- 1Department of Cardiopulmonary Function, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2Department of Cardiology, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 3International Medical Center, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, Henan, China
Objective: To analyze the application value of wearable adhesive Patch ECG monitors combined with transesophageal electrophysiological study (TEPS) in the diagnosis of palpitations of unknown origin.
Methods: This was a retrospective study of patients with suspected arrhythmia who were admitted to Henan Provincial People's Hospital between October 2021 and July 2023 due to recurrent paroxysmal palpitations of unknown origin, with or without accompanying symptoms such as dizziness, amaurosis, and syncope. All patients underwent TEPS. Those who did not exhibit arrhythmia during the TEPS were selected for Patch ECG monitoring, which lasted several weeks (depending on the duration of symptom capture). The results of TEPS, Patch ECG monitors, and clinical diagnoses were observed and recorded. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value (NPV) for diagnosing palpitations of unknown origins was analyzed based on clinical diagnostic outcomes for (1) TEPS alone, (2) Patch ECG monitoring in patients with negative TEPS results, and (3) the combination of both methods.
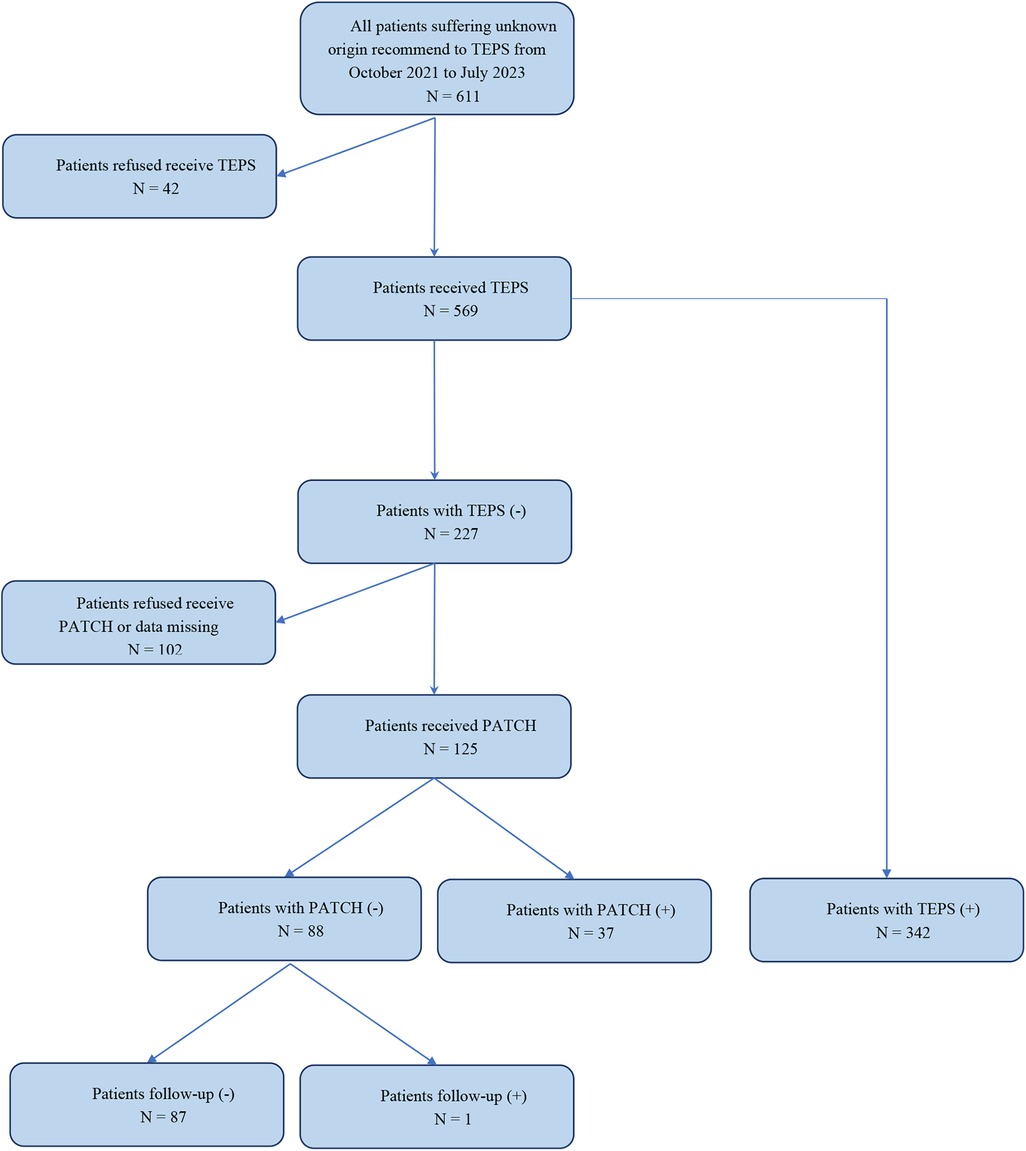
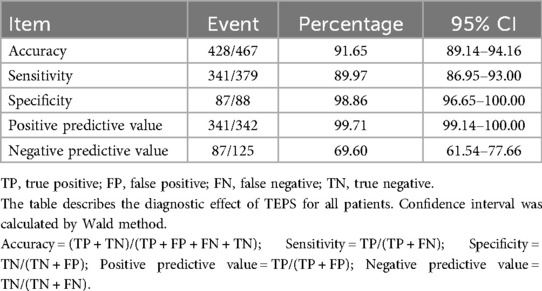
Results: A total of 569 patients were included in this study. The TEPS results exhibited that 227 of the 569 patients did not detect arrhythmias and 342 detected arrhythmias. Of the 569 patients, 102 refused to undergo Patch ECG monitors, and 467 patients completed the entire study process. Among them, 379 cases (66.61%) were clinically diagnosed as arrhythmias. TEPS shows good performance in most evaluation indices except NPV (69.60%, 95% CI, 61.54%–77.66%). The combined diagnosis was strongly consistent with clinical diagnosis. The accuracy, sensitivity, and NPV of TEPS combined with Patch ECG monitors in the diagnosis of palpitations of unknown origin were significantly higher than those of TEPS alone.
Conclusion: Wearable adhesive patch ECG monitors combined with TEPS can enhance the diagnostic efficiency of palpitations of unknown origin.
1 Introduction
Palpitations are among the most common subjective symptoms of patients presenting to primary care providers and cardiologists, characterized by an individual's subjective experience of an unpleasant awareness of the heart beating forceful, rapidly, regularly, or irregularly (1). Patients may also describe this sensation as a rapid fluttering or pounding in the chest or neck areas. The experience of palpitations can vary significantly among patients, ranging from a mild, transient sensation to a more intense and sustained feeling of discomfort. Some may even perceive their heart as pounding against their chest wall, creating a sensation that can be both alarming and disruptive to their daily activities.
The most common causes of palpitations include heart disease, physical diseases with endocrine and metabolic abnormalities, mental disorders, and drug side effects (2). For certain patients experiencing palpitations, clinicians can ascertain the underlying cause through a comprehensive medical history assessment, meticulous physical examination, 12-lead ECG, Holter monitors and pertinent laboratory investigations. However, in patients with an undetermined etiology, establishing a diagnosis poses considerable challenges (1, 3). When utilizing Rest 12-lead ECG and Holter monitors, clinicians can encounter difficulties in precisely diagnosing the underlying cause of palpitations with an unknown source, primarily due to constraints imposed by their limited observation windows. Wearable adhesive Patch ECG monitors, based on 24-h Holter monitors, are designed for long-term patient monitoring using a compact, waterproof, and high-capacity ECG recorder. This allows the detection of occasional abnormal electrical activity, providing an accurate and reliable foundation for diagnosing various arrhythmias (4–6).
Transesophageal electrophysiological study (TEPS) is a well-established procedure that employs esophageal pacing leads to indirectly stimulate and monitor the heart's electrical activity. Pacing leads are inserted through either the nose or mouth and positioned in the esophagus, eliminating the need for skin punctures, blood vessel invasion, or direct heart access. This method offers a relatively non-invasive alternative for pacing and assessing the heart without the requirement of fluoroscopy, strict sterile precautions, or cardiac catheterization (7–10). TEPS can not only induce tachycardia by different stimulation methods and evaluate the risk stratification of pre-excitation bypass, but also effectively terminate the onset of supraventricular tachycardia (SVT), atrial flutter, and avoid aggravation and deterioration of the original arrhythmia (11–20). The 2019 ESC Guidelines for managing patients with supraventricular tachycardia endorses the use of esophageal electrocardiogram for the differential diagnosis of narrow QRS tachycardia (19). As a lower-cost diagnostic and treatment option with fewer operational requirements, it is often utilized in many primary healthcare institutions and developing countries (9–11, 13–16, 20).
Currently, Patch ECG monitors and TEPS have been applied separately in the diagnosis of palpitations of unknown origin, and they have been proven to have certain diagnostic efficacy (21, 22). However, there are still missed diagnoses and misdiagnoses when applied alone. Combining these methods could improve the diagnostic efficiency of palpitations of unknown origin, but there are few relevant studies. Therefore, this study analyzed the application value of Patch ECG monitors combined with TEPS for the diagnosis of palpitations of unknown origin, aiming to provide a reference for the improvement of diagnostic methods.
2 Data and methods
2.1 General data
Patients with unexplained palpitations who were recommended for TEPS and Patch ECG monitors based on clinical evaluation between October 2021 and July 2023 were included in this study. The criteria for inclusion and exclusion were carefully established to ensure accuracy.
The inclusion criteria were (1) presence of recurrent paroxysmal palpitations, with or without accompanying symptoms such as dizziness, amaurosis, syncope, or suspicion of an underlying arrhythmia; (2) abrupt manifestation and sudden or gradual cessation of symptoms lasting from seconds to minutes or even hours (onset and relief may occur with or without a consistent triggering factor); (3) completion of initial comprehensive evaluation yielding negative results, including a thorough review of medical history, detailed physical examination, ECG, cardiac ultrasound imaging, and various laboratory tests ruling out other potential causes; (4) absence of antiarrhythmic drugs or other medications that could affect the diagnosis; and (5) obtaining written informed consent.
Exclusion criteria were (1) presence of significant esophageal conditions or severe nasal pathologies; (2) severe myocardial ischemia, cardiac enlargement, or heart failure; (3) severe hypertension, defined as blood pressure exceeding 200/110 mmHg; (4) refusal to continue using the Patch ECG monitors after completing the TEPS process; (5) inability to obtain valid data from Patch ECG monitors due to various factors such as skin allergies, intense physical exertion, detachment of patches, or occurrence of severe non-cardiac adverse events requiring immediate medical attention during the monitoring period; and (6) lack of follow-up records for accurate diagnosis.
A total of 569 patients were initially included in this study; 91 patients who did not exhibit arrhythmia induced by TEPS declined further Patch ECG monitoring, and 11 patients discontinued due to adverse events such as skin allergies were excluded, resulting in the final inclusion of 467 patients in the study (see more details in Figure 1). Among the 467 patients, 211 were males and 256 were females; their ages ranged 10–83 years old, with a mean age of 43.43 ± 17.17 years old. Clinical manifestations contained paroxysmal palpitations in all subjects, dizziness in 50, amaurosis in 2, and syncope in 2 cases. Previous history included smoking in 102 cases, drinking in 207 cases, and radiofrequency ablation in 10 cases. Complications contained coronary heart disease in 44 cases, hypertension in 99 cases, diabetes in 86 cases, cerebrovascular disease in 6 cases, kidney disease in 7 cases, congenital heart disease in 9 cases, hyperlipidemia in 46 cases, and tumors in 3 cases. This study was approved by the Ethics Committee of Henan Provincial People's Hospital.
2.2 Research methods
2.2.1 TEPS
DF-5A cardiac electric stimulator and 7F quadrupole esophageal pacing lead were purchased from Suzhou Dongfang Electronic Instrument Factory, Suzhou, China. The study was conducted according to Noninvasive Cardiac Electrophysiology Diagnostic and Therapeutic Technique-Basic and Clinic (23).
The steps were as follows: (1) Contraindications were excluded, and informed consent was obtained; (2) A 7 F esophageal pacing lead was inserted through a nostril into the esophagus with the patient in a supine position without sedation. The depth of insertion of the pacing lead was typically determined based on the distance between the nasal tip and the distal end of the esophageal pacing lead, which was calculated using the formula: (height + 200)/10 + 2 cm (Figure 2A); (3) The positioning of the esophageal pacing leads were further adjusted based on atrial wave (A), ventricular wave (V) size, and A/V ratio observed in the esophageal electrocardiogram. (Figure 2B); (4) After determining atrial pacing thresholds, various pacing maneuvers were performed with minimal stimulus strength required for consistent atrial capture to minimize any discomfort caused by pacing. The pacing maneuvers included single, double, and triple atrial extra stimuli, including RS2, S1S2, S1S2S3 reverse scan, and S1S1 step-up stimulation, introduced incrementally during sinus rhythm until reaching either atrial or AV node effective refractory period or inducing specific ECG phenomena or arrhythmia. The entire process was recorded in detail; (5) If no arrhythmia was induced and contraindications were ruled out, isoproterenol 0.02–0.04 μg/(kg min) was administered to increase heart rate by 25%–50%, followed by repeating aforementioned stimulation procedures.
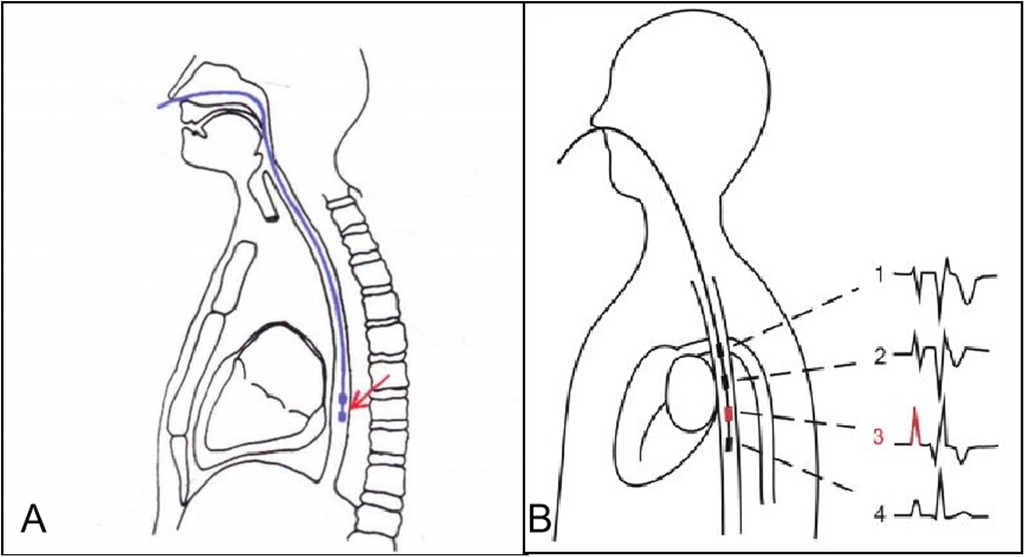
Figure 2. Position of the esophageal pacing lead and the best esophageal electrocardiogram. (A) Position of the esophageal pacing lead. (B) The best esophageal electrocardiogram is the third one.
Two experienced ECG chief physicians analyzed the TEPS results. If the results were inconsistent, the conclusion was given after discussion. Diagnostic criteria referred to previous results (23, 24).
2.2.2 Patch ECG monitors
Patch ECG monitors were applied to patients who did not exhibit symptom-related arrhythmia induced by TEPS. The device utilized in this study was procured from Hangzhou Proton Technology Co., Ltd., China (model CarePatch ECG-P01).
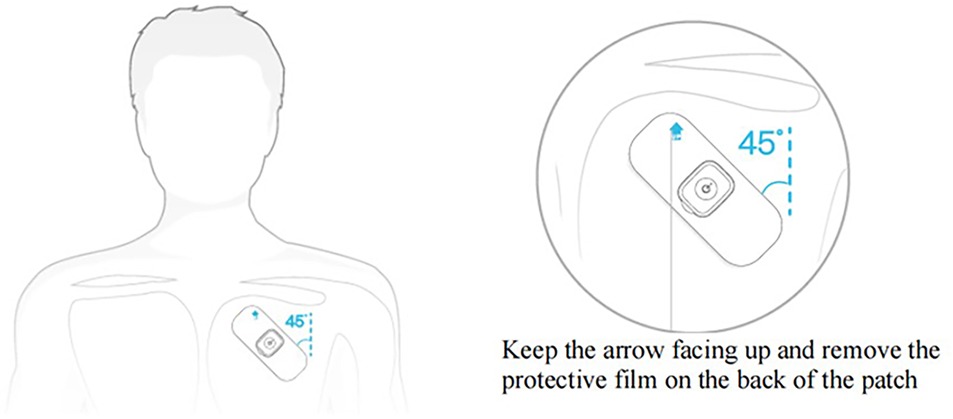
The steps were as follows: (1) Prior to commencing the measurement, remove any hair from the upper region of the left chest and cleanse the skin with warm water; (2) Attach the Patch ECG monitors to the left chest wall (Figure 3); (3) Provide patients with a symptom diary to document suspected arrhythmia symptoms, including their onset and duration. Depending on when symptoms occurred, patients could then wear it for several weeks.
The results of Patch ECG monitors were independently interpreted by two experienced chief physicians to observe the occurrence of arrhythmia (19, 24). If their conclusions were inconsistent, the final conclusion was given after discussion.
2.3 Clinical diagnosis of arrhythmias
Cardiologists determined the occurrence of arrhythmias based on the patient's medical history (attack triggers, frequency, onset and cessation patterns, symptoms during attacks, previous similar episodes and family history, underlying diseases, and medication history), physical examination findings (such as heart rate, rhythm, and abnormal heart sounds), as well as results obtained from TEPS, Patch ECG monitor (23, 24). All patients included in this study underwent TEPS, and induced tachycardia consistent with clinical symptoms could be initially diagnosed and managed clinically with subsequent follow-up. Patients without induced tachycardia used Patch ECG monitors for several weeks, depending on the timing of palpitations onset, and a preliminary diagnosis was possible if palpitations are accompanied by corresponding ECG abnormalities. Clinical follow-up lasted from 11 to 34 months, during which some patients underwent intracardiac electrophysiological study (EPS) for diagnostic clarification and received radiofrequency catheter ablation (RFCA).
2.4 Statistical methods
Normality of measurement data was assessed by the Shapiro–Wilk test. Continuous variables were expressed through the mean ± standard deviation or medians with interquartile ranges (IQRs) depending on the distribution. Analysis of the comparisons between the two groups was determined by Student's t-test or Mann–Whitney U test for normal distributed or non-normal distributed continuous variables. The percentage of cases described the count data. Binary data or sample constituent ratio data was tested by the χ2 test. The accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and 95% confidence interval of all statistical criteria were calculated by the Wald method to evaluate the diagnostic value of all methods. The missing data of the categorical variables were imputed by the dominant category and the continuous variables by the median. P < 0.05 was considered significant. Statistical analysis was performed using a commercially available software package (SPSS Statistical Software Version 25.0).
3 Results
3.1 TEPS results
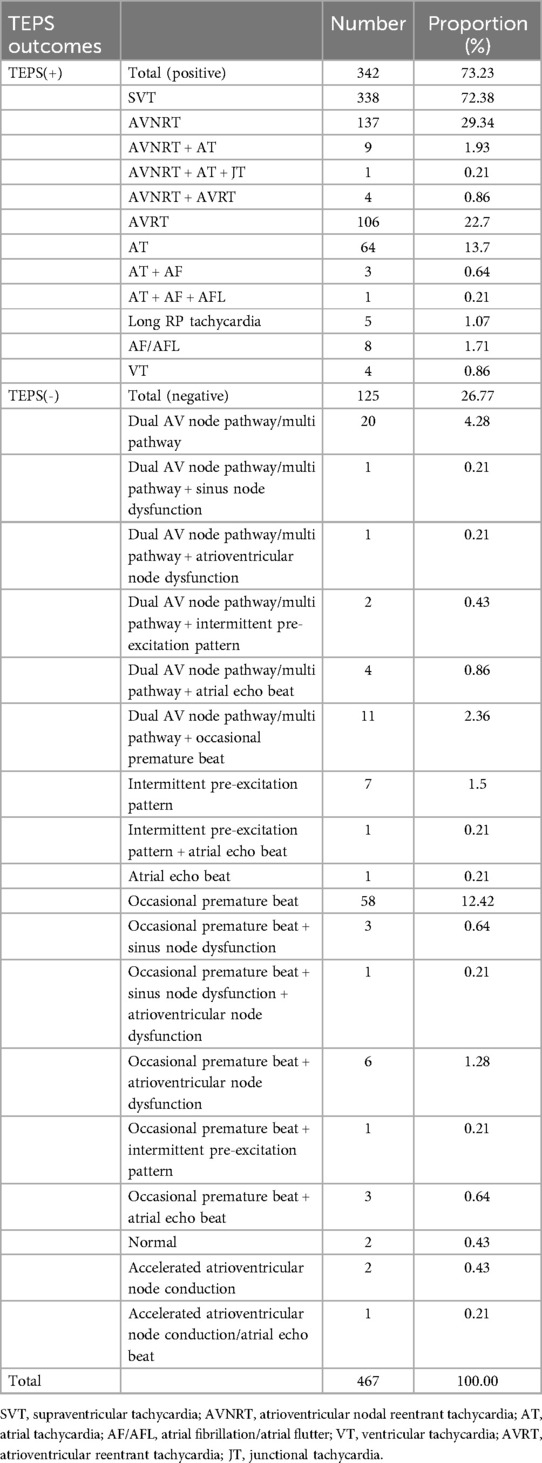
The TEPS results exhibited that 342 of the 467 patients induced arrhythmias consistent with the same clinical symptoms as the TEPS(+) group, in which the detection rate of supraventricular arrhythmias was high, accounting for 72.38%, and the detection rate of ventricular tachycardia (VT) was low, accounting for only 0.86%. Of the 467 patients, 125 did not induce arrhythmias consistent with clinical symptoms as the TEPS(-) group, with results including completely normal and some specific cardiac phenomena, and some minor arrhythmias (Table 1).
3.2 Patch ECG monitors results
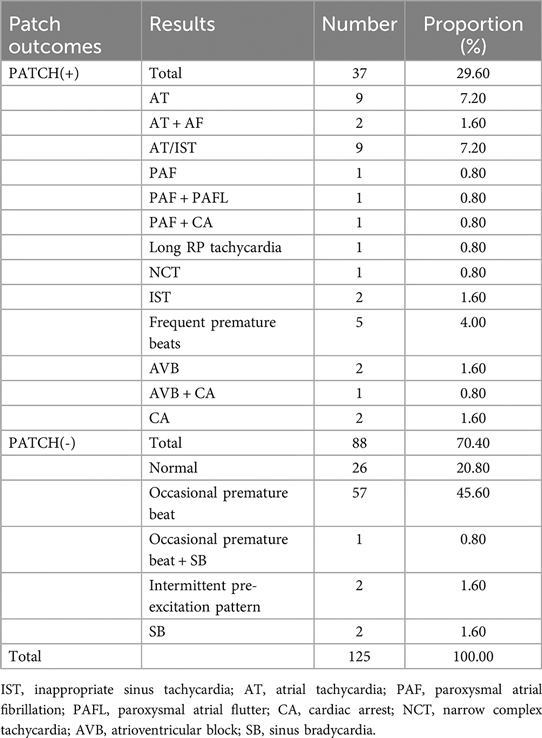
The Patch ECG monitors were worn by 125 TEPS(-) patients for a duration ranging from 2 to 6 weeks; 37 of the 125 patients detected arrhythmias consistent with the same clinical symptoms as the Patch(+) group, in which the detection rate of atrial tachycardia and inappropriate sinus tachycardia was high, accounting for 22.13%. Of the 125 patients, 88 did not detect arrhythmias consistent with clinical symptoms as the Patch(-) group, including completely normal and some specific cardiac phenomena, some minor arrhythmias (Table 2).
3.3 Clinical diagnostic results of TEPS in the diagnosis of all patients suffering unexplained palpitations
A total of 379 patients were diagnosed with arrhythmia according to clinical evaluation and follow-up, and a total of 167 patients underwent EPS and RFCA, including 157 cases of SVT, 8 cases of paroxysm atrial fibrillation and atrial flutter (PAF/PAFL), and 2 cases of VT. Among 467 participants with TEPS, results were positive in 342, for an accuracy of 91.65% (95% CI, 89.14%–94.16%). Meanwhile, TEPS showed good sensitivity (89.97%, 95% CI, 86.95%–93.00%), specificity (98.86%, 95% CI, 96.65%–100.00%), and PPV (99.71%, 95% CI, 99.14%–100.00%). However, the NPV of TEPS did not show good performance (69.60%, 95% CI, 61.54%–77.66%). Details of the diagnostic testing are provided in Table 3.
3.4 Clinical diagnostic results of Patch ECG monitors in the diagnosis of patients with negative results of TEPS
Among 125 participants who accepted Patch ECG monitors, the result was positive in 37, with an accuracy of 99.20% (95% CI, 97.64%–100.00%). The sensitivity of Patch ECG monitors for the patients was 97.37% (95% CI, 92.28%–100.00%). Both specificity and PPV were 100.00% (95% CI, 100.00%–100.00%). NPV was 98.86% (95% CI, 96.65%–100.00%). Details of the diagnostic testing are provided in Table 4.
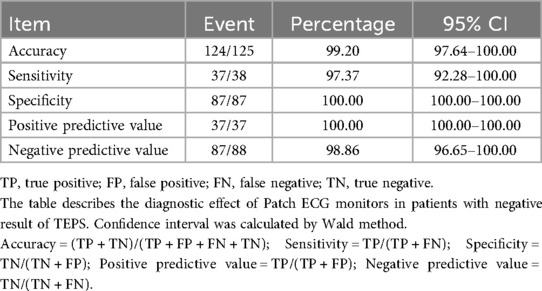
Table 4. Accuracy, sensitivity, specificity, PPV, and NPV of patch ECG monitors in patients with negative result of TEPS.
3.5 Clinical diagnostic results of TEPS combined with Patch ECG monitors in the diagnosis of all patients suffering unexplained palpitations
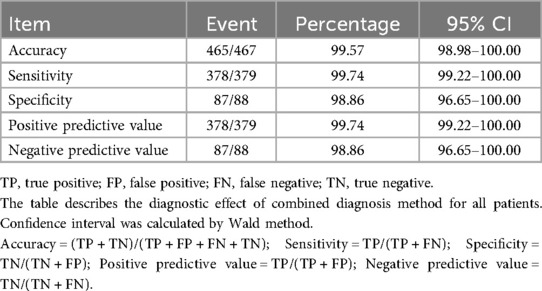
The combined diagnosis method showed better performance than TEPS alone. The accuracy (99.57%, 95% CI, 98.98%–100.00%), sensitivity (99.74%, 95% CI, 99.22%–100.00%), and NPV (98.86%, 95% CI, 96.65%–100.00%) were significantly higher than TEPS. Meanwhile, specificity (98.86%, 96.65%–100.00%) and PPV (99.74%, 99.22%–100.00%) still have a good performance. Details of the diagnostic testing are provided in Table 5.
4 Discussion
Current guidelines recommend ambulatory ECG monitoring as the most vital tool for diagnosing palpitations of unknown origin (4, 25). As a type of ambulatory ECG monitoring, Patch ECG monitors recently have been widely used in clinical practice because of their advantages of being non-invasive, having a prolonged observation period, and being relatively reliable recording. This device effectively records a wide array of abnormal ECG signals in the most natural state of patients, significantly enhancing the accuracy of disease diagnosis (26–33).
Previous studies indicate a duration of up to 1–2 weeks of Patch ECG monitoring yields a high rate of arrhythmia identification, with the diagnostic rate of palpitations during 1–4 weeks of Patch ECG monitoring varied between 70% and 85% (4, 33). Diagnostic efficacy is limited primarily by the need to wait for symptom recurrence (4). The use of single or three-channel formats in Patch ECG monitors also makes it challenging to discern specific types of captured arrhythmias. For symptomatic patients with challenging diagnosis, and a strong suspicion of arrhythmia, current recommendation suggest considering Loop record implantation or intracardiac EPS (2). However, ESP may not be suitable for all individuals due to its invasiveness, peripheral vascular issues, cardiac complications, the longer recovery period, and other factors.
In comparison to EPS, TEPS was selected for patient evaluation in our study due to its relatively non-invasive, economical, convenient, and acceptable accuracy. Current guidelines recommend esophageal electrocardiogram as a useful reference point for distinguishing narrow QRS tachycardia, demonstrating its clinical application value (19). Reported inducibility rates with this method for supraventricular tachycardia range from 73% to 98.5% (34–36), and the results regarding inducibility and tachycardia mechanism showing excellent correlation with findings on subsequent EPS (12, 37). Among the 342 patients in the TEPS(+) group, a total of 165 patients underwent EPS and RFCA, including 157 cases of SVT, 6 cases of PAF/PAFL, as well as 2 cases of VT. Apart from providing a specific diagnosis for SVT, TEPS demonstrated high diagnostic accuracy across different types of inducibility. According to our study, TEPS exhibits good overall diagnostic efficacy and holds certain diagnostic values for suspected paroxysmal supraventricular tachycardia in patients (Figure 4). This conclusion aligns with previous studies. However, it is important to note that the negative predictive value is low, and approximately 30.4% of patients may be missed, leading to potential diagnostic and therapeutic bias.
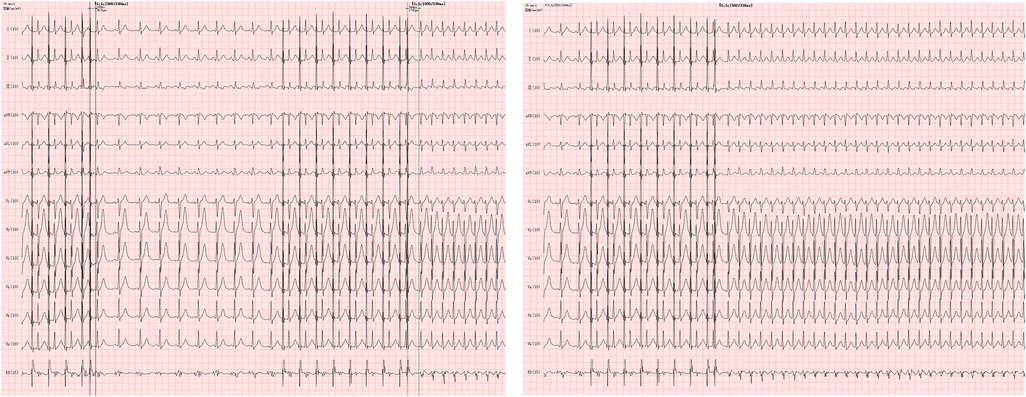
Figure 4. TEPS records of a 32-year-old male patient. The patient was presented with prolonged S2R jump induced by S1S2 stimulation for 176ms in TEPS, and SVT was also induced. Slow-fast atrioventricular nodal reentrant tachycardia (SFAVNRT) was confirmed by esophageal electrocardiogram (EB lead) and body surface electrocardiogram, which was verified by EPS.
Since the combination of Patch ECG monitors and TEPS in the diagnosis of palpitations of unknown origin may yield accurate diagnostic results, their combination was employed in this study. Depending on the time of onset of palpitations, patients without induced tachycardia in TEPS required several weeks of Patch ECG monitoring. There are several findings from the combined diagnosis.
First, the results demonstrate that Patch ECG monitors can effectively detect certain types of palpitations such as autonomic atrial tachycardia, inappropriate sinus tachycardia (or postural tachycardia) (Figure 5), paroxysmal atrial fibrillation, atrial flutter, and some bradyarrhythmia associated with automaticity, which were missed by TEPS. According to the follow-up, two cases underwent PAF/PAFL radiofrequency ablation and two cases underwent pacemaker implantation in the Patch(+) group. Due to the global coronavirus epidemic during the study period, autonomic dysfunction or underlying neo-coronavirus infection, stress, and anxiety could explain the more challenging-to-diagnose arrhythmias identified in this study, which aligns with findings from previous studies (38–40). Thus, Patch ECG monitors can effectively identify patients who have been underdiagnosed and prevent treatment delays.
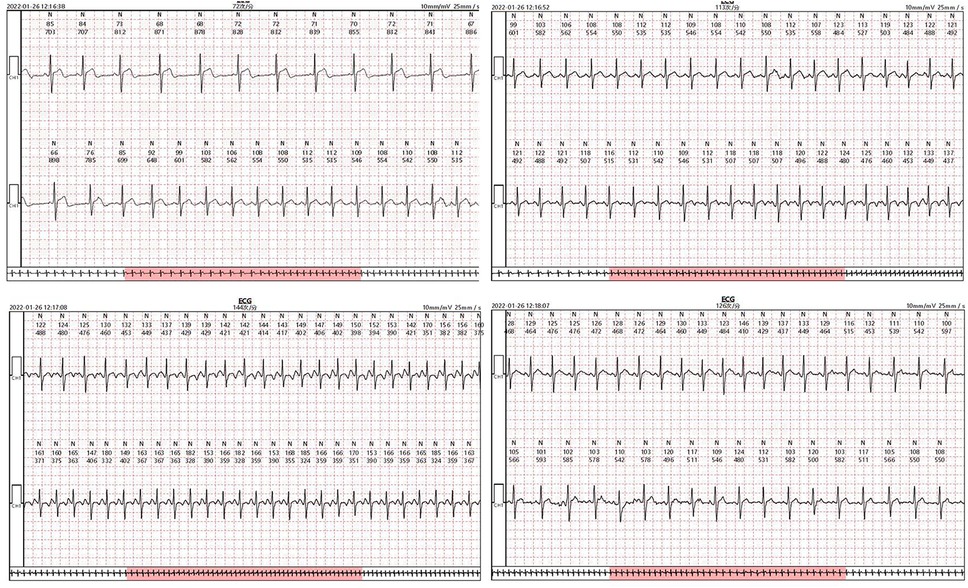
Figure 5. Patch ECG fragments of a 17-year-old male patient diagnosed with inappropriate sinus tachycardia. The Patch ECG monitors recorded the patient's spontaneous symptoms during a resting state, aiding in the detection of misdiagnoses by TEPS.
Second, only one patient with atrioventricular nodal reentrant tachycardia was missed; in this case, TEPS detected only dual atrioventricular nodal pathways, and the occurrence of tachycardia was documented by the Patch ECG monitors in the fourth week (Figure 6). This finding suggests the diagnosis of atrioventricular reentrant tachycardia and atrioventricular reentrant tachycardia could be ruled out if the TEPS results are non-inducible and there is no jump phenomenon, pre-excitation, or atrial echo beat. This is consistent with the study of Michel et al. (9).
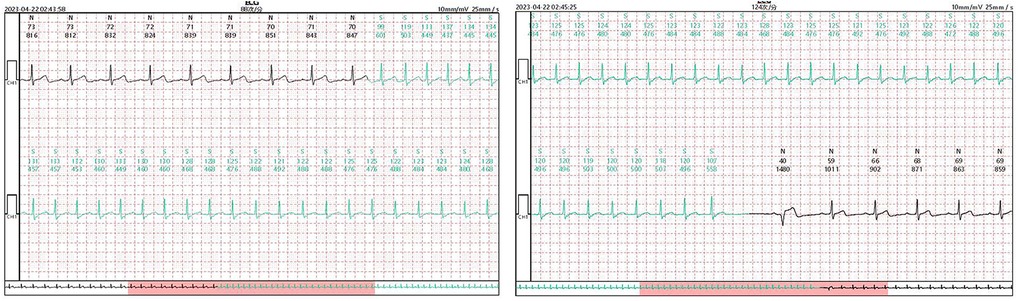
Figure 6. Patch ECG fragments of a 45-year-old female patient diagnosed with Supraventricular tachycardia. TEPS detected only dual atrioventricular nodal pathways, while the occurrence of tachycardia was documented by the Patch ECG monitors in the fourth week.
Third, although typical symptoms were detected in some patients, no corresponding arrhythmia was detected in the result analysis, and the diagnosis of arrhythmia-related diseases was also excluded; it was recommended to seek medical treatment in the departments of respiration and neurology.
Our results exhibited that the consistency, accuracy, sensitivity, and negative predictive value of the combined diagnosis of the two methods were higher than that of the TEPS alone, suggesting that the Patch ECG monitors combined with TEPS can elevate the diagnostic efficiency of palpitations of unknown origin. The reasons may be as follows: The combined application of Patch ECG monitors and TEPS can make up for each other's shortcomings. For example, TEPS has a good diagnostic value in inducing tachycardia involving a reentry mechanism and triggering mechanism using atrial program stimulation and drug stimulation. It provides very detailed information for the definite diagnosis of arrhythmia. In addition, it is undeniable that a substantial number of patients display negative TEPS findings. Before the implementation of Patch ECG monitors, the identification of these patients resided in a “murky territory,” thereby intensifying the strain on patients’ diagnostic clarity, psychological state, and financial stability. Our study utilized Patch ECG monitors to analyze patients in the TEPS(-) group, bridging the diagnosis gap. The detection rate of inappropriate sinus tachycardia, automatic atrial tachycardia, paroxysmal atrial fibrillation, and atrial flutter can be improved by Patch ECG monitors, and thus the diagnostic efficiency can be improved. The combined diagnosis method is conducive to diminishing the occurrence of excessive medical treatment and alleviating the patient's medical pressure and psychological pressure.
The researcher's hospital has a large volume of patient visits and high levels of patient compliance. Moreover, the hospital possesses ample relevant diagnostic expertise to investigate supplementary research on initial TEPS-negative diagnoses. Based on the above research advantages, we explored the possibility of combining TEPS with Patch ECG monitors, which reduces the possibility of missed diagnosis of the disease to a certain extent on the basis of lowering the patients’ medical cost and pain of examination and provides a feasible solution for the future diagnosis of clinical palpitation.
In the future, we could also explore the use of wearable ECG devices such as Apple Watch or ALIVECOR/Kardiamobile to replace Patch ECG monitors, which could extend the observation period and improve patient compliance, thereby improving the disease diagnosis rate (41, 42). Moreover, we will investigate the scoring system and further explore the implementation of the corresponding diagnostic methods to improve the degree of diagnostic standardization.
5 Limitations
The study was a retrospective cohort study, limiting the ability to address issues related to patient selection and sample size calculation through experimental design. Further studies should use randomized controlled trials. In addition, the diagnosis of the TEPS(+) group was established based on a comparison between induced tachycardia and patient-reported symptoms. No further Patch ECG monitors were employed within the TEPS(+) group, and only a subset of patients consented to undergo a more rigorous verification process involving EPS. Meanwhile, some patients of TEPS(-) group declined to undergo Patch ECG monitors due to milder symptoms and adverse reactions associated with wearing, which had an impact on the study findings. This influenced the overall outcomes of the study, potentially skewing the data and making it less representative of the broader population with similar symptoms. These factors should be considered when interpreting the results and designing future research in this field.
6 Conclusion
Our findings support the efficient diagnostic capabilities of TEPS in identifying paroxysmal supraventricular tachycardia using various programmed stimuli. In addition, combining Patch ECG monitors and TEPS can effectively reduce missed diagnoses associated with TEPS and enhance the diagnostic efficiency of palpitations of unknown origin.
Data availability statement
Due to patient privacy concerns, the raw data supporting the conclusions of this article will be made available after approved by the Ethics Committee of Henan Provincial People's Hospital.
Ethics statement
The studies involving humans were approved by Ethics Committee of Henan Provincial People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RY: Writing – review & editing, Writing – original draft, Formal Analysis, Data curation. LY: Writing – original draft, Resources. QZ: Writing – original draft, Resources. SW: Writing – original draft. JX: Writing – review & editing, Resources.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1469108/full#supplementary-material
References
1. Zimetbaum P, Josephson ME. Evaluation of patients with palpitations. New Engl J Med. (1998) 338(19):1369–73. doi: 10.1056/NEJM199805073381907
2. Giada F, Raviele A. Clinical approach to patients with palpitations. Card Electrophysiol Clin. (2018) 10(2):387–96. doi: 10.1016/j.ccep.2018.02.010
3. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. (1996) 100(2):138–48. doi: 10.1016/S0002-9343(97)89451-X
4. Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, et al. 2017 Ishne-Hrs expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. (2017) 14(7):e55–96. doi: 10.1016/j.hrthm.2017.03.038
5. Murali S, Brugger N, Rincon F, Mashru M, Cook S, Goy J-J. Cardiac ambulatory monitoring: new wireless device validated against conventional Holter monitoring in a case series. Front Cardiovasc Med. (2020) 7:587945. doi: 10.3389/fcvm.2020.587945
6. Bocharov M, Stasiuk V, Osyodlo V, Ryzhenko T, Malanin V, Chumachenko D, et al. Assessment of the activities physiological cost of the defense forces officers in Ukraine using miniature ECG device. Front Cardiovasc Med. (2023) 10:1239128. doi: 10.3389/fcvm.2023.1239128
7. Gallagher JJ, Smith WM, Kerr CR, Kasell J, Cook L, Reiter M, et al. Esophageal pacing: a diagnostic and therapeutic tool. Circulation. (1982) 65(2):336–41. doi: 10.1161/01.CIR.65.2.336
8. Volkmann H, Kühnert H, Dannberg G. Electrophysiological evaluation of tachycardias using transesophageal pacing and recording. Pace. (1990) 13(12 Pt 2):2044–7. doi: 10.1111/j.1540-8159.1990.tb06939.x
9. Michel M, Renaud C, Chiu-Man C, Gross G, Jaeggi E. Postnatal recurrence and transesophageal inducibility of prenatally treated fetal supraventricular tachycardia. Heart Rhythm. (2022) 9:1–7. doi: 10.1016/j.hrthm.2022.04.013
10. Chen J, Lin F, Zuo P, Li S, Lin L, Wang B, et al. Transesophageal electrophysiology study in the diagnosis of dual atrioventricular nodal nonreentrant tachycardia. Ann Noninvasive Electrocardiol. (2021) 27(1):e12845. doi: 10.1111/anec.12276
11. Koca S, Pac FA, Kavurt AV, Cay S, Mihcioglu A, Aras D, et al. Transesophageal and invasive electrophysiologic evaluation in children with Wolff-Parkinson-white pattern. Pacing Clin Electrophysiol. (2017) 40(7):808–14. doi: 10.1111/pace.13100
12. Aykan HH, Özer S, Karagöz T, Akın A, Gülgün M, Alehan D, et al. Comparison of transesophageal and intracardiac electrophysiologic studies for the diagnosis of childhood supraventricular tachycardias. Pediatr Cardiol. (2015) 36(7):1429–35. doi: 10.1007/s00246-015-1179-4
13. Koutsampasopoulos K, Pliatsika M, Vogiatzis I. Transesophageal overdrive pacing in a patient with atrial tachycardia and Beta -thalassemia major. A challenging simplicity. Med Arch. (2020) 74(4):309–11. doi: 10.5455/medarh.2020.74.309-311
14. Ijaz S, Meier I, Overholt E, Williams M, Sadana N, Lockwood D. Transesophageal atrial pacing for termination of atrial tachycardia during pregnancy. J Am Coll Cardiol. (2019) 73(9):2800. doi: 10.1016/s0735-1097(19)33406-0
15. Prasad D, Steinberg J, Snyder C. Comparison of cost-effectiveness of direct current cardioversion, transesophageal pacing and digoxin in the conversion of atrial flutter in neonates. J Am Coll Cardiol. (2014) 63(12):A427. doi: 10.1016/j.jacc.2010.03.112
16. Toni L, Blaufox AD. Transesophageal evaluation of asymptomatic Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. (2012) 35(5):519–23. doi: 10.1111/j.1540-8159.2012.03339.x
17. Rhodes LA. Conversion of atrial flutter in pediatric patients by transesophageal atrial pacing a safe, effective, minimally invasive procedure. Am Heart J. (1994) 130:323–7. doi: 10.1016/0002-8703(95)90448-4
18. Ajisaka H, Hiraki T, Ikeda H, Kubara I, Yoshida T, Ohga M, et al. Direct conversion of atrial flutter to sinus rhythm with low-output, short-duration transesophageal atrial pacing. Clin Cardiol. (1997) 20(9):762–6. doi: 10.1002/clc.4960200910
19. Zaza A, Suwalski P, Sarquella-Brugada G, Sacher F, Lambiase PD, Kuck K-H, et al. 2019 Esc guidelines for the management of patients with supraventricular tachycardiathe task force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (esc). Eur Heart J. (2020) 41(5):655–720. doi: 10.1093/eurheartj/ehz467
20. Qin C, He T, Li S. Successful risk stratification of a patient with ventricular preexcitation by improved transesophageal electrophysiological study. Ann Noninvasive Electrocardiol. (2021) 26(5):e12882. doi: 10.1111/anec.12882
21. Locati ET, Moya A, Oliveira M, Tanner H, Willems R, Lunati M, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the Synarr-Flash study. Europace. (2016) 18(8):1265–72. doi: 10.1093/europace/euv311
22. KoŻluk E, Piątkowska A, Szumowski Ł, Walczak F. Clinical significance of pharmacological provocation during diagnostic transesophageal atrial pacing in patients with “heart palpitation”. Europace. (2001) 2(Supple1):A22. doi: 10.1016/eupace/2.Supplement_1.A22-a
23. Xu Y, Li Z, Yang X. Noninvasive Cardiac Electrophysiology Diagnostic and Therapeutic Technique-Basic and Clinic (2017).
24. Issa ZF, Miller JM, Zipes DP. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. 2nd ed. Netherlands: Elsevier (2012).
25. Madan P, Singh V, Singh DP, Diwakar M, Pant B, Kishor A. A hybrid deep learning approach for Ecg-based arrhythmia classification. Bioengineering. (2022) 9(4):152. doi: 10.3390/bioengineering9040152
26. Dilaveris PE, Antoniou CK, Caiani EG, Casado-Arroyo R, Climent AΜ, Cluitmans M, et al. Esc working group on E-cardiology position paper: accuracy and reliability of electrocardiogram monitoring in the detection of atrial fibrillation in cryptogenic stroke patients. Eur Heart J Digit Health. (2022) 3(3):341–58. doi: 10.1093/ehjdh/ztac026
27. Francisco-Pascual J, Cantalapiedra-Romero J, Pérez-Rodon J, Benito B, Santos-Ortega A, Maldonado J, et al. Cardiac monitoring for patients with palpitations. World J Cardiol. (2021) 13(11):608–27. doi: 10.4330/wjc.v13.i11.608
28. Bolourchi M, Silver ES, Muwanga D, Mendez E, Liberman L. Comparison of Holter with Zio Patch electrocardiography monitoring in children. Am J Cardiol. (2020) 125(5):767–71. doi: 10.1016/j.amjcard.2019.11.028
29. Yenikomshian M, Jarvis J, Patton C, Yee C, Mortimer R, Birnbaum H, et al. Cardiac arrhythmia detection outcomes among patients monitored with the Zio Patch system: a systematic literature review. Curr Med Res Opin. (2019) 35(10):1659–70. doi: 10.1080/03007995.2019.1610370
30. Pradhan S, Robinson JA, Shivapour JK, Snyder CS. Ambulatory arrhythmia detection with Zio® Xt Patch in pediatric patients: a comparison of devices. Pediatr Cardiol. (2019) 40(5):921–4. doi: 10.1007/s00246-019-02089-0
31. Cheung CC, Kerr CR, Krahn AD. Comparing 14-day adhesive patch with 24-h Holter monitoring. Future Cardiol. (2014) 10:319–22. doi: 10.2217/fca.14.24
32. Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. (2014) 127(1):95.e11–e17. doi: 10.1016/j.amjmed.2013.10.003
33. Rosenberg MA, Samuel M, Thosani A, Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. (2013) 36(3):328–33. doi: 10.1111/pace.12053
34. Brembilla-Perrot B, Spatz F, Khaldi E, de la Chaise AT, Le Van D, Pernot C. Value of esophageal pacing in evaluation of supraventricular tachycardia. Am J Cardiol. (1990) 65(5):322–30. doi: 10.1016/0002-9149(90)90296-D
35. Guarnerio M, Furlanello F, Vergara G, Inama G, Del Greco M, Accardi R, et al. Electropharmacological testing by transoesophageal atrial pacing in inducible supraventricular tachyarrhythmias. A good approach for selection of long-term anti-arrhythmic therapy. Eur Heart J. (1992) 13(6):763–9. doi: 10.1093/oxfordjournals.eurheartj.a060253
36. Pehrson SM, Blomström-Lundqvist C, Ljungström E, Blomström P. Clinical value of transesophageal atrial stimulation and recording in patients with arrhythmia-related symptoms or documented supraventricular tachycardia–correlation to clinical history and invasive studies. Clin Cardiol. (1994) 17(10):528–34. doi: 10.1002/clc.4960171004
37. Kesek M, Sheikh H, Bastani H, Blomström P, Lundqvist CB. The sensitivity of transesophageal pacing for screening in atrial tachycardias. Int J Cardiol. (2000) 72(3):239–42. doi: 10.1016/S0167-5273(99)00191-6
38. Chadda KR, Blakey EE, Huang CLH, Jeevaratnam K. Long COVID-19 and postural orthostatic tachycardia syndrome- is dysautonomia to be blamed? Front Cardiovasc Med. (2022) 9:860198. doi: 10.3389/fcvm.2022.860198
39. Mouram S, Pannone L, Gauthey A, Sorgente A, Vergara P, Bisignani A, et al. Incidence and predictors of cardiac arrhythmias in patients with COVID-19. Front Cardiovasc Med. (2022) 9:908177. doi: 10.3389/fcvm.2022.908177
40. Mariani MV, Pierucci N, Forleo GB, Schiavone M, Bernardini A, Gasperetti A, et al. The feasibility, effectiveness and acceptance of virtual visits as compared to in-person visits among clinical electrophysiology patients during the COVID-19 pandemic. J Clin Med. (2023) 12(2). doi: 10.3390/jcm12020620
41. Rad AB, Kirsch M, Li Q, Xue J, Sameni R, Albert D, et al. A crowdsourced AI framework for atrial fibrillation detection in apple watch and Kardia Mobile Ecgs. Sensors. (2024) 24(17):5708. doi: 10.3390/s24175708
Keywords: palpitations of unknown origin, transesophageal electrophysiological study, wearable adhesive Patch ECG monitors, diagnosis, cardiac electrophysiology
Citation: Yang R, Yang L, Zhang Q, Wang S and Xu J (2024) Wearable Patch ECG monitors and transesophageal electrophysiological study for diagnosing palpitations of unknown origin. Front. Cardiovasc. Med. 11:1469108. doi: 10.3389/fcvm.2024.1469108
Received: 23 July 2024; Accepted: 15 October 2024;
Published: 13 November 2024.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Vincenzo Mirco La Fazia, Texas Cardiac Arrhythmia Institute, United StatesLi-Da Wu, Nanjing Medical University, China
Copyright: © 2024 Yang, Yang, Zhang, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinyi Xu, eGp5ZWNnQHp6dS5lZHUuY24=
 Ruike Yang1
Ruike Yang1 Jinyi Xu
Jinyi Xu