- 1Department of Cardiology, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
- 2Health Management Center, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
Objective: Levosimendan and Sacubitril/Valsartan are both potent pharmacotherapeutic agents in the clinical management of heart failure characterized by reduced ejection fraction. However, the limited efficacy of monotherapy and the lack of extensive clinical experience with combination therapy necessitate further investigation. This study aimed to evaluate the therapeutic effects of combining levosimendan with sacubitril/valsartan on chronic heart failure with reduced ejection fraction, specifically through a meta-analysis of studies conducted in China.
Methods: Cochrane systematic evaluation method was used to complete data retrieval from the following related databases: (1) Wanfang database; (2) CNKI China Academic Journal Network; (3) Wipo Full-text Database of Chinese Sci-tech journals; (4) PubMed; (5) Medline; (6) Chinese Biomedical Literature Database; (7) Web of Science; and (8) Google Scholar database. We searched for studies published up to December 2021. Data were extracted from applicable articles. Meta-analyses were performed to assess the left ventricular ejection fractions (LVEF) level, NT-proBNP level, Clinical efficacy, and the left ventricular end-diastolic dimension (LVEDD) level outcomes, following PRISMA 2020 guidelines.
Results: A total of five randomized controlled trials (RCTs) comprising 398 patients were included, half of the patients for levosimendan combined with Sacubitril/Valsartan and half of the patients for control groups. The effective rate in experimental group was significantly higher than that in control group [Peto-OR = 3.08, 95% CI (1.83, 5.19), P < 0.05]. The LVEF level after treatment in the experimental group was significantly higher than that in the control group [MD = 5.51, 95% CI (4.25, 6.76), P < 0.05]. After treatment, the LVEDD level in the experimental group was lower than that in the control group [MD = −3.83, 95% CI (−7.60, −0.05), P < 0.05]. There was a statistically significant difference in the N terminal pro B type natriuretic peptide (NT-proBNP) level between the two groups after treatment, where the value for the experimental group was lower than that for the control group [SMD = −2.68, 95% CI (−3.94, −1.43), P < 0.05].
Conclusion: Meta-analysis results showed that levosimendan combined with Sacubitril/Valsartan has a better therapeutic effect on heart failure with reduced ejection fraction and is beneficial for improving cardiac function. The main mechanism for this may be related to the pharmacological action of levosimendan.
Introduction
Heart failure is referred to as cardiac function failure caused by a variety of heart diseases. Its main characteristics include inadequate cardiac output, reduced tissue perfusion volume, and pulmonary circulation blood stasis (1). With changing lifestyles and population aging, in recent years, the incidence of heart failure is gradually increasing despite the continuous improvement in the related diagnosis and treatment level, and heart failure still has a high mortality rate with its three-year survival rate remains comparable to that of malignant tumors (2). How to treat heart failure effectively is a common concern for physicians. Sacubitril/Valsartan is a compound preparation, that has been widely used in the treatment of heart failure. It can effectively inhibit the over-activation of the renin-angiotensin-aldosterone system while protecting the heart function and is a first-line treatment drug recommended by the related guidelines (3). Levosimendan is a non-digitalis-positive inotropic drug, which can effectively improve the sensitivity of myocardial contractility to calcium ions and then achieve the effect of improving myocardial contractility (4, 5). Therefore, a meta-analysis of the therapeutic effect of levosimendan and Sacubitril/Valsartan on chronic heart failure patients with reduced ejection fraction was conducted to provide a theoretical basis for this combined treatment in china.
Materials and methods
Search strategy
The following relevant databases were searched: (1) Wanfang Database; (2) CNKI China Academic Journals Network; (3) Weipa Chinese Full-text Database of Scientific and Technological Journals; (4) PubMed; (5) Medline; (6) Chinese Biomedical Literature Database; (7) Web of Science; and (8) Google Scholar Database. The search terms included the following: (1) chronic heart failure; (2) decreased ejection fraction; (3) levosimendan; (4) Sacubitril/Valsartan. The search keywords included the following: (1) chronic heart failure; (2) decreased ejection fraction; (3) levosimendan; and (4) Sacubitril/Valsartan. Studies closely related to the above keywords were included in the analysis. Databases were searched for studies published up to December 2021. Additionally, the reference lists of review papers and every publication retrieved were manually searched for any additional published articles. Data quality assessment and relevant data extraction from all the included literature were completed by two researchers using a double-blind method of Cochrane systematic review.
Study selection
All randomized controlled trials (RCTs) evaluating the efficacy of levosimendan and Sacubitril/Valsartan for the treatment of heart failure with reduced ejection fraction were included. Patients of chronic heart failure who had New York Heart Association (NYHA) functional classification of III or IV, received conventional anti-heart failure treatment. Patients in control group were treated with Sacubitril/Valsartan and in experimental group were treated with Sacubitril/Valsartan combined with levosimendan. The exclusion criteria were as follows: the experimental design is not rigorous; the source data are wrong and repeatedly reported; the diagnosis and efficacy judgment criteria are not standardized; use of the in vitro or animal experiments; and the study object data are missing.
Data extraction
Data extraction was completed after retrieving relevant literature by two reviews independently. Each reviewer first read the titles of the articles and the abstracts, removed the reports that were inconsistent with the inclusion criteria, and then carefully read the whole text, further eliminating unsuitable manuscripts. and any disagreement was resolved by a third researcher.
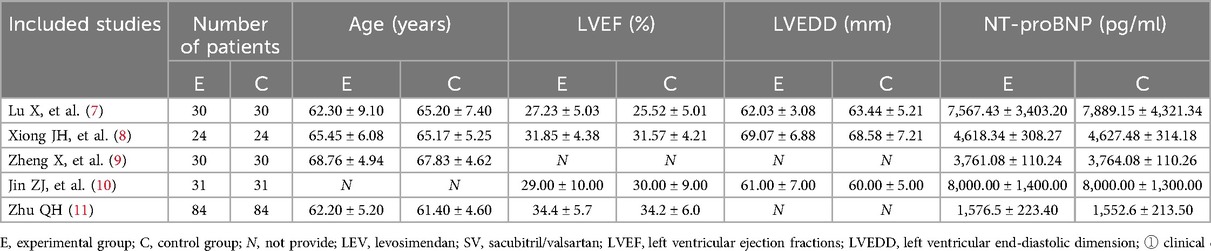
The final decision about the inclusion of the manuscript was then made. The following data were extracted from each study: the first author's name, publication year, regimes for intervention and control groups, number of patients in each group, and drug duration of administration, The basic observation indicators included the following: (1) clinical efficacy (6): clinical symptoms and signs are significantly improved, left ventricular ejection fractions (LVEF) is ≥50% or increased by ≥6%, NYHA class increased by ≥2 are excellent; clinical symptoms and signs have improved, LVEF is 45%−49% or increased by 3%−5%, and NYHA class increased by 1 are effective; clinical symptoms, signs, LVEF, and NYHA class without improvement are ineffective. total effectiveness = (excellent + effective)/total number of people × 100%. (2) LVEF, left ventricular terminal diastolic diameter (LVEDD), and NT-proBNP before and after treatment.
Quality assessment
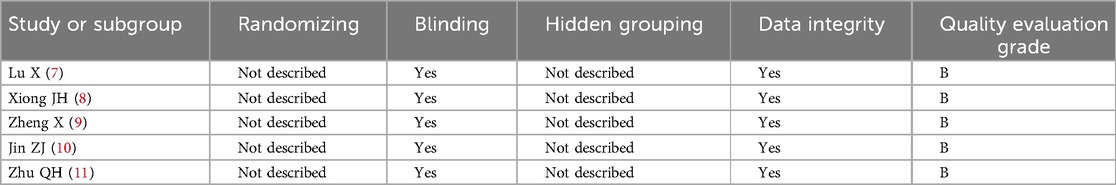
The quality of the included studies (randomized controlled trials, RCTs) was independently evaluated by two authors (Che Li and Zhenliang Chu), and any differences were resolved through discussion and adjudication. A quality evaluation of the included literature was carried out according to the bias risk assessment criteria, these included addressing whether grouping was random, whether blinding was used, whether blinding was hidden and whether the study results data were complete. Grade A was assigned to a study when all of the above criteria were met. Grade B was assigned if some of the above criteria were met. Grade C was assigned if almost none of the above criteria were satisfied.
Statistical analysis
The data analysis was performed with the RevMan 5.3 software, which describes the outcome indicators of categorical variables using risk ratio (RR) and its 95% confidence interval (CI). At the same time, the heterogeneity analysis was completed using the I2 test. No heterogeneity was present when the I2 was <50%. The fixed-effect and random effects models were then implemented. The test level was α=0.05.
2 Results
2.1 Basic features of the included studies
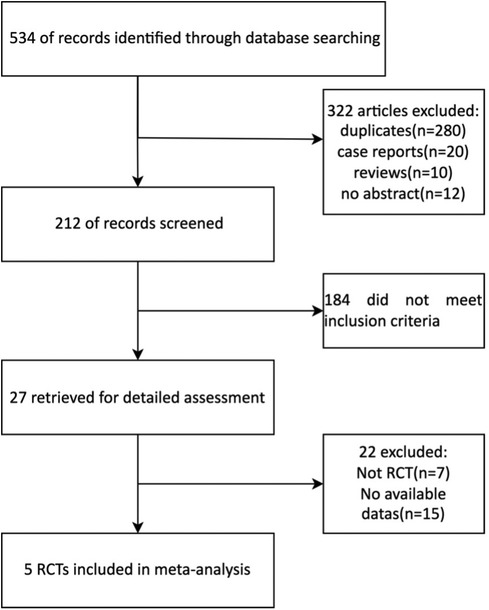
A total of 20 Chinese and 7 English reports were preliminarily obtained using the search keywords. After reading the questions and report contents, 15 Chinese and 7 English manuscripts did not meet the standard and were excluded. As a result, 5 articles were included in the final analysis with a total of 398 patients (Figure 1, Table 1).
2.2 Methodological evaluation of the quality of the included literature quality
All of the included literature were RCTs with a quality evaluation grade of B (Table 2).
2.3 Contrast in total effective rate between two groups
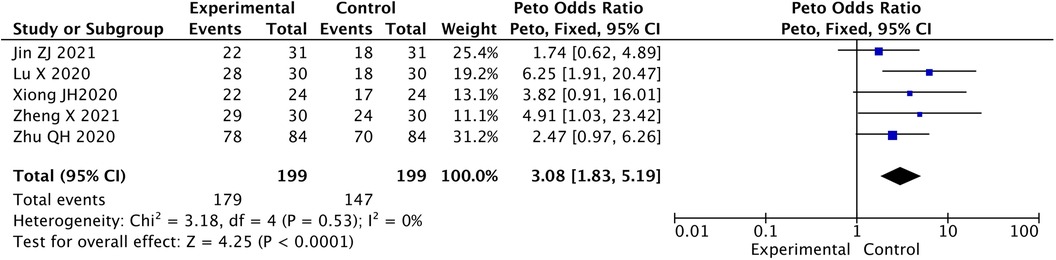
The effective rate in experimental group was significant higher than that in control group [Peto-OR = 3.08, 95% CI (1.83, 5.19), P < 0.05] (Figure 2).
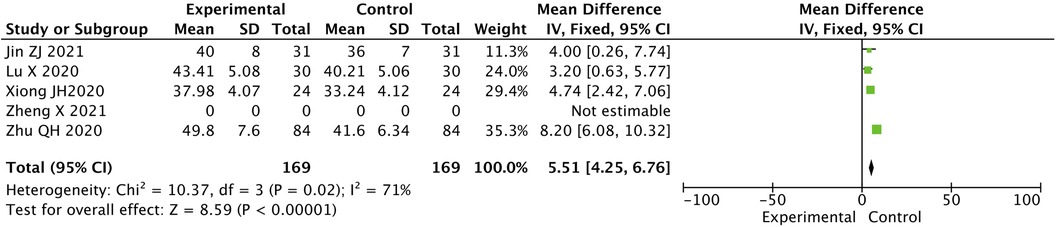
2.4 Contrast in LVEF level between two groups after treatment
The difference in the LVEF level in the two groups after treatment was statistically significant. The value for the experimental group was higher than that for the control group [MD = 5.51, 95% CI (4.25, 6.76), P < 0.05] (Figure 3).
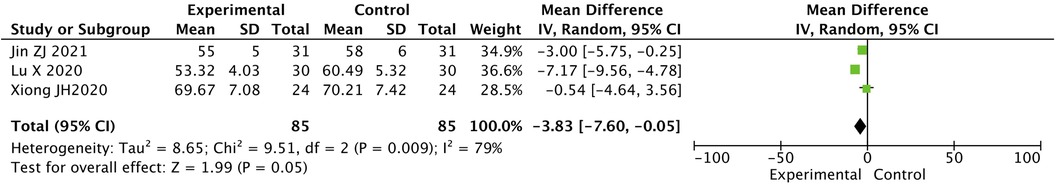
2.5 Contrast in LVEDD level between two groups after treatment
The LVEDD level in the two groups after treatment was statistically significantly different. The value for the experimental group was lower than that for the control group [MD = −3.83, 95% CI (−7.60, −0.05), P < 0.05] (Figure 4).
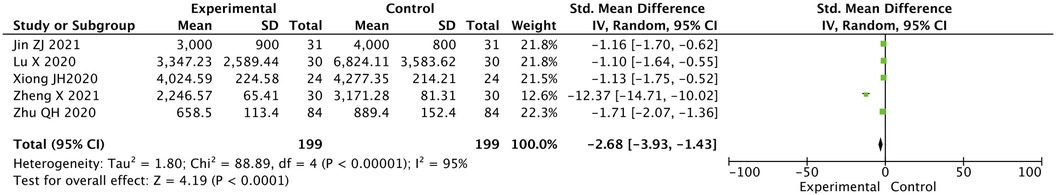
2.6 Contrast in NT-proBNP level between two groups after treatment
The NT-proBNP level in the two groups after treatment was statistically significantly different, the value for the experimental group was lower than that for the control group [SMD = −2.68, 95% CI (−3.94, −1.43), P < 0.05] (Figure 5).
3 Discussion
Some studies have suggested that abnormal activation of the sympathetic nervous system and the endocrine system may be involved in the occurrence and development of heart failure, ultimately leading to pathological changes such as ventricular remodeling and myocardial fibrosis (12), further resulting in impaired ventricular diastolic function and increased risk of arrhythmias and sudden cardiac death (13). Increasingly, research indicates that the effectiveness and safety of sacubitril/valsartan and levosimendan are good in the treatment of heart failure patients with reduced ejection fraction (14), but there is limited experience with the combination therapy of both drugs. In this analysis, we reviewed five studies, including 398 heart failure patients with reduced ejection fraction, and conducted a meta-analysis. The results showed that the combination of levosimendan and sacubitril/valsartan was more effective in treating heart failure with reduced ejection fraction and contributed to the improvement of patients’ cardiac function.
In the 2017 American College of Cardiology/American Heart Association/American Heart Failure Society of America (ACC/AHA/HFSA) guidelines, sacubitril/valsartan was recommended as the standard therapy for heart failure patients (15). The results of this study have shown that the experimental group had a higher overall effectiveness rate compared to the control group, indicating that the combination of sacubitril/valsartan with levosimendan is more effective in treating heart failure with reduced ejection fraction. This finding is consistent with previous related research (16). The analysis suggests that using sacubitril/valsartan alone lacks effective regulation of myocardial enzymes, resulting in reduced efficacy. Levosimendan, on the other hand, can compensate for this deficiency to some extent by enhancing myocardial contractility and calcium ion sensitivity, thereby regulating myocardial enzyme levels. The combination of the two drugs has a synergistic effect, leading to improved clinical treatment outcomes. Additionally, the LVEF levels in the experimental group were higher than those in the control group, while LVEDD and serum NT-proBNP levels were lower than in the control group. This reflects that levosimendan in combination with sacubitril/valsartan can effectively enhance the cardiac function of heart failure patients with reduced ejection fraction. The reason for this is that levosimendan can effectively increase the sensitivity of myocardial cells to calcium ions, thereby enhancing myocardial contractile function without affecting the cellular calcium mechanism. This can conserve ATP function, reduce total peripheral vascular resistance, and pulmonary artery pressure, and further improve heart function. Other studies have reported that levosimendan can effectively dilate blood vessels, reduce peripheral vascular resistance, and thereby reduce the cardiac load, ultimately leading to improved heart function (17).
This study has certain limitations. For instance, it did not analyze the cardiac index and adverse reactions of patients before and after treatment, which may result in some limitations in the study findings. The study population was limited to China, and the treatment experience in countries and regions outside of China was insufficient. The sample size included in the study was relatively small, with a limited number of patients in each study. Therefore, due to the limited number of original studies, the small number of patients in each subgroup, and significant differences in study designs, the statistical power of this study's results was weakened. In future research, efforts should be made to increase the sample size and expand the analysis of relevant indicators to obtain more comprehensive and reliable data.
Conclusion
The combination of levosimendan and sacubitril/valsartan is more effective in the treatment of heart failure with reduced ejection fraction and contributes to the improvement of patients’ cardiac function. Therefore, the clinical application of sacubitril/valsartan in combination with levosimendan is recommended for heart failure patients with reduced ejection fraction. It is imperative to undertake extensive multicenter randomized controlled trials to corroborate these meta-analysis findings, rendering them suitable for practical clinical adoption.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CL: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. JZ: Data curation, Writing – original draft. BZ: Writing – original draft, Formal Analysis. JX: Supervision, Writing – review & editing, Formal Analysis. ZC: Writing – review & editing, Conceptualization, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Science and Technology Bureau of Jiaxing City, Zhejiang, China (grant No. 2022AD30017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rørth R, Jhund PS, Yilmaz MB, Kristensen SL, Welsh P, Desai AS, et al. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail. (2020) 13(2):e006541. doi: 10.1161/CIRCHEARTFAILURE.119.006541
2. Wang Y, Ma X. Relationship between changes of electrocardiogram indexes in chronic heart failure with arrhythmia and serum PIIINP and BNP. Exp Ther Med. (2020) 19(1):591–6. doi: 10.3892/etm.2019.8269
3. Solomon SD, Vaduganathan M, Claggett B L, Packer M, Zile M, Swedberg K, et al. Sacubitril/valsartan across the Spectrum of ejection fraction in heart failure. Circulation. (2020) 141(5):352–61. doi: 10.1161/CIRCULATIONAHA.119.044586
4. Bouchez S, Fedele F, Giannakoulas G, Gustafsson F, Harjola VP, Karason K, et al. Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Ther. (2018) 32(6):617–24. doi: 10.1007/s10557-018-6838-2
5. Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, Almenar L, et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail. (2018) 20(7):1128–36. doi: 10.1002/ejhf.1145
6. Lee S, Oh J, Kim H, Ha J, Chun KH, Lee CJ, et al. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end-stage of renal disease. ESC Heart Fail. (2020) 7(3):1125–9. doi: 10.1002/ehf2.12659
7. Lu X, Jin ZT, Ding LP, Wang CZ, Zhang LJ, Zhang LN, et al. Efficacy of combination of sacubitril valsartan sodium and levosimendan in the treatment of refractory heart failure. Chin J Integr Med. (2020) 25(05):420–4. doi: 10.3969/j.issn.1007-5410.2020.05.004
8. Xiong JH. Effect of levosimendan combined with sacubitril and valsartan sodium in the treatment of chronic heart failure and the influence on hemodynamics. Shang Med Pharm J. (2021) 42(20):16–9. doi: 10.3969/j.issn.1006-1533.2021.20.005
9. Zheng X, Liu ZG, Dang Q, Dong J, Li JJ, Ren X. Effect of levosimendan combined with sacubitril valsartan in the treatment of elderly patients with chronic heart failure. Curr Med Res Pract. (2021) 6(21):62–4. doi: 10.19347/j.cnki.2096-1413.202121020
10. Jin ZJ, Fu YP, Liu LB, Tie CM, He SL, Hu JF, et al. Efficacy of levosimendan combined with sacubitril and valsartan in the treatment of senile refractory heart failure. China Modern Doctor. (2021) 59(25):47–50. doi: 10.3969/j.issn.1673-9701.2021.25.0047.04
11. Zhu QH. Efficacy of levosimendan combined with sakubatra on clinical efficacy and left ventricular ejection fraction in patients with heart failure. Chin J Pharm Econ. (2021) 16(08):55–8. doi: 10.12010/j.issn.1673-5846.2021.08.012
12. Han R, Li K, Li L, Zhang L, Zheng H. Expression of microRNA-214 and galectin-3 in peripheral blood of patients with chronic heart failure and its clinical significance. Exp Ther Med. (2020) 19(2):1322–8. doi: 10.3892/etm.2019.8318
13. Barutaut M, Fournier P, Peacock WF, Evaristi MF, Dambrin C, Caubère C, et al. sST2 adds to the prognostic value of gal-3 and BNP in chronic heart failure. Acta Cardiol. (2020) 75(8):739–47. doi: 10.1080/00015385.2019.1669847
14. Akhtar MS, Hassan Q, Afzal O, Altamimi A, Hassan MZ, Sharma AK, et al. Comparative efficacy of levosimendan, ramipril, and sacubitril/valsartan in isoproterenol-induced experimental heart failure: a hemodynamic and molecular approach. Curr Mol Pharmacol. (2023) 16(6):629–39. doi: 10.2174/1874467215666220919104526
15. Wang S, Wang Y, Deng Y, Zhang J, Jiang X, Yu J, et al. Sacubitril/valsartan: research progress of multi-channel therapy for cardiorenal syndrome. Front Pharmacol. (2023) 14:1167260. doi: 10.3389/fphar.2023.1167260
16. Vaduganathan M, Claggett BL, Desai AS, Anker SD, Perrone SV, Janssens S, et al. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol. (2020) 75(3):245–54. doi: 10.1016/j.jacc.2019.11.003
Keywords: levosimendan, sacubitril/valsartan, reduced ejection fraction, chronic heart failure, heart function
Citation: Li C, Zheng J, Zhang B, Xu J and Chu Z (2024) Meta-analysis of a controlled study of levosimendan combined with Sacubitril/Valsartan for the treatment of heart failure with reduced ejection fraction in China. Front. Cardiovasc. Med. 11:1469457. doi: 10.3389/fcvm.2024.1469457
Received: 23 July 2024; Accepted: 12 September 2024;
Published: 3 October 2024.
Edited by:
Maria Concetta Pastore, University of Siena, ItalyReviewed by:
Jingjing Rong, Hunan Provincial People’s Hospital, ChinaChao Feng, Zhejiang University, China
Copyright: © 2024 Li, Zheng, Zhang, Xu and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenliang Chu, Y2h1emhsQHpqeHUuZWR1LmNu
†These authors have contributed equally to this work
 Che Li
Che Li Jifeng Zheng1,†
Jifeng Zheng1,† Zhenliang Chu
Zhenliang Chu