- 1Department of Cardiology and Internal Intensive Care Medicine, Heart Center Munich-Bogenhausen Munich Municipal Hospital Group, Munich, Germany
- 2Department of Cardiology, Cardio Centrum Düsseldorf, Düsseldorf, Germany
- 3Department of Cardiology, Cardiology Practice, Traunreut, Germany
Objectives: The occurrence of sudden cardiac death (SCD) in competitive athletes has led to a discussion about appropriate preparticipation screening models. The role of an electrocardiogram (ECG) in routine testing remains controversial in current guidelines. Furthermore, data on cardiac findings and the prognostic utility of screening strategies in young female elite ice hockey is scarce.
Methods: Female elite ice hockey players were enrolled in the open prospective “General Evaluation Program for Arrhythmia-Related Death in Athletes” (GEPARD) registry from 2008 to 2018. A staged preparticipation screening was performed. The main goal was to determine the prevalence of SCD conditions and identify effective screening tools. The secondary aim was to study baseline results and follow-ups on a unique subgroup of young female ice hockey players.
Results: A total of 88 female ice hockey players, mean age 16 ± 1 years, were prospectively enrolled. The prevalence of conditions potentially leading to SCD during competition was 3.4% (3/88). The 12-lead ECG led to the diagnosis of one congenital long QT and one acute myocarditis and showed a number needed to screen of 44, with a specificity of 98%. One athlete demonstrated a relevant pericardial effusion on echocardiography, which was related to acute toxoplasmosis. No cases of SCD occurred during long-term follow-up.
Conclusion: The subgroup of young female ice hockey players showed a notable prevalence of athletes “at risk” of 3.4%, which indicates the importance of preparticipation screening that features a 12-lead ECG as the most important component.
Introduction
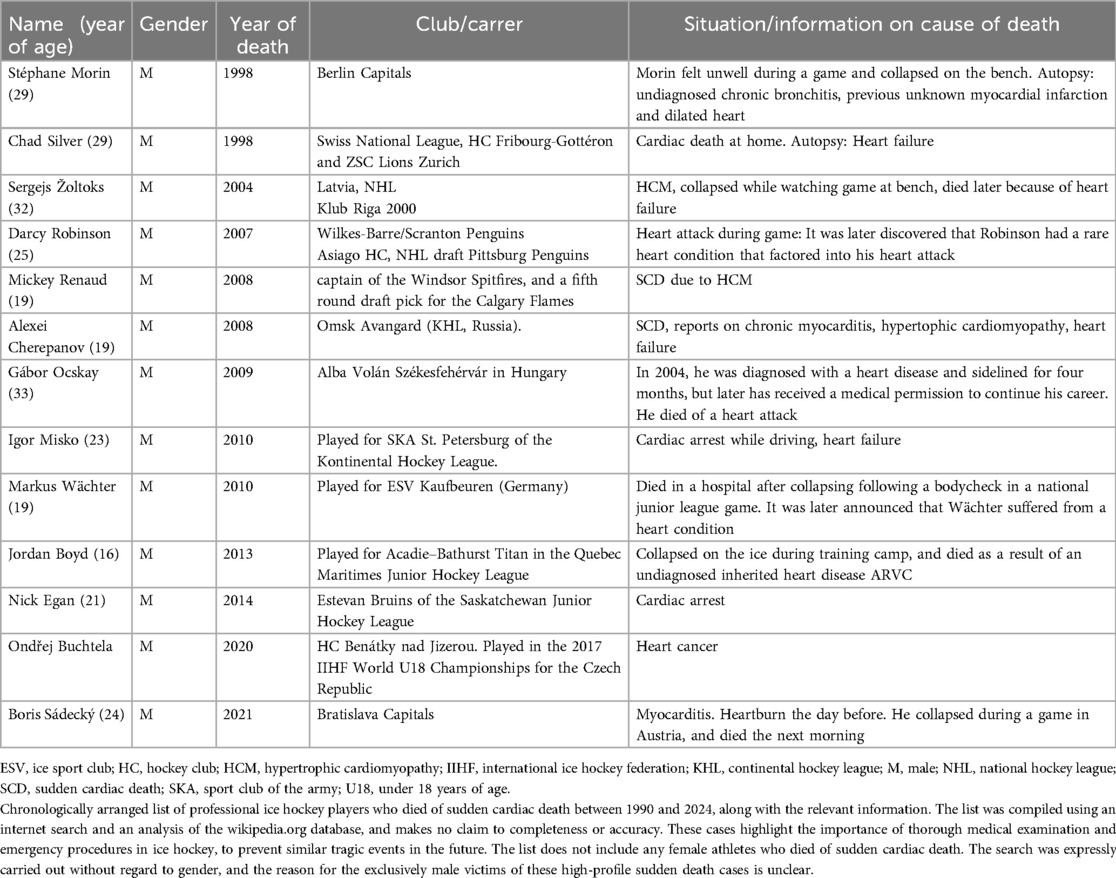
Sudden cardiac death (SCD) in competitive sports is a profoundly tragic event, typically affecting young, seemingly healthy athletes (1). Since 1999, when two professional male ice hockey players tragically died from SCD during a sport event, SCD has become a significant topic of concern in the professional ice hockey community (Table 1).
There is already substantial data on the increased risk, pathogenesis, and appropriate prevention of sudden cardiac death in competitive sports. However, most of these studies have predominantly focused on male athletes (1–3). Due to the increasing proportion of women in competitive sport, a gender-specific discussion is necessary. As existing studies showed women to have a 2–25 times lower risk of SCD than men (3–6), there may be less awareness of preparticipation screening for SCD in female sports.
Several studies indicate that the profile of women experiencing SCD differs from that of men in terms of underlying causes, clinical presentations, and outcomes. This necessitates further investigation to gather more data regarding the functionalities and performance of the female heart in competitive sports. Consequently, this will aid in developing appropriate preparticipation tests for all athletes (7, 8).
In this field there is an ongoing debate about the role of the 12-lead ECG as a fixed part of preparticipation screening between the American Heart Association (AHA), which is opposed due to the cost and the risk of false positive results (9–11), and the European Society of Cardiolgy (ESC), which clearly supports the use of the 12-lead ECG in screening after studies showed a significant reduction in SCD in athletes following its introduction as a mandatory screening test (1, 3, 12). This question might also need to be revisited with regard to gender differences.
The main objective of the study was to evaluate a preparticipation testing model to detect cardiovascular abnormalities that could potentially result in SCD and to define their prevalence in the special subgroup of female adolescent athletes. The secondary objective was to provide a unique baseline data-set of young female elite ice hockey players collected during preparticipation screening, and to provide follow-up data.
Methods
Study design
The “General Evaluation Program for Prevention of Arrhythmia Related Deaths in Athletes” (GEPARD) is an open, prospective, observational registry including competitive athletes who undergo preparticipation screening. The present study enrolled and analyzed all adolescent female ice hockey players referred from the German Female National Ice Hockey Team between 2008 and 2018. The study was approved by the ethical committee of the Bavarian Chamber of Physicians and follows the principles of the Declaration of Helsinki. All participants had to sign an informed consent form.
Study protocol
The GEPARD protocol follows a preparticipation screening model, which consisted of three stages (Figure 1) and includes a similar diagnostic workup for assessing athletes with arrhythmias and/or suspected cardiomyopathies as previously described (13, 14).
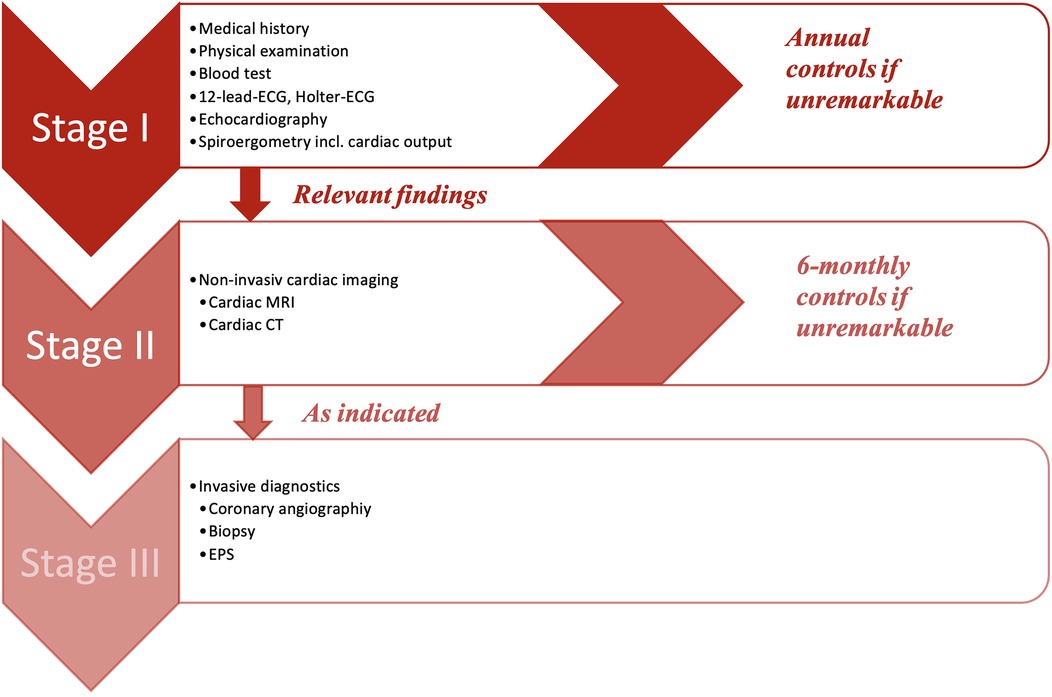
Figure 1. GEPARD multi-stage screening model. CT, computed tomography; ECG, electrocardiogram; EPS, electrophysiology study; MRI, magnetic resonance imaging.
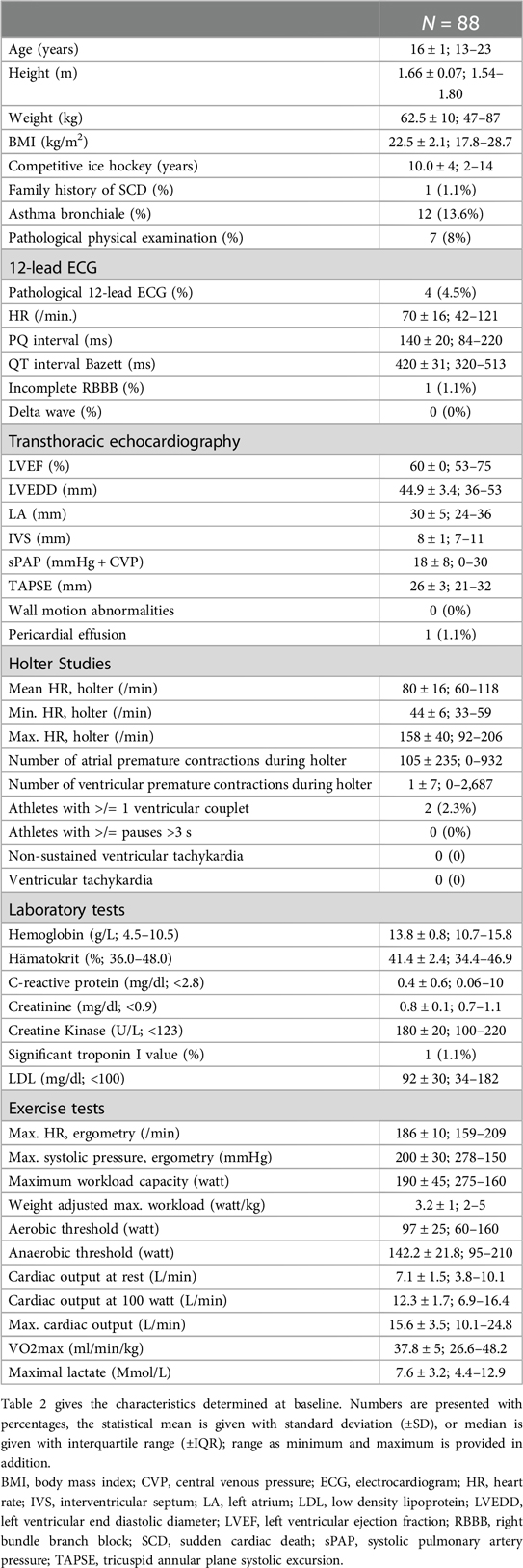
Every player had to undergo stage I, which included the evaluation of the medical history, a physical examination, a blood test, a 12-lead-ECG, a ≥18 h of Holter-ECG and a transthoracic echocardiography. In addition to screening for SCD during stage I, a spiroergometry was also performed with an Innocor® inert gas rebreathing system by COSMED. This was done to provide additional practice control and to assess the level of physical performance, including maximum oxygen consumption (VO2max), aerobic and anaerobic thresholds, and cardiac output. A step-up protocol was applied with 3-min intervals starting at 50 W with 25 W steps of increase. Measurements of CO was performed at baseline, sub-maximum, and maximum workload. Lactate measurements were taken at the end of each step. ECG monitoring was performed continuously throughout the examination. The recordings were printed out at the end of each stage.
If no abnormalities were detected, the athlete was cleared for sport and competition. In case of any suspicious findings, stage II of the study protocol became applicable, which contained further non-invasive cardiac imaging like cardiac magnetic resonance imaging (MRI) or computed tomography (CT) as indicated. Stage III included all invasive further diagnostic approaches (i.e., cardiac catheterization, electrophysiological study, myocardial biopsy), and was performed as clinically indicated.
Data analysis
Data was collected and analyzed on the secure institutional server using Microsoft Access, Microsoft Excel and IBM SPSS (USA). In accordance with the Shapiro-Wilk test, continuous variables are expressed as means with standard deviations (SD) or as medians with quartiles. Categorical data are shown as numbers and percentages.
Results
Participants
Eighty-eight elite female ice hockey players were enrolled between 01/2008 and 01/2018, at a median age of 16 (Table 2). The median time of follow-up from first screening to last contact was 13 years. Completeness of follow-up (FU) with at least ≥12 months individual follow-up was 100%. Main exercise related parameters are shown in Figure 2. All players included in this study were members of the German Female National Ice Hockey Teams.
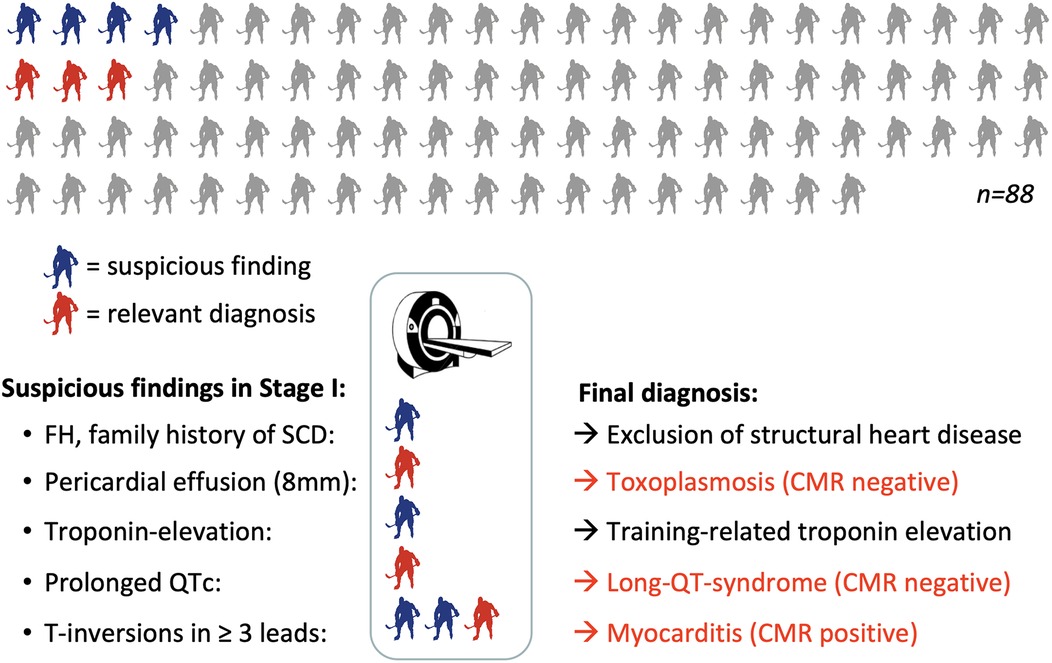
Figure 2. Graphical summary of the final results including cardiac MRI. Grey: player without pathologies at stage I; red: players with relevant diagnosis; blue = players with pathological findings in stage I showing normal results at stage II. CMR, cardiac magnetic resonance imaging; FH, family history; SCD, sudden cardiac death.
Screening for SCD
In stage I of the screening model, seven of 88 athletes (8%) showed relevant findings (Figure 2). Following the study protocol, stage II of the screening model became applicable and one further non-invasive diagnostic approach was performed, which was a cardiac magnetic resonance (CMR) in all of the seven cases. Stage III of the study protocol (invasive diagnostics) was neither indicated nor performed in any of the participants.
At stage I, ECG alterations were the main relevant finding (4/7, 57%). Three of them had significant T-negativity in ≥3 ECG leads (Figure 3), so the cardiac MRI as part of stage II was applied and lead to the diagnosis of myocarditis in one athlete (Figure 3) while the other 2 did not show any pathologies. The player with confirmed myocarditis was advised to take a break from sports practice, treated with nonsteroidal anti-inflammatory drugs and angiotensin-converting-enzyme inhibitors and controlled after 4–6 weeks and 3 months. One out of four athletes with pathological ECG showed a significant prolonged QT interval (QT 536 ms, QTc Bazett 513 ms; Figure 4). In combination with a positive family history for prolonged QT intervals, a congenital Long-QT-syndrome was diagnosed and consequently an exclusion from competitive sports was recommended. The cardiac MRI (stage II) in this player showed no relevant findings.
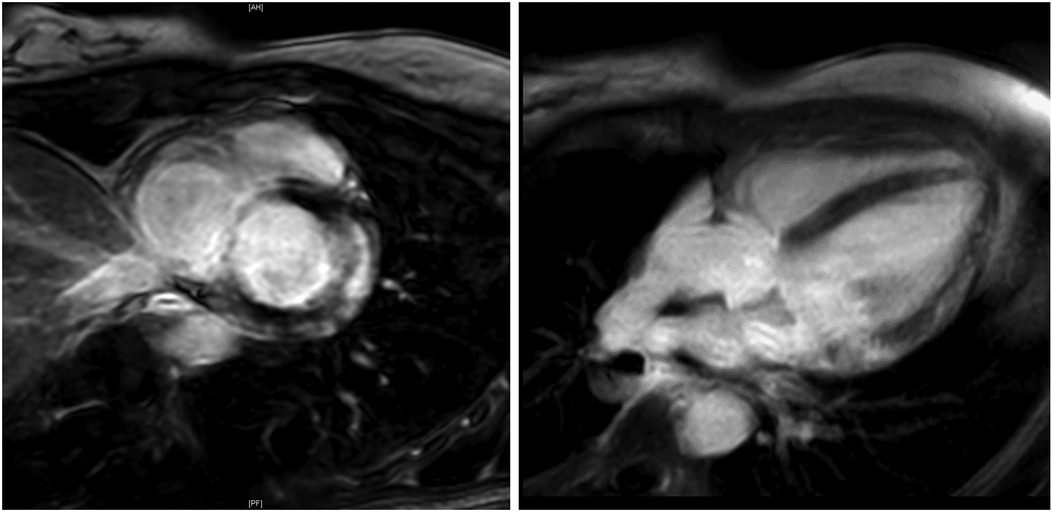
Figure 3. Cardiac MRI. Cardiac MRI of a young female ice-hockey player demonstrating late gadolinium enhancement of the lateral wall and global edema, following ECG-detected T-wave inversions, strongly suggesting myocarditis. MRI, magnetic resonance imaging.
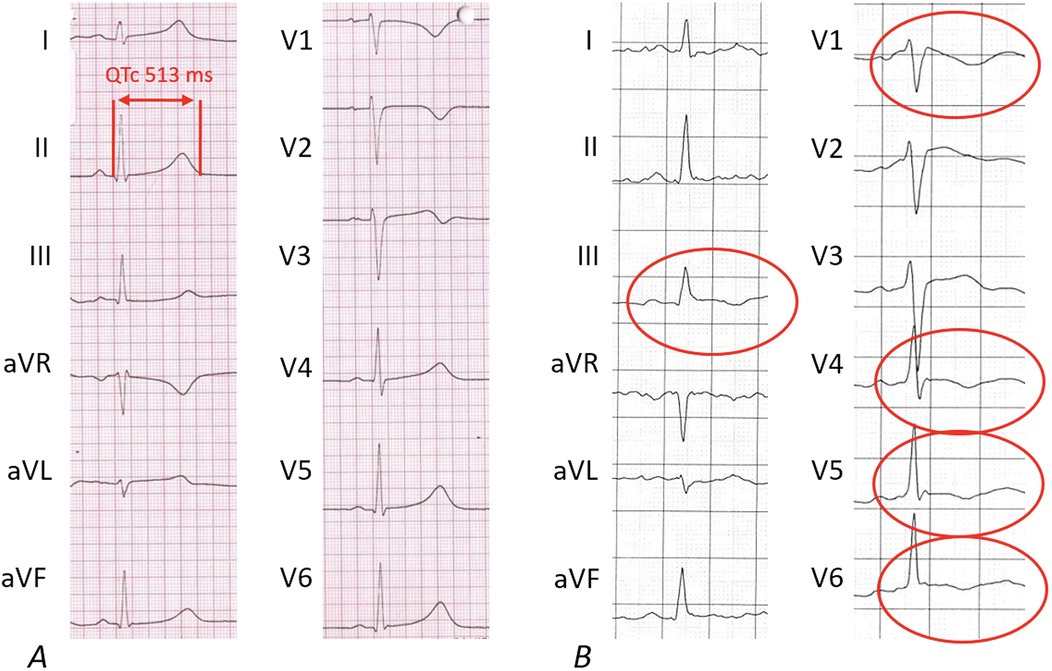
Figure 4. Pathological findings of 12-lead-electrocardigrams. The pathological 12-lead-ECGs from 2 out of 4 players lead to relevant diagnoses. (A) Shows the ECG of an athlete with a newly diagnosed Long-QT ECG pattern; (B) shows inverted T-waves in more than 2 leads leading to the final diagnosis of acute myocarditis following cardiac MRI.
There were no pathological electrocardiographic findings noted during spiroergometry.
In one athlete, the echocardiography (stage I) showed an asymptomatic pericardial effusion of a maximum of 8 mm. In this case, the cardiac MRI excluded myocarditis and revealed pericarditis. The potential cause of the pericarditis was found by serological examination: Positive IgM-antibody tests for toxoplasmosis with low IgG avidity were found. The player was treated with antibiotics, controlled with echocardiography several times and cleared for training and competition after full resolution of the pericardial effusion and seroconversion. One player with mildly elevated Troponin I, which was most likely related to excessive physical training, showed no pathologic findings in the following examinations and was cleared for training and competition. The family history showed familiar hyperlipidemia combined with history of SCD in one participant, who was also allowed to continue with competitive sports as all other screening examinations showed no relevant results.
In this study, the number needed to screen (NNTS) for the ECG to detect cardiovascular pathologies potentially leading to SCD was 44 with a sensitivity of 67% and specificity of 98%. Together with the echocardiography, the cumulative NNTS was 29. Two out of four participants (50%) showed false positive results at the 12-lead-ECG, but were allowed to continue with competitive sports as stage II excluded relevant pathologies.
The overall prevalence of conditions associated with an increased risk of SCD, which required a sport practice break or even an exclusion from competitive sports, was 3/88 players (3.4%). Four out of seven players (57%) with pathological findings in stage I showed no relevant finding in stage II and where allowed to continue with sports.
Spiroergometry, Holter-ECG, echocardiography
Table 2 and Figure 5 depicts all important parameters of spiroergometry, lactate testing, Holter-ECG and echocardiography.
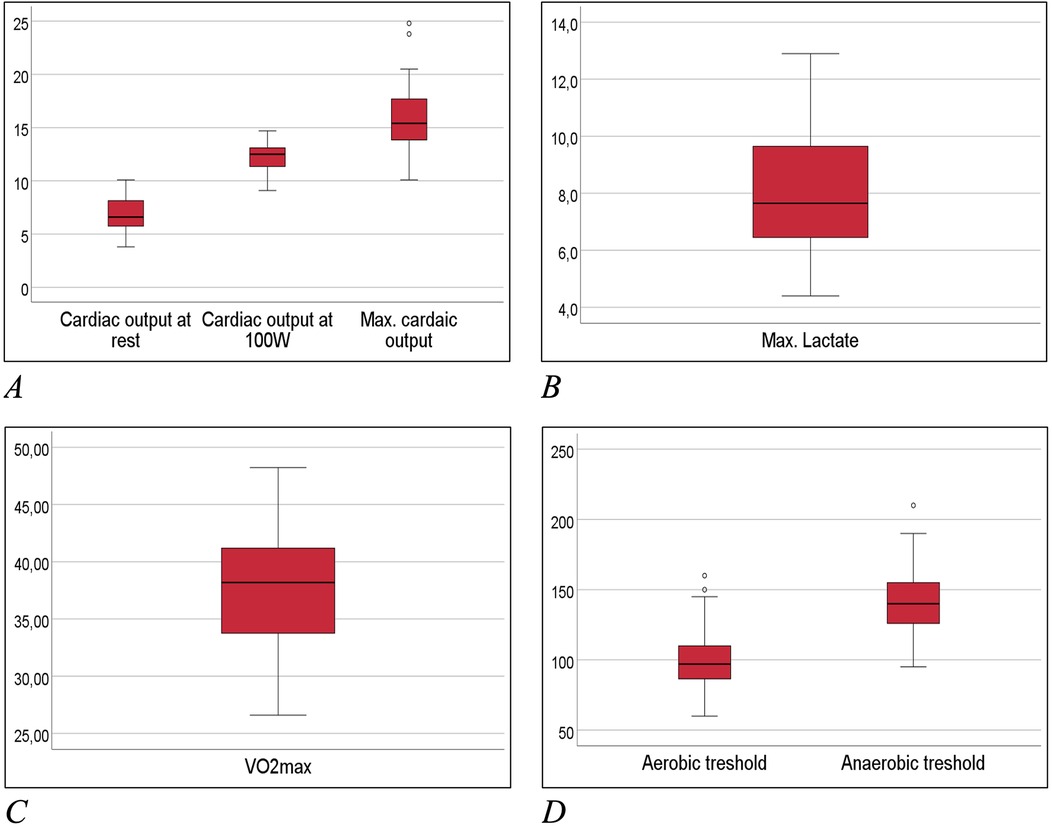
Figure 5. Box-whisker-plots of main exercise related measures. (A) Cardiac output at spiroergometry in ml/min; (B) lactate at maximum workload in mmol/L; (C) maximum oxygen uptake (Vo2max) in ml/min/kg; (D) aerobic and anaerobic threshold in watt.
Follow-up results
No case of SCD occurred during the total follow up of 13 years and 968 athlete years. One athlete (1/88, 1.1%) experienced acute pulmonary embolism during competition following long-distance traveling and was counted as MACCE, one athlete died from non-cardiac cause (cancer).
Discussion
Risk of SCD in female athletes
The preparticipation screening revealed a prevalence of 3.4% out of 88 participants showing 3 different pathologies that are associated with an increased risk of SCD. Considering the unique characteristics of our study population, which includes only young elite female ice hockey players, this rate appears to be relatively high. However, this finding aligns with results from young male athletes (3, 15, 16), demonstrating that preparticipation in sports to detect potential risks for sudden cardiac death is a highly relevant issue for women as well. This emphasizes the importance of conducting preparticipation tests regularly, regardless of gender.
In this population (peri-)myocarditis was the most common cardiac pathology followed by channelopathy (Long-QT), which is different from the most common causes of SCD in male athletes (2, 3). This gender difference in the etiology of sudden cardiac death in sports was also postulated in other studies, which suspected channelopathies as the most common causes in female athletes, while HCM predominates in men (2, 3, 7). Differences in hormonal mechanisms, sympathetic tone, catecholamine release, and the prevalence of hereditary diseases may result in varying causes and incidence rates of Sudden Cardiac Death (SCD) between male and female athletes (6, 8, 17).
In our study, mild and asymptomatic myocarditis emerged as the most prevalent pathological finding during screening. Considering that myocardial inflammation is relatively common in athletes and can lead to serious complications, including SCD, a comprehensive evaluation of the patient, including MRI and even endomyocardial biopsy, is crucial for effective management. This thorough assessment provides vital information for safely determining sports eligibility and estimating prognosis (18). For instance, a 2012 study by Gruen et al. indicated that 9.9% of patients with biopsy-confirmed myocarditis experienced SCD during a 5-year follow-up (19).
12-lead-ECG in preparticipation testing
This analysis emphasizes the crucial role of the 12-lead ECG in pre-participation testing to identify athletes at potential risk for Sudden Cardiac Death (SCD). With a reasonable number needed to screen (NNS 44), and high overall specificity of 98%, it substantiates its usage for detecting young female athletes who might be at risk for SCD.
Without the use of the ECG, neither the athlete with acute myocarditis nor the one with Long QT Syndrome would have been identified as “at risk” using only the other diagnostic tools available in Stage I.
Two out of four participants with pathological findings in the 12-lead EKG were able to continue participating in sports since no relevant findings were discovered during the additional examinations in stage I and II. This supports the idea that multi-stage screening models can aid in preventing false disqualifications.
Indeed, the effectiveness of the ECG largely depends on the expertise of the physician interpreting the results. Therefore, it is crucial to employ consistent ECG interpretation criteria during preparticipation screening (20, 21).
Further examinations in preparticipation testing and in staged protocols
In addition to the 12-lead EKG, medical history, and clinical status, a transthoracic echocardiography is only recommended for patients with known structural heart diseases or as deemed necessary (22). However, the player with the pericardial effusion related to a toxoplasmosis would not have been detected without the use of the echocardiography as a “baseline” test at stage I during the screening. This supports a generous use of the echocardiography to improve the sensitivity of preparticipation screening models.
During Stage II, an MRI was performed on all athletes who exhibited any pathologies in Stage I. This resulted in MRIs being administered to 7 out of 88 athletes (13%), with one case confirming myocarditis. This rate appears relatively high, suggesting a need for stricter MRI indications within general pre-participation screening models. This would ensure applicability and effectiveness across all sport types.
A cardiac computed tomography (a stage II option) was not administered in this young population due to the absence of any indication.
According to the present recommendations, the ECG exercise test and a Holter-ECG can also be added to preparticipation screening as indicated (e.g., for athletes with symptoms or in elderly athletes >40) but as expected in the present young female subgroup, both did not contribute to the diagnosis of SCD related cardiac conditions.
Reference data for female ice hockey athletes
Reference data for the baseline evaluation of female elite ice hockey players in preparticipation screening is rarely available. The present results helps to define standards in young female athletes and may be cited as reference values for healthy subject evaluation.
The spiroergometry by inert gas rebreathing (Innocor®) and the lactate tests as part of stage I of the protocol were not able to improve the sensitivity of screening for athletes “at risk for SCD” and could be omitted in further preparticipation screenings beyond academic studies. However, they can be useful for training control. To the best of our knowledge, this is the first report presenting VO2max, cardiac output under exercise and an aerobic and anaerobic threshold in the special group of young (median age 16 years) female ice hockey players. Certainly, there exists data on average reference values for endurance tests in elite athletes and hockey players, but these are primarily for male athletes over the age of 20 (23, 24). In the present study, VO2max was 37.8 ± 5 ml/min/kg in females elite ice hockey players at the mean age of 16 years. In comparison, in male ice hockey players at the age of 24, the VO2max was higher (51.8 ± 3 ml/min/kg) (24). Diameters and dynamic measurements from echocardiography as part of stage I match to normal reference values in adults (25, 26) but do not exceed them like as consequence of training-related cardiovascular adaptations (e.g., enlargement of LV dimensions, wall thickness) seen in male ice hockey players and athletes of other sports (27, 28). This might be explained by the very young population and the cardiac impact of testosterone in male athletes (8).
Therefore, the existing dataset could be used for comparison in future analyses of similar subgroups, and may also serve as a clinically relevant reference for the evaluation of young female patients.
Limitations
The main limitation of the study is the small sample size and the short follow-up time, especially because SCD in young female athlete is known to have a low incidence rate. As the analyzed population was quite specific including only young female ice hockey players (median age 16), the results might not be generalizable to other sports, age, or gender. No comparative group exists with non-screened athletes. Fortunately, no SCD occurred in the follow-up of this study.
Thus, we are unable to link our findings directly to the critical endpoint of mortality. We can only hypothesize that the screening model detected precursory irregularities related to sudden cardiac death (SCD), especially since no SCD occurred during the follow-up period of the study.
Conclusions
This study highlights the need for comprehensive preparticipation screening, especially 12-lead ECG, in young female elite ice hockey players to detect potential risk for sudden cardiac death (SCD). In this group, there is a significant 3.4% prevalence of conditions which could lead to SCD. Both ECG and echocardiography proved effective in detecting cardiac conditions like congenital Long QT syndrome and myocarditis. Subsequent cardiac MRI application also enabled accurate evaluations. No SCD cases occurred during follow-up, demonstrating the screening's effectiveness. The study underlines the need for ongoing vigilance and tailored screenings considering female athletes' unique SCD pathophysiological profiles and etiologies. The findings advocate for including comprehensive cardiac screening in young female athletes' preparticipation evaluations, enhancing their safety across all sports disciplines.
Data availability statement
The datasets presented in this article are not readily available due to the sensitive nature of the clinical data involved in this study, and in accordance with the ethical standards and institutional guidelines. This decision is made to ensure the privacy and confidentiality of patient information, as well as to comply with the institutional policies on data protection and patient consent. Researchers interested in accessing the raw data for verification purposes or for conducting further analyses are encouraged to contact the corresponding author. Requests to access the datasets should be directed toZmxvcmlhbi5zdHJhdWJlQG11ZW5jaGVuLWtsaW5pay5kZQ==.
Ethics statement
The studies involving humans were approved by Bavarian Chamber of Physicians (Bayerische Landesärztekammer, BLAEK). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AM: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, Visualization. JP: Data curation, Formal Analysis, Writing – review & editing, Supervision. SM: Formal Analysis, Writing – review & editing, Investigation, Visualization. MB: Investigation, Writing – review & editing, Conceptualization, Data curation, Project administration, Supervision. MB: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. BS: Investigation, Writing – review & editing. AS-K: Investigation, Writing – review & editing. UP: Investigation, Writing – review & editing. UD: Writing – review & editing, Conceptualization, Supervision. SR: Writing – review & editing, Investigation. EH: Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Validation. FS: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – review & editing, Formal Analysis, Investigation, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Primary data was collected during preparticipation tests at the Munich Clinic Bogenhausen, Munich Clinic gGmbH without external funding. FS received honoraria for lectures from Astra Zeneca, Bristol-Myers-Squibb, Korese GmbH, Medtronic GmbH, and Philips KODEX EPD, Wikonect, outside the submitted work. EH is head of the department; the department received compensation for participation in clinical research trials outside the submitted work from Abbott, Bayer, Biotronik, Boehringer Ingelheim, Edwards, Elixier, Medtronic, and Stentys. The hospital received funding for educational projects from Biotronik, Boston Scientific, and Shockwave, outside the submitted work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. (2006) 296:1593–601. doi: 10.1001/jama.296.13.1593
2. Emery MS, Kovacs RJ. Sudden cardiac death in athletes. JACC Heart Fail. (2018) 6:30–40. doi: 10.1016/j.jchf.2017.07.014
3. Mont L, Pelliccia A, Sharma S, Biffi A, Borjesson M, Terradellas JB, et al. Preparticipation cardiovascular evaluation for athletic participants to prevents sudden death: author’s reply. Europace. (2017) 19:883–63. doi: 10.1093/europace/eux055
4. Bogle BM, Ning H, Mehrotra S, Goldberger JJ, Lloyd-Jones DM. Lifetime risk for sudden cardiac death in the community. J Am Heart Assoc. (2016) 5(7):e002398. doi: 10.1161/JAHA.115.002398
5. Peterson DF, Kucera K, Thomas LC, Maleszewski J, Siebert D, Lopez-Anderson M, et al. Aetiology and incidence of sudden cardiac arrest and death in young competitive athletes in the USA: a 4-year prospective study. Br J Sports Med. (2021) 55:1196–203. doi: 10.1136/bjsports-2020-102666
6. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. (2022) 43:3997–4126. doi: 10.1093/eurheartj/ehac262
7. Finocchiaro G, Westaby J, Bhatia R, Malhotra A, Behr ER, Papadakis M, et al. Sudden death in female athletes: insights from a large regional registry in the United Kingdom. Circulation. (2021) 144:1827–9. doi: 10.1161/CIRCULATIONAHA.121.055535
8. Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T, et al. Sex differences in cardiac arrhythmia: a consensus document of the European heart rhythm association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. (2018) 20:1565ao. doi: 10.1093/europace/euy067
9. Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, et al. Assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12−25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. Circulation. (2014) 130:1303–34. doi: 10.1161/CIR.0000000000000025
10. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. (2015) 132:e267–72. doi: 10.1161/CIR.0000000000000238 26527714
11. Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. (2007) 115:1643–455. doi: 10.1161/CIRCULATIONAHA.107.181423
12. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. (2021) 42:17–96. doi: 10.1093/eurheartj/ehaa605
13. Russo AD, Compagnucci P, Casella M, Gasperetti A, Riva S, Dessanai MA, et al. Ventricular arrhythmias in athletes: role of a comprehensive diagnostic workup. Heart Rhythm. (2022) 19:90–9. doi: 10.1016/j.hrthm.2021.09.013
14. Russo AD, Compagnucci P, Zorzi A, Cavarretta E, Castelletti S, Contursi M, et al. Electroanatomic mapping in athletes: why and when. An expert opinion paper from the Italian society of sports cardiology. Int J Cardiol. (2023) 383:166–74. doi: 10.1016/j.ijcard.2023.05.013
15. Asif IM, Harmon KG. Incidence and etiology of sudden cardiac death: new updates for athletic departments. Sports Health. (2017) 9:268–79. doi: 10.1177/1941738117694153
16. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. (2015) 36:2793–867. doi: 10.1093/eurheartj/ehv316
17. Lorenzini M, Norrish G, Field E, Ochoa JP, Cicerchia M, Akhtar MM, et al. Penetrance of hypertrophic cardiomyopathy in sarcomere protein mutation carriers. J Am Coll Cardiol. (2020) 76:550–9. doi: 10.1016/j.jacc.2020.06.011
18. Compagnucci P, Volpato G, Falanga U, Cipolletta L, Conti MA, Grifoni G, et al. Myocardial inflammation, sports practice, and sudden cardiac death: 2021 update. Medicina (Kaunas). (2021) 57(3):277. doi: 10.3390/medicina57030277
19. Grun S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. (2012) 59:1604–15. doi: 10.1016/j.jacc.2012.01.007
20. Morrison B, Mohammad A, Oxborough D, Somauroo J, Lindsay S, Drane AL, et al. The 12-lead electrocardiogram of the elite female footballer as defined by different interpretation criteria across the competitive season. Eur J Sport Sci. (2022) 22:1475–83. doi: 10.1080/17461391.2021.1966103
21. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. (2018) 39:1466–80. doi: 10.1093/eurheartj/ehw631
22. Joisten CHA, Bauer P, Baum E, Behrens M, Berrisch-Rahmel S, Berrsche G, et al. DGSP—s2k-Leitlinie sportmedizinische vorsorgeuntersuchung. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). AWMF-Registernummer: 066-002. (2024). https://register.awmf.org/assets/guidelines/066-002l_S2k_Sportmedizinische-Vorsorgeuntersuchung_2024-04.pdf
23. Gharbi Z, Dardouri W, Haj-Sassi R, Chamari K, Souissi N. Aerobic and anaerobic determinants of repeated sprint ability in team sports athletes. Biol Sport. (2015) 32:207–12. doi: 10.5604/20831862.1150302
24. Roczniok R, Stanula A, Maszczyk A, Mostowik A, Kowalczyk M, Fidos-Czuba O, et al. Physiological, physical and on-ice performance criteria for selection of elite ice hockey teams. Biol Sport. (2016) 33:43–8. doi: 10.5604/20831862.1180175
25. Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2017) 18:1301–10. doi: 10.1093/ehjci/jex244
26. Harkness A, Ring L, Augustine DX, Oxborough D, Robinson S. Tool to improve qualitative assessment of left ventricular systolic function. Echo Res Pract. (2020) 7:1–8. doi: 10.1530/ERP-19-0053
27. Churchill TW, Petek BJ, Wasfy MM, Guseh JS, Weiner RB, Singh TK, et al. Cardiac structure and function in elite female and male soccer players. JAMA Cardiol. (2021) 6:316–25. doi: 10.1001/jamacardio.2020.6088
Keywords: preparticipation screening, sudden cardiac death, prevention, athletes, ice hockey, females
Citation: Mohl A, Pongratz J, Muxel S, Berger M, Berr M, Schneider B, Schlichting-Knoob A, Platz U, Dorwarth U, Rogowski S, Hoffmann E and Straube F (2024) Preparticipation screening in young female elite ice hockey players. Front. Cardiovasc. Med. 11:1461028. doi: 10.3389/fcvm.2024.1461028
Received: 7 July 2024; Accepted: 5 December 2024;
Published: 23 December 2024.
Edited by:
Flavio D’Ascenzi, University of Siena, ItalyReviewed by:
Paolo Compagnucci, Marche Polytechnic University, ItalyMelissa Tracy, Rush University Medical Center, United States
Copyright: © 2024 Mohl, Pongratz, Muxel, Berger, Berr, Schneider, Schlichting-Knoob, Platz, Dorwarth, Rogowski, Hoffmann and Straube. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florian Straube, Zmxvcmlhbi5zdHJhdWJlQG11ZW5jaGVuLWtsaW5pay5kZQ==
†These authors have contributed equally to this work
 Alexander Mohl1,†
Alexander Mohl1,† Janis Pongratz
Janis Pongratz Michael Berr
Michael Berr Ellen Hoffmann
Ellen Hoffmann Florian Straube
Florian Straube