- 1Department of Thyroid Breast Surgery, The Affiliated Yixing Hospital of Jiangsu University, Wuxi, China
- 2Department of Hand and Foot Surgery, Affiliated Huishan Hospital of Xinglin College, Nantong University, Wuxi Huishan District People's Hospital, Wuxi, China
Aim: We aim to investigate the association between body roundness index (BRI) and abdominal aortic calcification (AAC) among middle aged and elderly US residents.
Methods: This cross-sectional study used data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014 cycle, including 3,079 middle-aged and elderly participants aged 40 and above. AAC scores for these participants were assessed using dual-energy x-ray absorptiometry (DXA). BRI was calculated from participants’ height and waist circumference, with all measurements conducted by trained surveyors using standardized methods. The relationship between BRI and AAC was analyzed using weighted multivariate logistic regression, adjusting for confounding variable. Additionally, restricted cubic splines (RCS) analysis was also employed.
Results: We found that those with AAC were significantly older and had a higher prevalence of smoking and chronic kidney disease (CKD) prevalence compared to those without AAC. Using weighted multivariable logistic regression, we determined that an increase of one unit in BRI was associated with a 22% higher risk of AAC. Additionally, higher BRI quartiles (Q2, Q3, Q4) showed significantly increased risks of AAC compared to the lowest quartile. Visualization using RCS indicated a gradual increase in AAC risk with higher BRI, which plateaued beyond a BRI of 7.2. This relationship was significant across different age and gender group.
Conclusion: There is a positive association between abdominal obesity (as measured by BRI) and AAC in the middle-aged and elderly population. This suggests the impact of abdominal obesity on vascular health and that this factor should be considered in public health strategies.
Introduction
Abdominal aortic calcification (AAC) is a common degenerative condition in the elderly (1, 2). About 20% of people over 60 years-old with AAC (3–5). The pathological characteristics of AAC include atherosclerosis and reduced elasticity of the abdominal aorta. To some extent, arterial calcification is a marker of aging, and AAC can reflect the overall state of arterial calcification throughout the body (6). This is significant for predicting adverse cardiovascular and cerebrovascular events. Besides elderly, there are many risk factors that can lead to arterial calcification (7). For example, in hypertensive patients whose blood pressure is not controlled within the normal range over a long period, the arterial walls are affected by the high blood pressure, greatly increasing the risk of AAC (8, 9). Individuals with obesity often have diabetes and hyperlipidemia, hyperlipidemia can cause vascular stiffening, which also increases the risk of AAC. In diabetes, abnormal fat metabolism leads to increased fat deposition on the vascular walls, thereby inducing AAC.
Obesity has become a major public health issue worldwide (10). Studies have shown that deaths related to overweight and obesity account for 11.1% of deaths from non-communicable diseases (11, 12). Increasing research confirms that abdominal obesity poses a greater risk (13, 14). Abdominal obesity, characterized by excessive fat accumulation in the abdomen leading to increased waist circumference, significantly raises the risk of cardiovascular and cerebrovascular diseases (15). Causes of abdominal obesity include prolonged sedentary behavior and genetic factors, and it needs to be controlled through diet and physical exercise (16). Waist circumference and hip circumference are often used to compensate for the inability of BMI to reflect body fat distribution. However, these measures cannot account for the larger waist or hip circumference in taller individuals. Therefore, an index that comprehensively assesses both body height and width is needed. In 2013, Thomas developed the Body roundness index (BRI) as a method to predict body fat percentage and visceral adipose tissue percentage, thereby predicting overall health risk (17).
However, relationship of abdominal obesity with AAC is still not very clear. We aim to investigate the relationship between abdominal obesity, reflected by the BRI, and AAC in the middle-aged and elderly population.
Methods
Study population
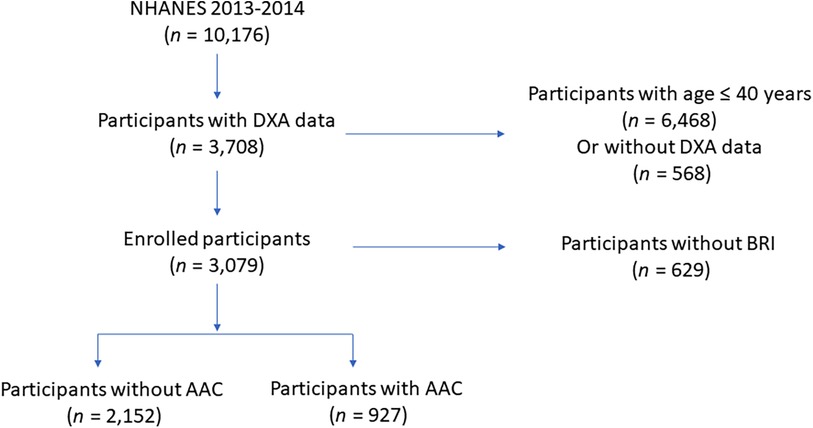
All of the enrolled participants were from National Health and Nutrition Examination Survey (NHANES), which aims to investigate the overall health and nutrition status of residents in US. Due to AAC scores can only be obtained in study circle of NHANES 2013–2014 and participants under the age of 40 lacked AAC scores data, we initially included 3,708 participants aged over 40 years old in NHANES 2013–2014 with AAC score eligible. Moreover, there were 69 participants without BRI measurement were excluded. After removing 69 participants with missing BRI measurement, 3,079 eligible middle aged and elderly participants were ultimately included in the present study to study the association between BRI and AAC. Population enrollment can be found in Figure 1.
BRI measurement
In NHANES, participants’ body height and waist circumference are measured by trained surveyors using standardized measurement methods. All measurement data undergo quality control and standardization processes to ensure accuracy and comparability of results. Consistent with previous studies, the formula for calculating BRI is as follows (17):
AAC scores
Dual-energy x-ray absorptiometry (DXA) was adopted to evaluate AAC. The measurements were derived from a lateral scan focused on the lumbar spine (vertebrae L1–L4) and were assessed using the Kauppila score system, spaning from 0 to 24, classifying AAC into absence of calcification (AAC = 0), moderate calcification (AAC > 0–6), and severe calcification (AAC > 6) categories. A Kauppila score surpassing 6 indicates substantial calcification, aligning with earlier research findings. Exclusions from DXA scanning in the NHANES survey encompassed individuals under 40 years old, pregnant women, those weighing over 450 pounds, and participants who had consumed barium in the previous week. DXA machines are widely available in clinical settings, this widespread availability makes it relatively easy to incorporate arterial calcification measurements into existing practices. DXA is generally more cost-effective compared to advanced imaging techniques like CT or MRI. Moreover, DXA employs low-dose x-rays, resulting in minimal radiation exposure compared to other imaging techniques like CT scans. However, DXA may have lower sensitivity compared to other imaging modalities like CT in detecting early or subtle arterial calcification. Besides, in areas where bone and arteries are closely aligned, such as the spine or pelvis, the overlap of bone and arterial calcification can complicate the interpretation of DXA results, leading to potential inaccuracies.
Covariates
Covariates were included in statistical analyses to control for potential confounding factors. We directly obtained participants’ demographical information through standardized demographic questionnaire. Blood pressure measurements was conducted by experienced examiners. Hypertensive status was categorized based on self-reported diagnosis, average systolic and diastolic blood pressure (140/90 mmHg), medication use, or laboratory tests. BMI was calculated using measured height and weight (kg/m2). Diabetes status was categorized as diabetic or non-diabetic based on self-reported diagnosis, medication use, or laboratory tests. Chronic kidney disease is categorized into five stages according to present clinical guidelines. Blood samples were obtained from participants after they had fasted for at least 8 h to determine the blood glucose, lipid, and other hematological parameters. Diabetes was assessed based on both historical diagnoses and newly identified cases. Participants with a fasting plasma glucose level of 7.0 mmol/L or higher were classified as having newly diagnosed diabetes. Moreover, Participants with an HbA1c level of 6.5% or higher were also diagnosed as participants with diabetes. We believe that retaining the original format of the data may more accurately reflect the actual situation. Similar to the weighted analysis methods used in previous research, we did not impute or exclude missing data.
Statistical methods
Data were presented as weighted means with corresponding 95% CI, or weighted proportions with 95% CI. Each participant was given a specific sampling weight, primary sampling unit (PSU), and stratum to ensure the production of accurate national estimates. The WTMEC2YR dataset contains the survey weights for all participants who underwent body measurements at a Mobile Exam Center. For our analysis, we calculated the sample weight applied these survey weights in our statistical analyses to generate estimates that accurately reflect the U.S. population. The population included in the present study represents 2,213,412 individuals in US. Baseline characteristics among continuous variables were compared using the adjusted Wald test, and for categorical variables, the Rao-Scott χ2 test was employed. The association between BRI and AAC was examined using weighted multivariate logistic analysis. Covariates including age, sex, and race/ethnicity, income, education levels, BMI, smoking, drinking, hypertension, CKD, eGFR, HbA1c, serum phosphorus, serum calcium, serum albumin were adjusted in logistic and restricted cubic splines (RCS) analysis. Moreover, RCS analysis was also employed. Statistical analyses were conducted using R, with significance set at two tailed p < 0.05.
Results
Baseline demographical and clinical characteristics
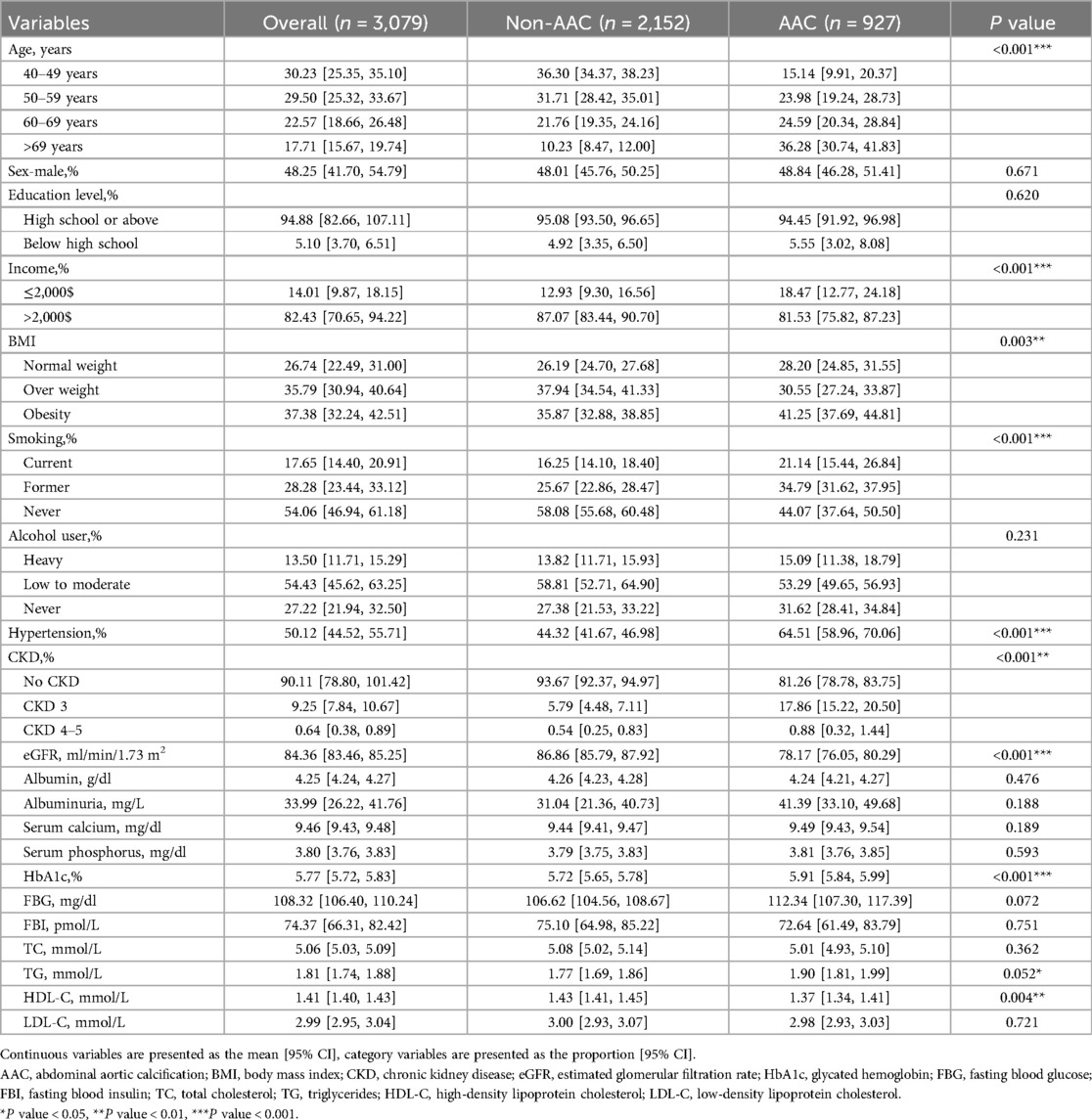
A total of 3,079 middle-aged and elderly participants over 40 years. Overall, participants over 60 years accounted for approximately 40.28% of the sample, with males constituting about 48.25%. According to the baseline data, participants with AAC were significantly older than those without AAC. However, there were no significant differences in gender composition among two groups. Similarly, no notable differences were observed regarding education level and alcohol consumption. Notably, smokers was more in the AAC group. Additionally, it is well known that AAC is significantly associated with chronic kidney disease (CKD); the prevalence of CKD was significantly higher in the AAC group. Furthermore, we collected detailed demographic data and comprehensive hematological indicators for both groups, as shown in Table 1.
Association between BRI and AAC
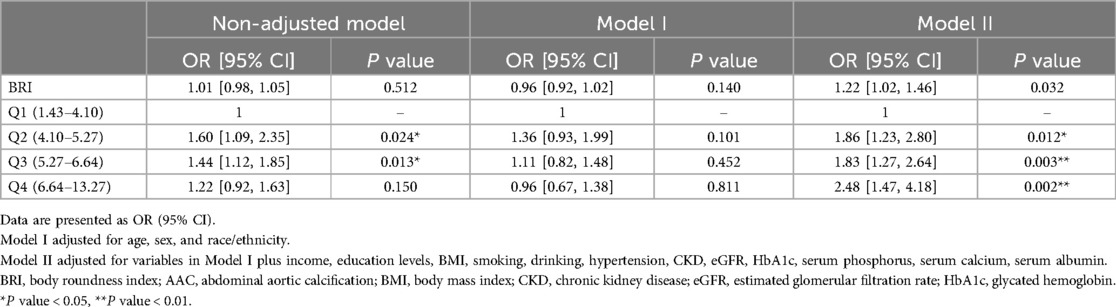
In this study, we utilized weighted multivariable logistic regression to investigate the relationship between BRI and AAC. We found that after adjusting for confounding variables, an increase of one unit in BRI was associated with a 22% higher risk of AAC (OR: 1.22; 95% CI: 1.02, 1.46). Additionally, compared to the BRI Q1 group, the risk of AAC in the BRI Q2, Q3, and Q4 groups was 1.86 (95% CI: 1.23, 2.80), 1.83 (95% CI: 1.27, 2.64), and 2.48 (95% CI: 1.47, 4.18) times higher, respectively (Table 2). Additionally, we used RCS to visualize the relationship between BRI and AAC (Figure 2). We found that as BRI increased, the risk of AAC gradually increased. Notably, after BRI exceeded 7.2, the trend of increasing AAC risk slowed down. However, our analysis indicates that there is no significant non-linear association between BRl and AAC, with the non-linear p value being 0.12, therefor we didn't carry out segmented logistic regression further. We also analyzed the relationship between BRI and AAC in different age and gender groups. It is worth noting that among individuals aged 40–49, the increase in AAC risk with increasing BRI was not significant. However, in both male and female populations, the risk of AAC increased significantly with higher BRI. However, no non-linear association was found between BRI and AAC among different populations.
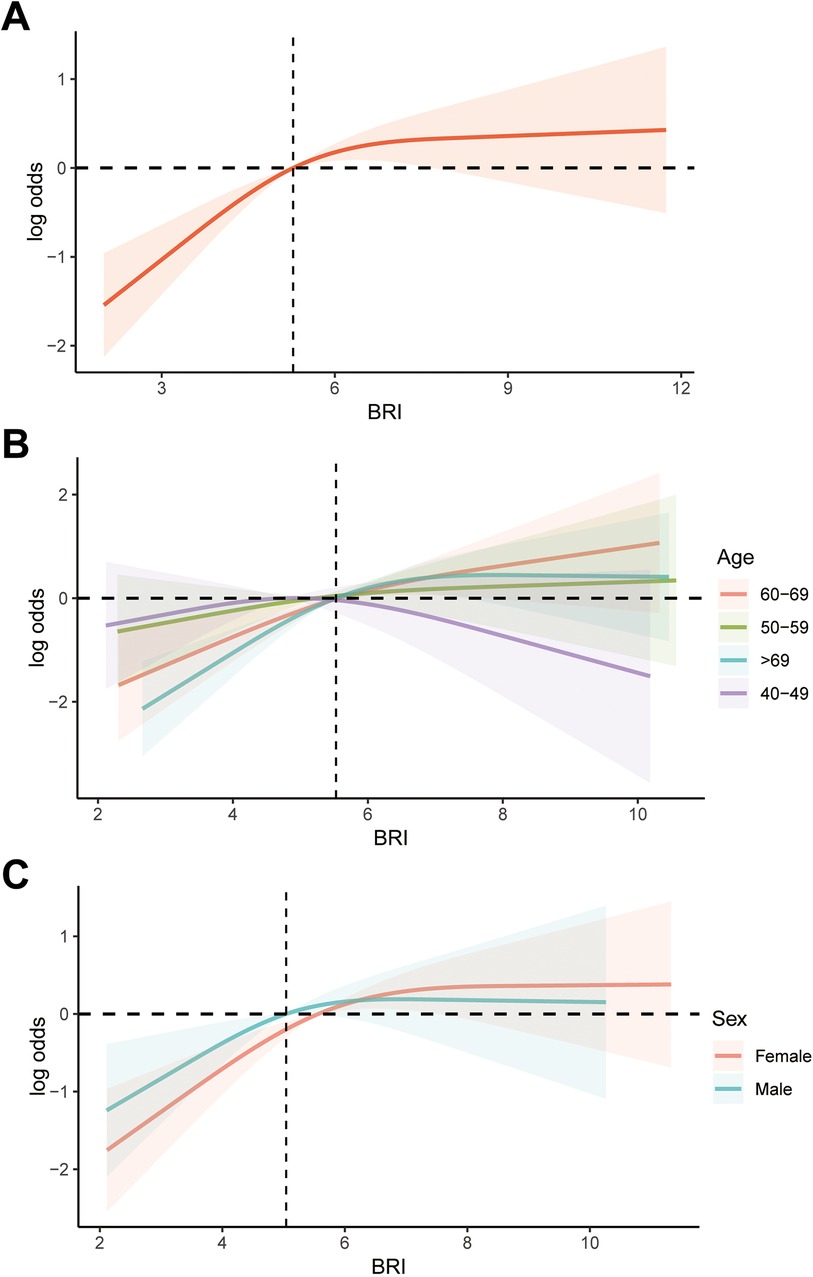
Figure 2. RCS analysis of the association between BRI and AAC. (A) Overall analysis; (B) subgroup analysis stratified by age; (C) subgroup analysis stratified by age gender. RCS, restricted cubic splines; BRI, body roundness index; AAC, abdominal aortic calcification.
Discussion
We utilized weighted analytical methods to investigate the relationship between the BRI, an indicator of abdominal obesity, and AAC among US residents. We found a positive association suggesting a close relationship between abdominal obesity and AAC.
Due to the limitations of traditional anthropometric measurements, such as BMI or WC, on evaluating of abdominal obesity, BRI has been applied in a large number of previous studies investigating abdominal obesity and its effects on the body. Zhang et al. tracked BRI trends in US adults over 20 years and its association with all-cause mortality. The investigators found that BRI, as a measure reflecting body fat distribution, increased steadily among participants. They also revealed a U-shaped relationship with mortality risk, higher risks were observed at both low and high BRI values compared to mid-range values. Their findings suggest BRI could serve as a valuable noninvasive tool for estimating mortality risk, potentially enhancing public health strategies pending further validation across diverse populations (18). Zhang et al. explored the association between BRI and depression also using NHANES data. BRI was found to correlate positively with depression prevalence among 18,654 adults aged ≥20 years. Adjusted analysis revealed an 8% increase in depression prevalence with each one-unit rise in BRI. The findings suggest BRI could be a valuable tool for predicting depression risk, though causal relationships couldn't be established due to the study's cross-sectional nature and absence of clinical depression diagnoses (19). Another study investigated the correlation between BRI and overactive bladder and confirmed a positive association (OR = 1.15, 95% CI = 1.12–1.17) between BRI and OAB. Moreover, subgroup analyses and ROC curves indicated BRI's superior predictive capability compared to other anthropometric indices, with gender-specific cutoffs (men: 5.151, women: 5.383) suggesting varying susceptibility (20). Experienced measurers in this study used standardized instruments to measure body measurements of participants included in the study. We calculated the Body Roundness Index for all participants using formulas established in previous research.
Considering AAC has a strong predictive capability for cardiovascular adverse events, many studies have conducted detailed research on the risk factors of AAC. A previous study found that participants aged 40 and above showed higher AAC prevalence with age, smoking, diabetes, and hypertension, however, the investigators did not find association between obesity and AAC. The investigators also found that engagement in moderate-to-vigorous physical activity, particularly occupational, was associated with lower AAC scores (β = 0.46, p < 0.001). Prolonged sedentary behavior correlated with higher AAC scores (β = 0.28, p = .023). Moreover, subgroup analyses underscored age and hypertension as influencing factors. The findings emphasize the cardiovascular benefits of moderate-to-vigorous physical activity and reduced sedentary time, especially for vulnerable populations (21). Numerous studies have shown that inflammation plays a crucial role in vascular calcification (22, 23), and abdominal obesity can lead to increased systemic inflammation levels in the body. Another study examined the relationship between the systemic immune-inflammation index and AAC, Multivariate logistic regression revealed a positive association, each 100-unit increase in SII correlated with a 4% higher risk of severe AAC. Participants in the highest SII quartile faced a 47% greater risk of severe AAC compared to the lowest quartile, with stronger effects observed in adults over 60 years old. These findings suggest SII could enhance AAC prevention strategies in the US adult population (24). Cai et al. explored the relationship between Life's Essential 8 (LE8), a measure of cardiovascular health, and AAC in adults aged ≥40 years. They found that higher LE8 scores correlated with lower AAC scores and prevalence, especially severe AAC. Adjustment for confounders showed significant associations, with nicotine exposure, blood glucose, and blood pressure components of LE8 demonstrating notable impacts (25). Currently, there is limited research on the relationship between body shape indices specific to abdominal obesity and AAC, and the relationship between waist circumference and AAC has not been reported. A previous cross-sectional study analyzed the association between body shape index (ABSI) and abdominal AAC using data from 3,140 participants aged 40–80 years from the 2013–2014 NHANES. The study found that higher ABSI was significantly associated with an increased risk of AAC (39.8% vs. 23.7%, P < 0.001), with multivariate logistic regression and ROC curves confirming that ABSI was independently linked to AAC and had favorable discriminant ability. However, ABSI is not specifically designed for abdominal obesity. In this study, we investigated the relationship between the BRI, a measure specifically for abdominal obesity, and AAC (26). In this study, we conducted a cross-sectional study to investigate the association between BRI and AAC in a large sample of middle-aged and elderly individuals. Abdominal obesity is characterized by an excess of visceral fat, which secretes pro-inflammatory cytokines such as TNF-α, IL-6, and CRP (27). These inflammatory mediators contribute to chronic low-grade inflammation, which promotes endothelial dysfunction and vascular inflammation (28). This inflammatory environment can lead to the activation of vascular smooth muscle cells and the promotion of their differentiation into osteoblast-like cells, which contributes to the calcification of the arterial walls. Visceral fat in abdominal obesity increases the production of ROS. Oxidative stress damages endothelial cells and promotes the accumulation of advanced glycation end-products (AGEs) in the vascular tissue. AGEs and oxidative stress can stimulate the expression of calcification-related proteins such as osteopontin and matrix Gla-protein, which contribute to vascular calcification (29, 30). This study may complement previous research on risk factors associated with AAC.
There are some limitations in the present study should be addressed, firstly, this study is a cross-sectional study, therefore, it is not possible to determine a causal relationship between BRI and AAC. More prospective studies are still needed. Secondly, all participants enrolled in this study were from the United States, so further research is needed to determine whether the findings of this study can be generalized to other countries and ethnic groups. Third, among all NHANES study cycles, only the 2013–2014 cycle included data related to AAC. Compared to other NHANES studies, this study had a limited amount of available research data. Therefore, the conclusions of this study require further confirmation through future works. Lastly, although our study focuses on AAC, it is essential to recognize that AAC differs significantly from other types of arterial calcification. AAC specifically refers to calcification within the abdominal aorta, which may have different pathophysiological mechanisms and clinical implications compared to calcification in other arteries, such as those in the coronary or cerebral circulation. Cardiovascular and cerebrovascular calcification are associated with distinct risk factors and disease processes, which may not be fully captured by assessing AAC alone.
Conclusion
In the present cross-sectional study enrolling participants from NHANES, we found that BRI, a novel measurement for evaluating abdominal obesity, was positively associated with AAC among US middle-aged and elderly participants. More attention should be paid to abdominal obesity for overall vascular health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the NCHS Ethics Review Board protects the rights and welfare of NHANES participants. The NHANES protocol complies with the U.S. Department of Health and Human Services Policy for the Protection of Human Research Subjects. NCHS IRB/ERC Protocol number: #2011-17. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. DL: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. XC: Conceptualization, Data curation, Investigation, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bartstra JW, Mali W, Spiering W, de Jong PA. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prev Cardiol. (2021) 28:1386–91. doi: 10.1177/204748732091989534647579
2. Szulc P. Abdominal aortic calcification: a reappraisal of epidemiological and pathophysiological data. Bone. (2016) 84:25–37. doi: 10.1016/j.bone.2015.12.00426688274
3. Golledge J. Abdominal aortic calcification: clinical significance, mechanisms and therapies. Curr Pharm Des. (2014) 20:5834–8. doi: 10.2174/138161282066614021219530924533938
4. Nadanaka S, Kitagawa H. EXTL2 Controls liver regeneration and aortic calcification through xylose kinase-dependent regulation of glycosaminoglycan biosynthesis. Matrix Biol. (2014) 35:18–24. doi: 10.1016/j.matbio.2013.10.01024176719
5. Raynor WY, Borja AJ, Zhang V, Kothekar E, Lau HC, Ng SJ, et al. Assessing coronary artery and aortic calcification in patients with prostate cancer using (18)F-sodium fluoride PET/computed tomography. PET Clin. (2022) 17:653–9. doi: 10.1016/j.cpet.2022.07.00936229106
6. Mazziotti G, Tupputi U, Ferrante G, Guglielmi G. Abdominal aortic calcification as a marker of relationship between atherosclerosis and skeletal fragility. J Clin Densitom. (2020) 23:539–42. doi: 10.1016/j.jocd.2020.05.00132536435
7. Desai MY, Cremer PC, Schoenhagen P. Thoracic aortic calcification: diagnostic, prognostic, and management considerations. JACC Cardiovasc Imaging. (2018) 11:1012–26. doi: 10.1016/j.jcmg.2018.03.02329976300
8. Jayalath RW, Mangan SH, Golledge J. Aortic calcification. Eur J Vasc Endovasc Surg. (2005) 30:476–88. doi: 10.1016/j.ejvs.2005.04.03015963738
9. Tao L, Miao L, Guo YJ, Liu YL, Xiao LH, Yang ZJ. Associations of body roundness index with cardiovascular and all-cause mortality: nHANES 2001–2018. J Hum Hypertens. (2024) 38:120–7. doi: 10.1038/s41371-023-00864-437752175
10. González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers. (2017) 3:17034. doi: 10.1038/nrdp.2017.34
11. Tomiyama AJ. Stress and obesity. Annu Rev Psychol. (2019) 70:703–18. doi: 10.1146/annurev-psych-010418-10293629927688
12. Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metab Clin Exp. (2019) 92:71–81. doi: 10.1016/j.metabol.2018.11.00530447223
13. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. (2006) 444:881–7. doi: 10.1038/nature05488
14. Geiker NRW, Astrup A, Hjorth MF, Sjödin A, Pijls L, Markus CR. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes Rev. (2018) 19:81–97. doi: 10.1111/obr.1260328849612
15. Carey DG. Abdominal obesity. Curr Opin Lipidol. (1998) 9:35–40. doi: 10.1097/00041433-199802000-000089502333
16. Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. (2018) 21:360–5. doi: 10.1097/mco.000000000000048529916924
17. Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring). (2013) 21:2264–71. doi: 10.1002/oby.2040823519954
18. Zhang X, Ma N, Lin Q, Chen K, Zheng F, Wu J, et al. Body roundness index and all-cause mortality among US adults. JAMA Netw Open. (2024) 7:e2415051. doi: 10.1001/jamanetworkopen.2024.1505138837158
19. Zhang L, Yin J, Sun H, Dong W, Liu Z, Yang J, et al. The relationship between body roundness index and depression: a cross-sectional study using data from the national health and nutrition examination survey (NHANES) 2011–2018. J Affect Disord. (2024) 361:17–23. doi: 10.1016/j.jad.2024.05.15338815765
20. Zhang Y, Song J, Li B, Wu Y, Jia S, Shu H, et al. Association between body roundness index and overactive bladder: results from the NHANES 2005–2018. Lipids Health Dis. (2024) 23:184. doi: 10.1186/s12944-024-02174-138867211
21. Sheng C, Huang W, Wang W, Lin G, Liao M, Yang P. The association of moderate-to-vigorous physical activity and sedentary behaviour with abdominal aortic calcification. J Transl Med. (2023) 21:705. doi: 10.1186/s12967-023-04566-w37814346
22. Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. (2018) 114:590–600. doi: 10.1093/cvr/cvy01029514202
23. Bessueille L, Magne D. Inflammation: a culprit for vascular calcification in atherosclerosis and diabetes. Cell Mol Life Sci. (2015) 72:2475–89. doi: 10.1007/s00018-015-1876-425746430
24. Xie R, Liu X, Wu H, Liu M, Zhang Y. Associations between systemic immune-inflammation index and abdominal aortic calcification: results of a nationwide survey. Nutr Metab Cardiovasc Dis. (2023) 33:1437–43. doi: 10.1016/j.numecd.2023.04.01537156667
25. Cai Z, Liu Z, Zhang Y, Ma H, Li R, Guo S, et al. Associations between life’s essential 8 and abdominal aortic calcification among middle-aged and elderly populations. J Am Heart Assoc. (2023) 12:e031146. doi: 10.1161/jaha.123.03114638063150
26. Li W, Wang Z, Li M, Xie J, Gong J, Liu N. Association between a body shape index and abdominal aortic calcification in general population: a cross-sectional study. Front Cardiovasc Med. (2022) 9:1091390. doi: 10.3389/fcvm.2022.109139036704474
27. Cavalcante RBM, Leão L, Tavares ABW, Lopes KG, Kraemer-Aguiar LG. Fat distribution and its correlation with insulin resistance, androgen markers, and proinflammatory cytokines in polycystic ovary syndrome. Horm Metab Res. (2024). doi: 10.1055/a-2386-928139226924
28. Machi JF, Altilio I, Qi Y, Morales AA, Silvestre DH, Hernandez DR, et al. Endothelial c-myc knockout disrupts metabolic homeostasis and triggers the development of obesity. Front Cell Dev Biol. (2024) 12:1407097. doi: 10.3389/fcell.2024.1407097
29. Martins MSA, Carneiro WF, Monteiro KS, Souza SP, Vianna A, Murgas LDS. Metabolic effects of physical exercise on zebrafish (Danio rerio) fed a high-fat diet. J Comp Physiol B. (2024). doi: 10.1007/s00360-024-01577-x
Keywords: BRI, AAC, elderly, middle age, NHANES, abdominal obesity
Citation: Wu J, Lu D and Chen X (2024) Association of body roundness index with abdominal aortic calcification among middle aged and elderly population: findings from NHANES. Front. Cardiovasc. Med. 11:1475579. doi: 10.3389/fcvm.2024.1475579
Received: 4 August 2024; Accepted: 23 September 2024;
Published: 10 October 2024.
Edited by:
Li-Da Wu, Nanjing Medical University, ChinaReviewed by:
Chuan Shao, Chongqing General Hospital, ChinaGou Ruoyu, Ningxia Medical University, China
Copyright: © 2024 Wu, Lu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Chen, c3RhZmY5ODRAeXhwaC5jb20=
†These authors have contributed equally to this work
 Ji Wu
Ji Wu Daojun Lu2,†
Daojun Lu2,†