- 1Department of Pharmacy, Kochi Medical School Hospital, Nankoku, Japan
- 2Department of Clinical Pharmacy Practice Pedagogy, Tokushima University Graduate School of Biomedical Sciences, Tokushima, Japan
- 3Department of Clinical Pharmacology and Therapeutics, Tokushima University Graduate School of Biomedical Sciences, Tokushima, Japan
- 4Department of Cardiology and Geriatrics, Kochi Medical School, Kochi University, Nankoku, Japan
- 5Department of Cardiology, Chikamori Hospital, Kochi, Japan
- 6Department of Cardiology, Kochi Prefectural Hatakenmin Hospital, Sukumo, Japan
- 7Department of Cardiology, Kochi Prefectural Aki General Hospital, Aki, Japan
- 8Department of Cardiology, Susaki Kuroshio Hospital, Susaki, Japan
Background: Constipation frequently affects heart failure patients because of medication side effects and physiological effects of the condition. Although recent speculation suggests that comorbid constipation may affect cardiovascular disease onset and survival rates, this relationship remains unclear. We examined the effect of comorbid constipation on the survival of patients with heart failure.
Methods: We conducted a multicenter prospective cohort study (the Kochi YOSACOI study) of patients hospitalized for acute decompensated heart failure. The influence of comorbid constipation on survival was evaluated using Cox regression analysis with 2-year survival as the index. Patients were divided into two groups based on the presence of comorbid constipation. The patient background was adjusted using propensity score matching, and the evaluation included assessing the 2-year survival and cardiovascular mortality occurrence using the log-rank test.
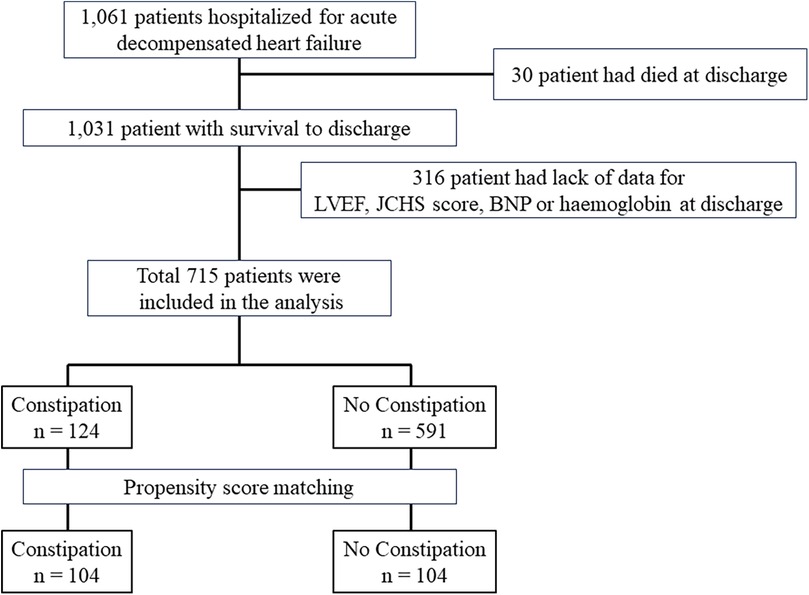
Results: Among 1,061 patients hospitalized for acute decompensated heart failure, 715 with complete data (124 with comorbid constipation and 591 without) were analyzed. Comorbid constipation was identified as a risk factor for poorer survival in the Cox regression model (hazard ratio: 1.90, 95% confidence interval: 1.3–2.8, P < 0.001). Propensity score matching included 104 patients in each group. Survival analysis using the log-rank test indicated worse survival (P = 0.023) and higher cardiovascular mortality (P = 0.043) in the comorbid constipation group.
Conclusion: Constipation can negatively affect the survival of patients with heart failure. Although the causal link between constipation and decreased survival remains unclear, identifying comorbid constipation is essential for identifying heart failure patients at a higher risk of poor outcomes.
1 Introduction
Cardiovascular disease is the leading cause of death globally, with heart failure (HF) being a serious condition characterized by high incidence and mortality rates (1). HF progresses through a series of exacerbations and remissions (2). The cost of treatment and diminishing quality of life (QOL) as the disease advances impose a significant economic burden on patients, families, and society (3). Therefore, to improve treatment and care for patients with HF, it is crucial to understand the factors associated with worsening prognosis. This understanding may help to identify the causes of rehospitalization and death (3). Several factors influence the prognosis of patients with HF. Heart-related factors, such as the left ventricular ejection fraction and atrial fibrillation, are known contributors (4, 5). Additionally, underlying conditions, such as diabetes, dyslipidemia, and hypertension, which are causes of cardiac diseases, play significant roles (6–8). Numerous studies have documented these factors, and international guidelines have emphasized the importance of adopting measures for patients with such comorbidities (6–9). Given that HF commonly affects older adults and is frequently associated with multiple underlying diseases (10), further research on comorbidities and their impact on HF is warranted.
Constipation usually presents with a decreased frequency of bowel movements and residual stools and is caused by organ-related factors, diet, lifestyle, and side effects of drugs (11). A Japanese study reported that 3.5% of the population suffers from constipation, with its prevalence worsening with age. Among individuals over 80 years old, the prevalence increases to 10% (12, 13). In developed countries, the population of patients with HF is aging, especially in Japan, which has the highest proportion of older adults aged 65 and over due to its long life expectancy and low birth rate (14). As a result, the average age of patients with HF in Japan is 79 years, which is higher than in other countries. This population is more likely to be affected by age-related diseases compared to patients in countries like the United States (average age 73 years) and Taiwan (average age 74 years), both of which are also developed countries (15). Moreover, constipation is more prevalent among patients with cardiovascular disease. A previous study in Japan reported a higher prevalence of 47% of constipation among patients hospitalized for cardiovascular conditions (16). Additionally, 33% of older adult patients with HF in the United States (aged 65 and older) reported a history of constipation (17). Recent research has shown that patients with comorbid constipation are at an increased risk of developing cardiovascular diseases such as coronary artery disease and ischemic stroke (16, 17). Furthermore, constipation has been linked to higher rates of rehospitalization among patients with HF (13). However, no studies have yet examined whether constipation impacts mortality in patients with HF. Therefore, this study aims to investigate the effect of constipation on mortality in patients with HF in Japan, where the older adult population is particularly high, to emphasize the importance of managing bowel movements as part of HF treatment.
2 Materials and methods
2.1 Patient population
This study used data from the Kochi Registry of Subjects with Acute Decompensated HF (YOSACOI) study, which registered 1,061 patients hospitalized for acute decompensated HF in Kochi, Japan, between May 2017 and December 2019. The dataset for this study was accessed on April 20, 2023. Patients were followed up until December 2021 to gather data on clinical outcomes, specifically focusing on all-cause mortality within a 2-year period. The details of the YOSACOI study have been explained in previous studies (18). The YOSACOI study involved collaboration among six hospitals that provide acute care for cardiovascular diseases in Kochi Prefecture, where the population of older adults aged ≥65 years constituted 35%. All participating hospitals undertook acute HF treatment according to standard guidelines (19). The eligibility criteria for enrolment were age ≥20 years and admission for acute decompensated HF (ADHF) at one of the participating hospitals. According to the Framingham criteria, ADHF is diagnosed based on the presence of at least two major criteria, including symptoms, physical examination, chest radiography, and echocardiographic findings, or one major and two minor criteria. Comorbid constipation was defined as the continuous use of laxatives from pre-admission to discharge.
This study was approved by the Ethics Committee of the Kochi Medical School (Approval No. 28–68). The study conformed to the principles outlined in the Declaration of Helsinki, and written informed consent was obtained from all patients or their families. Confidentiality and anonymity of patient data were maintained throughout.
2.2 Study design and statistical analysis
The researchers collected data from the participating hospitals during the enrolment period. We obtained information regarding patient demographics, HF etiology, medical history, HF symptoms, vital signs at discharge, laboratory data, echocardiographic data, other clinical parameters, and discharge prescriptions. The patients' nutritional status was assessed using the Geriatric Nutritional Risk Index (GNRI), a simple indicator of nutritional status in older adults, calculated using the following formula: GNRI = 14.89 × serum albumin (g/dl) + 41.7 × body weight (kg)/ideal body weight (kg) (20). Renin-angiotensin system (RAS) inhibitor use was defined as the prescription of an angiotensin-converting enzyme inhibitor or angiotensin-II receptor antagonist. Angiotensin receptor neprilysin inhibitors and sodium-glucose cotransporter-2 inhibitors were not approved in Japan during the registration period (21).
A flowchart depicting the patient selection process is shown in Figure 1. Among the initial patient cohort (n = 1,061), 30 patients who were discharged owing to death, and 316 patients with missing data on left ventricular ejection fraction (LVEF), the Japanese version of the Cardiovascular Health Study (J-CHS) score, brain natriuretic peptide (BNP) level, and medications were excluded. The remaining 715 cases were analyzed in this study. First, the 715 patients were divided into two groups based on whether they died within 2 years of enrolment, and the patient backgrounds of each group were compared. Second, univariate and multivariate Cox regression analyses were performed to evaluate the adjusted risk ratios of the variables in 715 patients included in the analysis. Factors with P < 0.1 on univariate analysis were used as explanatory variables for multivariate Cox regression analysis. The number of events per variable in the Cox regression analyses was set according to previous studies (22), with an event-to-variable ratio of 9.7. Third, patients were divided into two groups based on the presence of comorbid constipation. Patient backgrounds were matched using propensity score matching. The factors used in propensity score matching were age, sex, GNRI, New York Heart Association (NYHA) class III/IV at discharge, systolic blood pressure (mmHg), hospitalization history, BNP, estimated glomerular filtration rate (eGFR), hemoglobin level, sodium level, LVEF, J-CHS score, comorbid atrial fibrillation, chronic obstructive pulmonary disease (COPD), diabetes, and the use of RAS inhibitors, β-blockers, mineralocorticoid receptor antagonists, loop diuretics, thiazide diuretics, and tolvaptan (8, 19, 21, 23–28). We selected these factors based on their reported associations with constipation and HF. After creating the propensity score, we conducted 1:1 nearest-neighbor matching of the logit of the propensity score using a caliper width of 0.2 standard deviations of the propensity score (29). Matching was performed without replacement, and cases that did not meet the matching criteria were excluded. Standardized differences were used to measure covariate balance, which was defined as an absolute value exceeding 1.96 × √2/n (30). After matching, 2-year mortality and mortality due to cardiovascular causes were compared using the log-rank test. The 2-year mortality rate for the non-constipation group was set at 81%, while the increased mortality rate due to constipation was set at 19%. Assuming a 1:1 composition for each group, with a two-sided alpha of 0.05% and 80% power, the required sample size for each group was 71 individuals.
Data are expressed as medians with interquartile ranges (IQR) for variables that are not normally distributed and as frequencies (percentages) for categorical variables. Differences in variables were analyzed using the Mann–Whitney U test. Fisher's exact test was used to analyze categorical data. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox regression analysis, and multivariate Cox regression analysis was used to examine the adjusted relative risk of the variables. Propensity score matching and sample size calculation for the log-rank test were performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria). All other statistical analyses were conducted using EZR version 1.51 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (31).
3 Results
3.1 Univariate analysis of factors potentially associated with two-year mortality
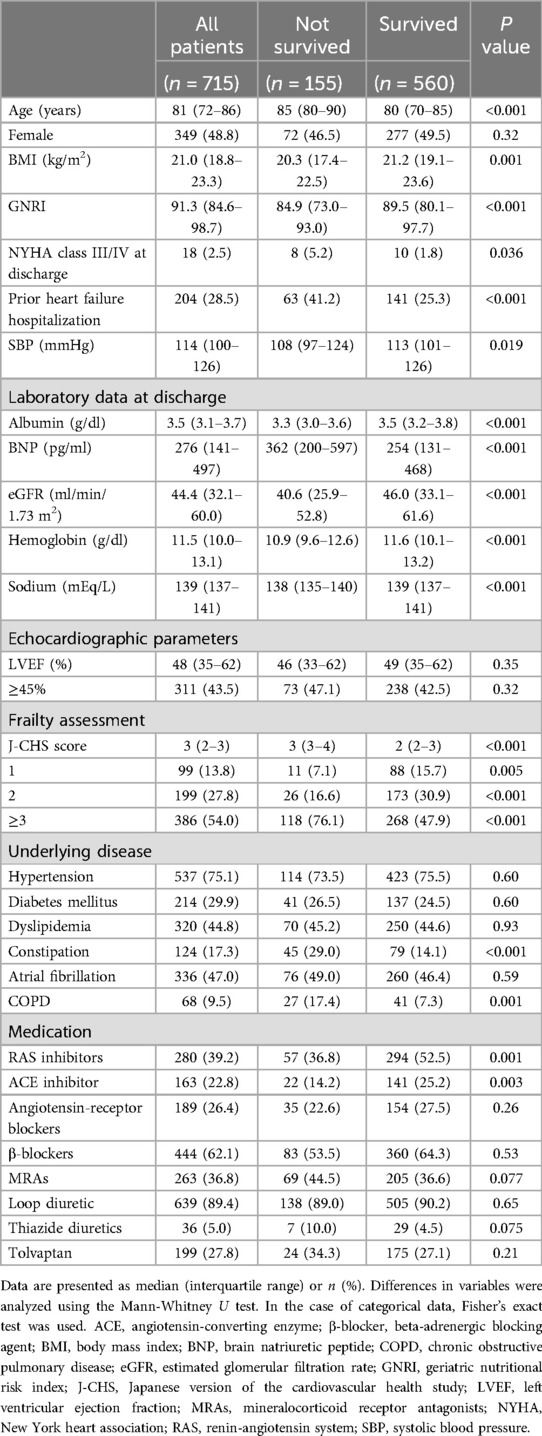
Among the 715 patients, 155 died during the 2-year follow-up period. Patients were categorized based on 2-year mortality, and differences in patient characteristics between groups were analyzed using univariate analysis. The results (Table 1) showed that patients who died were older [median (IQR): 85 (80–90) vs. 80 (70–85) years, P < 0.001], had lower GNRI [median (IQR): 84.9 (73.0–93.0) vs. 89.5 (80.1–97.7), P < 0.001], lower frequency of NYHA class III/IV at discharge [8 (5.2%) vs. 10 (1.8%) cases, P = 0.036], lower frequency of prior hospitalization with HF [63 (41.2%) vs. 141 (25.3%) cases, P < 0.001], higher BNP [median (IQR): 362 (200–597) vs. 254 (131–468), P < 0.001], lower eGFR [median (IQR): 40.6 (25.9–52.8) vs. 46.0 (33.1–61.6), P < 0.001], lower sodium level [median (IQR): 138 (135–140) vs. 139 (137–141), P < 0.001], higher J-CHS score [median (IQR): 3 (3–4) vs. 2 (2–3), P < 0.001], higher frequency of constipation [45 (29.0%) vs. 79 (14.1%) cases, P < 0.001], higher frequency of COPD [27 (17.4%) vs. 41 (7.3%) cases, P = 0.001], and more frequent use of RAS inhibitors [57 (36.8%) vs. 294 (52.5%) cases, P = 0.001].
3.2 Cox regression analysis of risk factors for all-cause mortality in 2 years
The results of multivariable Cox regression analysis, as shown in Table 2, revealed that underlying COPD (HR: 2.07, 95% CI: 1.28–3.35, P = 0.003), underlying constipation (HR: 1.86, 95% CI: 1.25–2.75, P = 0.002), higher J-CHS score (HR: 1.31, 95% CI: 1.12–1.54, P = 0.001), older age (HR: 1.06, 95% CI: 1.03–1.08, P < 0.001), and lower sodium level (HR: 0.90, 95% CI: 0.86–0.95, P < 0.001) were independently associated with all-cause mortality within 2 years (Table 2).
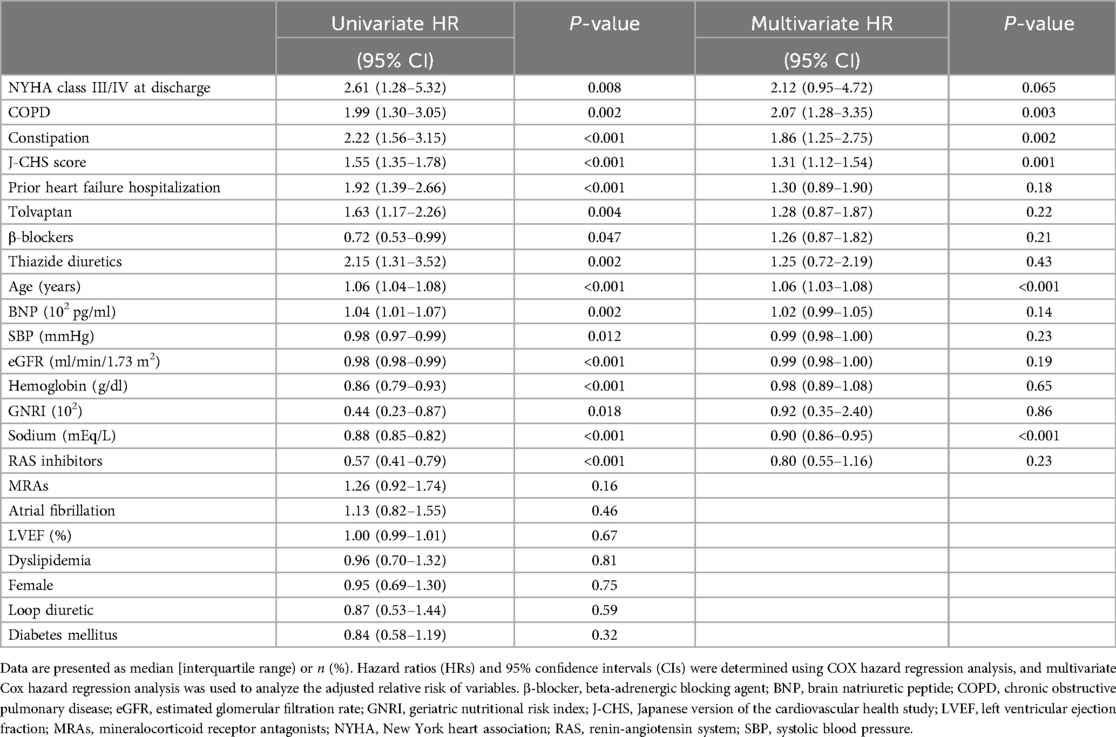
Table 2. Cox regression analysis of the risk factors for all-cause death (2 years) in patients with heart failure.
3.3 Baseline characteristics of the patients based on underlying constipation
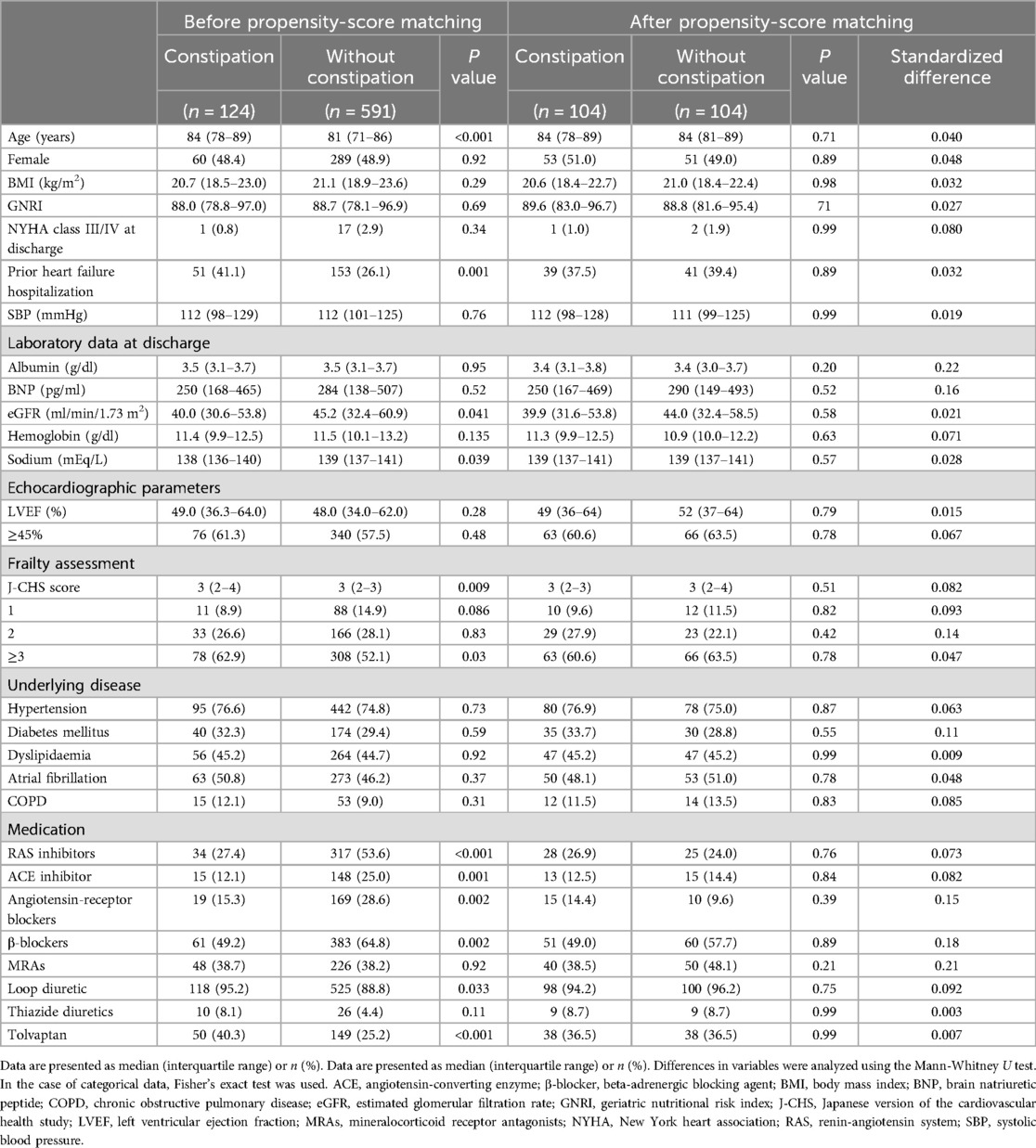
After 1:1 matching, equal numbers of patients with underlying constipation (n = 104 per group) were matched, and the cut-off value of the absolute standardized difference for imbalance was 0.27. Across the baseline covariates, the absolute standardized differences ranged from 0.003–0.22, indicating that the mean and frequency of continuous and dichotomous variables were similar in both matched groups (Table 3). Log-rank tests showed that the group with comorbid constipation had a higher incidence of all-cause mortality (P = 0.023) and cardiovascular death (P = 0.043) than the non-comorbid constipation group (Figure 2).
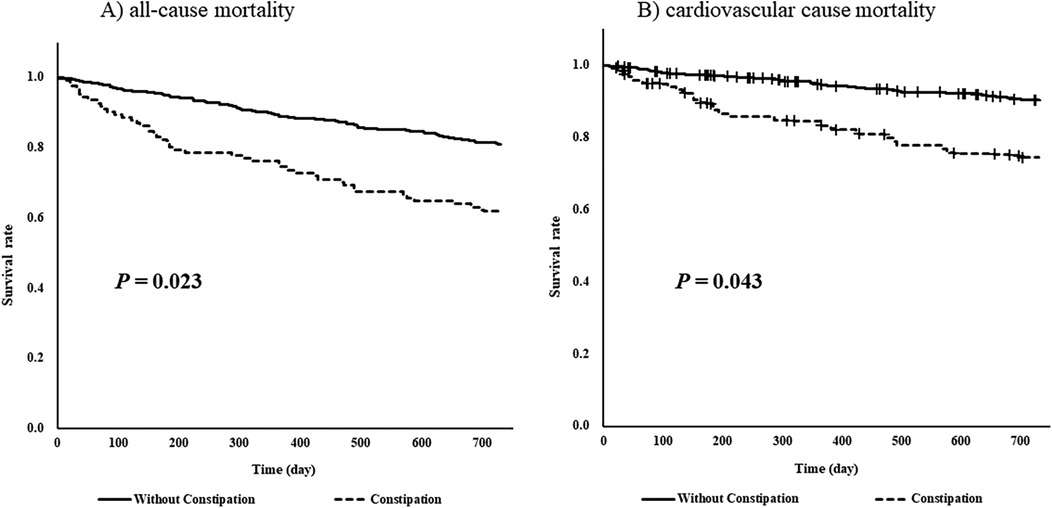
Figure 2. Log-rank tests of (A) all-cause mortality and (B) cardiovascular cause mortality for 730 days (2 years) by underlying constipation stratified by propensity-score matching.
3.4 Discussion
To the best of our knowledge, this is the first study to investigate the effect of constipation on the prognosis of patients with HF. The results of Cox regression analysis suggested that constipation may increase the risk of all-cause mortality in patients with HF. Furthermore, when life expectancy was compared using propensity score matching after dividing patients according to constipation, patients with HF and constipation showed a higher incidence of all-cause and cardiovascular mortality rates.
Constipation is a prevalent condition (32). Historically, constipation was regarded as a benign, non-life-threatening condition (33). However, recent studies have linked constipation to an increased risk of mortality (17). Furthermore, underlying constipation may cause complications or worsen the comorbidities (16, 34). Observational studies have indicated a higher likelihood of cardiovascular events such as stroke and myocardial infarction in individuals with underlying constipation (16, 35). This finding suggests a potential impact on the occurrence or exacerbation of other cardiovascular diseases, including HF. Nevertheless, no study has specifically examined the influence of a history of constipation on the prognosis of patients with HF. Therefore, our study aimed to investigate the association between constipation and prognosis in patients with HF. Cox regression analysis revealed that a history of constipation was a risk factor for early death. Furthermore, to examine the impact of constipation on the prognosis of patients with HF, we divided patients into groups based on the presence of constipation, matched patient backgrounds using propensity score matching, and evaluated the survival rate. Our results suggest that underlying constipation may significantly increase all-cause and cardiovascular mortality rates.
Several hypotheses have been proposed to explain the mechanisms by which constipation worsens symptoms of HF. First, straining during defecation can elevate blood pressure, potentially triggering cardiovascular events such as arrhythmias, acute coronary events, and aortic dissection (36). Elevated blood pressure also imposes an increased cardiac workload on patients with HF, potentially worsening their prognosis.
Second, changes in intestinal flora due to constipation can lead to an increase in inflammatory mediators (16). In patients with HF, hypoperfusion of the viscera leads to intestinal ischemia and edema, which has been proposed as a hypothetical mechanism by which the intestinal barrier function is compromised, and bacteria and their metabolites enter the systemic circulation (37). This process triggers both local and systemic inflammatory responses. Furthermore, gut microbiota contributes to endothelial dysfunction by promoting the upregulation of inflammatory signaling and the migration of leukocytes to endothelial cells through metabolites such as trimethylamine and uremic toxins (38–40). Endothelial dysfunction is a central factor in the pathogenesis of various cardiac disorders, including HF. It induces oxidative stress, endothelial impairment, increased secretion of adipokines, inflammatory cytokines (e.g., IL-6, TNF-α), endothelin-1, and fibroblast growth factors, as well as reduced nitric oxide production (41). These alterations in inflammatory cytokines exacerbate cardiac metabolism and diastolic function, thereby promoting the development and progression of cardiovascular diseases, including HF, and worsening their symptoms (41, 42). Therefore, if constipation negatively impacts the pathogenesis of HF, early intervention for constipation—such as pharmacotherapy, diet and exercise, and appropriate bowel management—may improve the prognosis of HF.
Constipation is common in patients with HF, and several hypotheses have been proposed to explain its prevalence in this population. First, gastrointestinal dysmotility resulting from an imbalance between sympathetic and parasympathetic activity plays a significant role (43). Increased sympathetic nervous system activity, which is characteristic of HF, reduces visceral circulation and, combined with a decreased cardiac output, can lead to intestinal ischemia. Studies have shown that blood flow to the intestines in patients with HF is reduced by approximately 30%–40% (44), impairing intestinal motility and contributing to constipation. In addition, this imbalance is also evident in HF, where heightened sympathetic nervous system activity places additional strain on the heart (45). Additionally, the high prevalence of constipation in patients with HF can be attributed to the aging population, as HF is more common among the older adults. Aging is a known risk factor for both HF and constipation, with physiological changes such as decreased gastrointestinal transit time and altered gut microbiota composition (46). In this study, the median age of patients with constipation was approximately 84 years, significantly older than that of patients without constipation [median (IQR): 84 (78–89) vs. 81 (71–86) years, P < 0.001]. This finding is consistent with previous observational studies (13). Another contributing factor is the reduction in total body water due to fluid restriction, a common management strategy in HF treatment (47). Diuretics, which are frequently prescribed to patients with HF, can decrease stool moisture content, exacerbating constipation (48). In this study, a higher proportion of constipated patients were prescribed diuretics [Loop diuretics: 118 (95.2%) vs. 525 (88.8%), P = 0.033; Tolvaptan: 50 (40.3%) vs. 149 (25.2%), P < 0.001], suggesting that diuretic use is a significant contributor to constipation in this population. Furthermore, intestinal permeability abnormalities resulting from hypoperfusion may lead to systemic inflammation, which can influence both HF progression and gastrointestinal function. These interconnected factors suggest a bidirectional relationship where constipation may exacerbate HF symptoms and vice versa. However, after analyzing patient backgrounds, including age and diuretic use, through propensity score matching, a higher mortality rate was observed among patients with constipation. This suggests that constipation may be an independent risk factor for increased mortality in patients with HF beyond the effects of diuretic use and other confounders.
3.5 Limitations
This study had a few limitations. First, it was an observational study. Although covariates were adjusted using propensity score matching to minimize selection bias, the findings should be interpreted with caution due to potential residual confounding and imbalances in baseline patient characteristics. Furthermore, propensity score matching cannot completely exclude all confounders, which may introduce selection bias. Therefore, the results must be verified in larger prospective studies or RCTs to ensure their validity and generalizability. Second, the symptoms associated with constipation were not comprehensively assessed in this observational study. Constipation was defined based on the use of constipation medication; therefore, future studies requiring evaluation of symptoms such as constipation severity should consider defining constipation according to symptom criteria such as the Rome III Criteria or other relevant standards (49). Third, the effect of the choice of constipation medication on the prognosis of HF has not yet been clarified. In this study, patients with constipation were administered different laxatives. Magnesium oxide was the most commonly prescribed agent (Supplementary Figure S1). However, hypermagnesemia reportedly negatively affects the prognosis of HF (50). Future studies should clarify the laxatives that are appropriate for use in patients with HF. Fourth, this study was unable to examine the patient's background regarding physical activity levels and dietary habits. These factors could have influenced both constipation and HF severity (51, 52). The absence of these confounding variables may affect the precision of our findings. Incorporating them into the analysis as confounding factors would have allowed for a more accurate examination of the relationship between constipation and HF outcomes. Future research should consider including physical activity and dietary assessments to enhance the validity of the results. Fifth, this study did not evaluate the severity of constipation. The severity of constipation, including factors such as frequency of defecation and duration of constipation episodes, may significantly influence HF outcomes and other comorbidities (53). Incorporating standardized measures of constipation severity into the analysis would enable a more precise assessment of its impact on HF prognosis. Future research should consider employing validated tools to grade constipation severity, thereby allowing for more accurate interclass comparisons and reducing potential variability among patients. Sixth, this study did not investigate the effect of constipation on quality of life (QOL). Previous studies have suggested that individuals with chronic constipation may experience reduced mental, social, and physical aspects of QOL compared with healthy individuals (33). However, the impact of constipation on QOL, specifically among patients with HF, has not been explored in this study. The absence of QOL assessment may limit the comprehensive understanding of how constipation affects overall patient well-being and disease management in HF. Therefore, future studies should incorporate validated QOL measurement tools to investigate this relationship and clarify the broader implications of constipation on the quality of life in HF populations.
In conclusion, this study suggests that constipation may negatively impact the prognosis of patients with HF. Although the causal relationship between constipation and worse prognosis in patients with heart failure is unknown, the presence of comorbid constipation should be considered when identifying patients with heart failure and poor prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Kochi Medical School. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TI: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KK: Investigation, Methodology, Validation, Writing – review & editing. KJ: Data curation, Methodology, Writing – review & editing. TH: Data curation, Investigation, Methodology, Writing – review & editing. TK: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. MO: Investigation, Writing – review & editing. KK: Investigation, Writing – review & editing. YN: Investigation, Writing – review & editing. TY: Investigation, Writing – review & editing. TF: Investigation, Writing – review & editing. EY: Investigation, Writing – review & editing. HK: Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing. YH: Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Kochi Prefecture Sponsorship Project, Bayer Yakuhin, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical, and Takeda Pharmaceutical Company.
Acknowledgments
Participating investigators from the study hospitals were Saho Tsumura, RN; Takayuki Maeda, PT (Kochi Medical School, Kochi University); Takako Fujita, MD; Hideyuki Matsuda, MD (Chikamori Hospital); Naoto Osawa, MD (Kochi Prefectural Hatakenmin Hospital); Masanori Kuwabara, MD (Kochi Prefectural Aki General Hospital); Mana Kusunose, MD (Susaki Kuroshio Hospital); and Yasumasa Kawada, MD (Japanese Red Cross Kochi Hospital). We thank all the physicians who made this study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fcvm.2024.1470216/full#supplementary-material
References
1. Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. (2021) 128:1421–34. doi: 10.1161/CIRCRESAHA.121.318172
2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. Corrigendum to: 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2021) 42:4901. doi: 10.1093/eurheartj/ehab670
3. Heidenreich PA, Fonarow GC, Opsha Y, Sandhu AT, Sweitzer NK, Warraich HJ, et al. Economic issues in heart failure in the United States. J Card Fail. (2022) 28:453–66. doi: 10.1016/j.cardfail.2021.12.017
4. Reddy YNV, Borlaug BA, Gersh BJ. Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation. (2022) 146:339–57. doi: 10.1161/CIRCULATIONAHA.122.057444
5. Branca L, Sbolli M, Metra M, Fudim M. Heart failure with mid-range ejection fraction: pro and cons of the new classification of heart failure by European society of cardiology guidelines. ESC Heart Fail. (2020) 7:381–99. doi: 10.1002/ehf2.12586
6. Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Med. (2017) 130:S40–50. doi: 10.1016/j.amjmed.2017.04.010
7. Nakagomi A, Seino Y, Kohashi K, Kosugi M, Endoh Y, Kusama Y, et al. Effects of statin therapy on the production of monocyte pro-inflammatory cytokines, cardiac function, and long-term prognosis in chronic heart failure patients with dyslipidemia. Circ J. (2012) 76:2130–8. doi: 10.1253/circj.cj-11-1123
8. Di Palo KE, Barone NJ. Hypertension and heart failure: prevention, targets, and treatment. Heart Fail Clin. (2020) 16:99–106. doi: 10.1016/j.hfc.2019.09.001
9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2023) 44:3627–39. doi: 10.1093/eurheartj/ehad195
10. Skrzypek A, Mostowik M, Szeliga M, Wilczynska-Golonka M, Debicka-Dabrowska D, Nessler J. Chronic heart failure in the elderly: still a current medical problem. Folia Med Cracov. (2018) 58:47–56. doi: 10.24425/fmc.2018.125703
11. Sharma A, Rao SSC, Kearns K, Orleck KD, Waldman SA. Review article: diagnosis, management and patient perspectives of the spectrum of constipation disorders. Aliment Pharmacol Ther. (2021) 53:1250–67. doi: 10.1111/apt.16369
12. Gallegos-Orozco JF, Foxx-Orenstein AE, Sterler SM, Stoa JM. Chronic constipation in the elderly. Am J Gastroenterol. (2012) 107:18–25. doi: 10.1038/ajg.2011.349
13. Namiuchi S, Tanita A, Sunamura S, Onodera K, Ogata T, Noda K, et al. Effect of constipation on readmission for heart failure in patients with acute heart failure. ESC Heart Fail. (2024) 11:819–25. doi: 10.1002/ehf2.14650
14. Statistics Bureau MoIAaC. Population of the Elderly (2021). Available online at: http://www.stat.go.jp/data/topics/topi1291.html (accessed December 2024).
15. Sundaram V, Nagai T, Chiang CE, Reddy YNV, Chao TF, Zakeri R, et al. Hospitalization for heart failure in the United States, UK, Taiwan, and Japan: an international comparison of administrative health records on 413,385 individual patients. J Card Fail. (2022) 28:353–66. doi: 10.1016/j.cardfail.2021.08.024
16. Ishiyama Y, Hoshide S, Mizuno H, Kario K. Constipation-induced pressor effects as triggers for cardiovascular events. J Clin Hypertens. (2019) 21:421–5. doi: 10.1111/jch.13489
17. Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Yamagata K, et al. Constipation and risk of death and cardiovascular events. Atherosclerosis. (2019) 281:114–20. doi: 10.1016/j.atherosclerosis.2018.12.021
18. Hamada T, Kubo T, Kawai K, Nakaoka Y, Yabe T, Furuno T, et al. Frailty in patients with acute decompensated heart failure in a super-aged regional Japanese cohort. ESC Heart Fail. (2021) 8:2876–88. doi: 10.1002/ehf2.13363
19. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
20. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. (2013) 77:705–11. doi: 10.1253/circj.cj-12-1091
21. Hamada T, Kubo T, Kawai K, Nakaoka Y, Yabe T, Furuno T, et al. Frailty interferes with the guideline-directed medical therapy in heart failure patients with reduced ejection fraction. ESC Heart Fail. (2023) 10:223–33. doi: 10.1002/ehf2.14163
22. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. (1995) 48:1503–10. doi: 10.1016/0895-4356(95)00048-8
23. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. (1999) 341:709–17. doi: 10.1056/NEJM199909023411001
24. Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:1178–95. doi: 10.1016/j.jacc.2019.12.059
25. Chen YJ, Sung SH, Cheng HM, Huang WM, Wu CL, Huang CJ, et al. Performance of AHEAD score in an Asian cohort of acute heart failure with either preserved or reduced left ventricular systolic function. J Am Heart Assoc. (2017) 6:e004297. doi: 10.1161/JAHA.116.004297
26. Kaneko H, Suzuki S, Goto M, Yuzawa Y, Arita T, Yagi N, et al. Geriatric nutritional risk index in hospitalized heart failure patients. Int J Cardiol. (2015) 181:213–5. doi: 10.1016/j.ijcard.2014.11.167
27. Rodriguez M, Hernandez M, Cheungpasitporn W, Kashani KB, Riaz I, Rangaswami J, et al. Hyponatremia in heart failure: pathogenesis and management. Curr Cardiol Rev. (2019) 15:252–61. doi: 10.2174/1573403X15666190306111812
28. Guder G, Stork S. COPD and heart failure: differential diagnosis and comorbidity. Herz. (2019) 44:502–8. doi: 10.1007/s00059-019-4814-7
29. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
30. Wickham RJ. Managing constipation in adults with cancer. J Adv Pract Oncol. (2017) 8:149–61. doi: 10.6004/jadpro.2017.8.2.3
31. Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
32. Bharucha AE, Pemberton JH, Locke GR 3rd. American gastroenterological association technical review on constipation. Gastroenterology. (2013) 144:218–38. doi: 10.1053/j.gastro.2012.10.028
33. Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. (2005) 23:461–76. doi: 10.2165/00019053-200523050-00006
34. Wei L, Ji L, Miao Y, Han X, Li Y, Wang Z, et al. Constipation in DM are associated with both poor glycemic control and diabetic complications: current status and future directions. Biomed Pharmacother. (2023) 165:115202. doi: 10.1016/j.biopha.2023.115202
35. Salmoirago-Blotcher E, Crawford S, Jackson E, Ockene J, Ockene I. Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med. (2011) 124:714–23. doi: 10.1016/j.amjmed.2011.03.026
36. Merkel IS, Locher J, Burgio K, Towers A, Wald A. Physiologic and psychologic characteristics of an elderly population with chronic constipation. Am J Gastroenterol. (1993) 88:1854–9.8237932
37. Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. (2021) 228:109–25. doi: 10.1016/j.trsl.2020.08.007
38. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
39. Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. (2015) 65:1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315
40. Maiuolo J, Carresi C, Gliozzi M, Mollace R, Scarano F, Scicchitano M, et al. The contribution of gut microbiota and endothelial dysfunction in the development of arterial hypertension in animal models and in humans. Int J Mol Sci. (2022) 23:3698. doi: 10.3390/ijms23073698
41. Drera A, Rodella L, Brangi E, Riccardi M, Vizzardi E. Endothelial dysfunction in heart failure: what is its role? J Clin Med. (2024) 13:2534. doi: 10.3390/jcm13092534
42. Gallo G, Savoia C. New insights into endothelial dysfunction in cardiometabolic diseases: potential mechanisms and clinical implications. Int J Mol Sci. (2024) 25:2973. doi: 10.3390/ijms25052973
43. Liu Z, Ge Y, Xu F, Xu Y, Liu Y, Xia F, et al. Preventive effects of transcutaneous electrical acustimulation on ischemic stroke-induced constipation mediated via the autonomic pathway. Am J Physiol Gastrointest Liver Physiol. (2018) 315:G293–301. doi: 10.1152/ajpgi.00049.2018
44. Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. (2014) 64:1092–102. doi: 10.1016/j.jacc.2014.06.1179
45. Dyavanapalli J. Novel approaches to restore parasympathetic activity to the heart in cardiorespiratory diseases. Am J Physiol Heart Circ Physiol. (2020) 319:H1153–H61. doi: 10.1152/ajpheart.00398.2020
46. Corella D, Ordovas JM. Aging and cardiovascular diseases: the role of gene-diet interactions. Ageing Res Rev. (2014) 18:53–73. doi: 10.1016/j.arr.2014.08.002
47. Kaplon-Cieslicka A, Soloveva A, Mareev Y, Cabac-Pogorevici I, Verbrugge FH, Vardas P. Hyponatraemia in heart failure: time for new solutions? Heart. (2022) 108:1179–85. doi: 10.1136/heartjnl-2021-320277
48. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion—a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2019) 21:137–55. doi: 10.1002/ejhf.1369
49. Rao SS, Meduri K. What is necessary to diagnose constipation? Best Pract Res Clin Gastroenterol. (2011) 25(1):127–40. doi: 10.1016/j.bpg.2010.11.001
50. Angkananard T, Anothaisintawee T, Eursiriwan S, Gorelik O, McEvoy M, Attia J, et al. The association of serum magnesium and mortality outcomes in heart failure patients: a systematic review and meta-analysis. Medicine. (2016) 95:e5406. doi: 10.1097/MD.0000000000005406
51. Wilson PB. Associations between physical activity and constipation in adult Americans: results from the national health and nutrition examination survey. Neurogastroenterol Motil. (2020) 32:e13789. doi: 10.1111/nmo.13789
52. Forootan M, Bagheri N, Darvishi M. Chronic constipation: a review of literature. Medicine. (2018) 97:e10631. doi: 10.1097/MD.0000000000010631
Keywords: heart failure, constipation, risk factors, propensity score matching, mortality
Citation: Ishida T, Kawada K, Jobu K, Hamada T, Kubo T, Okazaki M, Kawai K, Nakaoka Y, Yabe T, Furuno T, Yamada E, Kitaoka H and Hamada Y (2025) Impact of comorbid constipation on the survival of patients with heart failure: a multicenter, prospective cohort study conducted in Japan. Front. Cardiovasc. Med. 11:1470216. doi: 10.3389/fcvm.2024.1470216
Received: 25 July 2024; Accepted: 26 December 2024;
Published: 14 January 2025.
Edited by:
Ping Li, Second Affiliated Hospital of Nanchang University, ChinaReviewed by:
Munkhtuya Tumurkhuu, Wake Forest Baptist Medical Center, United StatesAkash Batta, Dayanand Medical College & Hospital, India
Copyright: © 2025 Ishida, Kawada, Jobu, Hamada, Kubo, Okazaki, Kawai, Nakaoka, Yabe, Furuno, Yamada, Kitaoka and Hamada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroaki Kitaoka, a2l0YW9rYWhAa29jaGktdS5hYy5qcA==
 Tomoaki Ishida
Tomoaki Ishida Kei Kawada
Kei Kawada Kohei Jobu1
Kohei Jobu1 Toru Kubo
Toru Kubo Hiroaki Kitaoka
Hiroaki Kitaoka Yukihiro Hamada
Yukihiro Hamada