- 1Department of Medicine, Cedar-Sinai Medical Center, Los Angeles, CA, United States
- 2Department of Radiation Oncology, Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 3Biostatistics Research Center, Samuel Oschin Comprehensive Cancer Center, Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 4Division of Medical Oncology, Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 5Department of Radiation Oncology, Brigham and Women’s Hospital/Dana-Farber Cancer Institute, Boston, MA, United States
- 6Department of Cardiology, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Osimertinib is first-line treatment for epidermal growth factor (EGFR)-mutated non-small cell lung cancer (NSCLC) and has been associated with cardiotoxicity. However, the nature of cardiac remodeling and associated risk factors remains incompletely understood. Retrospective analysis of NSCLC patients with ≥1 echocardiogram post-osimertinib between 2007 and 2022 was performed. The cumulative incidence of grade ≥2 cardiac common terminology criteria for adverse events (CTCAE) was estimated and Fine and Gray regressions performed (non-cardiac death as competing risk). Eighty-five patients [mean [interquartile range, IQR], 68 [60–75] years; 67% female; 12% with pre-existing heart conditions] met inclusion criteria. With a median follow up of 34.7 months, the 2-year cumulative incidence of grade ≥2 and grade ≥3 cardiac events were 19.2% and 8.5%, respectively. There was an increased risk of grade ≥2 cardiac CTCAE with pre-existing arrhythmia [hazard ratio(HR) 3.90, 95%CI, 1.11–13.72; p = 0.034] and higher body mass index (HR 1.07, 95%CI, 1.00–1.14; p = 0.04). Following osimertinib (vs. baseline), the median QTc was prolonged (451 vs. 437 ms; p < 0.001) and LVEF ≤50% was more common (10.6% vs. 5.3%; p = .046). Osimertinib treatment was associated with QTc prolongation and reduced LVEF. BMI was identified as a potentially modifiable risk factor for osimertinib-associated cardiotoxicity, worthy of further study.
Introduction
Lung cancer mortality has been declining over the past two decades driven in part by advances in systemic therapies (1–3). However, lung cancer survivors face increasing risk of cardiovascular disease (CVD) from both pre-existing risk factors as well as excess risk from cancer therapies. Patients with lung cancer have the highest prevalence of concomitant CVD compared to patients with other malignancies, with more than 40% harboring pre-existing CVD (4). Among lung cancer survivors, CVD is the leading cause of death (5). Further, a number of epidemiologic studies have demonstrated shared risk factors between lung cancer and CVD (4, 6, 7). In addition to these baseline and shared risk factors, lung cancer treatment often includes multi-modality treatment—radiotherapy, cytotoxic chemotherapy, immune checkpoint inhibitors, and/or tyrosine kinase inhibitors—for which treatment-associated cardiac toxicities are observed and associated with pre-existing cardiovascular co-morbidities (8).
For epidermal growth factor (EGFR)-mutated non-small cell lung cancer (NSCLC), osimertinib is an oral, third-generation EGFR tyrosine kinase inhibitor (EGFR-TKI) that selectively inhibits EGFR mutations and T790M resistance mutations, and is the first-line systemic therapy for advanced disease and as adjuvant therapy in patients with resected disease (2, 9, 10). While osimertinib has been shown to improve survival outcomes compared to other systemic therapies (earlier generation TKIs such as erlotinib or gefitinib, or platinum therapy plus pemetrexed, respectively) (9, 11), it has been associated with higher rates of cardiotoxicity compared to earlier generation EGFR-TKIs (9, 12, 13). Indeed, a recent pharmacovigilance study using the FDA Adverse Events Reporting System (FAERS), reported that osimertinib (compared to standard therapies) was associated with higher rates of grade 3 or greater cardiac events such as QTc prolongation, cardiac failure, a decline in left ventricular ejection fraction (LVEF), among others (14). However, given the voluntary nature of the reporting and limited toxicity details, it may not accurately reflect the true incidence and extent of toxicity and additional studies characterizing osimertinib-associated cardiac events are warranted.
Together, the above studies highlight the significance of osimertinib-associated cardiac toxicity and underscore the need for more nuanced understanding of the cardiac remodeling that occurs with such therapy. To this end, we developed a single institution, retrospective cohort with detailed echocardiographic and electrocardiographic profiling in patients treated with osimertinib to investigate rates of cardiovascular toxicity and to identify predictive factors associated with cardiac events and mortality.
Methods
Study design
This was a retrospective analysis of patients with NSCLC treated with osimertinib between 2007 and 2022 at Cedar-Sinai Medical Center (Los Angeles, California). Patients were identified using a natural language search engine, DEEP-6 AI (Pasadena, California), with key terms “lung cancer”, “osimertinib”, and “echocardiogram.” Eligible patients included those with any stage NSCLC treated with osimertinib with at least one echocardiogram available after the initiation of osimertinib (Supplementary Figure 1). This study complied with the prinicples of the Declaration of Helsinki and was approved by the Cedars-Sinai Medical Center institutional review board with a waiver of informed consent.
Cardiovascular events and follow-up
An in-depth manual review of the electronic medical record including past medical history, notes (consultation, follow-up, emergency department visits, and admissions), and diagnostic/imaging reports was used to identify cardiac events. Cardiovascular adverse events were defined according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (15). Event types/groups included heart failure, left ventricular ejection fraction (LVEF) reduction, myocardial infarction, arrhythmia (e.g., supraventricular tachycardia, atrial fibrillation, etc), QTc prolongation, valvular disease, pericardial disease. By CTCAE, grade 2 generally means non-urgent medical intervention required, grade 3 is symptomatic and/or urgent intervention required (i.e., hospitalization), grade 4 is life-threatening consequences or urgent intervention required, while grade 5 is death.
Twelve-lead electrocardiogram (ECG) and echocardiogram data were extracted from patients within 3 years prior to osimertinib administration and at any time after osimertinib was started. In patients with multiple ECGs and echocardiograms, the most recent study before and after osimertinib administration was used for analysis. Echocardiography studies were performed and interpreted by a multitude of observers and the original diagnostic reports were utilized for analysis and not re-interpreted for this study. QTc prolongation, calculated by Bazett formula (16), was defined as >445 ms in men and >460 ms in women. Patients were classified to have left ventricular hypertrophy by ECG if they met either the modified Cornell or Sokolow-Lyon criteria (17, 18). The endpoints of grade ≥2 (or grade ≥3) cardiac events and all-cause mortality were defined as time from the start of osimertinib to the date of the first cardiac CTCAE, or death, whichever occurred first.
Statistical analysis
Comparisons between patients with and without cardiac events were made using analysis of variance or Wilcoxon rank-sum test for continuous variables and chi-square test or Fisher's exact test for categorical variables as appropriate. Data are presented as number of patients (%) for categorical variables and mean [± standard deviation (SD)] or median [interquartile range (IQR)] for continuous variables. Median follow-up was calculated using the reverse Kaplan-Meier method (19). Cumulative incidence estimates of cardiac events were estimated using non-cardiac death as a competing risk. Cumulative incidence estimates of all-cause mortality was calculated as 1—Kaplan Meier method (20). Univariate and multivariable analyses of cardiac events and all-cause mortality were carried out using a Fine-Gray proportional subdistribution hazards regression model (21) and a Cox proportional hazards model (22), respectively. Model assumptions were assessed by examining interaction effects between variables of interest and functions of time and scaled Schoenfeld residuals (23). Multivariable analyses were performed using a stepwise variable selection procedure based on Akaike Information Criterion (24). In the multivariable analysis, multicollinearity was assessed using tolerance and the variance inflation factor. Since hypothesis testing of unique baseline and post-Osimertinib ECG and echocardiogram parameters was limited to simple comparative analysis in this exploratory study, multiple testing correction was not deemed necessary. Analyses were performed using R package version 4.3.0 with two-sided tests at a significance level of 0.05 (25).
Results
Clinical characteristics
Of approximately 500 patients assessed for study eligibility, 85 met inclusion criteria (Supplementary Figure 1). The median age was 68 years [interquartile range (IQR), 60–75 years], 67.1% patients were female (Table 1). The cohort was comprised of 64.7% white and 15.3% Hispanic/Latin(x) individuals. In total, at baseline 49.4% of patients had hypertension, 36.5% with hyperlipidemia, 40.5% with history of tobacco use (only n = 2 current smokers), 16.5% with diabetes mellitus, and 11.8% with coronary heart disease. Most patients (82.1%) had metastatic (Stage IV) disease at the time of starting osimertinib. Osimertinib was used as first-line therapy in 36.2% of patients, as our cohort was treated in eras both prior to and after approval of osimertinib as first line therapy. Most patients (54.9%) were treated with cytotoxic chemotherapy, 25.3% with other EGFR inhibitors, 10.5% with immune checkpoint inhibitors, and 32.5% with thoracic radiotherapy.
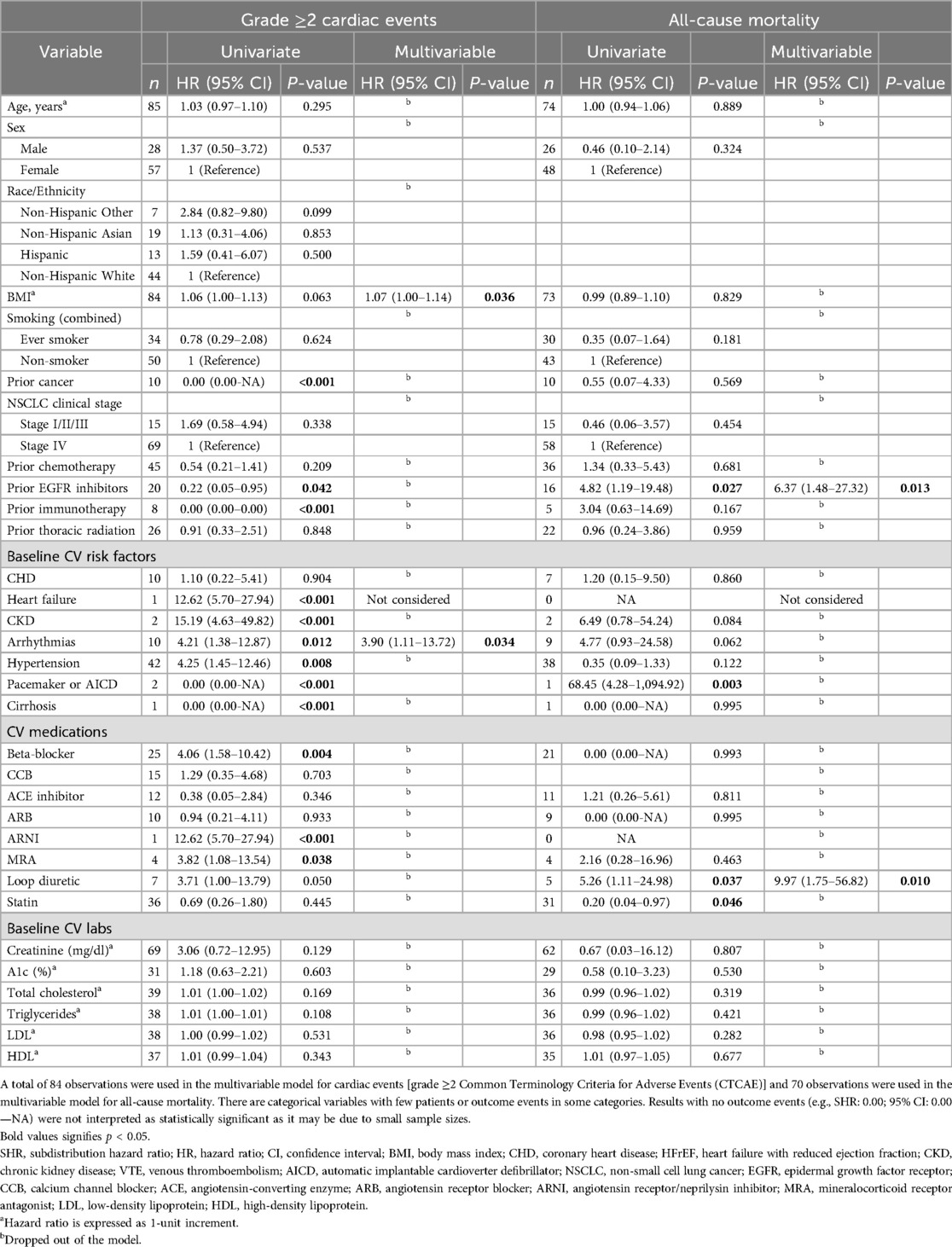
Baseline characteristics were compared between patients who experienced any grade ≥2 cardiac CTCAE (n = 17) vs. those who did not (n = 68; Table 1). Patients with cardiac events were more likely to have chronic kidney disease (CKD) (11.8% vs. 0%; p = 0.038), hypertension (76.5% vs. 42.7%; p = 0.013), and be on a beta-blocker (58.8% vs. 22.1%; p = 0.003) compared to those who did not experience cardiac events. No significant differences were observed between groups in the usage of other CV medications, including statins, angiotensin-converting enzyme (ACE) inhibitors, and mineralocorticoids (p > 0.05).
Analysis of cardiac events
With a median follow-up of 34.7 months (95% CI: 26.2–41.5 months), 17 of 85 patients (20.0%) experienced one or more grade ≥2 cardiac CTCAE with a median time to first event of 24.2 months (IQR: 10.8–42.7 months). The 2-year cumulative incidences of grade ≥2 and grade ≥3 cardiac CTCAE were 19.2% (95% CI: 11%–29%) and 8.5% (95% CI: 3.4%–16.5%), respectively (Figure 1). There were 27 grade ≥2 cardiac CTCAE, including QTc prolongation (n = 10), EF decline from baseline (of ≥5% if symptomatic or ≥10% if asymptomatic) to ≤50% (n = 5), new-onset moderate-to-severe valvular regurgitation or stenosis (n = 5), supraventricular tachycardia requiring medication prescription or hospitalization (SVT, n = 3), and pericardial tamponade (n = 1). Eight patients experienced severe toxicities with six grade 3 [n = 3 QTc prolongation (≥501 ms), n = 2 SVT, n = 1 EF decline to 20%–39% with ≥20% decrease from baseline], and two grade 4 (n = 1 EF decline to <20%, n = 1 pericardial tamponade) CTCAE. To note, the patient with pericardial tamponade did not have fluid cytology analyzed and had Stage IV disease—thus cancer-related effusion was a possible etiology. There were no cardiovascular deaths/grade 5 cardiac CTCAE. On multivariable analysis, there was an increased risk of grade ≥2 cardiac CTCAE with pre-existing arrythmia [subdistribution hazard ratio (SHR) 3.9; 95% CI: 1.1–13.7; p = 0.034] and BMI (SHR 1.07/unit; 95% CI: 1.00–1.14; p = 0.036) (Table 2). There was no impact of race/ethnicity on the risk of cardiac events (p > 0.05).
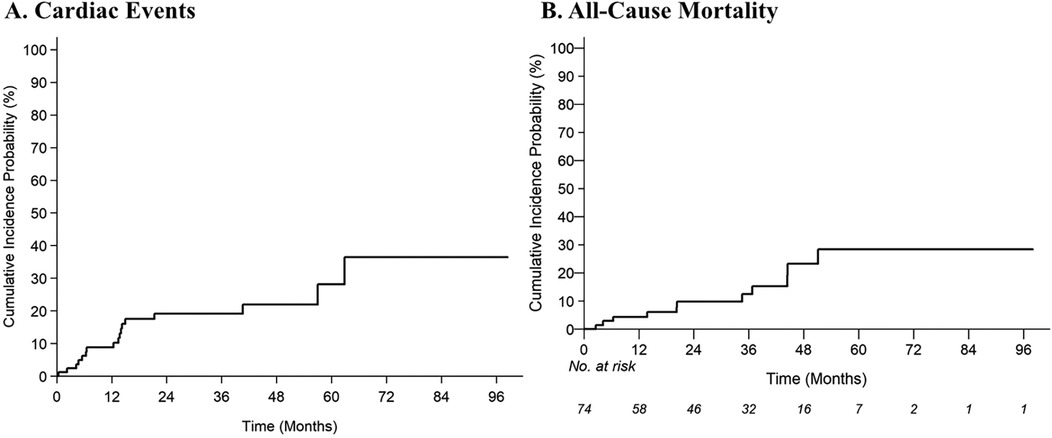
Figure 1. Cumulative incidence estimates of (A) grade ≥2 cardiac common terminology criteria for adverse events (CTCAE), and (B) all-cause mortality.
Analysis of all-cause mortality
With a median follow-up of 34.7 months, 13% of patients died (8% of lung cancer and 5% of known non-cardiac cause). The 2-year estimate of all-cause mortality was 9.8% (95% CI: 3.91–18.98). On multivariable analysis, prior EGFR inhibitors (HR 6.37; 95% CI: 1.48–27.32; p = 0.013) and use of loop diuretics (HR 9.97; 95% CI: 1.75–56.82; p = 0.010) were associated with an increased risk of all-cause mortality (Table 2).
Dynamic electrocardiogram and echocardiographic changes
There were 64 patients with ECGs prior to osimertinib and 73 patients with ECGs after osimertinib initiation (Table 3). The most common ECG change that occurred with Osimertinib treatment was QTc prolongation. The average QTc length was significantly prolonged from 437 (IQR, 422–450 ms) to 451 (IQR, 432–474) ms after osimertinib administration (p < 0.001). Grade 3 QTc prolongation (≥501 ms) occurred in three patients, grade 2 QTc prolongation (481–500 ms) occurred in seven patients, and grade 1 QTc prolongation (450–480 ms) occurred in 18 patients. No statistically significance changes were observed in other ECG parameters, including rhythm changes or conduction abnormalities.
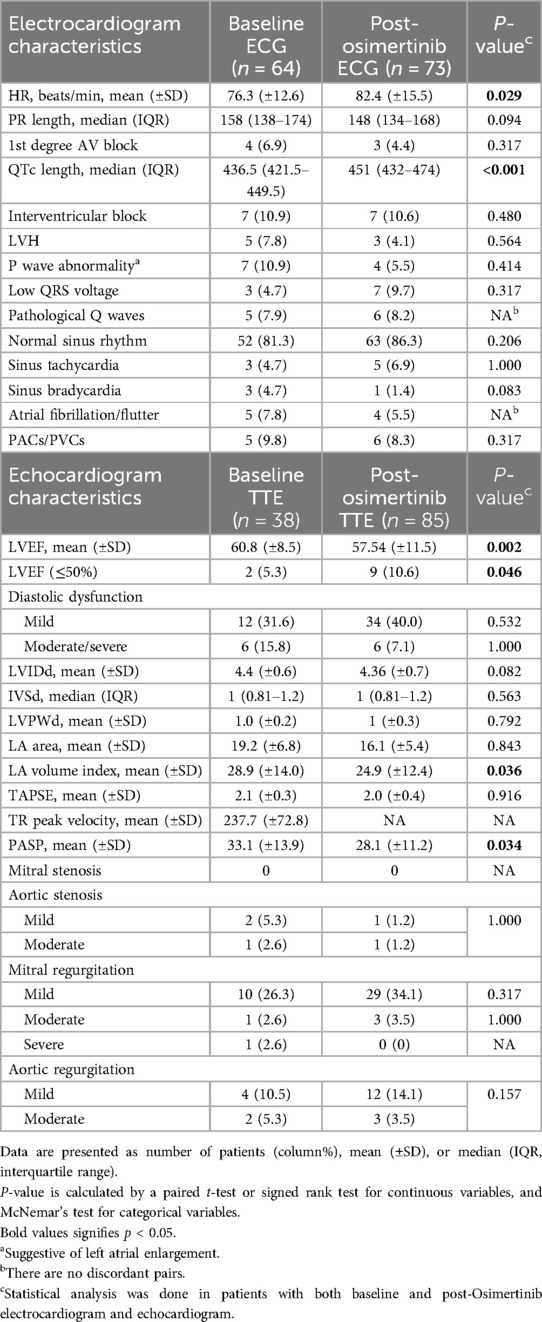
Table 3. Electrocardiogram and echocardiogram characteristics at baseline and post-osimertinib therapy.
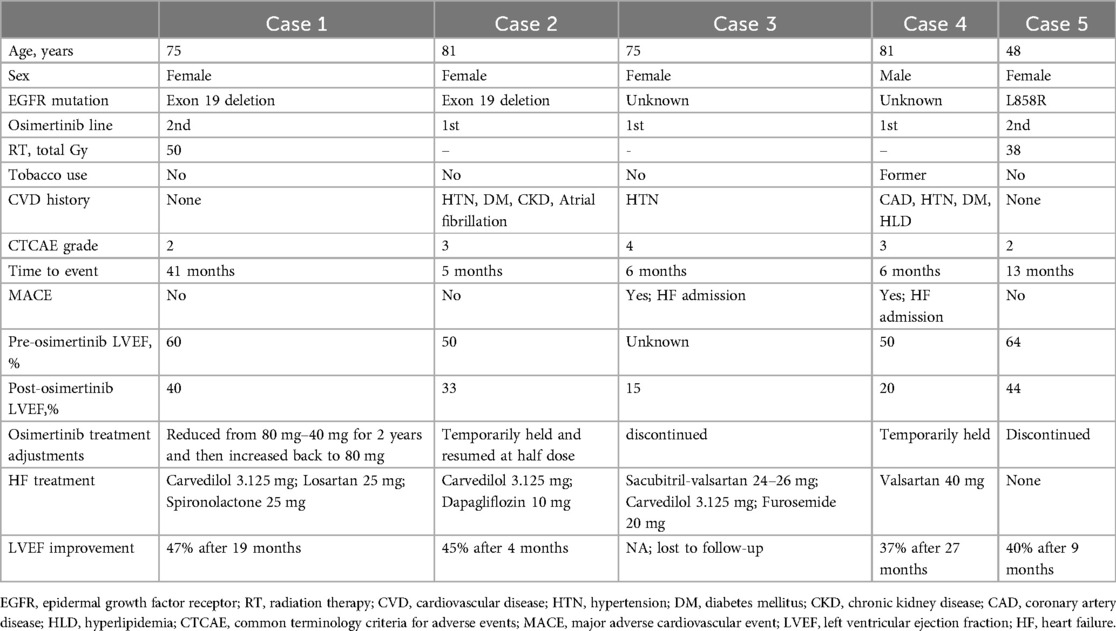
Among 85 patients with at least one echocardiogram after osimertinib initiation, 38 had at least one echocardiogram prior to treatment (Table 3). The median time between the start of osimertinib and follow-up echocardiogram completion was 17.6 months (95% CI: 13.7–21.4). When comparing post- vs. pre-osimertinib echocardiographic variables, there was a significant decline in LVEF from 60.8 ± 8% vs. 57.5 ± 11% (p = 0.002), and in particular—LVEF ≤50% was observed with increased frequency following osimertinib treatment (10.6% vs. 5.3% of patients; p = .046). Grade 4 LV dysfunction (LVEF reduction to <20%) occurred in one patient, grade 3 LV dysfunction (LVEF decline to 20%–39% with ≥20% decrease from baseline) occurred in one patient, and grade 2 LV dysfunction (LVEF decline by ≥10% from baseline to 40%–50%) occurred in three patients. The five patients with osimertinib-associated left ventricular dysfunction are described Table 4. Of these five patients, three had some degree of recovery but had persistently low LVEF (case #1, 2, and 4), while one patient had worsening of LVEF despite discontinuation of osimertinib (case #5), and one patient was lost to follow-up (case #3). In addition, post-osimertinib left atrial (LA) area was significantly increased in patients with cardiac events vs. those without cardiac events (18.8 ± 5.0 cm2 vs. 15.3 ± 5.3 cm2, p = 0.029, Supplementary Table 1). Grade 2 valvular disease occurred in five (6%) patients in the cohort: three patients developed moderate aortic regurgitation, one developed moderate mitral regurgitation, and one developed moderate tricuspid regurgitation. No significant differences were observed in other echocardiographic parameters.
Discussion
In this retrospective single-center study of 85 patients with NSCLC treated with osimertinib and who received at least one echocardiogram following osimertinib initiation, we made the following observations: (1) this patient cohort is at very high risk of CV events with 2-year cumulative incidence of grade ≥2 CV CTCAE of 19% and grade ≥3 CV CTCAE of 8.5%; (2) The most common CV adverse events with osimertinib therapy were QTc prolongation, LV dysfunction (with or without heart failure symptoms), progressive valvular disease, and SVTs; (3) there appears to be a latency in the manifestation of CV events with a median time to first event of 24.2 months (IQR: 10.8–42.7 months); and (4) pre-existing arrythmia and BMI were identified as risk factors for cardiac events following osimertinib treatment. A strength of this study is the availability of serial electrocardiogram and echocardiographic analysis in one of the largest NSCLC cohorts that builds upon recent studies (14, 26, 27) Additionally, BMI was identified as a modifiable risk factor for osimertinib-associated CV toxicity, and validated in further studies, represents an opportunity for risk mitigation in patients who receive osimertinib.
Several issues warrant further discussion. First, our observation of a nearly 20% 2-year cumulative incidence of grade ≥2 cardiac CTCAE rate is higher than several recent reports. A single-center retrospective cohort study from Japan reported 5% incidence of grade 3 or higher CTCAE (26). In the prior study, the number of patients who had echocardiograms was small (n = 36) and the baseline cardiovascular risk profile vastly differed from the US population, where cardiovascular co-morbidities are significantly more prevalent. Indeed, our study observed a higher prevalence of hypertension, diabetes, and prior/current smoking use in the US cohort, which might have contributed to the increased rate of osimertinib-associated cardiotoxicity. Further, a retrospective analysis on the FDA Adverse Events Reporting System (FAERS), a pharmacovigilance database, showed that osimertinib (vs. standard therapies) was associated with higher rates of grade ≥3 cardiac events such as QTc prolongation, cardiac failure, a decline in left ventricular ejection fraction (LVEF), among others (14). However, this study has several limitations, including heterogeneity in individual reporting and details of the adverse events (e.g., grade of toxicity), as well as unknown baseline characteristics. In addition, because FAERS is a voluntary reporting system, it likely is an inaccurate estimate of the true incidence of cardiac events.
LV dysfunction is well-described toxicity of osimertinib therapy and is similarly observed in the current study with incidence of 6% among those patients who have had echocardiogram performed post-osimertinib initiation (12–14.) The characterization of the true prevalence of LV dysfunction in osimertinib treated patients is limited by the inconsistent echocardiographic monitoring practices across centers. A small single-center series of 17 patients with LV dysfunction suspected to be causally linked to osimertinib demonstrated that only in half of the cases was echocardiogram performed as part of screening strategy and the majority of echocaridograms were performed due to symptoms (28). Similalry, this inconsistent echocardiographic monitoring limits our understanding of the timing of onset of toxicities, so that monitoring is implemented at the most vulnerable window. In our cohort of patients, of the five cases of LV dysfunction observed, three had some degree of improvement in their LVEF on subsequent echocardiograms after initiation of guideline-directed medical therapy (Table 4).
Similar to other reports (12–14), the most common ECG change observed in the current study following osimertinib therapy was QTc prolongation (median 14 ms). Importantly, however, clinically significant polymorphic ventricular arrhythmias are infrequently witnessed until the QTc interval exceeds 500 ms (29). The majority of observed events (25/28) were grade 1 (450–480 ms) and 2 (481–500 ms), while three patients developed grade 3 QTc prolongation (>500 ms)—though there were no episodes of torsade de pointes, polymorphic ventricular tachycardia, or other clinical consequence of QTc prolongation. However, several case reports have described osimertinib-induced ventricular arrhythmias (30–32.) Thus, regular clinical monitoring should be performed, including ECG surveillance—particularly if other QTc prolonging drugs are used (anti-emetics, antibiotics), correcting electrolyte imbalances, and discontinuing other unnecessary QTc prolongaing medications. Further, temporary drug interruption and resumption at a lower daily dose is recommended in patients who develop grade 3 QTc prolongation (33).
While the precise mechanism of osimertinib-associated cardiotoxicity has yet to be elucidated, in vitro studies have shown that osimertinib can inhibit human epidermal growth factor receptor 2 (HER2) (34), which is important in maintaining cardiac function (35). Given that anti-HER2 therapies (such as trastuzumab) are also associated with cardiac toxicity (36), this suggests that off target effects may potentially contribute to the enhanced toxicity profile of this drug. Further, given the increasing use of osimertinib in the curative setting, there is a growing need for improved baseline CV risk stratification, early cardiac event detection, and adequate surveillance of cardiotoxicity. In fact, the comparable rates of LV dysfunction observed with osimertinib vs. HER2 antagonists (37–41) calls for diligent echocaridograhic monitoring in osimertinib-treated patients with protocols that mirror those of HER2 therapies. In recognition of this toxicity, the European Society of Cardiology Cardio-Oncology guidelines recommend baseline echocardiogram in patients prior to starting osimertinib (Class I, Level B evidence) and consideration of performing echocardiograms every 3 months while patients are maintained on osimertinib (Class IIA, Level B evidence). Our studies and others are a call for action in the oncology and cardio-oncology community—namely, for a call for more systematic monitoring for LV dysfunction, valvular disease progression, and QTc prolongation for patients on osimertinib therapy, particularly as we enter an era of increased osimertinib use as curative-intent therapy in patients with non-metastatic disease.
This study has several limitations. Given its retrospective nature, this could under- (or over-) estimate the true incidence of cardiac dysfunction, including in patients with limited follow-up due to competing risk or medical care received locally. In addition, being a tertiary referral center where more medically complex patients are evaluated, could also over-estimate the incidence of cardiac dysfunction. Further, this cohort is a subset that underwent echocardiograms, which may impart a selection bias as these patients may be at higher CV risk than those that did not, particularly in the retrospective setting. There were fewer echocardiograms available prior to osimertinib compared to during treatment, which may lead to overestimation of changes in echocardiographic metrics. Similarly, given the variable timing of echocardiograms across the cohort, the most recent study was analyzed—though this may not fully capture dynamic changes over time. Additionally, while prior thoracic radiotherapy was not associated with cardiac event risk in this study, the impact of cardiac substructure radiation dose exposure could be a contriobuting factor (42), which is not fully captured here given the small numbers of patients treated with RT and small event numbers. Similarly, given the high baseline cardiovascular risk and exposure to multiple potential cardio-toxic cancer therapies, the observed cardiac events may not be solely attributed to Osimertinib and may reflect several contributing risk factors. Further, given the limited sample size, the impact of concurrent cardiovascular medical therapies could not be fully assessed. Lastly, as this was a sample size of convenience, the treatment years spanned 15 years and may not be fully generalizable to a modern treatment cohort.
Together, these findings underscore the importance of early referral to cardiology or cardio-oncology for patients who develop osimertinib-associated cardiotoxicity. Additionally, in our study there was a significant increase in LA area and a trend toward increased left atrial volume index (LAVI) post-osimertinib among patients with cardiac events (vs. no cardiac events) (Supplementary Table 1). These remodeling changes may predispose patients to developing arrhythmias, such as atrial fibrillation—which has been associated with the osimertinib cardiac toxicity spectrum (14). Longitudinal studies of longer duration will be needed to characterize the long-term sequelae of treatment with Osimertinib.
Conclusion
In this retrospective cohort study, cardiac events were common after Osimertinib treatment, with nearly a 20% 2-year cumulative incidence of grade ≥2 cardiac CTCAE and 9% 2-year cumulative incidence of grade ≥3 cardiac CTCAE. Importantly, several electrocardiogram and echocardiographic changes were observed, including QTc prolongation and reduced LVEF. BMI was identified as a potentially modifiable risk factor for Osimertinib-associated cardiac events, worthy of further study. These findings highlight the need for optimized risk mitigation approaches following Osimertinib treatment, including identifying patients who might benefit from intensified surveillance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Cedar Sinai Medical Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this was a retrospective study.
Author contributions
JL: Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. JG: Data curation, Methodology, Writing – original draft, Writing – review & editing. OP: Writing – original draft, Writing – review & editing, Data curation. AS: Data curation, Writing – original draft, Writing – review & editing. KS: Data curation, Writing – original draft, Writing – review & editing. SK: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. AN: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. MK: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. AM: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. BH: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. KR: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. KS: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. RM: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. AN: Conceptualization, Formal Analysis, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. KA: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. KA reports funding from the Garber Award for Cancer Research.
Conflict of interest
MK reported receiving personal fees from Theragenics, Alessa, GTMedical, and Springer, outside the submitted work. RM reports consulting with AstraZeneca, ViewRay, Novartis, Sio Capital Mgmt, Varian Medical Systems; advisory board with ViewRay, AstraZeneca; grant funding from AstraZeneca, ViewRay. KA reported receiving honoraria from OncLive outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1485033/full#supplementary-material
References
1. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
2. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. (2020) 383:1711–23. doi: 10.1056/NEJMoa2027071
3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
4. Al-Kindi SG, Oliveira GH. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo Clin Proc. (2016) 91:81–3. doi: 10.1016/j.mayocp.2015.09.009
5. Abdel-Rahman O. Risk of cardiac death among cancer survivors in the United States: a SEER database analysis. Expert Rev Anticancer Ther. (2017) 17:873–8. doi: 10.1080/14737140.2017.1344099
6. Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, et al. Ideal cardiovascular health is inversely associated with incident cancer: the atherosclerosis risk in communities study. Circulation. (2013) 127:1270–5. doi: 10.1161/CIRCULATIONAHA.112.001183
7. Pursnani A, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Guideline-Based statin eligibility, cancer events, and noncardiovascular mortality in the framingham heart study. J Clin Oncol. (2017) 35:2927–33. doi: 10.1200/JCO.2016.71.3594
8. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: eSMO consensus recommendations. Ann Oncol. (2020) 31:171–90. doi: 10.1016/j.annonc.2019.10.023
9. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
10. Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohé C, et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med. (2023) 389:137–47. doi: 10.1056/NEJMoa2304594
11. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
12. Ewer MS, Tekumalla SH, Walding A, Atuah KN. Cardiac safety of osimertinib: a review of data. J Clin Oncol. (2021) 39:328–37. doi: 10.1200/JCO.20.01171
13. Thein KZ, Swarup S, Ball S, Quirch M, Vorakunthada Y, Htwe KK, et al. Incidence of cardiac toxicities in patients with advanced non-small cell lung cancer treated with osimertinib: a combined analysis of two phase III randomized controlled trials. Ann Oncol. (2018) 29(Suppl 8):viii500. doi: 10.1093/annonc/mdy292.011
14. Anand K, Ensor J, Trachtenberg B, Bernicker EH. Osimertinib-induced cardiotoxicity: a retrospective review of the FDA adverse events reporting system (FAERS). JACC CardioOncol. (2019) 1:172–8. doi: 10.1016/j.jaccao.2019.10.006
15. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Bethesda, MD: National Institutes of Health (2017). Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
16. Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. (1997) 2:177–94. doi: 10.1111/j.1542-474X.1997.tb00325.x
17. Molloy TJ, Okin PM, Devereux RB., Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. (1992) 20:1180–6. doi: 10.1016/0735-1097(92)90376-X
18. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. (1949) 37:161–86. doi: 10.1016/0002-8703(49)90562-1
19. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. (1996) 17:343–6. doi: 10.1016/0197-2456(96)00075-X
20. Kalbfleisch JD, Prentice RL. Statistical Analysis of Failure Time Data. Hoboken, NJ: Wiley & Sons, Incorporated (2011).
21. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. (1999) 94:496–509. doi: 10.1080/01621459.1999.10474144
22. Cox DR. Regression models and life-tables. J R Stat Soc. (1972) 34:187–202. doi: 10.1111/j.2517-6161.1972.tb00899.x
23. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. (1994) 81:515–26. doi: 10.1093/biomet/81.3.515
24. Yamashita T, Yamashita K, Kamimura R. A stepwise AIC method for variable selection in linear regression. Commun Stat Theory Methods. (2007) 36:2395–403. doi: 10.1080/03610920701215639
25. Core Team R. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2023).
26. Kunimasa K, Kamada R, Oka T, Oboshi M, Kimura M, Inoue T, et al. Cardiac adverse events in EGFR-mutated non-small cell lung cancer treated with osimertinib. JACC CardioOncol. (2020) 2:1–10. doi: 10.1016/j.jaccao.2020.02.003
27. Kobat H, Elkonaissi I, Foreman E, O’Brien M, Dorak MT, Nabhani-Gebara S. Investigating the efficacy of osimertinib and crizotinib in phase 3 clinical trials on anti-cancer treatment-induced cardiotoxicity: are real-world studies the way forward? J Oncol Pharm Pract. (2023) 29:646–62. doi: 10.1177/10781552221077417
28. Franquiz MJ, Waliany S, Xu AY, Hnatiuk A, Wu SM, Cheng P, et al. Osimertinib-associated cardiomyopathy in patients with non-small cell lung cancer. JACC CardioOncol. (2023) 5:839–41. doi: 10.1016/j.jaccao.2023.07.006
29. Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. (2013) 88:315–25. doi: 10.1016/j.mayocp.2013.01.013
30. Ikebe S, Amiya R, Minami S, Ihara S, Higuchi Y, Komuta K. Osimertinib-induced cardiac failure with QT prolongation and torsade de pointes in a patient with advanced pulmonary adenocarcinoma. Int Canc Conf J. (2021) 10:68–71. doi: 10.1007/s13691-020-00450-2
31. Kondo M, Kisanuki M, Kokawa Y, Gohara S, Kawano O, Kagiyama S, et al. Case report: QT prolongation and abortive sudden death observed in an 85-year-old female patient with advanced lung cancer treated with tyrosine kinase inhibitor osimertinib. Front Cardiovasc Med. (2021) 8:655808. doi: 10.3389/fcvm.2021.655808
32. Luo J, Zhou B, Yang J, Qian H, Zhao Y, She F, et al. Recurrent ventricular arrhythmias and heart failure induced by osimertinib- a case report. Front Cardiovasc Med. (2024) 11:1423647. doi: 10.3389/fcvm.2024.1423647
33. Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
34. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. (2014) 4:1046–61. doi: 10.1158/2159-8290.CD-14-0337
35. Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. (2007) 116:954–60. doi: 10.1161/CIRCULATIONAHA.107.690487
36. Florido R, Smith KL, Cuomo KK, Russell SD. Cardiotoxicity from human epidermal growth factor receptor-2 (HER2) targeted therapies. J Am Heart Assoc. (2017) 6(9):e006915. doi: 10.1161/JAHA.117.006915
37. Gonciar D, Mocan L, Zlibut A, Mocan T, Agoston-Coldea L. Cardiotoxicity in HER2-positive breast cancer patients. Heart Fail Rev. (2021) 26:919–35. doi: 10.1007/s10741-020-10072-8
38. Castillo C, Camejo N, Etcheverria C, Ferradaz J, Ferreira A, Fontan A, et al. Trastuzumab-induced cardiotoxicity in early breast cancer over a 10-year period in Uruguay. Medicine (Baltimore). (2022) 101:e29927. doi: 10.1097/MD.0000000000029927
39. Alowais SA, Luk SO, Kim EB, Alsuhebany N, Zangardi M. Late-onset cardiotoxicity in patients with HER2-positive metastatic breast cancer receiving trastuzumab-based therapy. J Oncol Pharm Pract. (2024) 30(6):992–8. doi: 10.1177/10781552231193149
40. Seferina SC, Boer M, Derksen MW, Berkmortel F, Kampen RJ, Wouw AJ, et al. Cardiotoxicity and cardiac monitoring during adjuvant trastuzumab in daily Dutch practice: a study of the southeast Netherlands breast cancer consortium. Oncologist. (2016) 21:555–62. doi: 10.1634/theoncologist.2015-0230
41. Tarantini L, Cioffi G, Gori S, Tuccia F, Boccardi L, Bovelli D, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Card Fail. (2012) 18:113–9. doi: 10.1016/j.cardfail.2011.10.015
42. Atkins KM, Chaunzwa TL, Lamba N, Bitterman DS, Rawal B, Bredfeldt J, et al. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol. (2021) 7:206–19. doi: 10.1001/jamaoncol.2020.6332
Keywords: osimertinib, lung cancer, cardiac toxicity, echocardiogram, EGFR
Citation: Le JN, Gasho JO, Peony O, Singh A, Silos KD, Kim S, Nguyen AT, Kamrava M, Mirhadi A, Hakimian B, Reckamp KL, Sankar K, Mak RH, Nikolova AP and Atkins KM (2024) Cardiac events and dynamic echocardiographic and electrocardiogram changes following osimertinib treatment in lung cancer. Front. Cardiovasc. Med. 11:1485033. doi: 10.3389/fcvm.2024.1485033
Received: 23 August 2024; Accepted: 6 December 2024;
Published: 16 December 2024.
Edited by:
Jose Luis Ramirez-GarciaLuna, McGill University Health Centre, CanadaCopyright: © 2024 Le, Gasho, Peony, Singh, Silos, Kim, Nguyen, Kamrava, Mirhadi, Hakimian, Reckamp, Sankar, Mak, Nikolova and Atkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katelyn M. Atkins, a2F0ZWx5bi5hdGtpbnNAY3Nocy5vcmc=
†These authors have contributed equally to this work
 Jonathan N. Le
Jonathan N. Le Jordan O. Gasho
Jordan O. Gasho Olivia Peony2
Olivia Peony2 Sungjin Kim
Sungjin Kim Mitchell Kamrava
Mitchell Kamrava Karen L. Reckamp
Karen L. Reckamp Kamya Sankar
Kamya Sankar Katelyn M. Atkins
Katelyn M. Atkins