- Vascular Anomaly Center, Department of Interventional Therapy, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Purpose: This study aimed to evaluate the efficacy and safety of ethanol embolization in treating traumatic arteriovenous fistulas (TAVFs).
Materials and methods: From March 2012 to April 2020, 42 consecutive patients (29.9 ± 15.1 years, range: 3–68 years) with peripheral TAVFs underwent ethanol embolization. All patients underwent clinical and imaging follow-ups (40.0 ± 25.9 months, range: 3–90 months). The mean time to onset of symptoms after trauma was 5.4 ± 5.9 months (range: 0.5–30 months). Among the patients, 27 (64.3%) reported that the TAVFs occurred after blunt trauma, 10 (23.8%) presented after penetrating trauma (with 4 patients involving penetration by infusion indwelling needles), and 3 (7.1%) had a history of surgery. Treatment effects, devascularization rates, and complications were evaluated at follow-ups conducted at 1–3 month intervals.
Results: Seventy-one embolization procedures were performed, with a mean of 1.6 ± 0.7 procedures per patient. Thirty-four patients received coil-assisted ethanol embolization. Absolute ethanol was used in all procedures, with an average volume of 7.1 ± 4.2 ml per procedure (range: 1–18 ml); 28 patients (28/42, 66.7%) received coil embolization in 36 procedures (36/71, 50.7%). Upon re-examination, 39 patients (92.9%) achieved 100% devascularization; of these, 29 patients (74.4%) with Schobinger stage II TAVFs improved to stage I or became asymptomatic. Overall, 30 cases (66.7%) achieved a complete response, while the other 12 cases (33.3%) showed a partial response. In addition, no major complications were observed postoperatively, apart from minor complications.
Conclusions: Coil-assisted ethanol embolization can effectively manage TAVFs with an acceptable risk of mild complications.
Introduction
Arteriovenous fistulas (AVFs) are abnormal channels between adjacent arteries and veins. Traumatic arteriovenous fistulas (TAVFs) refer to AVFs caused by trauma (1). The most common cause of TAVFs is penetrating injuries from sharp instruments (2). With the widespread development of various new percutaneous vascular puncture techniques, medically induced injuries are becoming a more common cause (3).
Patients with TAVFs tend to have a history of trauma (4). Due to prolonged abnormal arteriovenous communication, local and distal hemodynamic disorders and disturbances occur in the lesion, leading to local tissue compression, hyperplasia, massive opening of the collateral circulation, and expansion of the bruit circulation. TAVFs are almost impossible to self-heal. To date, various treatment options for TAVFs have been introduced, including surgical resection, ligation of feeding arteries, and interventional therapies (5–7). Inadequate surgical resection or ligation of only the supply artery, which ultimately reconstructs the flow pattern of the TAVFs due to induced preferential dilatation of collateral vessels, makes the treatment ineffective (8). Developments in interventional radiology have revolutionized previous treatment methods and have produced satisfactory results in the treatment of congenital arteriovenous malformations (AVMs). Coil-assisted ethanol embolization has demonstrated better long-term clinical and radiological results with an acceptable risk of morbidity compared to mechanical embolization alone (granular material, n-butyl cyanoacrylate, Onyx). It has evolved as the primary modality for managing congenital AVMs (9, 10). However, the suitability of the technique for TAVFs still needs to be explored. The purpose of the present study was to retrospectively assess the safety and effectiveness of ethanol embolization in the treatment of TAVFs.
Materials and methods
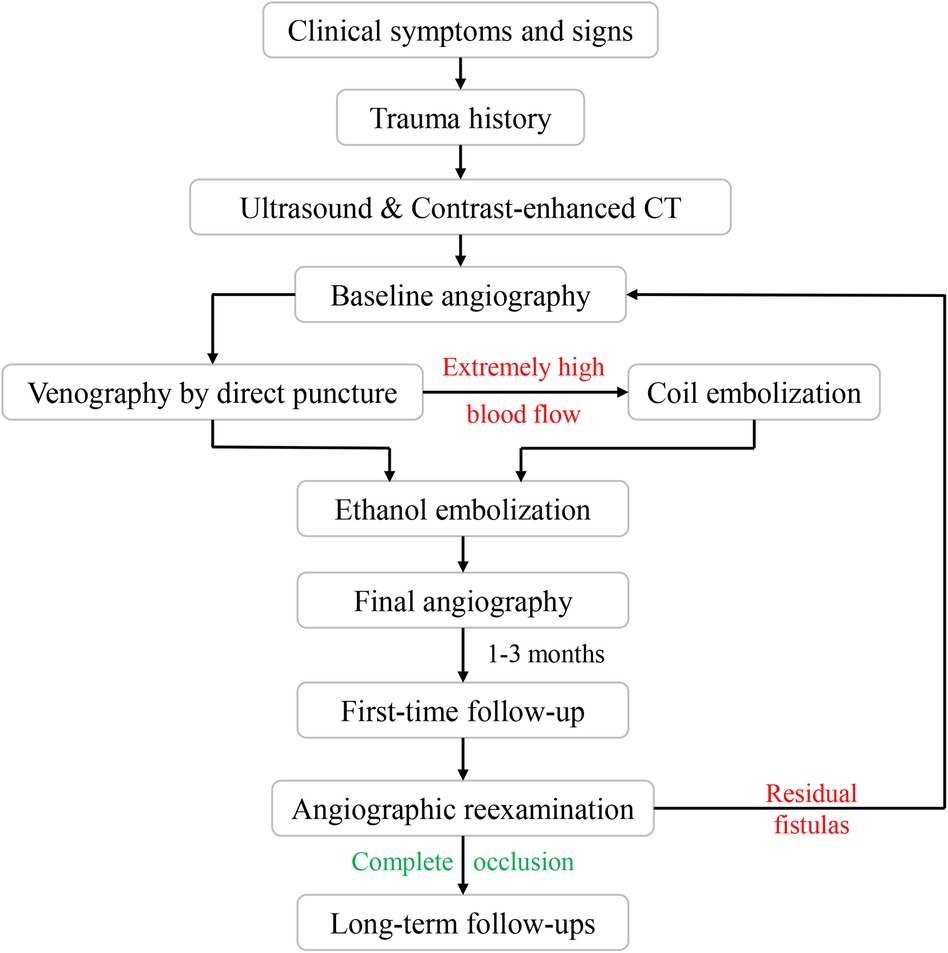
This study received approval from the Institutional Review Board of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine. Informed consent was obtained from all patients who participated in the study. The flow diagram is shown in Figure 1.
Patients
A retrospective review of medical records, photographs, and radiological imaging results of 42 consecutive patients who underwent ethanol embolization between March 2012 and April 2020 was performed. Forty-two patients were included, comprising 19 ales and 23 females, with a mean age of 29.9 ± 15.1 years (range: 3–68 years). The mean time to morbidity after trauma was 5.4 ± 5.9 months (range: 0.5–30 months). Of the 42 patients, 23 (54.8%) had previously undergone unsuccessful treatments, such as incomplete surgical resection, transarterial embolization, and sclerotherapy, at other hospitals (Table 1).
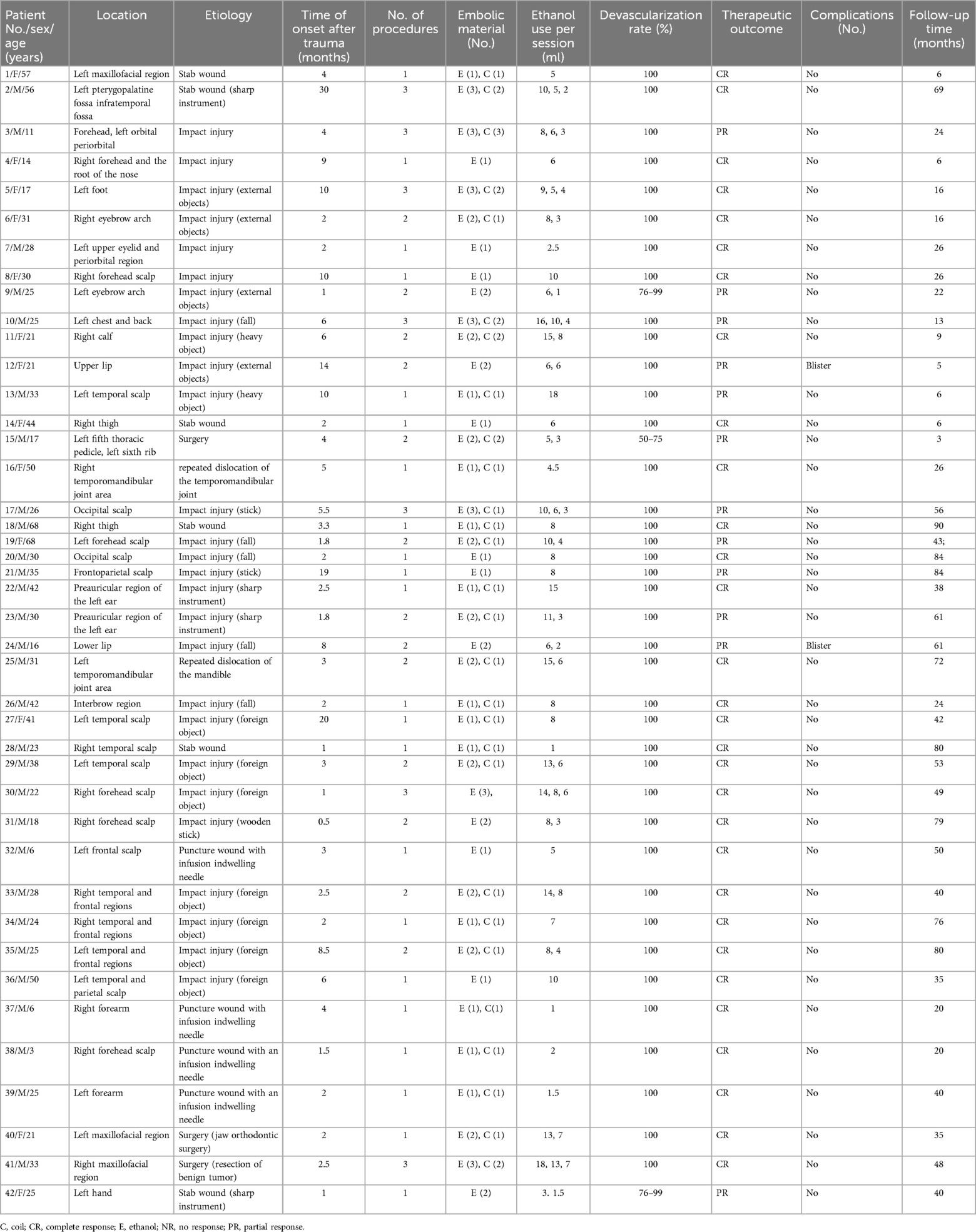
Table 1. Clinical data and outcomes of ethanol embolization in treating patients with traumatic arteriovenous fistulas.
Of the 42 patients, 27 (64.3%) reported that the TAVFs developed after blunt trauma, 10 (23.8%) presented after penetrating trauma (with 4 patients experiencing penetration with infusion indwelling needles), 3 (7.1%) had a history of surgery, and 2 (4.8%) caused by repeated dislocation of the temporomandibular joint. All outpatients were initially diagnosed based on clinical manifestations, followed by a color duplex ultrasound to evaluate the hemodynamic characteristics of vascular lesions. Preoperative contrast-enhanced computed tomography (CT) was used to make a definite diagnosis and assess the anatomical features of TAVFs in detail.
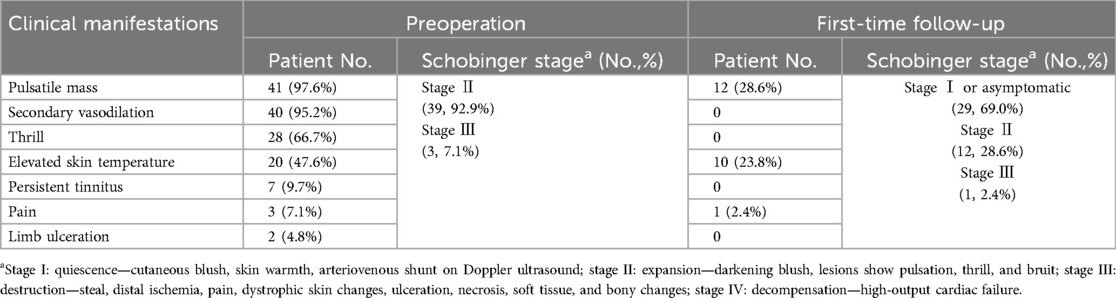
The indications for treatment include the presence of subjective clinical symptoms that make daily life uncomfortable (e.g., swelling, troublesome tinnitus, pulses), complications (e.g., secondary varicose veins), and progressive enlargement over time. The clinicians recorded the patients’ sex, age, lesion location, previous treatments, clinical findings, and Schobinger stage (11).
Angiographic and embolization techniques
According to previous reports, ethanol embolization was initiated under general anesthesia to control pain (12). Briefly, baseline angiograms were obtained using the femoral artery approach to assess the extent and hemodynamic characteristics of TAVFs and to determine whether transarterial or direct puncture access would be used (Figures 2A,B).
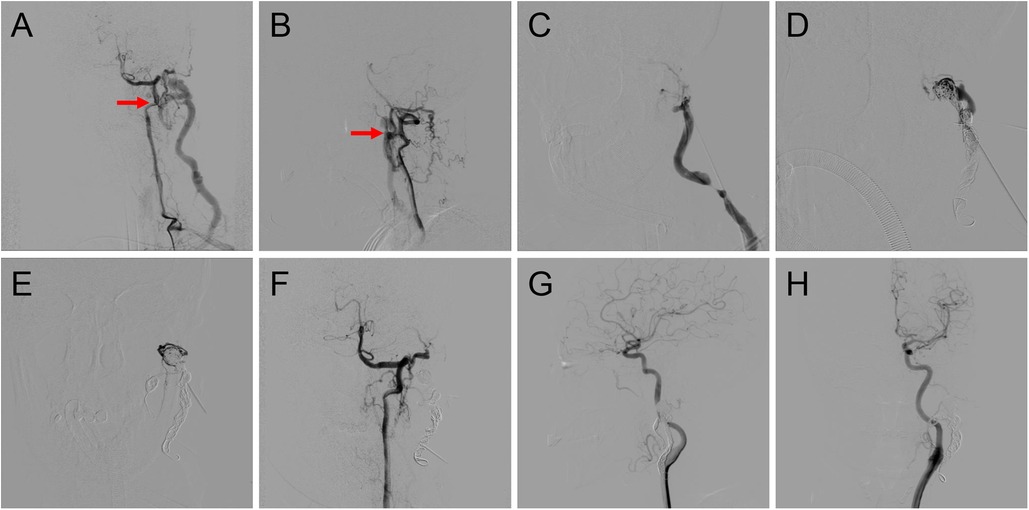
Figure 2. Procedure of coil-assisted ethanol embolization of traumatic arteriovenous fistulas. (A,B) Anteroposterior and lateral views of baseline angiography. (C) Direct puncture of the nidus. (D) Configuration of coils. (E) Angiography indicating the reduction of blood flow. (F) Final angiography after ethanol injection showing 100% devascularization. (G,H) Anteroposterior and lateral views of angiography 1.5 years postoperatively. Red arrows: arteriovenous shunts.
A dominant outflowing vein (DOV) of TAVF is defined as the dilated vein originating from the nidus with the maximum and fastest flow (13). If angiography identified the DOV (Figure 2C), we penetrated directly with an 18-G puncture needle. A 2.1-F microcatheter (Asahi, Seto, Japan) was then inserted into the DOV, and venography was used to confirm the correct location of the microcatheter. Next, the three-dimensional mechanically detachable coils (Micro Therapeutics, Irvine, CA, USA) and synthetic fiber-attached stainless steel coils (Cook Medical, Bloomington, IN) were delivered via the microcatheters until repeat venography represented decreased blood flow (Figures 2D,E). For patients without a significant DOV, coils were placed in the nidus.
Absolute ethanol was injected at a dose of 0.1 ml/kg body weight per injection, with a maximum of 1 ml/kg body weight per procedure. An angiogram was performed 5–10 min after each ethanol injection to determine whether the AVFs were effectively embolized. If the nidus was still noted on angiography, repeated ethanol injections were required (Figure 2F).
Postoperative management
Management after ethanol embolization included intravenous infusion of fluids and dexamethasone. The dose of intravenous dexamethasone (0.1 mg/kg every 8 h for the first 3 days) was gradually reduced over 7 days after ethanol embolization to reduce swelling. If hemoglobinuria was observed postoperatively, hydration was provided with intravenous lactated Ringer's solution (2,000 ml or 30 ml/kg for children weighing <60 kg). Ranitidine (Zantac; Sanofi, Hangzhou, China) was administered to prevent gastric or duodenal ulceration.
Follow-up modality
Patients were followed up at regular intervals of 1–3 months after the initial treatment with physical examinations. The color duplex ultrasound or contrast-enhanced CT was performed for outpatients. Angiographic re-examination was regularly carried out during the patient's first follow-up. Angiography was also recommended if the clinical outcomes of symptoms and signs of the patients improved or worsened during long-term follow-ups (Figures 2E,F). Additional embolotherapy was required if residual fistulas were detected on angiography.
Evaluation of clinical outcomes
The devascularization of the TAVFs was evaluated by comparing preoperative and re-examined angiography results. Two independent radiologists assessed the degree of devascularization, classifying it into four levels: 100%, 76%–99%, 50%–75%, and <50% (Figures 3, 4). Therapeutic outcomes were classified into complete response (complete resolution of symptoms with 100% devascularization of the AVFs), partial response (improvement of clinical symptoms with 50%–99% devascularization of the AVFs), no response (no change in clinical symptoms or signs with <50% devascularization of the AVFs), and worsening (deterioration of clinical symptoms regardless of the devascularization of the AVFs). Complete and partial responses were judged to have practical therapeutic benefits.
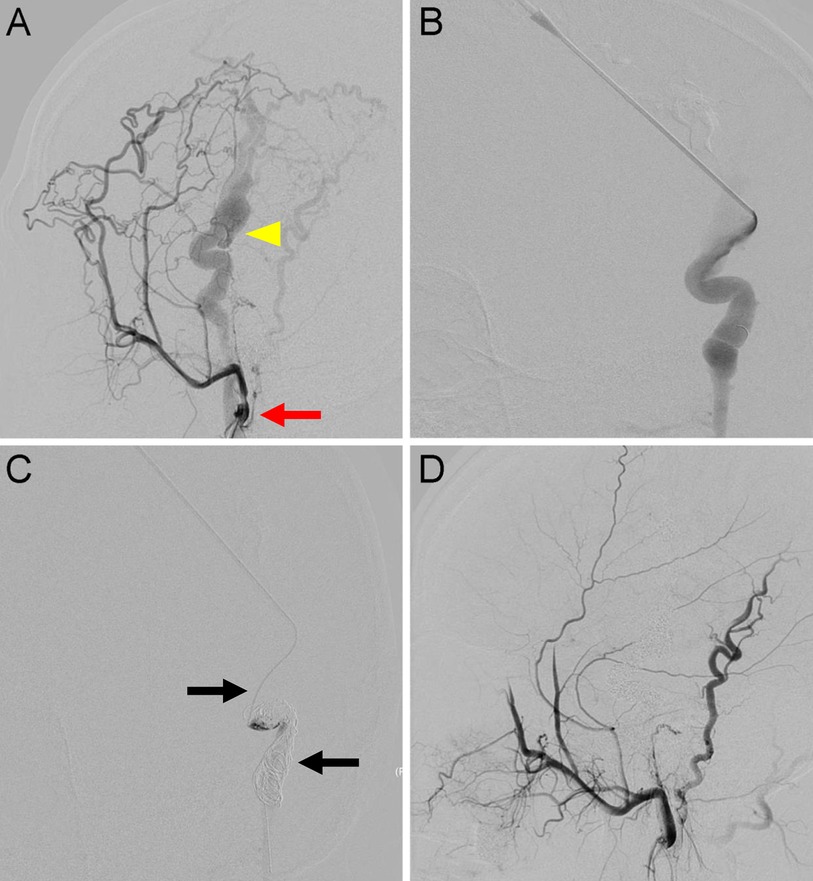
Figure 3. Traumatic arteriovenous fistulas located at the scalp. (A) Anteroposterior view of a left external carotid angiography showing AVFs (arrow) DOV (arrowhead) around the parietal area. (B) For high-flow lesions with DOVs, an 18-gauge needle was used to percutaneously puncture the DOV. (C) A 2.1-F microcatheter (upper arrow) was introduced through the needle into the dilated venous sac. The detachable coils (lower arrow) were then released after confirmation of no migration in the distal end of the draining veins. (D) Final angiography showing 100% devascularization.
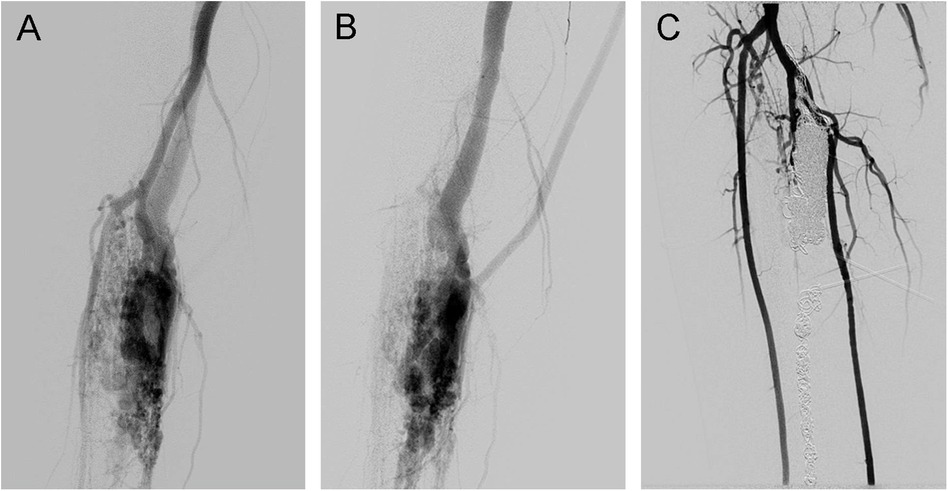
Figure 4. Traumatic arteriovenous fistulas located at the lower extremity. (A) Angiography in the arterial phase, (B) angiography in the venous phase, and (C) angiography showing 100% devascularization after coil deployment and ethanol injection.
The detected complications, classified as minor or major, related to the embolization procedure were analyzed. Minor complications included non-permanent adverse sequelae, such as transient nerve injury or spontaneously healed skin necrosis. Major complications included permanent adverse sequelae or death, all of which need medical intervention.
All data are reported as the mean and standard deviation (SD).
Results
Baseline data of patients with traumatic arteriovenous fistulas
The locations of TAVFs of the present cohort were as follows: head and neck (33/42, 78.6%), lower extremities (4/42, 9.5%), upper extremities (3/42, 7.1%), and trunk (2/42, 4.8%) (Table 1). Patients’ clinical manifestations included pulsatile mass (41/42; 97.6%), secondary vasodilation (40/42; 95.2%), trill (28/42, 66.7%), elevated skin temperature (20/42, 47.6%), persistent tinnitus (7/42; 16.7%), pain (3/42; 38.1%), and limb ulceration (2/42; 4.8%). According to the Schobinger classification, 39 out of 42 patients (92.9%) were classified as Schobinger stage II, whereas 3 patients (7.1%) were classified as Schobinger stage III (Table 2).
Embolization modality of patients with traumatic arteriovenous fistulas
In total, 71 embolization procedures were conducted, with a mean of 1.6 ± 0.7 procedures per patient. Among the 42 patients, 20 (47.6%) underwent a single embolization session, 15 (35.7%) underwent two sessions, and 7 (26.7%) underwent three sessions. Notably, 28 patients (28/42, 66.7%) had extremely high-blood flow TAVFs, for which coil embolization was carried out in 36 procedures (36/71, 50.7%). Absolute ethanol was administered in all procedures, with an average volume of 7.1 ± 4.2 ml per procedure (1–18 ml) (Table 1).
Postoperative outcomes of patients with traumatic arteriovenous fistulas
Angiography showed that 39 patients (92.9%) achieved 100% devascularization, while 2 patients (4.8%) achieved 76%–99% devascularization; the remaining patient had 50%–75% devascularization (2.4%). Among 39 patients initially diagnosed with Schobinger stage II, 29 (74.4%) improved to stage I (or became asymptomatic). Two out of three (66.7%) patients with stage III TAVFs improved to stage II after coil-assisted ethanol embolization. No recurrence was observed during imaging or clinical follow-ups (Table 2). According to angiographic re-examination and clinical manifestations, 30 cases (66.7%) achieved a complete response, while the other 12 (33.3%) showed a partial response (Table 1).
No major complications were reported. Thirty-four patients (34/42, 81.0%) developed focal swelling at the treatment area postoperatively, which subsided within 2 weeks. Two patients (2/42, 4.8%) developed blisters on their lesions shortly after treatment, which recovered spontaneously after 1 week. No patient experienced superficial skin necrosis and transient hemoglobinuria. Also, no abnormal feelings or neurological dysfunction associated with the embolization procedure were noted. Finally, no procedure-related mortality occurred during the perioperative phase for any of the patients.
Regular follow-up was achieved in all patients, with an average duration of 40.0 ± 25.9 months (range: 3–90 months) (Table 1).
Discussion
Managing TAVFs remains challenging, with limited available reports. This study demonstrates that ethanol embolization of TAVFs produces satisfactory clinical and radiographic results with few complications.
Congenital AVMs are characterized by a vascular developmental defect in the differentiation of the primitive capillary plexus during fetal angiogenesis, owing to genetic variations associated with MAP2K1 and GNA11 mutations (14, 15). Congenital AVFs are deemed as a simple form of AVMs (16). On the other hand, TAVFs are vascular anomalies usually secondary to trauma or invasive procedures, including biopsy, surgical intervention, and placement of intravenous catheters (17–19). We found that TAVFs were more frequently located in the head and neck, which is different from previous reports that TAVFs are more common in the extremities (20). The onset of initial symptoms varied from weeks to years, depending on the mode and degree of injury, indicating that the development of the lesions secondary to trauma is dissimilar (21).
Trauma promotes the formation of AVFs via direct and indirect potential approaches, establishing direct communication between the walls of veins and arteries in proximity by damaging their walls. During the healing process, small bridging vessels develop via proliferation of endothelial cells and angiogenesis, facilitated by a variety of secreted vascular growth factors (22). TAVFs usually result from deep penetrating injuries that cause damage to arterial and venous walls. This injury is thought to initiate the growth of endothelial progenitor cells, known as angioblastic rest, and cause arterial recruitment in the affected area (22). TAVFs have been reported, but controversy exists about whether the trauma acts as an independent factor or merely a trigger for the disintegration of pre-existing fistulous embryonic connections (23). In addition, it remains unclear whether the variated gene lies in TAVFs, needing further investigation.
Treatment strategies for TAVFs include embolization, surgical resection, or a combination. Historically, surgical resection is the primary treatment for TAVFs, often at the expense of esthetics and function. However, the complexity of head and neck anatomy and potential massive blood loss during the operation hindered a complete resection, further accelerating lesion expansion and complicating future treatment. In comparison, endovascular therapy has gained popularity. Various embolic agents are available, such as coils, ethanol, n-Butyl cyanoacrylate (NBCA), Onyx, polyvinyl alcohol (PVA), etc. The insufficient destruction of the nidus is the disadvantage of NBCA or Onyx, leading to recanalization and recurrence (24, 25). Our previous reports have confirmed the potent efficacy of absolute ethanol in treating peripheral AVMs (26, 27). Unlike NBCA or Onyx, high-concentration ethanol exerts a unique denaturation effect on proteins. By destroying vascular endothelial cells, ethanol disrupts the angiogenesis engine, eliminating the possibility of recanalization (13). Nevertheless, ethanol embolization still confronts obstacles to popularization. One of the most notable reasons is radiolucency of ethanol. Clinicians have to make a “blind shot” when injecting ethanol under digital subtraction angiography (DSA), increasing the risk of ethanol reflux into normal vasculature and causing tissue necrosis. To solve this dilemma, our team has been researching and developing radiopaque ethanol injections (28).
During ethanol embolization of high-flow TAVFs, the embolic efficiency depends on adequate contact time with the nidus. In managing high-flow congenital AVMs with dilated draining veins, previous reports have shown that coil placement can increase ethanol exposure to endothelial cells of the nidus by reducing the rate of arteriovenous shunting, yielding satisfactory therapeutic outcomes and decreasing the risk of complications, such as ethanol-related necrosis and cardiopulmonary collapse (29, 30). The results showed that the disappearance of signs and symptoms and satisfactory devascularization on angiogram achieved after coil embolization indicate that the treatment of congenital AVMs can be applied to TAVFs in the head and neck region. When we analyzed the treatment results, the complete and partial responses were better than those observed for congenital AVMs. The complete and partial response rates of 66.7% and 33.3%, respectively, were comparable to previous reports (10, 30, 31). Therefore, coil-assisted ethanol embolization therapy could be an effective treatment option for TAVFs.
This study has several limitations. First, this study presents a retrospective report. A prospective study is needed for a more precise assessment. Second, the location of the lesions and pathogenic factors differ among patients. At last, the sample size is relatively small. More patients with TAVFs are supposed to be included in our future research.
Conclusion
In conclusion, TAVFs are more common in the head and neck region than in other body parts. Coil-assisted ethanol embolization achieved safe and effective outcomes in treating TAVFs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Shanghai Ninth People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Validation, Writing – original draft. QH: Writing – review & editing. DW: Resources, Supervision, Writing – review & editing. LS: Methodology, Supervision, Writing – review & editing. MW: Data curation, Resources, Writing – review & editing. XF: Conceptualization, Project administration, Supervision, Writing – review & editing. XY: Formal Analysis, Investigation, Software, Visualization, Writing – original draft.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Transverse Research Project of Shanghai Ninth People’s Hospital (No. JYHX2022007) and the Rare Disease Registration Platform of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. JYHJB202301).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nagpal K, Ahmed K, Cuschieri R. Diagnosis and management of acute traumatic arteriovenous fistula. Int J Angiol. (2008) 17(4):214–6. doi: 10.1055/s-0031-1278313
2. Hegarty MM, Angorn IB, Gollogly J, Baker LW. Traumatic arteriovenous fistulae. Injury. (1975) 7(1):20–8. doi: 10.1016/0020-1383(75)90054-6
3. Lindgren A, Ahmed SU, Bodani V, Chung E, Agid R, Barazarte HA, et al. Embolization techniques of spontaneous direct carotid-cavernous fistulae: a single-center experience. Neuroradiology. (2024) 66(9):1625–33. doi: 10.1007/s00234-024-03389-w
4. Maher SM, Rabee HM, Takrouri MS, Al-Salman MM. Traumatic arteriovenous fistula. Ann Thorac Surg. (1997) 63(6):1792–4. doi: 10.1016/S0003-4975(97)00370-6
5. Sahin M, Yucel C, Kanber EM, Ilal Mert FT, Bicakhan B. Management of traumatic arteriovenous fistulas: a tertiary academic center experience. Ulus Travma Acil Cerrahi Derg. (2018) 24(3):234–8. doi: 10.5505/tjtes.2017.49060
6. Kuy S, Rossi PJ, Seabrook GR, Brown KR, Lewis BD, Rilling WS, et al. Endovascular management of a traumatic renal-caval arteriovenous fistula in a pediatric patient. Ann Vasc Surg. (2014) 28(4):1031.e1–5. doi: 10.1016/j.avsg.2013.04.028
7. Davis AJ, Nelson PK. Arteriovenous fistula of the scalp secondary to punch autograft hair transplantation: angioarchitecture, histopathology, and endovascular and surgical therapy. Plast Reconstr Surg. (1997) 100(1):242–9. doi: 10.1097/00006534-199707000-00036
8. Johnson AB, Richter GT. Surgical considerations in vascular malformations. Tech Vasc Interv Radiol. (2019) 22(4):100635. doi: 10.1016/j.tvir.2019.100635
9. Zheng L, Su L, Wang D, Wang Z, Wen M, Yang X, et al. Ethanol embolization of lingual arteriovenous malformations: positive experience in 52 patients during 11 years. J Vasc Surg. (2019) 72(2):651-7.e4. doi: 10.1016/j.jvs.2019.08.286
10. Fernandez-Alvarez V, Suarez C, de Bree R, Nixon IJ, Makitie AA, Rinaldo A, et al. Management of extracranial arteriovenous malformations of the head and neck. Auris Nasus Larynx. (2019) 47(2):181–90. doi: 10.1016/j.anl.2019.11.008
11. Shen Y, Su L, Wang D, Fan X. Overview of peripheral arteriovenous malformations: from diagnosis to treatment methods. J Interv Med. (2023) 6(4):170–5. doi: 10.1016/j.jimed.2023.10.006
12. Shen Y, Wang D, Fan X, Zheng L, Su L, Yang X. Ethanol embolization of arteriovenous malformations in the buttock: ten-year experiences in diagnoses and treatment options. Orphanet J Rare Dis. (2024) 19(1):195. doi: 10.1186/s13023-024-03205-x
13. Shen Y, Wang D, Wen M, Di R, Fan X, Su L, et al. Coil-assisted ethanol embolotherapy for refractory head and neck arteriovenous malformations with onyx recrudescence: 10-year experiences. J Vasc Surg Venous Lymphat Disord. (2023) 11(6):1219–30. doi: 10.1016/j.jvsv.2023.07.006
14. Qiao C, Richter GT, Pan W, Jin Y, Lin X. Extracranial arteriovenous malformations: from bedside to bench. Mutagenesis. (2019) 34(4):299–306. doi: 10.1093/mutage/gez028
15. Couto JA, Huang AY, Konczyk DJ, Goss JA, Fishman SJ, Mulliken JB, et al. Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am J Hum Genet. (2017) 100(3):546–54. doi: 10.1016/j.ajhg.2017.01.018
16. Griauzde J, Wilseck ZM, Chaudhary N, Pandey AS, Vercler CJ, Kasten SJ, et al. Endovascular treatment of arteriovenous malformations of the head and neck: focus on the Yakes classification and outcomes. J Vasc Interv Radiol. (2020) 31(11):1810–6. doi: 10.1016/j.jvir.2020.01.036
17. Pandey NN, Kumar S. Delayed presentation of a post-traumatic lower extremity arteriovenous fistula. Indian J Med Res. (2020) 152(Suppl 1):S69. doi: 10.4103/ijmr.IJMR_2049_19
18. An R, Li J, Zhu T, Zhang Y, Li Y, Li L, et al. Traumatic iliac arteriovenous fistula treated with the Amplatzer Vascular Plug II: a case report and literature review. Vasc Endovascular Surg. (2024) 58(5):535–9. doi: 10.1177/15385744231225375
19. Scuracchio P, Achkar R, Dias L, Oliveira M, Casella I, Presti C, et al. A rare vascular injury in a blood donor after whole blood donation. Transfusion. (2024) 64(3):546–9. doi: 10.1111/trf.17725
20. Asensio JA, Dabestani PJ, Miljkovic SS, Wenzl FA, Kessler JJ 2nd, Kalamchi LD, et al. Traumatic penetrating arteriovenous fistulas: a collective review. Eur J Trauma Emerg Surg. (2022) 48(2):775–89. doi: 10.1007/s00068-020-01574-z
21. Adame NJ, Bayless P. Carotid arteriovenous fistula in the neck as a result of a facial laceration. J Emerg Med. (1998) 16(4):575–8. doi: 10.1016/S0736-4679(98)00037-7
22. Brahmbhatt A, Remuzzi A, Franzoni M, Misra S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. (2016) 89(2):303–16. doi: 10.1016/j.kint.2015.12.019
23. Hong JT, Lee SW, Ihn YK, Son BC, Sung JH, Kim IS, et al. Traumatic pseudoaneurysm of the superficial temporal artery treated by endovascular coil embolization. Surg Neurol. (2006) 66(1):86–8. doi: 10.1016/j.surneu.2005.10.022
24. Do YS, Park KB. Special consideration for intraosseous arteriovenous malformations. Semin Intervent Radiol. (2017) 34(3):272–9. doi: 10.1055/s-0037-1605368
25. Yakes WF. Use of multiple sclerosant agents in vascular malformation management: a world in endovascular confusion and chaos. J Vasc Interv Radiol. (2015) 26(10):1494–6. doi: 10.1016/j.jvir.2015.06.002
26. Yu-Chen S, Xin-Yu L, Li-Xin S, Xi-Tao Y. A fibular defect resembling a moth-eaten cavity. Br Med J. (2023) 383:e076551. doi: 10.1136/bmj-2023-076551
27. Su L, Yang X, Wang Z, Wen M, Fan X, Wang D. Eradication of the nidus in arteriovenous malformations with a dominant outflow vein in the lower extremities using coils and absolute ethanol. J Vasc Surg Venous Lymphat Disord. (2023) 11(4):809–15. doi: 10.1016/j.jvsv.2022.10.019
28. Shen YC, Wang DM, Yang XT, Wang ZF, Wen MZ, Han YF, et al. Novel radiopaque ethanol injection: physicochemical properties, animal experiments, and clinical application in vascular malformations. Mil Med Res. (2024) 11(1):39. doi: 10.1186/s40779-024-00542-7
29. Ugajin A, Fujii H, Fujita A, Nakamura H, Fujisaki A, Sugimoto H. Transvenous embolization for a huge pelvic arteriovenous malformation associated with prominent outflow veins. Radiol Case Rep. (2020) 15(3):285–91. doi: 10.1016/j.radcr.2019.12.013
30. Soulez G, Gilbert P, Giroux MF, Racicot JN, Dubois J. Interventional management of arteriovenous malformations. Tech Vasc Interv Radiol. (2019) 22(4):100633. doi: 10.1016/j.tvir.2019.100633
Keywords: arteriovenous fistula, trauma, embolization, ethanol, coil
Citation: Shen Y, Han Q, Wang D, Su L, Wen M, Fan X and Yang X (2024) Coil-assisted ethanol embolization of traumatic arteriovenous fistulas: a 10-year retrospective study. Front. Cardiovasc. Med. 11:1449480. doi: 10.3389/fcvm.2024.1449480
Received: 15 June 2024; Accepted: 20 August 2024;
Published: 3 September 2024.
Edited by:
Pasqualino Sirignano, Sapienza University of Rome, ItalyReviewed by:
Marcello Andrea Tipaldi, Sapienza University of Rome, ItalyGianluca Faggioli, University of Bologna, Italy
Gemmi Sufali University of Bologna, Italy, in collaboration with GF
Copyright: © 2024 Shen, Han, Wang, Su, Wen, Fan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xindong Fan, ZmFueGluZG9uZ0BhbGl5dW4uY29t; Xitao Yang, eGl0YW8xMjM0NTZAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yuchen Shen
Yuchen Shen Qianyun Han†
Qianyun Han† Deming Wang
Deming Wang Lixin Su
Lixin Su Xindong Fan
Xindong Fan Xitao Yang
Xitao Yang