- 1Department of Nuclear Medicine, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 2Department of Ultrasonography, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 3Medical Records Statistics Room, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Purpose: Left ventricular ejection fraction (LVEF) strongly predicts cardiac events. However, conflicting findings exist regarding the prognostic value of the LVEF reserve (ΔLVEF) when measured by gated single-photon emission computed tomography myocardial perfusion imaging (SPECT G-MPI). In particular, data related to the prognostic value of ΔLVEF when measured by SPECT in patients with reduced LVEF are scarce. In this study, we aimed to evaluate the prognostic value of ΔLVEF when acquired by SPECT G-MPI in patients with coronary artery disease (CAD) and a LVEFStress < 60%.
Methods: We retrospectively recruited 260 consecutive patients diagnosed with CAD by coronary angiography (CAG) and a LVEFStress < 60%, as determined by SPECT G-MPI. These patients were followed up for 33.4 ± 7.6 months. The patients were divided into two groups (ΔLVEF > 0% and ΔLVEF ≤ 0%), and survival analyses were conducted. The primary endpoints were major adverse cardiac events (MACEs), a composite of all-cause death, nonfatal myocardial infarction, unplanned coronary revascularization, and hospitalization for unstable angina.
Results: We observed 69 MACEs (26.5%). The cumulative incidence of MACEs in patients with ΔLVEF ≤ 0% was significantly higher than in patients with ΔLVEF > 0% (P = 0.042). Multivariate Cox regression further revealed that a ΔLVEF ≤ 0% represented an independent predictor of MACEs (adjusted hazard ratio [HR]: 1.276; 95% confidence interval [CI]: (1.006, 1.618), P = 0.045). Adding a ΔLVEF ≤ 0% to traditional myocardial perfusion and function variables evaluated by MPI significantly improved the ability to predict MACEs (P = 0.044).
Conclusions: Determining ΔLVEF by SPECT G-MPI was associated with MACEs and improved risk stratification compared to prediction models based on traditional perfusion and functional parameters in CAD patients with left ventricular dysfunction, particularly those with no or mild myocardial ischemia.
1 Introduction
Coronary artery disease (CAD) is associated with high morbidity and mortality rates worldwide (1). Prognostic assessment is critical when deciding to treat patients with CAD and formulating prevention strategies. The main method used for the stratification of risk among patients with CAD is the evaluation of stress-induced myocardial ischemia, often by single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) (2). Essentially, gated MPI (G-MPI) enables the simultaneous assessment of the distribution of myocardial perfusion and cardiac function. Risk stratification can be enhanced by applying multiple parameters acquired by G-MPI, including myocardial perfusion data and functional information. A range of key factors, such as enlarged ventricular volume, the presence of transient ischemic dilatation (TID), and, in particular, reduced left ventricular ejection fraction (LVEF), have been identified as independent risk factors for adverse outcomes in patients with CAD (3).
LVEF is the preferred variable for evaluating LV systolic function (4). Furthermore, a reduction in LVEF reserve (ΔLVEF), defined as LVEFStress minus LVEFRest (5), has been associated with ischemic contractile dysfunction (6, 7). Previous studies utilizing 82Rb positron emission tomography (PET) myocardial perfusion imaging demonstrated that ΔLVEF represented an independent predictor of major adverse cardiac events (MACEs) (5, 8). Nevertheless, the prognostic value of ΔLVEF, as measured by SPECT G-MPI (9–11) has yet to be fully evaluated. Besides, most studies did not specifically focus on patients with cardiac dysfunction (10–12). Furthermore, the incremental prognostic value of an abnormal ΔLVEF in patients with reduced LVEFStress has yet to be investigated. In addition, research has shown that the extent and severity of myocardial ischemia can both influence the prognosis and a large area of ischemia (>10%/LV) is considered to be a key indicator of revascularization for patients with CAD (13). There is a significant paucity of data relating to the prognostic value of ΔLVEF in patients with varying degrees of myocardial ischemia, especially in patients with no or mild myocardial ischemia.
Therefore, this study aimed to evaluate the prognostic value of ΔLVEF, as determined by SPECT G-MPI, in patients diagnosed with CAD and in a high-risk group of patients with left ventricular dysfunction (LVEFStress < 60%). In addition, we analysed the prognostic value of ΔLVEF in patients with different degrees of myocardial ischemia.
2 Methods
2.1 Study population
Between October 2016 and December 2019, we retrospectively screened the medical records of all consecutive patients attending Anzhen Hospital for suspected CAD and who had undergone stress-rest SPECT G-MPI and a subsequent invasive coronary angiogram (CAG).
The British Society of Echocardiography recently defined the normal reference interval for LVEF as ≥ 55% (14). Reference values of LVEF are unlikely to be universally applicable across different imaging modalities and may vary among ethnic groups. According to our recent study (15), we treated a LVEFStress < 60% on SPECT G-MPI as indicative of impaired left ventricle systolic function.
Patients were included if they had: (1) a LVEFStress < 60% on SPECT G-MPI, (2) underwent invasive CAG within three months of SPECT G-MPI, and (3) had significant stenosis of the left main coronary artery and/or stenosis of at least one major coronary artery. The ethics committee of Anzhen Hospital approved the study protocol.
2.2 Coronary angiography
CAG was performed using either the femoral or radial approach using the standard Judkins method. Two experienced interventional cardiologists blinded to the study's objective and design performed an analysis of the Arteriography. Significant stenosis was defined as luminal narrowing ≥50% in the left main coronary artery and/or ≥70% in the major epicardial coronary arteries. Stenosis in the left main stem was defined as a two-vessel disease. Decisions relating to revascularization, as well as the choice of revascularization method, were made at the discretion of the cardiologist.
2.3 SPECT G-MPI
All patients underwent SPECT G-MPI following the two-day stress/rest protocol described in our previous study (16). Stress was induced by physical exertion on an ergometer bicycle or by pharmacological intervention with adenosine. In this protocol, 99mTc-sestamibi (radiochemical purity > 95%, injected dose of 740–925 MBq) was administered intravenously at peak stress. Perfusion images were captured over 8 min using a dual-headed Siemens Camera (Siemens Symbia Intevo 16 Systems) with a multifocal (SMART ZOOM) collimator. Images were reconstructed using flash 3D mode and displayed as horizontal short-axis and vertical long-axis slices.
A 17-segment model was applied by two experienced physicians who were unaware of the clinical data (17). Next, the total perfusion defect (TPD), which represents the total extent of reversible (ischemia) and fixed (scar) defects, was quantified and expressed as a percentage of the involved left ventricle.
Quantitative ECG-gated SPECT was analysed by QGS software (Cedars Sinai Medical Center, Los Angeles, CA, USA). The LVEF, end-systolic volume (ESV), and end-diastolic volume (EDV) were calculated post-stress and at rest. Subsequently, we calculated ΔLVESV (ΔLVESV = LVESVStress - LVESVRest), ΔLVEDV (ΔLVEDV = LVEDVStress - LVEDVRest), and ΔLVEF (ΔLVEF = LVEFStress - LVEFRest). As reported previously, an abnormal LVEF reserve was defined as ΔLVEF ≤ 0% (11, 18–20). TID was described as a stress/rest left ventricle volume ratio ≥ 1.2 (21), including EDV and ESV (TIDEDV and TIDESV).
2.4 Follow-up
Follow-up was performed by consulting the electronic medical record system in the hospital and by contacting patients or their relatives by telephone. The primary outcome was the occurrence of MACEs, including all-cause death, nonfatal myocardial infarction, unplanned coronary revascularization, and hospitalization for unstable angina (22). Patients were censored after the first event or at the end of the follow-up period. During the follow-up period, unplanned coronary revascularization is defined as any unexpected coronary revascularization, including percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG). We identified a diagnosis of unstable angina according to the ESC guidelines (13), and an expert was consulted when uncertain of a diagnosis.
2.5 Statistical analysis
Normally distributed continuous variables are presented as mean ± standard division, while non-normally distributed continuous variables are presented as median and interquartile range (Q1 to Q3). Categorical variables are presented as numbers (%). For all continuous variables, means were evaluated by the unpaired t-test or the Mann-Whitney U-test. Categorical variables were compared between groups using the chi-squared test or Fisher's exact test, as appropriate.
The cumulative incidence of MACEs was estimated using the Kaplan-Meier method and compared using the log-rank test. Landmark analyses were performed using a landmark point of 2 year and beyond 2 years. Independent prognostic factors associated with MACEs were determined by univariate and multivariate Cox regression, performed stepwise backward. The ΔLVEF ≤ 0% was incorporated as a time-varying covariate in Cox models. All variables were first assessed by univariate Cox proportional hazards regression analysis. Only variables with a statistically significant association with the cumulative incidence of MACEs (P < 0.05) were included in the multivariate model. Results are presented as hazard ratios (HRs) and 95% confidence intervals (95% CIs). In addition, we evaluated the incremental prognostic value of predicting MACEs by MPI results and LVEF reserve in comparison baseline, including age, sex and body mass index (BMI), based on calculated global χ2 values. P < 0.05 was defined as statically significant. All data were analysed using SPSS version 26 for Windows (IBM SPSS Statistics 26; NY, USA).
3 Results
3.1 Baseline clinical characteristics
A total of 8,844 consecutive patients with known or suspected CAD who underwent SPECT G-MPI were preliminarily enrolled. Among these patients, only 641 underwent invasive coronary angiography within three months. Moreover, the gated data of 92 patients was unavailable, and no significant stenosis was found in 141 patients. Additionally, from the 408 patients who were eligible for analysis, we excluded 148 patients for one of the following reasons: (1) LVEFStress ≥ 60% on SPECT G-MPI (n = 86), (2) acute myocardial infarction (MI) (< 8 weeks, n = 6), and (3) rheumatic valvar disease (n = 14). In addition, 42 patients (10%) were lost during follow-up. Thus, 260 consecutive patients were finally enrolled in the final analysis (Figure 1).

Figure 1. Flow diagram showing initial selection of cohort and excluded patients. CAD, coronary artery disease; SPECT G-MPI, gated single-photon emission computed tomography myocardial perfusion imaging; CAG, coronary angiography; LVEF, left ventricular ejection fraction.
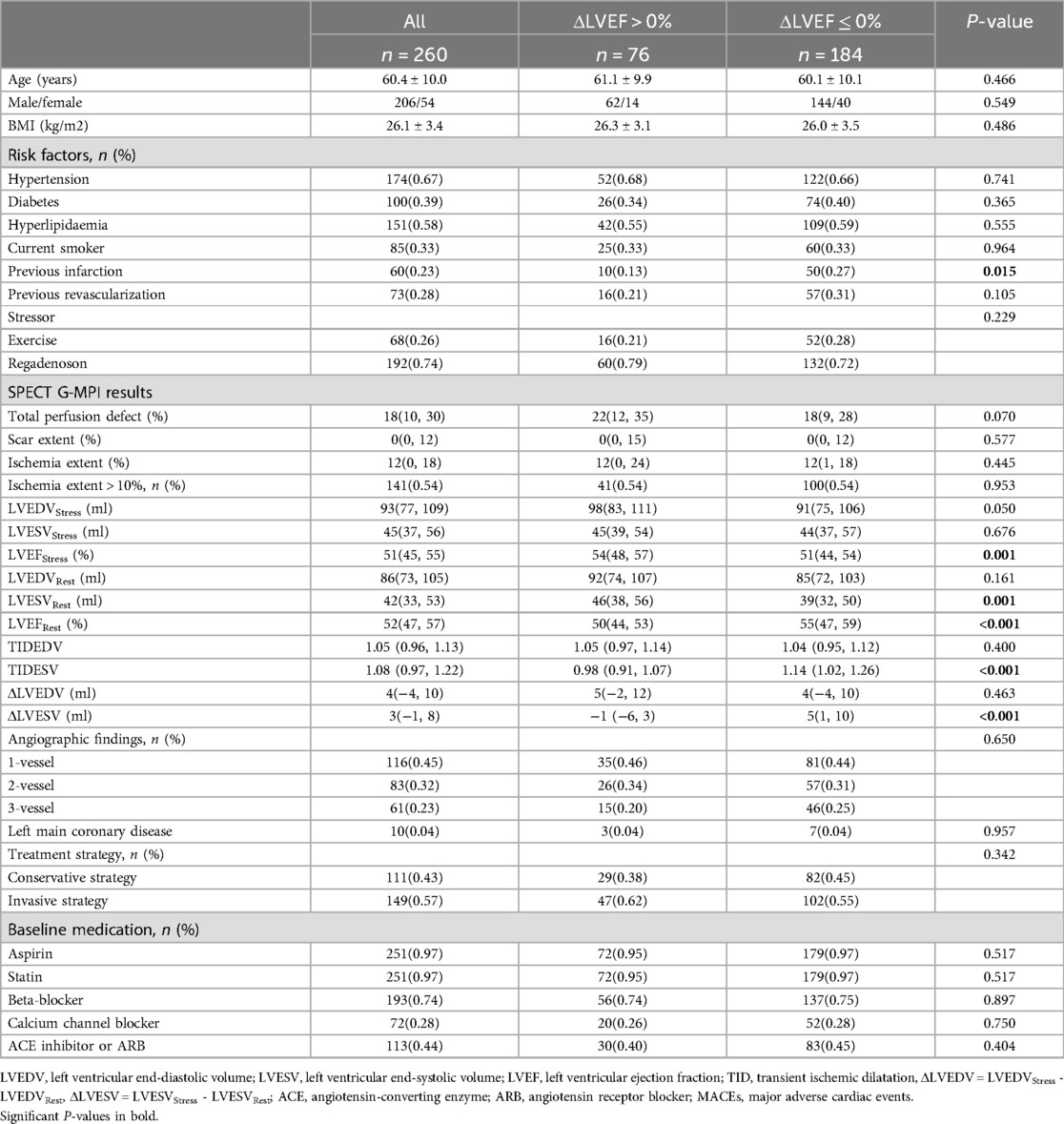
Of the 260 patients (age 60.4 ± 10.0 years, 206 male), 76 had an ΔLVEF > 0% and 184 had an ΔLVEF ≤ 0%. The baseline characteristics of the two groups are reported in Table 1. There was no significant difference (P > 0.05) between the two groups in terms of baseline characteristics, including age, gender, BMI, hypertension, diabetes, hyperlipidaemia, current smoker status, and previous revascularization. Compared with patients with an ΔLVEF > 0%, a history of prior myocardial infarction was more common in patients with an ΔLVEF ≤ 0% (P = 0.015).
An equivalent proportion of patients underwent exercise or pharmacological stress testing in the two groups (P = 0.229), and no significant differences were observed between the two groups in terms of TPD, scarring, ischemia, ischemia >10%, LVEDVStress, LVESVStress, LVEDVRest, TIDEDV and ΔLVEDV. The ΔLVEF ≤ 0% group exhibited a higher LVEFRest than the ΔLVEF > 0% group (P < 0.001), whereas LVEFStress was higher in the ΔLVEF > 0% group (P = 0.001). Patients with ΔLVEF ≤ 0% had a smaller LVESVRest (P = 0.001) and a greater TID-ESV (P < 0.001) and ΔLVESV (P < 0.001) than patients with ΔLVEF > 0%. There was no significant difference between the two groups regarding angiographic findings, treatment strategy, and medications.
3.2 Clinical outcomes
During a mean follow-up period of 33.4 ± 7.6 months, we recorded 69 MACEs (26.5%), including 10 all-cause deaths, 2 myocardial infarctions, 28 coronary revascularizations, and 29 hospitalizations for unstable angina. The ΔLVEF ≤ 0% group had a significantly increased event rate for the primary endpoint of MACEs (P = 0.027). However, when individual MACEs were analysed separately, no significant differences were observed between the two groups (P > 0.05) (Table 2).
As depicted in Figure 2A, the cumulative incidence of MACEs in patients with an ΔLVEF of ≤ 0% (22.7% ± 7.9%) was significantly higher than that in patients with an ΔLVEF >0% (15.4% ± 4.0%) (P = 0.042). Landmark analysis was performed at 2 years and beyond 2 years (Figure 2B). At 2 years, there was no significant difference in cumulative incidence of MACEs between two groups. Beyond 2 years, the cumulative incidence of MACEs in the ΔLVEF ≤ 0% group (11.6% ± 4.6%) was significantly higher than that in the ΔLVEF > 0% group (0%) (P = 0.001). In addition, considering the guideline (23) by The British Society of Echocardiography, a “normal” LVEF is defined as ≥55%, the sensitivity analysis was conducted using a 55% as a cutoff point. We compared the cumulative incidence of MACEs between the two groups in patients with LVEFStress < 55% (n = 181) and LVEFRest < 55% (n = 160). In patients with LVEFStress < 55%, the cumulative incidence of MACEs revealed differences, but these did not reach statistical significance (P = 0.188). In patients with LVEFRest < 55%, the differences achieved statistical significance (P = 0.045) (Supplementary Figure S1 and Supplementary Figure S2).
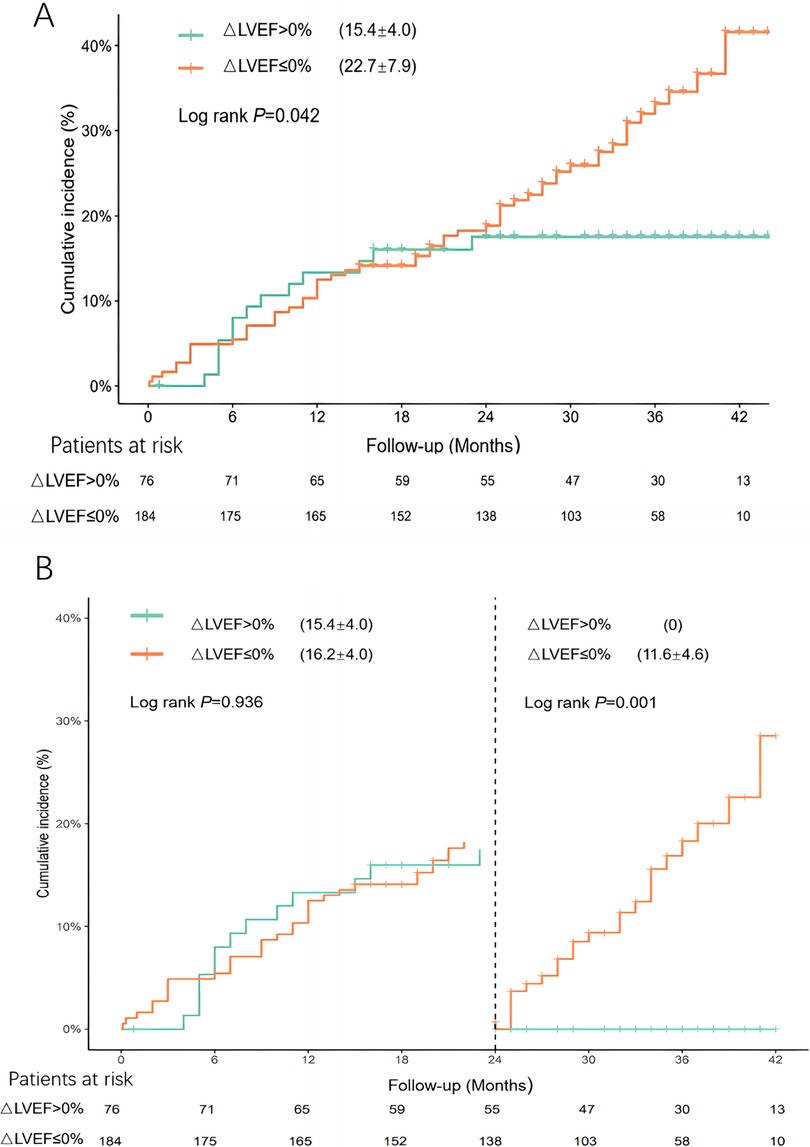
Figure 2. (A) Cumulative incidence of MACEs in patients with different LVEF reserves. (B) Landmark analyses were performed using a landmark point of 2 year and beyond 2 years.
Figure 3 compares the rate of MACEs between different LVEF reserves in patients with no or mild myocardial ischemia (extent of ischemia ≤ 10%) and moderate to severe myocardial ischemia (extent of ischemia > 10%). In patients with no or mild myocardial ischemia, the incidence of MACEs in the ΔLVEF ≤ 0% group (25.3%) was significantly higher than that in the ΔLVEF >0% group (8.6%) (P = 0.039). However, no significant difference was detected between the LVEF reserve groups in patients with moderate to severe myocardial ischemia (P = 0.263).

Figure 3. Comparison of the incidence of MACEs between different LVEF reserves in patients with no or mild myocardial ischemia (extent of ischemia ≤ 10%) and moderate to severe myocardial ischemia (extent of ischemia > 10%).
3.3 MACE prediction by univariate and multivariate Cox regression analysis
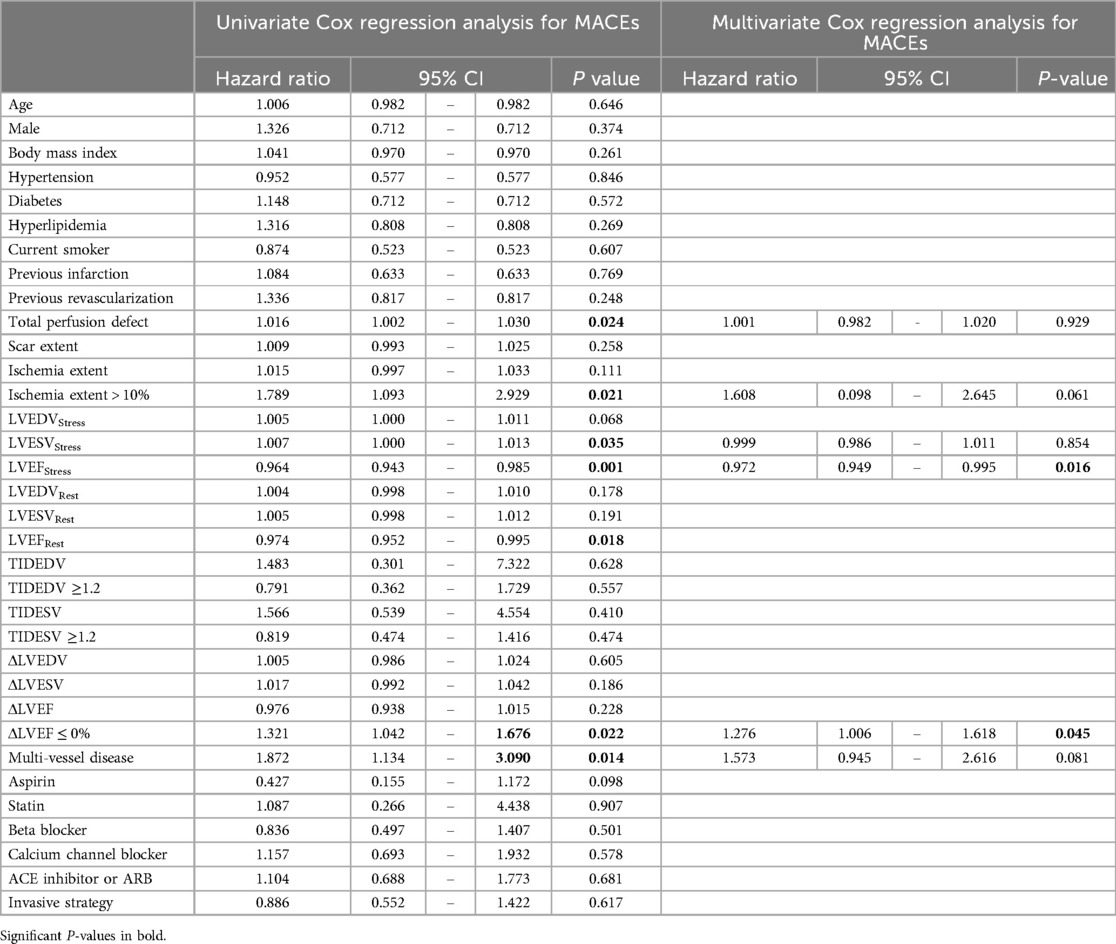
Univariate Cox regression analysis revealed that TPD, an extent of ischemia > 10%, LVESVStress, ΔLVEF ≤ 0%, and multivessel disease were all independent predictors for MACEs. However, LVEFStress and LVEFRest were identified as independent negative predictors. Multivariate Cox analysis showed that LVEFStress [adjusted HR: 0.972; 95% CI: 0.949, 0.995, P = 0.016] was an independent negative predictor while an ΔLVEF ≤ 0% [adjusted HR: 1.276; 95% CI: 1.006, 1.618, P = 0.045] was an independent positive predictor of MACEs (Table 3).
3.4 Incremental prognostic value of LVEF reserve
Figure 4 illustrates the global χ2 value for the prediction of MACEs. The global χ2 for Model 2 (Baseline + TPD) increased significantly from Baseline (Age, Sex and BMI, P = 0.036). The global χ2 for Model 3 (Model 2 + LVESVStress) did not significantly improve the prediction of MACEs (P = 0.456). The trend of an increase in global χ2 for Model 4 (Model 3 + LVEFStress) compare to Model 3 was observed but did not reach statistical significance (P = 0.058). The global χ2 for Model 5 (Model 4 + the extent of ischemia > 10%) was significantly higher than that for Model 4 (P = 0.016). Finally, the global χ2 for Model 6 (Model 5 + ΔLVEF ≤ 0%) was significantly higher than that for Model 5 (P = 0.044). A typical case is presented in Figure 5.
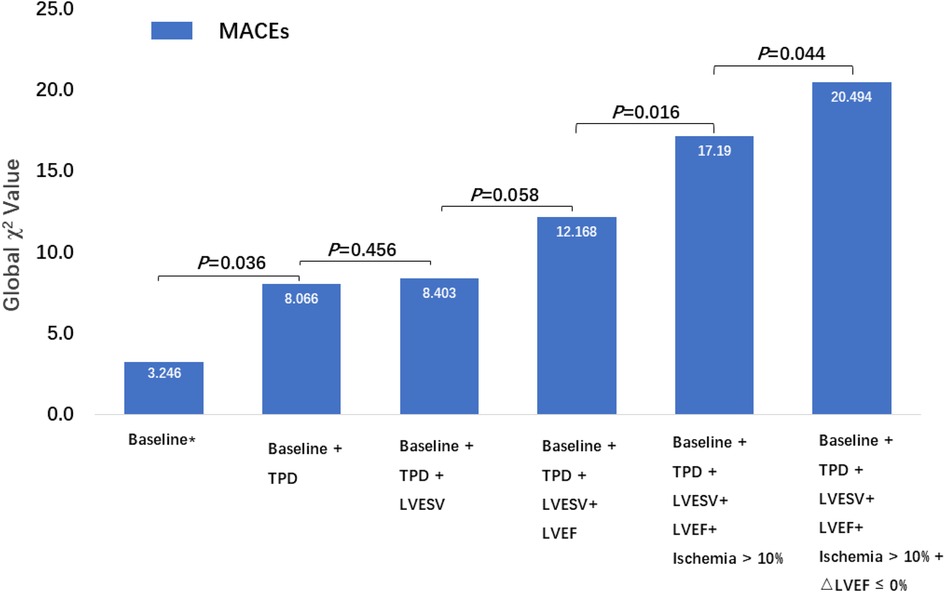
Figure 4. Incremental prognostic value of MPI variables, including TPD, LVESVStress, LVEFStress, an extent of ischemia >10%, and LVEF reserve, for MACEs in patients with CAD and with a LVEFStress < 60%. * Baseline including age, sex and BMI.
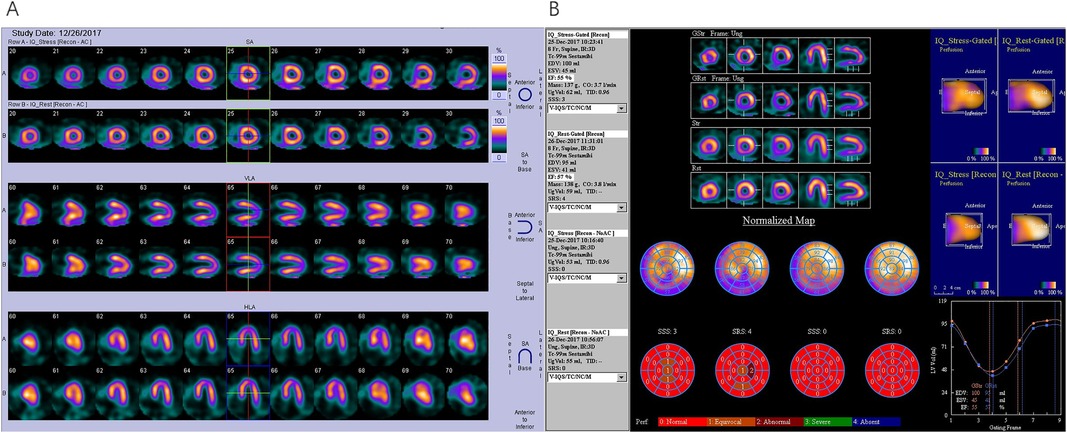
Figure 5. SPECT G-MPI in a 61-year-old male with CAD and with a history of PCI. (A) Perfusion imaging showing mild stress-induced ischemia in the apical inferior. (B) Analysis of cardiac function parameters revealed a LVEFStress of 55%, a LVEFRest of 57%, and a ΔLVEF = LVEFStress – LVEFRest = −2%. Subsequent coronary angiography revealed the absence of significant stenosis in the LM and LAD, patency of stent in LCX, and 100% occlusion of RCA. After a failed attempt of PCI in RCA, the patient was given medical therapy, and acute myocardial infarction was detected after 2.5 years of follow-up. PCI, percutaneous coronary intervention; LM, left main stem; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
4 Discussion
This study aimed to evaluate the prognostic value of ΔLVEF, as determined by SPECT G-MPI in patients with CAD, to predict MACEs. Our results indicated that in patients with a LVEFStress < 60%, an ΔLVEF ≤ 0% was identified as independent predictors of MACEs by multivariate Cox regression analysis. Furthermore, in patients with no or mild myocardial ischemia, the incidence of MACEs in the ΔLVEF ≤ 0% group was significantly higher than in the ΔLVEF > 0% group. Moreover, adding ΔLVEF to the traditional perfusion and functional variables of MPI significantly improved the discriminatory power to predict MACEs. Our results were generally consistent when left ventricular systolic dysfunction was defined as LVEFRest < 55%.
LVEF has been a key variable for the diagnosis and management of heart failure. In our study, we specifically focussed on LVEFStress < 60% because the latest recommendations by the British Society of Echocardiography (14) and the American Society of Nuclear Cardiology (24) state that the cut-off value for a “normal” LVEF is 55%. However, differences have been identified in terms of sex, age, and ethnicity. For example, there is a clear difference in LVEF between Europeans and Asians. The predicted values for Europeans are known to be significantly lower than those for East Asians. Specifically, for both sexes (at the age of 50 years), the lower reference value of LVEF for Europeans was 6% lower than that for East Asians. Furthermore, tenfold more Europeans than East Asians were found to have an LVEF < 50% (25). Unfortunately, while the LVEF criteria are applicable and appropriate for European populations, there is a significant scarcity of available data relating to LV function parameters acquired by gated MPI in the Chinese population. In addition, we cannot ignore the wide limits of agreement between echocardiography and SPECT G-MPI when determining LVEF (26). In routine clinical practice, we recommend monitoring borderline LVEF to avoid delay or missing high-risk patients. Our recent study (15) provided insights into the normal reference values of LVEFStress when measured by D-SPECT G-MPI in both women and men, which were 70 ± 8% and 68 ± 7%, respectively. Therefore, our centre gives considerable attention to patients with LVEFStress < 60%. Meanwhile, the results were generally consistent when left ventricular systolic dysfunction was defined as LVEFRest < 55%, but not when defined as LVEFStress < 55%. Indeed, in some centres, the stress-only strategy, or stress-first strategy, has been implemented to reduce costs and enhance the efficacy of testing (27, 28). Therefore, an appropriate expansion of the criteria for LVEFStress reduction aligns with clinical practice.
Previous evidence showed that a reduced ΔLVEF, as determined by 82Rb PET MPI, serves as a marker for ischemic contractile dysfunction (7) and is associated with an increased risk of cardiac events (5) and all-cause mortality (18). However, the existing literature describes inconsistent findings concerning the predictive significance of ΔLVEF when determined by SPECT MPI (9, 10, 12). In a previous study, Smith et al. (12) demonstrated that an abnormal LVEF reserve was not associated with an increased risk of the primary outcome. One possible explanation for this difference is that most patients in the study reported by Smith et al. (12) underwent a single-day protocol. In contrast, a two-day protocol was used in the present study as per our routine clinical practice. In the single-day protocol, the rest examination was performed approximately three hours after the stress examination, possibly leading to an underestimation of the alteration in LVEF, particularly in patients with severe ischaemia who may have experienced prolonged stunning. In addition, our landmark analysis revealed the effect of ΔLVEF on long-term prognosis. Specifically, in our cohort of patients with coronary stenosis and left ventricular systolic dysfunction, 23 MACEs were observed beyond 2 years, a large proportion (78.3%) of whom underwent incomplete revascularization (n = 2) and conservative strategies (n = 16). BARI-2D (29) found in high-risk patients, including those with reduced LVEF and extensive coronary disease, the five-year risk of death/MI/stroke were significantly lower among those undergoing revascularizations when compared with the group of medical therapy alone. In particular, the survival curve showed a significant increase in the difference in event rates after 2 years. Similarly, STICH trial (30) reported a significant benefit began to accrue after 2 years when comparing CABG and medical therapy in patients with heart failure. Our results strongly correlated with the above reports. We speculate that the absence of LVEF reserve may indicate a declining cardiac reserve, and that coronary artery stenosis and progressive myocardial ischemia may contribute to this poor prognosis in the later stages.
In a previous study, Gomez et al. (9) defined an abnormal ΔLVEF as a reduction of <5% in LVEF in post-stress images. This criterion was derived from a previous study (31) that proposed a 5% threshold for ΔLVEF when distinguishing between normal and abnormal responses. The study demonstrated that a ΔLVEF of 5% provided the highest diagnostic accuracy (sensitivity 52%, specificity 83%) for detecting multivessel CAD. Nevertheless, the most extensive cohort study to date (10), featuring 10,275 patients who underwent SPECT-MPI, revealed that an increase of 1% in LVEF reserve was significantly and independently associated with a lower incidence of MACEs, including cardiac death and myocardial infarction [HR: 0.98; 95% CI: 0.97, 0.99, P = 0.003]. Thus, additional clarification is needed to enable a more significant prognostic capability for patient outcomes. Within our present cohort, only 9.2% (n = 24) of patients exhibited an ΔLVEF of ≥5%, thus indicating that an ΔLVEF of ≤0%, rather than an ΔLVEF of ≤5%, represents a crucial and autonomous prognostic marker, thereby aligning with recent research (11, 18, 19). However, the prognostic value of ΔLVEF, as determined by SPECT MPI, has not been reported in a high-risk cohort with a reduced LVEF. This study is the first to report that an ΔLVEF ≤ 0% was an independent predictor of MACEs in patients with a LVEFStress < 60%. This finding provides a valuable point of reference for guiding future clinical practice.
Previous research established the importance of myocardial ischemia for determining therapeutic strategies. Patients with no to mild ischemia were categorised as low risk, for whom a conservative treatment approach was considered to be appropriate. In contrast, patients with moderate to severe ischemia were recommended for revascularization to improve their prognosis (32, 33). To the best of our knowledge, there is a significant scarcity of data relating to the prognosis of ΔLVEF in patients with varying degrees of myocardial ischemia. Smith et al. (12) previously performed subgroup analysis for patients with large areas of ischemia (≥10%/LV) and determined no significant difference in the incidence of primary outcomes compared to those with and without LVEF reserve. These findings are consistent with those arising from our present analysis. Unfortunately, the study lacked data on patients with no to mild ischemia. Our results suggest, for the first time, that the combination of ΔLVEF with the extent of myocardial ischemia could enhance risk stratification in patients with CAD. Notably, patients with no to mild myocardial ischemia were considered to have a favourable prognosis, whereas those with an ΔLVEF ≤ 0% exhibited a relatively high risk of MACEs.
A large area of myocardial ischemia has been confirmed to be associated with poor outcomes in CAD patients. Its prognostic effect is very strong and significant. In our cohort, a total of 44 MACEs were observed in patients with moderate to severe myocardial ischemia, with 75% (n = 33) occurring within 2 years. Our results reveal that the influence of the ischemia on prognosis was significantly greater than that of ΔLVEF in a short term. The influence of ΔLVEF on outcomes has gradually become more apparent with the progression of the disease. In fact, the mechanism of the prognostic significance of ΔLVEF is not very clear at present. We observed that the ΔLVEF ≤ 0% group exhibited a higher LVEFRest than the ΔLVEF > 0% group, whereas LVEFStress was higher in the ΔLVEF > 0% group. Furthermore, the 79.5% of impaired LVEFStress (≤50%) (23) was included in the ΔLVEF ≤ 0% group (vs. ΔLVEF > 0% group, P = 0.005). In contrast, a slightly higher proportion of individuals with supra-normal left ventricular ejection fraction (snLVEF) (LVEFRest ≥ 65%) were found in the ΔLVEF ≤ 0% group (n = 9, P = 0.050), compared to ΔLVEF > 0% group (n = 0). The snLVEF is considered to be associated with a poor prognosis (34), but the mechanism is unclear. We speculate that the combination of potential functional abnormalities in the resting state and impaired cardiac reserve, which presents a poor response to stress, may lead to a reduced ΔLVEF and posing a risk of long-term poor prognosis. Further research into this potential relationship is needed.
To our knowledge, only one previous study investigated the incremental value of ΔLVEF for predicting MACEs beyond the conventional variables of MPI. Otaki et al. (11) recruited 151 patients undergoing same-day rest/stress SPECT G-MPI. Early stress imaging was initiated 2 min after the injection of regadenoson, followed by late-stress acquisition. This study demonstrated that adding ΔLVEF during early stress enhanced the combined model of age, a prior history of PCI, and TPD (P < 0.001). The annualised MACEs rates during the late-stress period exhibited variances between patients with an ΔLVEF < 0% (6.7%) and an ΔLVEF ≥ 0% (4.9%), although these differences were not statistically significant. However, the sample size of this previous study was limited and focused explicitly on preserved LVEFStress, unlike our current study. Furthermore, Otaki et al. did not analyse the traditional parameters of MPI, except for TPD. It is widely acknowledged that larger perfusion defects, reduced ejection fraction, and larger ventricular volume predict adverse cardiac events (10, 35). Our current findings concur with these earlier findings. Based on our current findings, we emphasize that in patients with left ventricular dysfunction, both stress and resting MPI parameters, including TPD, ischemia, and LVEF, particularly ΔLVEF, may provide valuable assistance for the further risk stratification of patients with CAD.
The European Society of Cardiology guidelines (13) published recently for managing chronic coronary syndromes (CCS), guide clinicians in choosing imaging techniques (36). Both functional and anatomical aspects must be considered in patients with suspected CCS, and the importance of non-invasive imaging for selecting patients to be referred for invasive angiography has been emphasized. In particular, functional assessment may be crucial for identifying the mechanisms behind myocardial ischemia and, eventually, angina, thus guiding symptomatic treatment (37). Speckle tracking echocardiography (STE) is a reliable and widely used imaging technique of recognized clinical value in several settings. This method uses the motion of ultrasound backscatter speckles within echocardiographic images to derive myocardial velocities and deformation parameters (38). Notably, global longitudinal strain (GLS) is considered an earlier marker of myocardial damage and predicts mortality in patients with CCS independently of LVEF (39). The myocardial deformation imaging might reveal subtle abnormalities that can be attributed to clinically relevant ischemic or ischemic memory (40). This ischemic memory may be considered relevant to myocardial stunning and the reduction of post-stress LVEF (41). Integrating multiple imaging modalities and attempting to reveal the pathophysiological mechanisms is an important direction for future research.
Some studies support the notion that the presence of TID can specifically indicate extensive or severe coronary artery disease (42). However, this study found that TID was not an independent predictor for MACEs, thus aligning with a large cohort study previously conducted by Kattoor et al. (10) who found that the prognostic value of ΔLVEF was higher than that of TID. The pathophysiology of TID remains controversial (43, 44), although the predominant hypothesis is that TID originates from either diffuse subendocardial hypoperfusion leading to an apparent increase in LV endocardial cavity size and/or stress-induced LV dysfunction (3). Although investigating a specific group of patients with LV dysfunction may influence the prognostic value of TID, it is noteworthy that we identified clear differences between the ΔLVEF groups for ΔLVESV and TIDESV but not for ΔLVEDV or TIDEDV, thus indicating an association between a reduction in post-stress LVEF and left ventricular systolic dysfunction.
5 Limitations
Our research is subject to several limitations that need to be considered. First, owing to its retrospective nature and the fact that this was a single-centre study with a relatively small sample size, there is potential for selection bias. To reduce the waiting list time for MPI, we did not perform rest studies in patients with normal stress-gated MPI in our laboratory. Therefore, even if more than 3,000 MPI studies were conducted per year, the number of populations in the current study was limited. Another limitation was the acquisition of gated MPI, which was performed after 60–90 min according to different stress or rest states. This implies that the acquisition of LVEFStress by SPECT was not derived during peak stress. However, we confirmed the predictive value of ΔLVEF in patients with a LVEFStress < 60%, particularly in those with no or mild myocardial ischemia. Our findings emphasise that combining perfusion and cardiac function parameters may enhance risk stratification.
6 Conclusions
In this pilot study, we found that when determined by SPECT G-MPI, ΔLVEF was independently associated with MACEs in CAD patients with LVEFStress < 60%, enhancing risk stratification for MACEs. Patients with no to mild myocardial ischemia were considered to have a favourable prognosis, whereas those with an ΔLVEF ≤ 0% exhibited a relatively high risk of MACEs. This is a pilot study with a small sample size, and further investigation and validation are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SZ: Conceptualization, Data curation, Writing – original draft. JM: Data curation, Methodology, Writing – review & editing. YZ: Data curation, Writing – review & editing. LL: Data curation, Writing – review & editing. XZ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Reference number: 82171994, 81871377), Beijing Municipal Natural Science Foundation (Reference: 7232040) and Beijing Municipal Administration of Hospitals (Reference: ZYLX202110).
Acknowledgments
The patients with LVEFStress < 60%, estimated by SPECT G-MPI, deserve sufficient attention. Patients with no to mild myocardial ischemia were considered to have a favorable prognosis, whereas those with ΔLVEF ≤ 0% exhibited a relatively high risk of MACEs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1480501/full#supplementary-material
Supplementary Figure S1 | Cumulative incidence of MACEs in patients with different LVEF reserves in patients with LVEFStress < 55%.
Supplementary Figure S2 | Cumulative incidence of MACEs in patients with different LVEF reserves in patients with LVEFRest < 55%.
Abbreviations
CABG, coronary artery bypass graft; CAD, coronary artery disease; CAG, coronary angiography; EDV, end-diastolic volume; ESV, end-systolic volume; LVEF, left ventricular ejection fraction; MACEs, major adverse cardiac events; MPI, myocardial perfusion imaging; PCI, percutaneous coronary intervention; PET, positron emission computed tomography; SPECT, single-photon emission computed tomography; TID, transient ischemic dilatation; TPD, total perfusion defect; ΔLVEF, left ventricular ejection fraction reserve.
References
1. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7
2. Moazzami K, Lima BB, Hammadah M, Ramadan R, Al Mheid I, Kim JH, et al. Association between change in circulating progenitor cells during exercise stress and risk of adverse cardiovascular events in patients with coronary artery disease. JAMA Cardiol. (2020) 5:147–55. doi: 10.1001/jamacardio.2019.4528
3. Bajaj NS, Singh S, Farag A, El-Hajj S, Heo J, Iskandrian AE, et al. The prognostic value of non-perfusion variables obtained during vasodilator stress myocardial perfusion imaging. J Nucl Cardiol. (2016) 23:390–413. doi: 10.1007/s12350-016-0441-3
4. Scatteia A, Silverio A, Padalino R, De Stefano F, America R, Cappelletti AM, et al. Non-invasive assessment of left ventricle ejection fraction: where do we stand? J Pers Med. (2021) 11:1153. doi: 10.3390/jpm11111153
5. Dorbala S, Hachamovitch R, Curillova Z, Thomas D, Vangala D, Kwong RY, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. (2009) 2:846–54. doi: 10.1016/j.jcmg.2009.04.009
6. Brown TLY, Merrill J, Volokh L, Bengel FM. Determinants of the response of left ventricular ejection fraction to vasodilator stress in electrocardiographically gated 82rubidium myocardial perfusion PET. Eur J Nucl Med Mol Imag. (2007) 35:336–42. doi: 10.1007/s00259-007-0603-2
7. Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a Rb-82 PET/CT study. J Nucl Med. (2007) 48:349–58.17332611
8. Thomas M, Sperry BW, Peri-Okonny P, Malik AO, McGhie AI, Saeed IM, et al. Relative prognostic significance of positron emission tomography myocardial perfusion imaging markers in cardiomyopathy. Circ Cardiovasc Imaging. (2021) 14:e012426. doi: 10.1161/CIRCIMAGING.121.012426
9. Gomez J, Golzar Y, Fughhi I, Olusanya A, Doukky R. The significance of post-stress decrease in left ventricular ejection fraction in patients undergoing regadenoson stress gated SPECT myocardial perfusion imaging. J Nucl Cardiol. (2018) 25:1313–23. doi: 10.1007/s12350-017-0802-6
10. Kattoor AJ, Kolkailah AA, Iskander F, Iskander M, Diep L, Khan R, et al. The prognostic value of regadenoson SPECT myocardial perfusion imaging: the largest cohort to date. J Nucl Cardiol. (2021) 28:2799–807. doi: 10.1007/s12350-020-02135-y
11. Otaki Y, Fish MB, Miller RJH, Lemley M, Slomka PJ. Prognostic value of early left ventricular ejection fraction reserve during regadenoson stress solid-state SPECT-MPI. J Nucl Cardiol. (2021) 29:1219–30. doi: 10.1007/s12350-020-02420-w
12. Smith P, Farag A, Bhambhvani P, Iskandrian A, Hage FG. Prognostic value of absent left ventricular ejection fraction reserve with regadenoson SPECT MPI. J Nucl Cardiol. (2020) 29:978–86. doi: 10.1007/s12350-020-02390-z
13. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
14. Harkness A, Ring L, Augustine DX, Oxborough D, Robinson S, Sharma V. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: a guideline from the British Society of Echocardiography. Echo Research & Practice. (2020) 7:G1–G18. doi: 10.1530/ERP-19-0053
15. Jingjing M, Jian J, Xiaofen X, Tiantian M, Zhi C, Junqi L, et al. Establishment of the normal reference values of left ventricular function parameters evaluated by CZT SPECT stress gated myocardial perfusion imaging in low-likelihood of stable coronary artery disease. Chin J Nuclear Med Molecular Imaging. (2023) 43:144–9. doi: 10.3760/cma.j.cn321828-20221123-00352
16. Li J, Yang X, Tian Y, Wei H, Hacker M, Li X, et al. Complete revascularization determined by myocardial perfusion imaging could improve the outcomes of patients with stable coronary artery disease, compared with incomplete revascularization and no revascularization. J Nucl Cardiol. (2019) 26:944–53. doi: 10.1007/s12350-017-1145-z
17. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation. (2002) 105:539–42. doi: 10.1161/hc0402.102975
18. Tamarappoo BK, Lee FL, Cerqueira M, Hachamovitch R. Independent prognostic value of left ventricular contractile reserve and chronotropic response in patients with reduced left ventricular ejection fraction undergoing vasodilator stress myocardial perfusion imaging with Rb-82 positron emission tomography. Eur Heart J Cardiovasc Imaging. (2018) 19:442–9. doi: 10.1093/ehjci/jex157
19. Miller RJH, Han D, Singh A, Pieszko K, Slomka PJ, Gransar H, et al. Relationship between ischaemia, coronary artery calcium scores, and major adverse cardiovascular events. Eur Heart J Cardiovasc Imaging. (2022) 23:1423–33. doi: 10.1093/ehjci/jeac082
20. Zhou YH, Lu Y, Meng JJ, Mou TT, Bai YJ, Zhang S, et al. Predictive value of left ventricular ejection fraction reserve assessed by SPECT G-MPI for major adverse cardiovascular event in patients with coronary artery disease. Chinese J Cardiol. (2023) 51:626–32. doi: 10.3760/cma.j.cn112148-20220919-00730
21. Mut F, Gaudiano MP, Kapitan M. Relationship between transient ischemic dilatation and changes in heart rate during gated SPECT acquisition in a low-risk population without perfusion defects. Nucl Med Commun. (2024) 45:581–8. doi: 10.1097/MNM.0000000000001852
22. Miller RJH, Hu LH, Gransar H, Betancur J, Eisenberg E, Otaki Y, et al. Transient ischaemic dilation and post-stress wall motion abnormality increase risk in patients with less than moderate ischaemia: analysis of the REFINE SPECT registry. Eur Heart J Cardiovasc Imaging. (2020) 21:567–75. doi: 10.1093/ehjci/jez172
23. Hudson S, Pettit S. What is 'normal' left ventricular ejection fraction? Heart. (2020) 106:1445–6. doi: 10.1136/heartjnl-2020-317604
24. Dorbala S, Ananthasubramaniam K, Armstrong IS, Chareonthaitawee P, DePuey EG, Einstein AJ, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. (2018) 25:1784–846. doi: 10.1007/s12350-018-1283-y
25. C. Echocardiographic Normal Ranges Meta-Analysis of the Left Heart. Ethnic-Specific normative reference values for echocardiographic LA and LV size, LV mass, and systolic function: the EchoNoRMAL study. JACC Cardiovasc Imaging. (2015) 8:656–65. doi: 10.1016/j.jcmg.2015.02.014
26. Jacobson AF, Narula J, Tijssen J. Analysis of differences in assessment of left ventricular function on echocardiography and nuclear perfusion imaging. Am J Cardiol. (2021) 156:85–92. doi: 10.1016/j.amjcard.2021.06.039
27. Han D, Hyun MC, Miller RJH, Gransar H, Slomka PJ, Dey D, et al. 10-year Experience of utilizing a stress-first SPECT myocardial perfusion imaging. Int J Cardiol. (2024) 401:131863. doi: 10.1016/j.ijcard.2024.131863
28. Kaminek M, Havel M, Kincl V, Henzlova L, Hudson L. The prognostic value of CZT SPECT stress myocardial blood flow (MBF) quantification-opportunity for stress-first/stress-only protocol. Eur J Nucl Med Mol Imaging. (2024) 51:344–5. doi: 10.1007/s00259-023-06531-7
29. Brooks MM, Chaitman BR, Nesto RW, Hardison RM, Feit F, Gersh BJ, et al. Clinical and angiographic risk stratification and differential impact on treatment outcomes in the bypass angioplasty revascularization investigation 2 diabetes (BARI 2D) trial. Circulation. (2012) 126:2115–24. doi: 10.1161/CIRCULATIONAHA.112.092973
30. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, et al. Coronary-Artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. (2016) 374:1511–20. doi: 10.1056/NEJMoa1602001
31. Hida S, Chikamori T, Tanaka H, Usui Y, Igarashi Y, Nagao T, et al. Diagnostic value of left ventricular function after stress and at rest in the detection of multivessel coronary artery disease as assessed by electrocardiogram-gated SPECT. J Nucl Cardiol. (2007) 14:68–74. doi: 10.1016/j.nuclcard.2006.10.019
32. Azadani PN, Miller RJH, Sharir T, Diniz MA, Hu LH, Otaki Y, et al. Impact of early revascularization on major adverse cardiovascular events in relation to automatically quantified ischemia. JACC Cardiovasc Imaging. (2021) 14:644–53. doi: 10.1016/j.jcmg.2020.05.039
33. Yong J, Tian J, Zhao X, Yang X, Zhang M, Zhou Y, et al. Revascularization or medical therapy for stable coronary artery disease patients with different degrees of ischemia: a systematic review and meta-analysis of the role of myocardial perfusion. Ther Adv Chronic Dis. (2022) 13:20406223211056713. doi: 10.1177/20406223211056713
34. Wehner GJ, Jing L, Haggerty CM, Suever JD, Leader JB, Hartzel DN, et al. Routinely reported ejection fraction and mortality in clinical practice: where does the nadir of risk lie? Eur Heart J. (2020) 41:1249–57. doi: 10.1093/eurheartj/ehz550
35. Nishimura T, Nakajima K, Kusuoka H, Yamashina A, Nishimura S. Prognostic study of risk stratification among Japanese patients with ischemic heart disease using gated myocardial perfusion SPECT: j-ACCESS study. Eur J Nucl Med Mol Imaging. (2008) 35:319–28. doi: 10.1007/s00259-007-0608-x
36. van der Bijl P, Stassen J, Bax JJ. Guideline-based use of cardiac imaging for chronic coronary syndromes. Eur Heart J. (2023) 44:159–61. doi: 10.1093/eurheartj/ehac630
37. Morrone D, Gentile F, Aimo A, Cameli M, Barison A, Picoi ME, et al. Cluster imaging of the Italian society of, perspectives in noninvasive imaging for chronic coronary syndromes. Int J Cardiol. (2022) 365:19–29. doi: 10.1016/j.ijcard.2022.07.038
38. Gherbesi E, Gianstefani S, Angeli F, Ryabenko K, Bergamaschi L, Armillotta M, et al. Myocardial strain of the left ventricle by speckle tracking echocardiography: from physics to clinical practice. Echocardiography. (2024) 41:e15753. doi: 10.1111/echo.15753
39. Edvardsen T, Asch FM, Davidson B, Delgado V, DeMaria A, Dilsizian V, et al. Non-Invasive imaging in coronary syndromes: recommendations of the European Association of Cardiovascular Imaging and the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. (2022) 35:329–54. doi: 10.1016/j.echo.2021.12.012
40. Asanuma T, Fukuta Y, Masuda K, Hioki A, Iwasaki M, Nakatani S. Assessment of myocardial ischemic memory using speckle tracking echocardiography. JACC Cardiovasc Imaging. (2012) 5:1–11. doi: 10.1016/j.jcmg.2011.09.019
41. Hannon MV, Schwartz RG. LVEF Reserve: state of the heart is a matter of time, jeopardy and ischemic memory. J Nucl Cardiol. (2022) 29:3461–5. doi: 10.1007/s12350-020-02461-1
42. Alama M, Labos C, Emery H, Iwanochko RM, Freeman M, Husain M, et al. Diagnostic and prognostic significance of transient ischemic dilation (TID) in myocardial perfusion imaging: a systematic review and meta-analysis. J Nucl Cardiol. (2018) 25:724–37. doi: 10.1007/s12350-017-1040-7
43. Emmett L, Ng A, Ha L, Russo R, Mansberg R, Zhao W, et al. Comparative assessment of rest and post-stress left ventricular volumes and left ventricular ejection fraction on gated myocardial perfusion imaging (MPI) and echocardiography in patients with transient ischaemic dilation on adenosine MPI: myocardial stunning or subendocardial hypoperfusion? J Nucl Cardiol. (2012) 19:735–42. doi: 10.1007/s12350-012-9571-4
Keywords: ejection fraction reserve, myocardial perfusion imaging, gated SPECT, prognosis, coronary artery disease
Citation: Zhang S, Meng J, Zhou Y, Lv L and Zhang X (2024) Prognostic value of the left ventricular ejection fraction reserve acquired by gated myocardial perfusion SPECT in patients with CAD and reduced stress LVEF. Front. Cardiovasc. Med. 11:1480501. doi: 10.3389/fcvm.2024.1480501
Received: 14 August 2024; Accepted: 26 September 2024;
Published: 10 October 2024.
Edited by:
Seokhun Yang, Seoul National University Hospital, Republic of KoreaReviewed by:
Luca Bergamaschi, University of Bologna, ItalyFeifei Zhang, First People’s Hospital of Changzhou, China
Copyright: © 2024 Zhang, Meng, Zhou, Lv and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Zhang, eGx6aGFuZzY4QDEyNi5jb20=
 Shuang Zhang
Shuang Zhang Jingjing Meng1
Jingjing Meng1 Xiaoli Zhang
Xiaoli Zhang