostic value in patients with vasovagal syncope regardless of age. Front. Cardiovasc. Med. 11:1495129. doi: 10.3389/fcvm.2024.1495129
Received: 24 October 2024; Accepted: 9 December 2024;
Published: 18 December 2024.
Edited by:
Robert Sheldon, University of Calgary, CanadaReviewed by:
Jiri Plasek, University Hospital Ostrava, CzechiaMartina Rafanelli, Careggi University Hospital, Italy
Copyright: © 2024 Wang, Xu, Qiu, Yang, Wang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanyu Gao, Z2FvY3k2ODAyMEAxNjMuY29t
 Jijing Wang
Jijing Wang Jinyi Xu1
Jinyi Xu1