- 1Department of Cardiology, Linping Hospital of Integrated Traditional Chinese and Western Medicine, Hangzhou, China
- 2Department of Cardiology, Jinan Integrated Traditional Chinese and Western Medicine Hospital, Jinan, China
- 3Department of Interventional Cardiology, Shandong Healthcare Group Zaozhuang Hospital, Zaozhuang, China
- 4Department of Interventional Cardiology, Zaozhuang Municipal Hospital, Zaozhuang, China
Background: The association between the Neutrophil-to-Lymphocyte Ratio (NLR) and the prognosis of Atrial Fibrillation (AF) has been extensively studied, yet clinical outcomes have varied. Consequently, this analysis was undertaken to explore the link between NLR and the prognostic markers of AF.
Methods: We conducted an exhaustive search across electronic databases, including PubMed, Embase, Web of Science, and the Cochrane Library, to investigate the correlation between the NLR and indicators of adverse clinical outcomes associated with AF from the database establishment date through March 31, 2024. In this study, the recurrence rate of AF was the primary outcome measure, while the secondary outcome measures were mortality, stroke, and left atrial thrombus. Odds ratio (OR), relative risk (RR), hazard ratio (HR) and standard mean difference (SMD) with a 95% confidence interval (CI) were integrated for assessment, and the stability of prognostic outcomes and publication bias were verified by sensitivity analysis and Egger's test, respectively. Subgroup analyses were performed to pinpoint the sources of heterogeneity.
Results: This analysis included 20 studies, encompassing a total of 59,256 patients. Our statistical analysis of both categorical and continuous variables revealed that an elevated NLR was significantly associated with increased risks in AF patients for recurrence (categorical variable: OR = 1.39, 95% CI = 1.21–1.60; continuous variable: SMD = 0.49, 95% CI = 0.24–0.74), mortality (categorical variable: OR = 1.87, 95% CI = 1.59–2.20), stroke (categorical variable: OR = 1.56, 95% CI = 1.13–2.17; continuous variable: SMD = 0.77, 95% CI = 0.63–0.91), and left atrial thrombus (categorical variable: OR = 1.87, 95% CI = 1.27–2.75; continuous variable: SMD = 0.59, 95% CI = 0.30–0.89). Subgroup analyses found that high NLR was significantly linked to AF recurrence when the NLR was >3. High NLR was significantly linked to the risk of stroke in AF when the NLR was ≤3.
Conclusions: This study suggested that a high NLR is significantly linked to prognostic risk markers of AF, and NLR may be an effective biomarker for the prognosis of AF in clinical practice.
Systematic Review Registration: PROSPERO (CRD42024530970).
1 Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias encountered in clinical practice. It may significantly increase the risk of stroke, heart failure, cardiac arrest, mortality and other adverse outcomes (1). Its incidence is on the rise annually, leading to a considerable deterioration in patients’ quality of life. Data from the Framingham Heart Study indicates that the prevalence of AF has increased twofold over the last five decades (1, 2). The estimated count of AF cases in the United States is anticipated to grow from 1.2 million to 2.6 million between 2010 and 2030 (3). Similarly, in Europe, the number of cases could hit 14 million by the year 2060 (4). At present, AF can be treated by drugs, radiofrequency ablation and surgical intervention, but its recurrence rate is still high (5, 6). For example, La Fazia et al. found in a clinical study that HIV+ AF patients had a persistently high mid- to long-term recurrence rate after undergoing catheter ablation (CA) (7). Although there are different treatments for AF currently, there remains a lack of upstream biomarkers to predict AF prognosis effectively. Therefore, it is still a challenge to pursue effective biomarkers that can predict the clinical outcomes of AF.
A variety of inflammatory indicators, such as interleukins (IL) and C-reactive protein (CRP) have been linked to the occurrence and outcomes of AF. Among them, the neutrophil-lymphocyte ratio (NLR), derived from the comparison of neutrophil to lymphocyte counts, has gained recognition as a robust predictor for the prognosis of AF (8). White blood cell (WBC) counts, along with their subtypes, serve as measures of inflammatory activity. Within the leukocyte spectrum, an elevated neutrophil level suggests a nonspecific inflammatory response, while lymphopenia is indicative of physiological stress and a diminished health condition. Consequently, the NLR offers insight into the equilibrium between these two cell types, shedding light on the body's stress and inflammatory profiles (9). Evidence showed that NLR has good predictive value for progression and clinical outcome of cardiovascular disorder (10). For instance, a significant correlation was identified between elevated preoperative NLR and the recurrence of AF following Cox-Maze IV procedure (CMP-IV) (11). Another large, randomized study reported that increased NLR is associated with a heightened risk of cardiovascular-related events and death in individuals with AF (12). Meanwhile, some studies have debated whether NLR could predict AF prognosis. For example, a clinical study reported that NLR levels did not have predictive value for AF recurrence based on the comparison of NLR levels before and after cryoballoon ablation (13). For this, this comprehensive and precise meta-analysis was conducted to identify the precise predictive function of NLR for AF.
Although recent meta-analyses of NLR on prognosis in AF have been published (14), they focused on a limited range of clinical outcomes. Therefore, this meta-analysis was performed to comprehensively integrate common outcome measures in AF, aiming to evaluate the predictive performance of NLR.
2 Materials and methods
2.1 Protocol and registration
The meta-analysis was duly registered in the Preferred Reporting Guidelines, International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42024530970). There were no protocol deviations from the current study. Furthermore, the research was executed in full compliance with the PRISMA2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (15).
2.2 Study selection
The study selection was guided by the following inclusion criteria: (1) subjects were patients with AF, including various types such as paroxysmal atrial fibrillation, persistent atrial fibrillation, long-standing persistent atrial fibrillation, and permanent atrial fibrillation; (2) the study encompassed a variety of research designs, including randomized controlled trials, cohort studies, and case-control studies.; (3) human subjects were involved; (4) test data included NLR; (5) reported AF outcome measures include recurrence rate, mortality, stroke, and left atrial thrombus, with OR, RR, or HR and 95% CI, as well as SMD, or information that allows for these calculations.”; (6) the study published in English. Exclusion criteria included: (1) case reports, letters, conference abstracts, reviews, and comments; (2) animal experiments; (3) studies with duplicated or overlapping data; and (4) non-English language articles.
2.3 Literature retrieval
An extensive search of online databases from PubMed, Embase, Web of Science, and the Cochrane Library was conducted from database establishment date through March 31, 2024. The keywords were neutrophil and Lymphocyte and ratio and atrial fibrillation. PubMed retrieval formula is as follows: ((((“Neutrophils"[Mesh]) OR ((((((((((((((Neutrophil) OR (Leukocytes, Polymorphonuclear)) OR (Leukocyte, Polymorphonuclear)) OR (Polymorphonuclear Leukocyte)) OR (Polymorphonuclear Leukocytes)) OR (Polymorphonuclear Neutrophils)) OR (Neutrophil, Polymorphonuclear)) OR (Polymorphonuclear Neutrophil)) OR (LE Cells)) OR (Cell, LE)) OR (LE Cell)) OR (Neutrophil Band Cells)) OR (Band Cell, Neutrophil)) OR (Neutrophil Band Cell))) AND ((“Lymphocytes"[Mesh]) OR (((((Lymphocyte) OR (Lymphoid Cells)) OR (Cell, Lymphoid)) OR (Cells, Lymphoid)) OR (Lymphoid Cell)))) AND (ratio)) AND ((“Atrial Fibrillation"[Mesh]) OR (((((((((((((((((((((((((Atrial Fibrillations) OR (Fibrillation, Atrial)) OR (Fibrillations, Atrial)) OR (Auricular Fibrillation)) OR (Auricular Fibrillations)) OR (Fibrillation, Auricular)) OR (Fibrillations, Auricular)) OR (Persistent Atrial Fibrillation)) OR (Atrial Fibrillation, Persistent)) OR (Atrial Fibrillations, Persistent)) OR (Fibrillation, Persistent Atrial)) OR (Fibrillations, Persistent Atrial)) OR (Persistent Atrial Fibrillations)) OR (Familial Atrial Fibrillation)) OR (Atrial Fibrillation, Familial)) OR (Atrial Fibrillations, Familial)) OR (Familial Atrial Fibrillations)) OR (Fibrillation, Familial Atrial)) OR (Fibrillations, Familial Atrial)) OR (Paroxysmal Atrial Fibrillation)) OR (Atrial Fibrillation, Paroxysmal)) OR (Atrial Fibrillations, Paroxysmal)) OR (Fibrillation, Paroxysmal Atrial)) OR (Fibrillations, Paroxysmal Atrial)) OR (Paroxysmal Atrial Fibrillations))). The complete retrieval formulas of the other databases are provided in Supplementary Table S1.
2.4 Data extraction
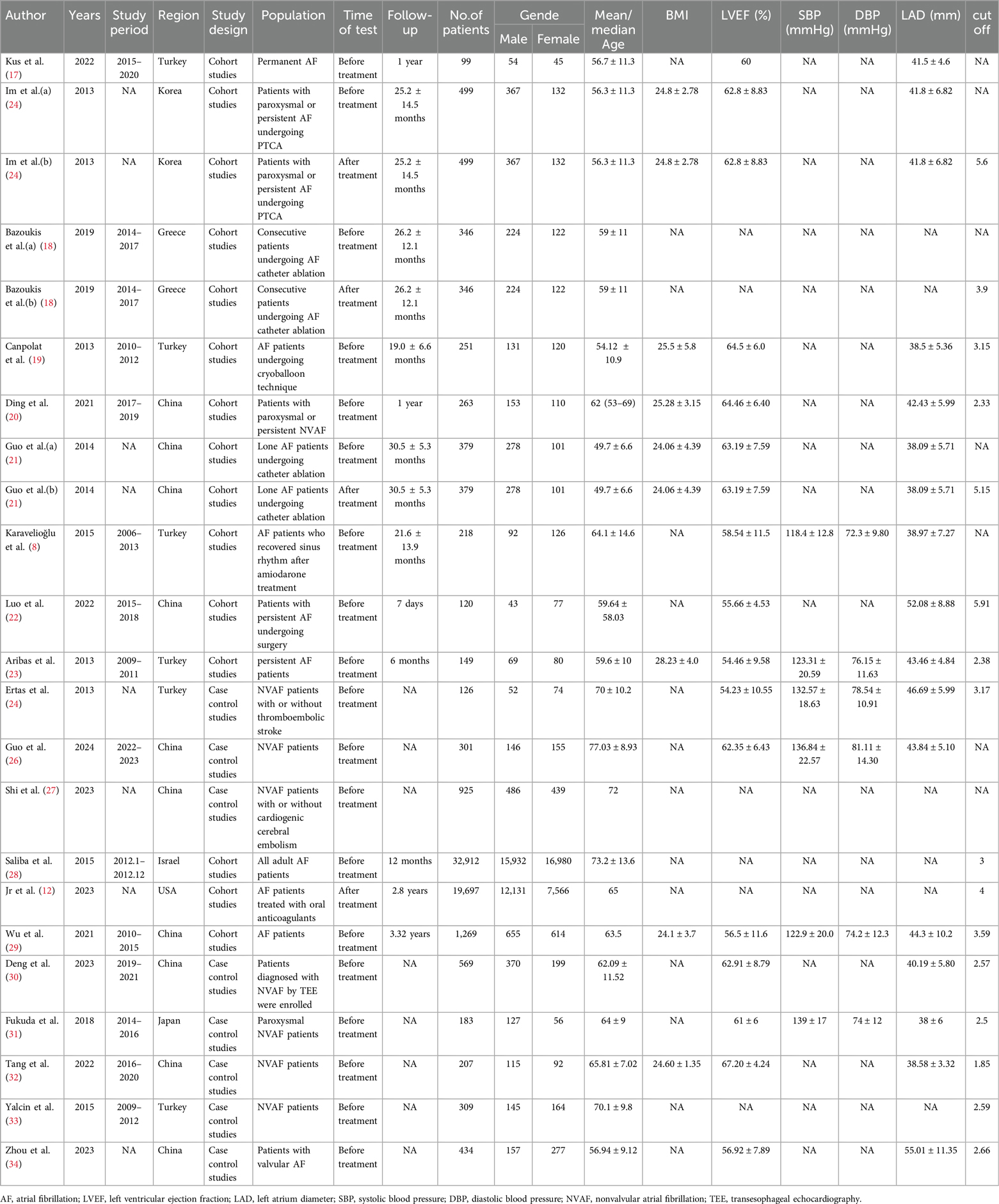
Two investigators (LP and DW) independently evaluated eligible studies and performed data extraction from those included studies. Any discrepancies were addressed through discussions with all contributing co-authors. The data extracted encompassed details such as first author name, publication year, study period, country/region, test timing, sample size, and subjects’ age, sex, body mass index (BMI), blood pressure, left ventricular ejection fraction (LVEF), and left atrial area, follow-up time, cut off, and OR or RR or HR, and 95% CI and SMD. The primary endpoint of interest was the recurrence of AF, while secondary endpoints included mortality, incidence of stroke, and the presence of left atrial thrombus.
2.5 Quality assessment of the studies included
The included study quality was appraised using the Newcastle-Ottawa Scale (NOS), with two independent evaluators (LP and LL) conducting the assessment (16) across three domains: selection (4 points), outcome and adequacy of follow-up (3 points), and comparability (2 points). The NOS scoring system allocates a total of 9 points, with studies earning 7 points or more classified as high quality.
2.6 Statistical analysis
Pooled effect values and 95% CI were determined by a random-effects model to estimate the prognostic effect of NLR in patients with AF. Categorical variables were pooled by OR and continuous variables were pooled by SMD. The heterogeneity between studies was evaluated by Higgins I2 and Cochran's Q test. I2 > 50% and P < 0.1 meant significant heterogeneity. Sensitivity analyses and subgroup analyses stratified by factors were used to confirm the robustness of prognostic outcome measures in AF and to explore potential sources of heterogeneity. We utilized funnel plots and conducted Egger's test to assess publication bias. All data were statistically analyzed using STATA 15.0 and Review Manager 5.4. A P-value < 0.05 was set as the threshold for statistical significance.
3 Results
3.1 Characteristics of the included studies
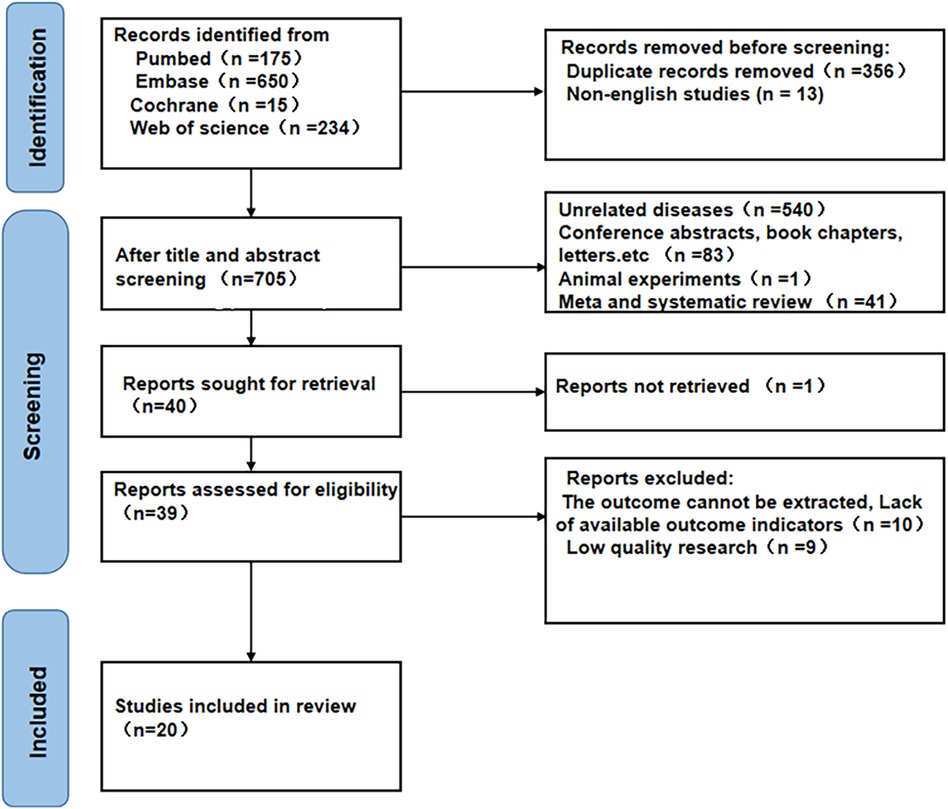
Initially, the search across the four databases yielded a total of 1,074 articles. Among them, 356 duplicated and 13 non-English articles were excluded, and after reading the titles and abstracts of the others, 665 studies were further excluded. The remaining 40 studies were then read in full. Of these, 20 studies were removed: nine due to low quality, one due to the absence of a retrieval report, four because NLR data could not be extracted, and six because the outcome measures were not specified. Ultimately, 20 studies (8, 12, 17–34) involving 59,256 subjects were included in this meta-analysis (Figure 1).
Among the 20 studies, nine originated from research conducted in China (20–22, 26, 27, 29, 30, 32, 34), six in Turkey (8, 17, 19, 23, 25, 33), one each in Greece (18), South Korea (24), the United States (12), Israel (28), and Japan (31). Three (18, 21, 24) of these studies had two cohorts, and therefore a total of 23 studies were included. Twenty-two studies were retrospective (8, 17–34), with the remaining one (12) being prospective. All studies were published within the timeframe from 2013 to 2024. Eighteen of these studies provided data on the NLR and dichotomous variable data on prognostic outcome measures in AF (8, 12, 17–25, 27–31, 33, 34), with one study (12) containing both dichotomous data on mortality and stroke and another one (21) containing two sets of dichotomous data before and after treatment. Eighteen studies (8, 17–22, 24–27, 30, 32–34) reported NLR and continuous variable data on prognostic outcome measures in AF, three (18, 21, 24) of which included two pre- and post-treatment cohort studies. Regarding the measurement of NLR, 16 studies (8, 17, 19, 20, 22, 23, 25–34) investigated NLR at baseline, one (12) investigated NLR after treatment, and three (18, 21, 24) investigated NLR both at baseline and after treatment, and the baseline characteristics are fully described in Table 1.
3.2 Study quality of the studies included
The NOS scores for the included studies ranged between 7 and 8 points, signifying a high level of study quality. Detailed NOS scores are presented in Supplementary Tables S2, S3.
3.3 Analysis results of meta-analysis
3.3.1 NLR and recurrence rate
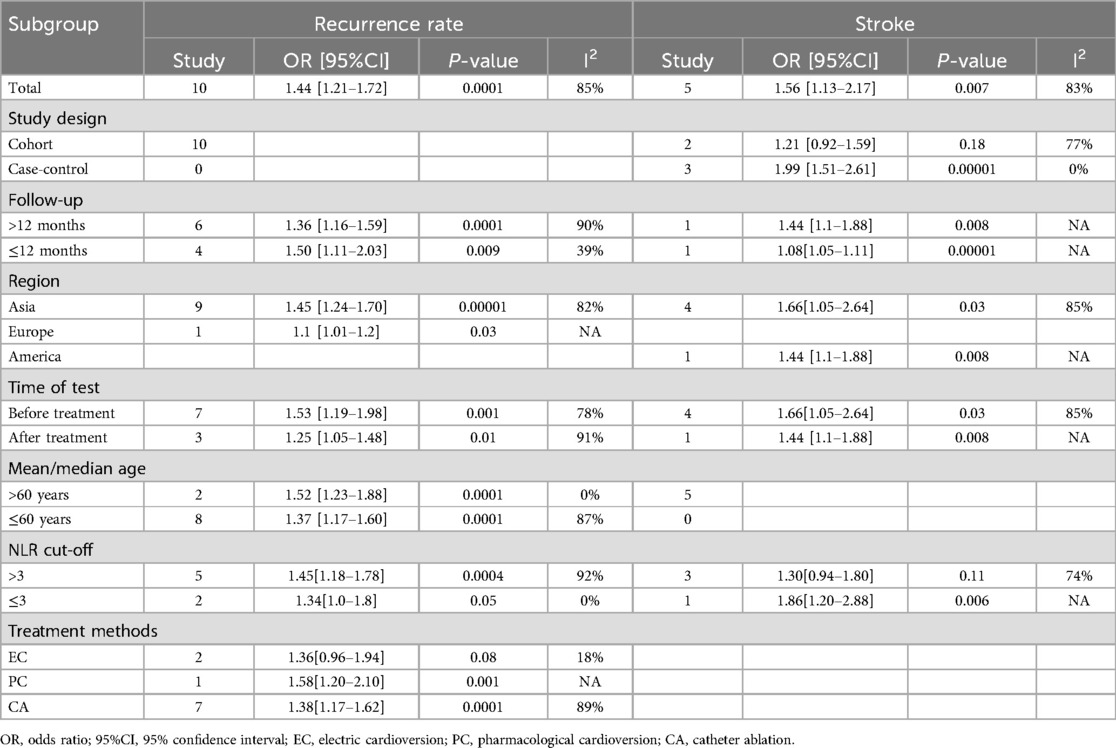
The relationship between NLR and recurrence rate (categorical variable) was investigated. Ten cohort studies were incorporated into the analysis in total, which exhibited significant heterogeneity (I2 = 84%, P < 0.00001) (Figure 2A). Of these studies, seven provided only baseline NLR values and three provided only post-treatment NLR values, the combined result was OR = 1.39, 95% CI = 1.21–1.60, P < 0.00001, indicating a higher NLR was significantly associated with higher AF recurrence rate (Figure 2A). Subgroup analyses were performed before and after treatment, follow-up time, country/region, age and NLR cut off. The findings indicated that there were also notable differences in NLR and AF recurrence rates before and after treatment, follow-up time, country/region, and age (P < 0.05 for all) (Table 2). In the treatment method subgroups, NLR remained a significant predictor of outcomes in the ablation subgroup (P < 0.05), but it was not a significant predictor in the cardioversion subgroup (OR = 1.36, 95% CI = 0.96–1.94, P = 0.08). Additionally, no significant heterogeneity was observed (I2 = 18%, P = 0.27). Taking 3 as the cut off of NLR, NLR > 3 was associated with AF prognosis (OR = 1.45, 95% CI = 1.18–1.78, P = 0.0004) and significant heterogeneity (I2 = 92%, P < 0.00001). NLR ≤ 3 was not significantly linked to the AF recurrence (OR = 1.34, 95% CI: 1.0–1.8; P = 0.05), without heterogeneity (I2 = 0%, p = 0.33). Additionally, we found that when the follow-up time was ≤12 months, age was >60 years, and the NLR cut off was ≤3, the heterogeneity in the cardioversion subgroup was all <50%, suggesting that factors such as follow-up time, age, and cut off may be the source of heterogeneity. The detailed data for the subgroup analysis are presented in Table 2.
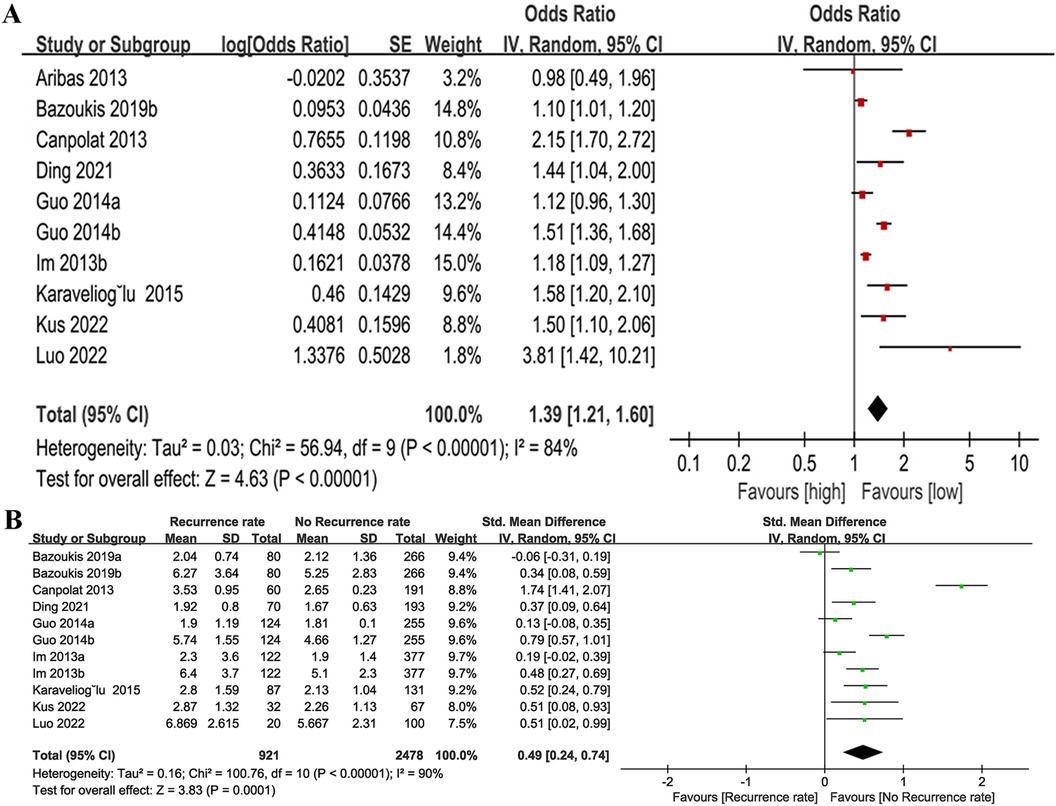
Figure 2. Forest plot of the association between NLR and recurrence rate as categorical variable (A) and continuous variable (B).
For recurrence rate (continuous variable) analysis (Figure 2B), 11 cohort studies involving 2,175 patients were included. Eight of these studies provided pre-treatment baseline NLR and three studies provided pre- and post-treatment NLR. A considerable degree of heterogeneity was observed in the studies included (I2 = 90%, P < 0.00001). The findings indicated that patients with recurrent AF had significantly elevated NLR values compared to those without recurrence (SMD = 0.49, 95% CI = 0.24–0.74, P = 0.0001).
3.3.2 NLR and mortality
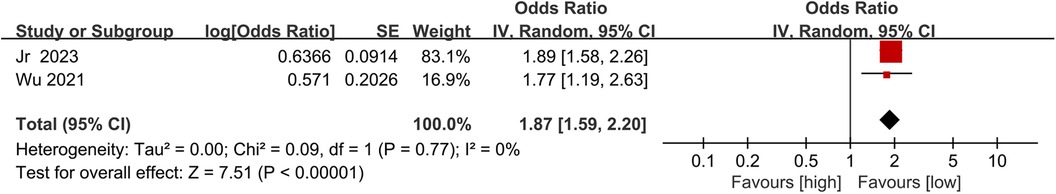
Two studies were incorporated to investigate the link between NLR and mortality: one was a prospective post-treatment study and the other was a retrospective pre-treatment study. No significant heterogeneity was observed between these two studies (I2 = 0%, p = 0.77). As shown in Figure 3, higher NLR was found to be significantly correlated with increased mortality in patients with AF (OR = 1.87, 95% CI = 1.59–2.20, P = 0.00001).
3.3.3 NLR and stroke
In total, five studies that explored the link between NLR and the risk of stroke (categorical variable) were included, of which four provided pre-treatment baseline NLR values while one provided only post-treatment NLR values, with significant heterogeneity observed (I2 = 83%, P < 0.0001). As shown in Figure 4A, high NLR was significantly related to stroke (OR = 1.56, 95% CI = 1.13–2.17, P = 0.007), showing that an elevated NLR is notably correlated with an increased likelihood of stroke in individuals with AF. Subgroup analyses showed that NLR was a significant predictor for the occurrence of stroke in AF patients both before and after treatment, as well as across different follow-up times and nation/region subgroups (P < 0.05 for all) (Table 2). Similarly, taking 3 as the cut off of NLR, we found that in the NLR ≤ 3 subgroup, NLR was significantly associated with the risk of stroke in AF patients (OR = 1.86, 95% CI: 1.20–2.88, P = 0.006). In contrast, in the NLR > 3 subgroup, a significant association was not observed (OR = 1.30, 95% CI = 0.94–1.80, P = 0.11), and significant heterogeneity was observed (I2 = 74%, P = 0.02). In addition, the subgroup analyses found that there was no significant correlation between high NLR and stroke in AF patients in the cohort studies (OR = 1.21, 95% CI: 0.92–1.59; P = 0.18), with significant heterogeneity observed (I2 = 77%, P = 0.04). In contrast, controlled studies demonstrated a considerable link between high NLR and stroke in AF (OR = 1.99, 95% CI = 1.51–2.61, P = 0.00001), with no significant heterogeneity observed (I2 = 0%, P = 0.73), which indicated that study type may be one of the sources of heterogeneity.
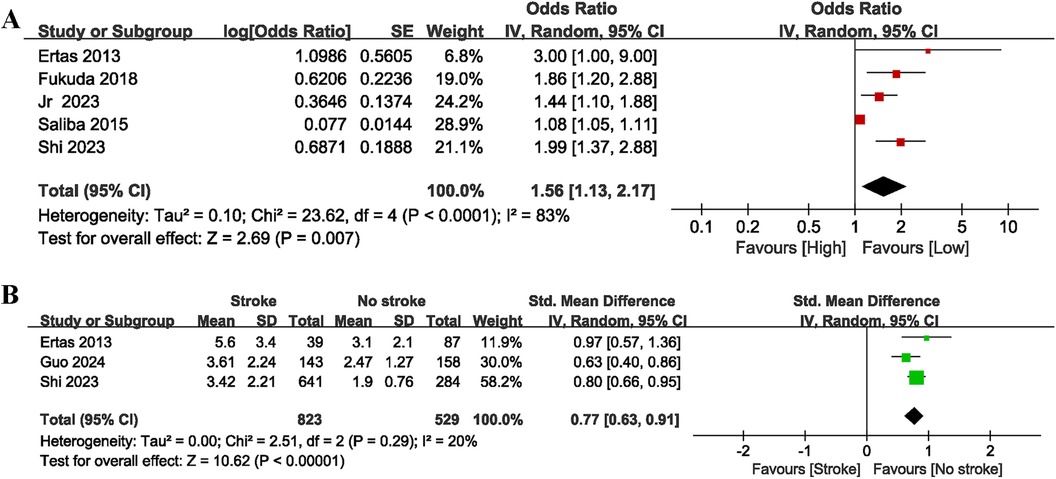
Figure 4. Forest plot of the association between NLR and stroke as categorical variable (A) and continuous variable (B).
For stroke (continuous variable) analysis (Figure 4B), three cohort studies involving 1,352 patients were included. These studies provided pre-treatment baseline NLR, with no significant heterogeneity (I2 = 20%, P = 0.29). The findings indicated that AF patients with stroke exhibited significantly higher NLR values compared to those without stroke (SMD = 0.77, 95% CI = 0.63–0.91, P = 0.00001).
3.3.4 NLR and left atrial thrombus
Three studies provided the relationship between NLR and left atrial thrombus (categorical variable), all of which were pre-treatment-controlled studies. No significant heterogeneity was detected in the studies (I2 = 40%, P = 0.19). As shown in Figure 5A, a high NLR was significantly related to left atrial thrombus (OR = 1.87, 95% CI = 1.27–2.75, P = 0.002), indicating that a higher NLR was significantly linked to an increased risk of left atrial thrombus in AF patients.
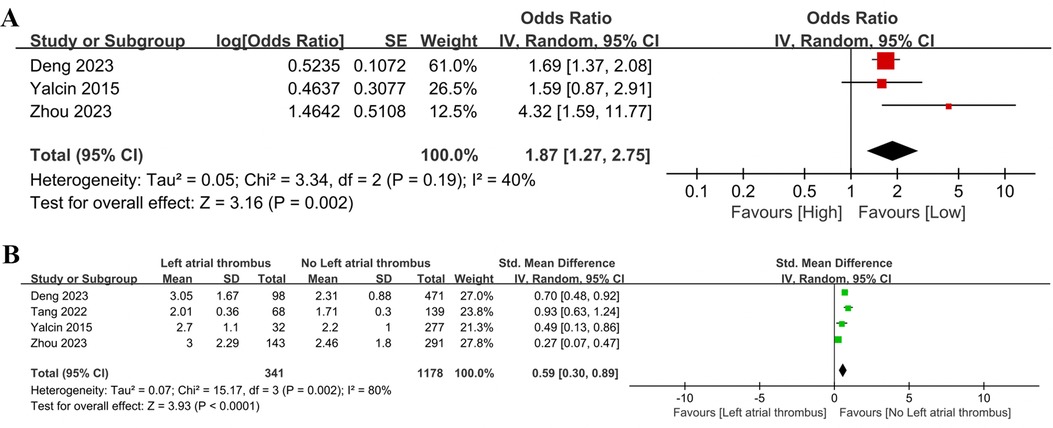
Figure 5. Forest plot of the association between NLR and left atrial thrombus as categorical variable (A) and continuous variable (B).
Four controlled studies involving 1,519 patients were included for left atrial thrombus analysis (continuous variable) (Figure 5B). A considerable level of heterogeneity was identified in the studies (I2 = 80%, P = 0.002). The analysis demonstrated that patients with AF who had left atrial thrombus exhibited significantly higher NLR values compared to those without (SMD = 0.59, 95% CI = 0.30–0.89, P = 0.0001).
3.4 Sensitivity analyses
The sensitivity analyses indicated that the outcomes of this meta-analysis were robust and reliable, as shown in Figures 6A–F. Additionally, the results for recurrence rate, stroke, and left atrial thrombus were not significantly influenced by any single included study. Due to the limited number of studies on mortality (n = 2), no sensitivity analysis was conducted for this outcome.
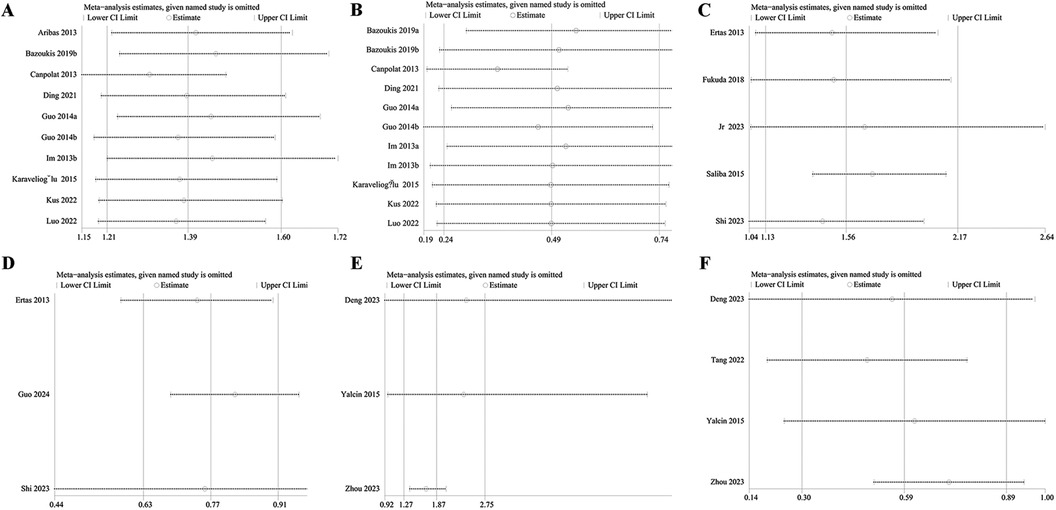
Figure 6. Sensitivity analysis of NLR and recurrence rate as categorical variable (A) and continuous variable (B); sensitivity analysis of NLR and stroke as categorical variables (C) and continuous variable (D) sensitivity analysis of NLR and left atrial thrombus as categorical variable (E) and continuous variable (F).
3.5 Publication bias of the studies included
Egger's test and Funnel plots were utilized to assess publication bias. As shown in Figures 7A–G, the funnel plots appeared symmetrical. The Egger's test results were as follows: P was 0.129 for recurrence rate (categorical variable), 0.314 for recurrence rate (continuous variable), 0.005 for stroke (categorical variable), 0.904 for stroke (continuous variable); 0.508 for left atrial thrombus (categorical variable), and 0.535 for left atrial thrombus (continuous variable).
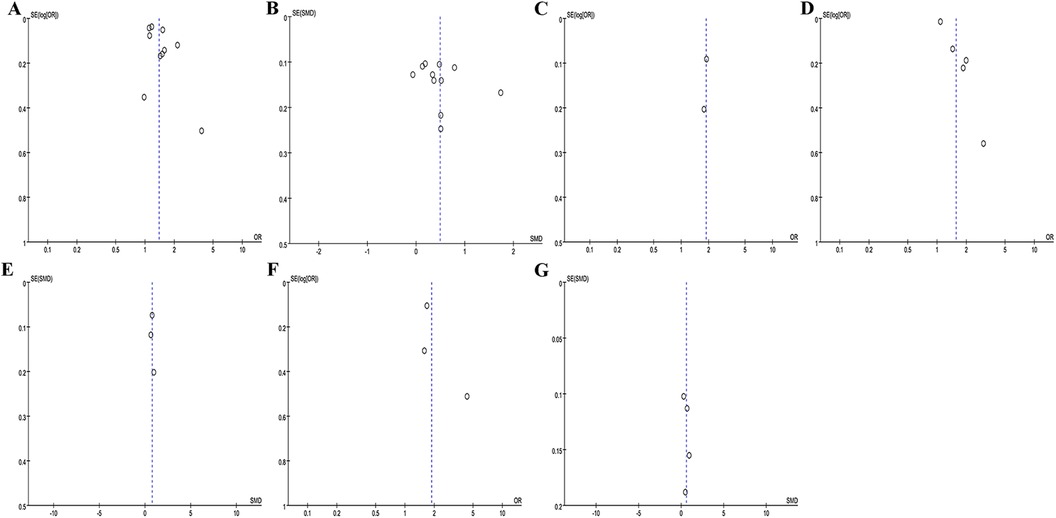
Figure 7. Funnel plot of the association between NLR and recurrence rate as categorical variable (A) and continuous variable (B); funnel plot of the association between NLR and mortality as categorical variable (C) funnel plot of the association between NLR and stroke as categorical variable (D) and continuous variable (E); funnel plot of the association between NLR and left atrial thrombus as categorical variable (F) and continuous variable (G).
4 Discussion
Over the last two decades, the occurrence and spread of atrial fibrillation have escalated, and this upward trend is anticipated to persist for the forthcoming three decades, particularly in nations or regions that possess a moderate socio-demographic index. AF has emerged as one of the most significant epidemic and public health challenges we face (35). The pathogenesis of AF is complex and it is caused by multiple factors such as fibrosis, oxidative stress, inflammation, prothrombotic state and genetics, and inflammation is linked to the initiation and perpetuation of AF, resulting in atrial structural abnormalities and electrophysiological changes (1). Recognizing the pivotal role of inflammation in the advancement of cardiovascular diseases, inflammatory biomarkers like the NLR have gained significant interest in recent years (36). Clinical studies have indicated that preoperative NLR is able to predict late recurrence of non-valvular atrial fibrillation (NVAF) (20). However, according to previous studies, the predictive value of NLR for AF patients is not consistent. For instance, in a prospective cohort study conducted by Aribas et al., it was determined that NLR did not serve as a predictor for the recurrence of AF in patients following cardiac surgery (23). Based on the controversy of predicting the prognosis of AF with NLR, we undertook a systematic and exhaustive meta-analysis to clarify that NLR can serve as an effective biomarker for predicting the prognosis of AF patients.
This meta-analysis suggests that high NLR is an important biomarker for poor prognosis in AF and is significantly associated with recurrence rate, mortality, stroke, and left atrial thrombus. Sensitivity analyses and tests for publication bias have substantiated the dependability of our findings. In conclusion, high NLR is an important prognostic biomarker in patients with AF. Undeniably, recent studies on the prognosis of NLR and AF have been published. For example, a meta-analysis by Lekkala et al. found a significant positive correlation between NLR and AF recurrence (14). Lu et al., in a meta-analysis that included 11 studies involving 35,221 patients, found that elevated NLR values were associated with a higher risk of stroke in AF patients (37). Previous meta-analyses have often focused primarily on AF recurrence rates and stroke, with limited reports on mortality and left atrial thrombus. Therefore, our study expanded on this by including all types of AF patients, incorporating 20 studies with a total of 59,256 patients. This analysis not only examined AF recurrence rates and stroke but also included mortality and left atrial thrombus, covering common clinical prognostic indicators. To the best of our knowledge, this is the latest and most comprehensive meta-analysis investigating NLR in the prognosis of AF patients.
During the past few years, various meta-analyses have similarly documented the prognostic function of NLR in various cardiovascular diseases (38, 39). In a meta-analysis by Vakhshoori et al, it was discovered that an elevated NLR level was associated with a notably higher risk of mortality in individuals with heart failure (38). Furthermore, Wang et al. found in their analysis that NLR was a predictive factor for mortality of all cause and cardiovascular events in patients who have undergone coronary angiography or cardiac revascularization procedures (39). In a meta-analysis by Perry et al., which included 12 studies involving 13,262 patients undergoing cardiac surgery, it was found that perioperative NLR is an independent predictor of both short-term and long-term postoperative mortality following cardiac surgery (40). In our analysis, we discovered a notably substantial prognostic influence exerted by NLR on AF, which is consistent with the findings in other cardiovascular diseases.
In order to provide a more detailed analysis, we performed subgroup assessments on AF recurrence and stroke-related factors to explore the relationship between a high NLR and stroke and AF recurrence. Since left atrial thrombus is a contraindication for catheter ablation in patients with AF, patients with left atrial thrombus were excluded by transesophageal echocardiography (TEE) before catheter ablation in our included studies. Due to the limited binary and continuous data on mortality and left atrial thrombus, we did not conduct a subgroup analysis for these outcomes. However, our statistical analysis of categorical variables revealed that a high NLR was significantly associated with an increased risk of mortality and left atrial thrombus in AF patients. The mechanism by which NLR influences the formation of left atrial thrombus in AF patients may be related to neutrophil extracellular traps (NETs) released by neutrophils, which promote thrombosis (41, 42). In addition, lymphocytes have also been shown to regulate thrombosis (43). Subgroup analyses showed significant differences in AF recurrence and stroke before and after treatment, across different follow-up durations, and between different countries/regions. In the treatment method subgroups, NLR remained a significant predictor of outcomes in the ablation subgroup, but it was not a significant predictor in the cardioversion subgroup. On the other hand, based on an NLR cut off of 3, patients with AF who exhibited high NLR experienced a notably higher rate of recurrence in the NLR > 3 subgroups, suggesting that if NLR cut off associated with recurrence rate is observed in clinical practice, NLR > 3 can be used as the observation threshold for AF recurrence. Canpolat et al. conducted a study on the role of preablation NLR in the recurrence of AF in 251 patients who underwent cryoballoon-based atrial fibrillation ablation. They reported that pre-ablation NLR > 3.15 was associated with a 2.5-fold increased risk of AF recurrence (19). In the subgroup of AF stroke, we found that in the NLR ≤ 3 subgroup, NLR was significantly associated with the risk of stroke in AF patients. It suggests that NLR ≤ 3 can be used as the observation threshold for AF stroke if the relationship between NLR levels and stroke in AF patients is observed in the clinical practice. However, due to the limited data in our study, it needs to be further confirmed if it could find a threshold range for AF recurrence and stroke in clinical practice.
NLR, an indicator of the combined inflammatory state of neutrophils and lymphocytes, is considered a reliable biomarker of systemic inflammation. A greater degree of inflammation in AF patients is indicated by higher NLR levels (21). Despite this, the precise mechanism linking the NLR with the prognosis of AF remains to be fully explained, it can be explained from the following aspects: First, neutrophils release a variety of proteolytic enzymes like elastase, myeloperoxidase, and acid phosphatase, resulting in destructive effects on cardiac tissue (44, 45). Second, neutrophils can also promote apoptosis and activate atrial fibroblasts by acting on cardiomyocytes, resulting in increased fibrous tissue (46, 47). Additionally, neutrophils contribute to endothelial dysfunction (ED), which has been shown to play a role in the development and worsening of AF. This may explain the poor prognosis in patients with high NLR values (48). In addition, lymphocytes play various roles in cardiac tissue injury and repair (49). Regulatory T lymphocytes (Tregs), through the increased secretion of inflammatory cytokines, can promote immune system activation and pathological remodeling (50). Cytotoxic lymphocytes have been shown to be associated with epicardial adipocyte death and subsequent AF-related fibrosis (51). In many cardiovascular diseases, a low lymphocyte count is considered an independent indicator of poor prognosis (52). In conclusion, NLR contains information about two leukocyte subtypes and reflects the balance between neutrophil and lymphocyte levels in the body. NLR may be more accurate than a single leukocyte count in predicting the occurrence of diseases and has more clinical predictive value (53).
This analysis has several limitations. First, most of the included studies were retrospective, resulting in inevitable selective bias and confounding factors in this study. Additionally, the majority of these studies were conducted in Asian countries, which might lead to regional selectivity bias. Further studies are needed to validate the prognostic relevance of NLR in non-Asian patients with AF. Thirdly, significant heterogeneity was found in the analysis of prognostic outcome measures among studies. While subgroup analyses in this study helped pinpoint sources of heterogeneity, the inconsistency in NLR cut offs used in the included studies likely contributed to it as well. Therefore, there is a continued need for multicenter, prospective trials to confirm the findings of our meta-analysis. Fourthly, due to significant differences in the treatment methods for the patients included in this study, and unavailable treatment methods from some studies on mortality, stroke, and left atrial thrombosis, and a limited number of studies on mortality and left atrial thrombosis, subgroup analysis by treatment methods is infeasible, which is also one of the limitations of this study. Further research is needed to address such problems.
5 Conclusions
To sum up, this meta-analysis revealed that a high NLR was significantly linked to adverse outcomes such as recurrence rate, mortality, stroke, and atrial thrombus in AF patients. This association remained consistent regardless of region and treatment timing, and the results were robust. The study's outcomes imply that, in clinical settings, NLR has potential as a prognostic biomarker for patients with AF, with cost-effectiveness and rich information. However, given the limitations of this study, including the predominance of retrospective studies, high heterogeneity, and potential selectivity bias, future research should focus on larger, multicenter, prospective clinical trials to ascertain the precise link between NLR and the AF patients’ prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LP: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. LL: Writing – review & editing, Writing – original draft. MC: Writing – original draft, Writing – review & editing. ZC: Writing – original draft, Writing – review & editing. DW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1461923/full#supplementary-material
References
1. Sagris M, Vardas EP, Theofilis P, Antonopoulos AS, Oikonomou E, Tousoulis D. Atrial fibrillation: pathogenesis, predisposing factors, and genetics. Int J Mol Sci. (2021) 23(1):6–24. doi: 10.3390/ijms23010006
2. Brundel B, Ai X, Hills MT, Kuipers MF, Lip GYH, de Groot NMS. Atrial fibrillation. Nature Reviews Disease Primers. (2022) 8(1):21. doi: 10.1038/s41572-022-00347-9
3. Khurshid S, Healey JS, McIntyre WF, Lubitz SA. Population-Based screening for atrial fibrillation. Circ Res. (2020) 127(1):143–54. doi: 10.1161/CIRCRESAHA.120.316341
4. Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi M, et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European union: the FAI project. Europace. (2019) 21(10):1468–75. doi: 10.1093/europace/euz141
5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. Corrigendum to: 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(40):4194. doi: 10.1093/eurheartj/ehab648
6. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14(10):e275–444. doi: 10.1016/j.hrthm.2017.05.012
7. La Fazia VM, Pierucci N, Mohanty S, Gianni C, Della Rocca DG, Compagnucci P, et al. Catheter ablation approach and outcome in HIV+ patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol. (2023) 34(12):2527–34. doi: 10.1111/jce.16076
8. Karavelioğlu Y, Karapınar H, Yüksel M, Memiç K, Sarak T, Kurt R, et al. Neutrophil to lymphocyte ratio is predictor of atrial fibrillation recurrence after cardioversion with amiodarone. Clin Appl Thromb Hemost. (2015) 21(1):5–9. doi: 10.1177/1076029613518368
9. Shao Q, Chen K, Rha SW, Lim HE, Li G, Liu T. Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Arch Med Res. (2015) 46(3):199–206. doi: 10.1016/j.arcmed.2015.03.011
10. Kim S, Eliot M, Koestler DC, Wu WC, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson heart study and modification by the duffy antigen variant. JAMA Cardiology. (2018) 3(6):455–62. doi: 10.1001/jamacardio.2018.1042
11. Jiang Z, Song L, Liang C, Zhang H, Tan H, Sun Y, et al. Machine learning-based analysis of risk factors for atrial fibrillation recurrence after cox-maze IV procedure in patients with atrial fibrillation and chronic valvular disease: a retrospective cohort study with a control group. Front Cardiovasc Med. (2023) 10:1140670. doi: 10.3389/fcvm.2023.1140670
12. Fagundes A Jr., Ruff CT, Morrow DA, Murphy SA, Palazzolo MG, Chen CZ, et al. Neutrophil-lymphocyte ratio and clinical outcomes in 19,697 patients with atrial fibrillation: analyses from ENGAGE AF- TIMI 48 trial. Int J Cardiol. (2023) 386:118–24. doi: 10.1016/j.ijcard.2023.05.031
13. Aksu T, Baysal E, Guler TE, Golcuk SE, Erden İ, Ozcan KS. Predictors of atrial fibrillation recurrence after cryoballoon ablation. J Blood Med. (2015) 6:211–7. doi: 10.2147/JBM.S81551
14. Lekkala SP, Mellacheruvu SP, Gill KS, Khela PS, Singh G, Jitta SR, et al. Association between preablation and postablation neutrophil-lymphocyte ratio and atrial fibrillation recurrence: a meta-analysis. J Arrhythm. (2024) 40(2):214–21. doi: 10.1002/joa3.12996
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
17. Kus G, Çagirci G, Bayar N, Özgünoglu EC, Güven R, Arslan S. Usefulness of systemic immune-inflammation index in predicting atrial fibrillation recurrence after direct current cardioversion. Biomark Med. (2022) 16(11):847–55. doi: 10.2217/bmm-2022-0120
18. Bazoukis G, Letsas KP, Vlachos K, Saplaouras A, Asvestas D, Tyrovolas K, et al. Simple hematological predictors of AF recurrence in patients undergoing atrial fibrillation ablation. J Geriatr Cardiol. (2019) 16(9):671–5. doi: 10.11909/j.issn.1671-5411.2019.09.008
19. Canpolat U, Aytemir K, Yorgun H, Şahiner L, Kaya EB, Kabakçı G, et al. Role of preablation neutrophil/lymphocyte ratio on outcomes of cryoballoon-based atrial fibrillation ablation. Am J Cardiol. (2013) 112(4):513–9. doi: 10.1016/j.amjcard.2013.04.015
20. Ding B, Liu P, Zhang F, Hui J, He L. Predicting values of neutrophil-to-lymphocyte ratio (NLR), high-sensitivity C-reactive protein (hs-CRP), and left atrial diameter (LAD) in patients with nonvalvular atrial fibrillation recurrence after radiofrequency ablation. Med Sci Monit. (2021) 27:e934569. doi: 10.12659/MSM.934569
21. Guo XY, Zhang S, Yan XL, Chen YW, Yu RH, Long DY, et al. Postablation neutrophil/lymphocyte ratio correlates with arrhythmia recurrence after catheter ablation of lone atrial fibrillation. Chin Med J. (2014) 127(6):1033–8. doi: 10.3760/cma.j.issn.0366-6999.20133001
22. Luo Y, Zhang J, Liu T, Yin Z, Jin Y, Han J, et al. The systemic-immune-inflammation index predicts the recurrence of atrial fibrillation after cryomaze concomitant with mitral valve surgery. BMC Cardiovasc Disord. (2022) 22(1):45. doi: 10.1186/s12872-022-02494-z
23. Aribas A, Akilli H, Gül EE, Kayrak M, Demir K, Duman Ç, et al. Can neutrophil/lymphocyte ratio predict recurrence of non-valvular atrial fibrillation after cardioversion? Anatol J Cardiol. (2013) 13(2):123–30. doi: 10.5152/akd.2013.036
24. Im SI, Shin SY, Na JO, Kim YH, Choi CU, Kim SH, et al. Usefulness of neutrophil/lymphocyte ratio in predicting early recurrence after radiofrequency catheter ablation in patients with atrial fibrillation. Int J Cardiol. (2013) 168(4):4398–400. doi: 10.1016/j.ijcard.2013.05.042
25. Ertas G, Sonmez O, Turfan M, Kul S, Erdogan E, Tasal A, et al. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. (2013) 324(1-2):49–52. doi: 10.1016/j.jns.2012.09.032
26. Guo J, Zhou Y, Zhou B. Development and validation of a new nomogram model for predicting acute ischemic stroke in elderly patients with non-valvular atrial fibrillation: a single-center cross-sectional study. Clin Interv Aging. (2024) 19:67–79. doi: 10.2147/CIA.S437065
27. Shi Y, Xuan C, Ji W, Wang F, Huang J, Li L, et al. Combination of platelet-to-lymphocyte ratio and D-dimer for the identification of cardiogenic cerebral embolism in non-valvular atrial fibrillation. Front Neurol. (2023) 14:1069261. doi: 10.3389/fneur.2023.1069261
28. Saliba W, Barnett-Griness O, Elias M, Rennert G. Neutrophil to lymphocyte ratio and risk of a first episode of stroke in patients with atrial fibrillation: a cohort study. J Thromb Haemostasis. (2015) 13(11):1971–9. doi: 10.1111/jth.13006
29. Wu S, Yang YM, Zhu J, Ren JM, Wang J, Zhang H, et al. Impact of baseline neutrophil-to-lymphocyte ratio on long-term prognosis in patients with atrial fibrillation. Angiology. (2021) 72(9):819–28. doi: 10.1177/00033197211000495
30. Deng Y, Zhou F, Li Q, Guo J, Cai B, Li G, et al. Associations between neutrophil-lymphocyte ratio and monocyte to high-density lipoprotein ratio with left atrial spontaneous echo contrast or thrombus in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. (2023) 23(1):234. doi: 10.1186/s12872-023-03270-3
31. Fukuda Y, Okamoto M, Tomomori S, Matsumura H, Tokuyama T, Nakano Y, et al. In paroxysmal atrial fibrillation patients, the neutrophil-to-lymphocyte ratio is related to thrombogenesis and more closely associated with left atrial appendage contraction than with the left atrial body function. Internal Medicine. (2018) 57(5):633–40. doi: 10.2169/internalmedicine.9243-17
32. Tang LX, Xia Y, Fang L. Correlation between left atrial thrombosis and neutrophil-to-lymphocyte ratio upon non-valvular atrial fibrillation. Clin Lab. (2022) 68(2):326–33. doi: 10.7754/Clin.Lab.2021.210409
33. Yalcin M, Aparci M, Uz O, Isilak Z, Balta S, Dogan M, et al. Neutrophil-lymphocyte ratio may predict left atrial thrombus in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. (2015) 21(2):166–71. doi: 10.1177/1076029613503398
34. Zhou Y, Song X, Ma J, Wang X, Fu H. Association of inflammation indices with left atrial thrombus in patients with valvular atrial fibrillation. BMC Cardiovasc Disord. (2023) 23(1):9. doi: 10.1186/s12872-023-03036-x
35. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. (2021) 16(2):217–21. doi: 10.1177/1747493019897870
36. Farah R, Samra N. Mean platelets volume and neutrophil to lymphocyte ratio as predictors of stroke. J Clin Lab Anal. (2018) 32(1):e22189. doi: 10.1002/jcla.22189
37. Lu M, Zhang Y, Liu R, He X, Hou B. Predictive value of neutrophil to lymphocyte ratio for ischemic stroke in patients with atrial fibrillation: a meta-analysis. Front Neurol. (2022) 13:1029010. doi: 10.3389/fneur.2022.1029010
38. Vakhshoori M, Nemati S, Sabouhi S, Yavari B, Shakarami M, Bondariyan N, et al. Neutrophil to lymphocyte ratio (NLR) prognostic effects on heart failure; a systematic review and meta-analysis. BMC Cardiovasc Disord. (2023) 23(1):555. doi: 10.1186/s12872-023-03572-6
39. Wang X, Zhang G, Jiang X, Zhu H, Lu Z, Xu L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis. (2014) 234(1):206–13. doi: 10.1016/j.atherosclerosis.2014.03.003
40. Perry LA, Liu Z, Loth J, Penny-Dimri JC, Plummer M, Segal R, et al. Perioperative neutrophil-lymphocyte ratio predicts mortality after cardiac surgery: systematic review and meta-analysis. J Cardiothorac Vasc Anesth. (2022) 36(5):1296–303. doi: 10.1053/j.jvca.2021.07.001
41. Carminita E, Crescence L, Panicot-Dubois L, Dubois C. Role of neutrophils and NETs in animal models of thrombosis. Int J Mol Sci. (2022) 23(3):1411–33. doi: 10.3390/ijms23031411
42. Behzadifard M, Soleimani M. NETosis and SARS-COV-2 infection related thrombosis: a narrative review. Thromb J. (2022) 20(1):13. doi: 10.1186/s12959-022-00375-1
43. Bennett JA, Mastrangelo MA, Ture SK, Smith CO, Loelius SG, Berg RA, et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat Commun. (2020) 11(1):3479. doi: 10.1038/s41467-020-17254-w
44. Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. (2010) 56(6):944–51. doi: 10.1373/clinchem.2009.142257
45. Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev. (2006) 17(4):225–33. doi: 10.1016/j.cytogfr.2006.04.003
46. Meulendijks ER, Al-Shama RFM, Kawasaki M, Fabrizi B, Neefs J, Wesselink R, et al. Atrial epicardial adipose tissue abundantly secretes myeloperoxidase and activates atrial fibroblasts in patients with atrial fibrillation. J Transl Med. (2023) 21(1):366. doi: 10.1186/s12967-023-04231-2
47. He L, Liu R, Yue H, Zhang X, Pan X, Sun Y, et al. Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct Target Ther. (2023) 8(1):279. doi: 10.1038/s41392-023-01497-2
48. Angkananard T, Anothaisintawee T, McEvoy M, Attia J, Thakkinstian A. Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int. (2018) 2018:2703518. doi: 10.1155/2018/2703518
49. Huang M, Huiskes FG, de Groot NMS, Brundel B. The role of immune cells driving electropathology and atrial fibrillation. Cells. (2024) 13(4). doi: 10.3390/cells13040311
50. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation. (2019) 139(2):206–21. doi: 10.1161/CIRCULATIONAHA.118.036065
51. Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J. (2017) 38(1):53–61. doi: 10.1093/eurheartj/ehv625
52. Vaduganathan M, Ambrosy AP, Greene SJ, Mentz RJ, Subacius HP, Maggioni AP, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. (2012) 5(6):750–8. doi: 10.1161/CIRCHEARTFAILURE.112.970525
Keywords: neutrophil-to-lymphocyte ratio, atrial fibrillation, prognosis, systematic review, meta-analysis
Citation: Peng L, Liu L, Chai M, Cai Z and Wang D (2024) Predictive value of neutrophil to lymphocyte ratio for clinical outcome in patients with atrial fibrillation: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1461923. doi: 10.3389/fcvm.2024.1461923
Received: 9 July 2024; Accepted: 16 September 2024;
Published: 26 September 2024.
Edited by:
Yap-Hang Chan, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Carlo Lavalle, Sapienza University of Rome, ItalySimon Salim, RSUPN Dr. Cipto Mangunkusumo, Indonesia
Copyright: © 2024 Peng, Liu, Chai, Cai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deqi Wang, d2RxMTk4MEAxMjYuY29t
 Lei Peng1
Lei Peng1 Deqi Wang
Deqi Wang