- 1Echocardiography Department, European Interbalkan Medical Center, Thessaloniki, Greece
- 2Department of Medicine and Surgery, University of Milano Bicocca, Milan, Italy
- 3Department of Cardiology, Istituto Cardiologico Italiano, IRCCS, Milan, Italy
- 4Structural and Valvular Center of Excellence, Marcus Heart Valve Center, Piedmont Heart Institute, Atlanta, GA, United States
- 5Department of Medical Biotechnology, University of Siena, Siena, Italy
- 6School of Medicine, Vita Salute San Raffaele University, Milan, Italy
- 7Unit of Advanced Imaging for Personalized Medicine, IRCCS Ospedale San Raffaele, Milan, Italy
- 8Unit of Cardiovascular Imaging, IRCCS San Raffaele Scientific Institute, Milan, Italy
Editorial on the Research Topic
Advances in heart valve imaging
Heart valve diseases (HVD) pose significant challenges for imaging specialists, especially when combined with other cardiac pathologies. The advent of transcatheter interventions necessitates a multidisciplinary “heart team,” including a skilled multi-modality imaging department. This team assesses patient suitability for various treatments, guides operations, and evaluates final results. It is now recognized that beyond the anatomical characteristics of the valves, parameters such as ventricular and atrial dimensions and performance, pulmonary pressures, and other comorbidities must be evaluated during pre-operative screening for valvulopathies.
Recent advances in three-dimensional echocardiography, 2D speckle-tracking, and cardiac CT and MRI have enhanced our understanding of the pathophysiology of a severe valvulopathy. Additionally, artificial intelligence (AI) and machine learning are becoming significant in screening and managing patients with HVD. This special issue explores the advances in imaging for HVD and the potential role of AI in the near future (Table 1).
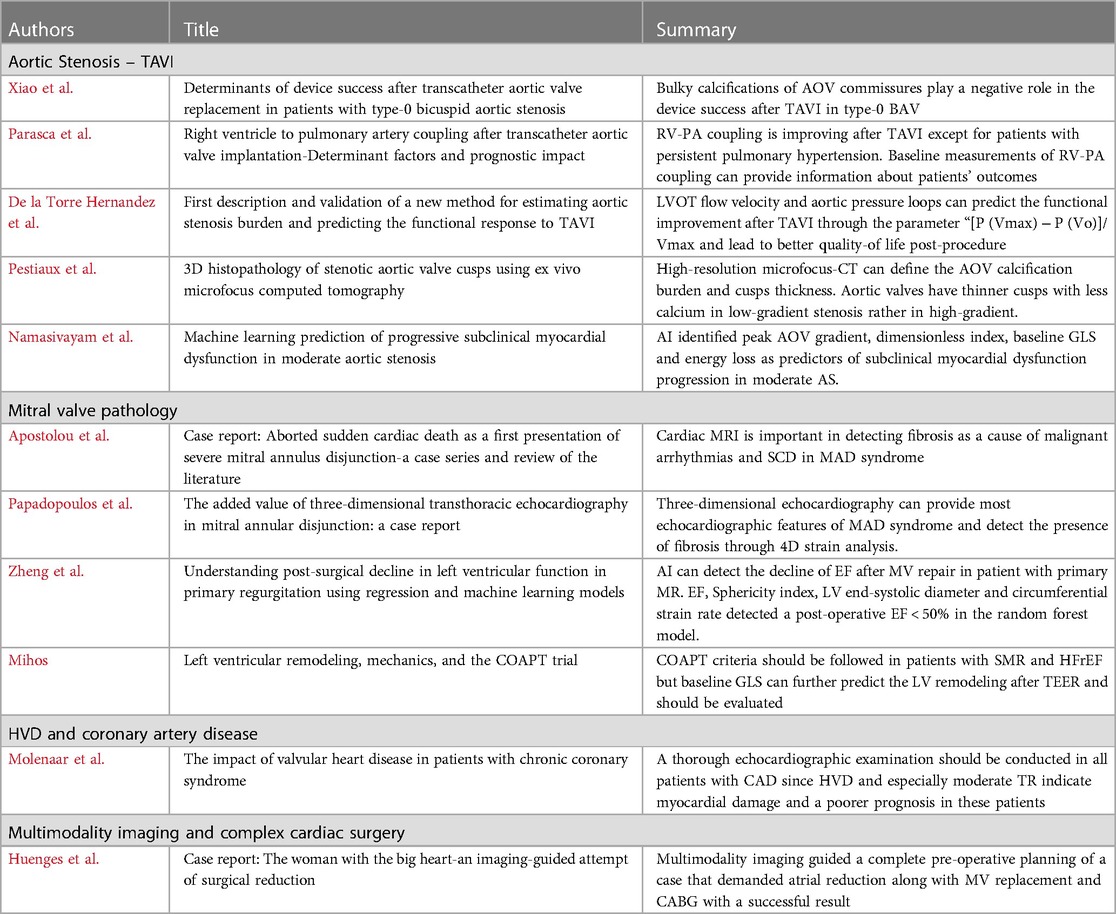
Transcatheter Aortic Valve Implantation (TAVI) is the most common transcatheter intervention with expanding indications, garnering attention from the cardiology community. Xiao et al. studied a small cohort of patients with type-0 bicuspid aortic valve and identified predictors of success after TAVI. They found that the ellipticity index of the aortic root and bulky calcifications of aortic commissures differed between success and failure subgroups. Bulky calcifications were determined by visual assessment using multidetector CT transverse planes and maximum intensity projections. Multivariate analysis revealed though that only bulky calcifications had negative correlation with device success post-TAVI. This study highlights the importance of calcification degree and distribution in managing patients with bicuspid aortic valve, suggesting that a larger patient series could further improve TAVI outcomes for this group.
Right ventricular (RV) dysfunction and pulmonary hypertension are crucial in determining TAVI outcomes. Parasca et al. showed that RV-pulmonary artery (PA) coupling improves after TAVI, except in patients with persistent pulmonary hypertension. They emphasized the importance of evaluating RV-PA coupling during TAVI screening. A baseline RV-free wall longitudinal strain (FWLS)/pulmonary artery systolic pressure (PASP) cutoff of 0.63 was able to differentiate between normal and impaired RV-PA coupling, providing valuable prognostic information and guiding treatment decisions to improve patient outcomes.
Predicting the response after TAVI is vital since 20% of patients continue to experience poor quality of life post-procedure. De la Torre Hernandez et al. validated a new method to assess aortic stenosis (AS) burden and functional outcome after TAVI. This method integrates left ventricular outflow tract flow velocity and aortic pressure, focusing on parameters like “[P (Vmax) − P (Vo)]/Vmax,” an independent predictor of functional improvement post-TAVI. This approach offers a more comprehensive hemodynamic assessment, potentially leading to better patient outcomes and quality of life post-procedure.
Understanding aortic stenosis mechanisms is crucial for treatment and patient outcomes. Pestiaux et al. analyzed the microstructure of calcified aortic valve cusps using high-resolution microfocus CT, demonstrating the calcification burden and cusp thickness. They found that aortic valves have thinner cusps with significantly less calcium in low-gradient vs. high-gradient patients. While not routine, this examination can enhance in vivo imaging protocols and clinical data interpretation.
AI plays a significant role in diagnosing structural heart diseases. Namasivayam et al. applied artificial neural networks to echocardiographic data from patients with moderate AS, identifying predictors of subclinical myocardial dysfunction progression. Key factors include peak gradient, dimensionless index (DI), baseline left ventricular global longitudinal strain (GLS), and energy loss. These parameters should be closely monitored in patients with moderate AS.
Mitral valve pathology, including new entities like mitral annular disjunction (MAD), is also under extensive research. Apostolou et al. described cases of aborted sudden cardiac death in patients with mitral valve prolapse and MAD, investigated using cardiac MRI, which revealed fibrosis associated with malignant arrhythmias. This highlights the significance of multimodality imaging and interdisciplinary collaboration for managing such patients. Papadopoulos et al. suggested that three-dimensional transthoracic echocardiography and 4D strain analysis might suffice to demonstrate MAD features and fibrosis presence, but this needs validation through larger studies.
AI has been tested in mitral valve diseases as well. Zheng et al. used regression and machine learning models to predict postoperative ejection fraction (EF) decline in primary mitral regurgitation (PMR) patients. The random forest model accurately detected patients with postoperative EF < 50%, including predictors like LVEF, LV sphericity index, LV end-systolic diameter (LVESD), and LV mid-systolic circumferential strain rate. Although further research is needed, this study suggests a more accurate preoperative assessment for these patients.
LV mechanics are crucial for outcomes after transcatheter edge-to-edge repair (TEER) in secondary mitral regurgitation (SMR) patients. Mihos emphasized the importance of GLS in predicting left ventricular remodeling, crucial for patient prognosis. Following the echocardiographic criteria from the COAPT trial, which demonstrated TEER benefits in heart failure with reduced ejection fraction (HFrEF) and SMR, is recommended. However, deformation metrics might provide additional data on TEER responders. Heart teams managing these patients should also consider guideline-directed medical therapy and cardiac resynchronization therapy (CRT) when indicated.
In a study of about 2,000 patients, Molenaar et al. found that HVD affect prognosis in coronary artery disease (CAD) patients. LV dysfunction and moderate or severe HVD were the main indicators of higher mortality. Moderate tricuspid regurgitation (TR) was the strongest mortality predictor in multivariable regression analysis. HVD often indicates a higher atherosclerotic burden and more significant CAD, leading to more common myocardial damage and poorer prognosis. A thorough echocardiographic examination should be conducted when screening CAD patients.
Cardiac surgery has also progressed, allowing for the efficient management of complex cases. Huenges et al. described treating a patient with a gigantic left atrium requiring mitral valve replacement and coronary artery bypass grafting (CABG). Thorough multi-modality imaging with echocardiography and cardiac CT facilitated a successful operation, including atrial reduction techniques and MV replacement.
In summary, advances in imaging and AI are transforming the management of HVD and improve patients’ outcomes through more accurate preoperative assessments and innovative treatment approaches.
Author contributions
KP: Writing – original draft. LB: Writing – review & editing. MV: Writing – review & editing. MC: Writing – review & editing. AP: Writing – review & editing. FA: Writing – review & editing. AE: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: heart valves, imaging, transcatheter interventions, artificial intelligence, structural heart diseases
Citation: Papadopoulos K, Badano LP, Vannan MA, Cameli M, Palmisano A, Ancona F and Esposito A (2024) Editorial: Advances in heart valve imaging. Front. Cardiovasc. Med. 11: 1450661. doi: 10.3389/fcvm.2024.1450661
Received: 17 June 2024; Accepted: 1 July 2024;
Published: 11 July 2024.
Edited and Reviewed by: Elena Aikawa, Harvard Medical School, United States
© 2024 Papadopoulos, Badano, Vannan, Cameli, Palmisano, Ancona and Esposito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos Papadopoulos, cGFwYWRvY2FyZGlvQGdtYWlsLmNvbQ==
 Konstantinos Papadopoulos
Konstantinos Papadopoulos Luigi P. Badano
Luigi P. Badano Mani A. Vannan
Mani A. Vannan Matteo Cameli
Matteo Cameli Anna Palmisano
Anna Palmisano Francesco Ancona
Francesco Ancona Antonio Esposito6,7
Antonio Esposito6,7