- 1Department of Cardiac Surgery, Yantai Yuhuangding Hospital, Yantai, China
- 2Department of Hematology, Yantai Yuhuangding Hospital, Yantai, China
- 3Department of Intensive Care Unit, Yantai Yuhuangding Hospital, Yantai, China
- 4Department of Pathology, Yantai Yuhuangding Hospital, Yantai, China
A 56-year-old man with a 5-year history of paroxysmal palpitations, which have worsened over the past year, was diagnosed with atrial fibrillation. During evaluation, transesophageal echocardiography revealed a left atrium (LA) tumoral mass attached to the atrial septal fossa ovale, with intra-tumoral blood flow and blood stream draining from the mass. Both coronary computed tomography angiography and coronary angiography demonstrated a coro-cameral fistula connection between the left circumflex artery (LCX) branch and the LA. In addition, they showed feeding arteries of the mass arising from the LCX. The patient underwent surgical resection of the LA mass and repair of the coronary artery fistula. Intraoperative exploration revealed a 1.7 cm × 1.0 cm jelly-like, brittle LA mass and confirmed a rupture of the supplying artery, leading to a coronary artery–left atrial fistula. Surgical ligation was executed to ensure complete sealing of the supplying coronary branch within the atrial septum. Histopathological examination confirmed the diagnosis of left atrial myxoma. The 6-month follow-up indicated no recurrence of the myxoma and restoration of sinus rhythm after radiofrequency ablation. In the literature, cases of a left circumflex artery branch–left atrial fistula due to rupture of the artery supplying a left atrial myxoma are rare.
1 Introduction
Primary cardiac tumors are exceedingly rare, representing a mere 0.03% of autopsy findings (1). Of these cases, an impressive 80% are benign, with myxomas accounting for nearly half of these benign tumors (2). Coronary artery fistulas (CAFs) are rare coronary anomalies characterized by abnormal connections between a coronary artery and a cardiac chamber or major vessels (3). CAFs can be congenital or acquired, with congenital types being the most common. Acquired CAFs can result from trauma, therapeutic procedures, or surgical interventions (4). To the best of our knowledge, the formation of a coronary-to-atrial fistula through a tumor has been rarely documented in the literature. Herein, we present a unique case of a left atrial myxoma leading to an acquired fistula between a branch of the left circumflex artery (LCX) and the left atrium (LA), caused by the rupture of the supplying artery within the myxomatous tissue.
2 Case report
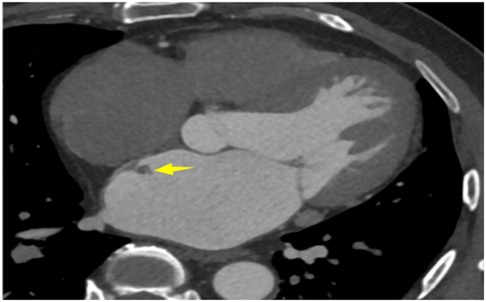
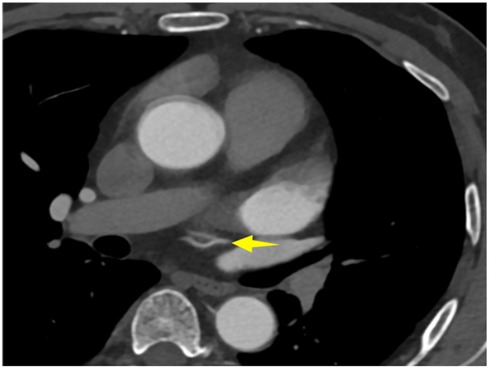
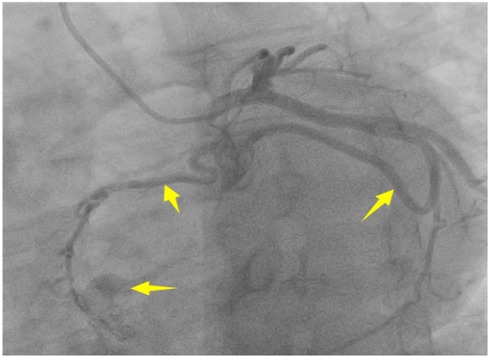
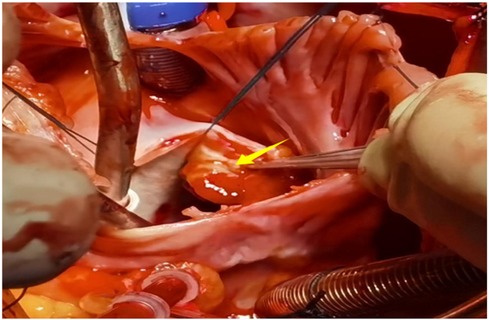
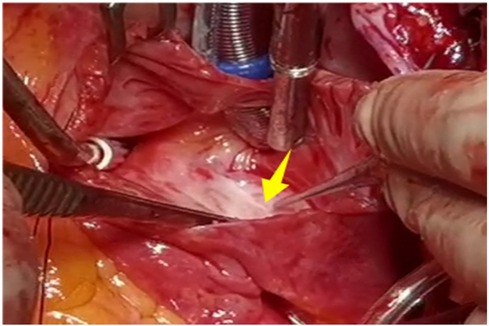
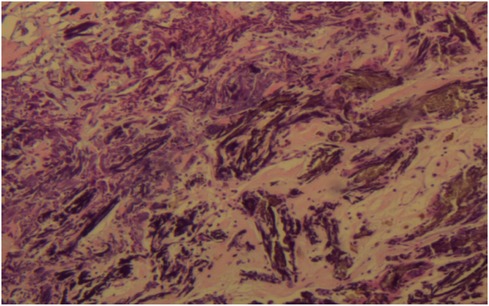
A 56-year-old man with a 5-year history of paroxysmal palpitations, which have worsened over the past year, was diagnosed with atrial fibrillation. His vital signs and test results were within the normal range at that time. During evaluation, two- (2D) and three-dimensional (3D) transesophageal echocardiography (TEE) revealed a 17.8 mm × 11 mm LA tumoral mass, attached to the atrial septal fossa ovale (Figure 1). Multiplane TEE identified the mass as a tumor with heterogeneous echogenicity, suggesting myxoma (Figure 2). Color Doppler images showed intra-tumoral blood flow and blood stream draining from the mass (Figure 3). Left atrium computed tomography angiography (CTA) showed no signs of thrombosis but did reveal a mass in the left atrium arising from the atrial septal (Figure 4). Coronary CTA suggested intra-tumoral neovascularization with vascular channels supplied by a branch of the LCX (Figure 5). Coronary angiography (CAG) revealed a coro-cameral fistulous connection between a branch of the LCX and the LA. Furthermore, it showed the feeding arteries of the mass arising from the LCX (Figure 6). The rest of the CAG was normal. The patient was scheduled for surgical resection of the LA mass and repair of the coronary fistula. Pre-cardiopulmonary bypass (CPB) TEE demonstrated a bloodstream spurting from the tumor with a peak velocity of 70 mm/s. Intraoperative exploration revealed a 1.7 cm × 1.0 cm jelly-like, brittle LA mass attached to the atrial septal fossa ovale (Figure 7). An abnormal coronary artery of the coronary artery fistula was located within the atrial septal muscle bundle and was approximately 1.0–1.5 mm in diameter (Figure 8). The intraoperative probe confirmed that the rupture of the feeding artery or its branches resulted in a coronary artery–left atrial fistula. The abnormal opening of the feeding artery was located at the upper end of the atrial septal incision (Figure 9). The LA mass was successfully resected via the right atrial approach using a standard hypothermic CPB. Surgical ligation was executed to ensure complete sealing of the supplying coronary branch within the atrial septum. The patient was transitioned from CPB with the support of a minimal dose of dopamine. Post-procedure TEE confirmed complete resection of the LA mass and no flow across the atrial septal. The postoperative course was uneventful, and transthoracic echocardiography (TTE) showed no abnormal flow in LA. Histopathological analysis confirmed the diagnosis of myxoma accompanied by calcification and hemosiderin accumulation, revealing a multinucleated giant cell response, extensive cardiac cell degeneration, and localized infiltration of chronic inflammatory cells into the interstitium (Figure 10). The 6-month follow-up showed no recurrence of myxoma and recovery of sinus rhythm after radiofrequency ablation.
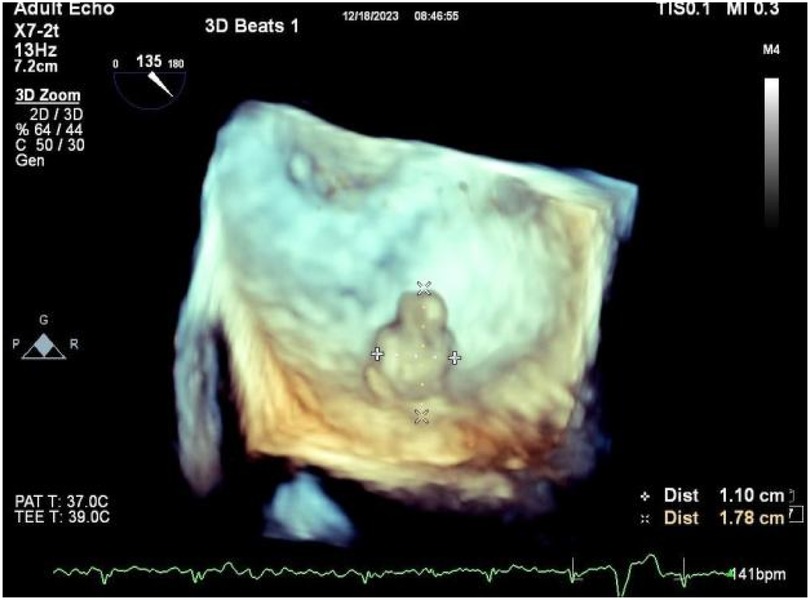
Figure 1. 3D TEE revealed a 17.8 mm × 11 mm LA tumoral mass, attached to the atrial septal fossa ovale.
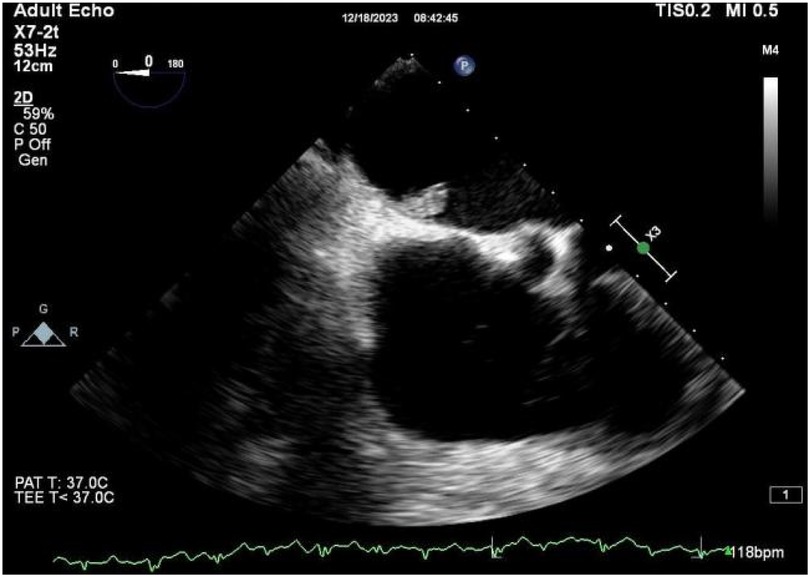
Figure 2. 2D TEE revealed a mass with heterogeneous echogenicity attached to the atrial septal fossa ovale.
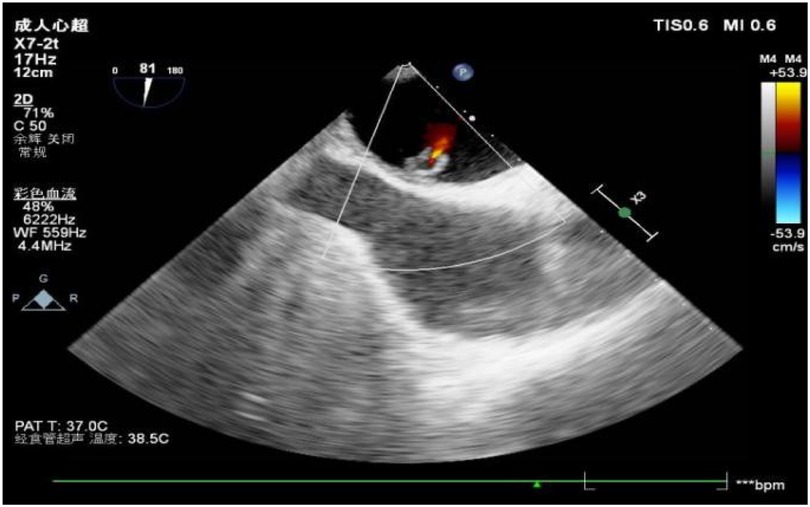
Figure 3. Color Doppler images showed intra-tumoral blood flow and blood stream draining from the mass.
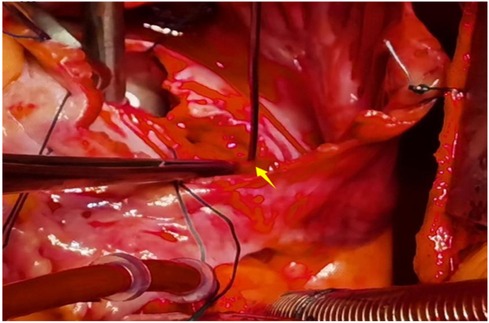
Figure 9. The abnormal opening of the feeding artery was located at the upper end of the atrial septal incision.
3 Discussion
The literature documents only a few rare cases of left circumflex artery branch-to-left atrial fistulas resulting from the rupture of a feeding artery within a left atrial myxoma. Myxomas are the most common benign primary tumors of the heart, representing 50% of such cases. They typically occur in the LA (85%), followed by the right atrium (10%) and the ventricles (5%) (5). Despite their benign nature, they require immediate surgical treatment as they carry a risk of embolic events that can be fatal. Angiographically identifiable neovascularization has been observed in 37%–56% of symptomatic cardiac myxomas, according to studies by Van Cleemput and Fueredi et al. (6–9). A small number of case series have reported the coronary steal phenomenon, which is caused by highly vascular cardiac tumors that receive their blood supply from the coronary arteries (12). In our patient, a branch of the LCX supplied blood to the myxoma. CAFs can be acquired or congenital, and they are usually considered rare coronary anomalies (10). Acquired CAFs are further categorized as spontaneous or traumatic. The most common CAFs are from the right coronary artery (RCA) (50%–55%), followed by the left coronary artery (LCA) (35%), with a small percentage (5%) arising from both coronaries. More than 90% of fistulas drain into the right heart chambers, while approximately 8% drain into the left heart chambers (11). Although systolic or diastolic murmurs are frequently observed in more than 50% of CAF patients, the patient in the present case report did not show any cardiac murmurs during physical examination, presumably due to the reduced size of the mass and the low flow rate of the arterial fistula. The combination of myxoma and CAF may promote coronary steal, although the patient was not investigated for ischemia (12). In this case, coronary fistula repair was performed simultaneously during the operation. The initial imaging modality employed to detect a cardiac mass is TTE, which provides information on the tumor's location, size, and appearance (13). Although TTE did not identify the feeding artery and fistula drainage site in our case, it could provide valuable indications of abnormal blood flow associated with the tumor (14). A subsequent CAG revealed a feeding artery originating from the coronary artery that supplies the tumor. In summary, in our case, the blood flow from the tumor manifested as an acquired CAF due to a ruptured feeding artery originating from the LCX that supplied the left atrial myxoma. The fistula was visualized as continuous flow on TEE, and CAG confirmed its course. To conclude, we report a rare case of a complicated atrial myxoma with a coro-cameral fistula arising from its feeding branch. Cardiac tumoral neovascularization may lead to the formation of CAF secondary to spontaneous vessel rupture after tumoral necrosis. Such fistulae may further complicate the course of the disease, potentially causing coronary steal and myocardial ischemia if the tumor-feeding branch is large.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Yantai Yuhuangding Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
J-QL: Conceptualization, Writing – original draft, Writing – review & editing. PD: Investigation, Writing – original draft, Writing – review & editing. Q-LW: Writing – review & editing. X-XL: Writing – review & editing. T-MS: Data curation, Writing – original draft, Writing – review & editing. PZ: Investigation, Writing – original draft, Writing – review & editing. Z-QZ: Investigation, Writing – original draft, Writing – review & editing. C-LL: Data curation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1445366/full#supplementary-material
References
1. Ha JW, Kang WC, Chung N, Chang BC, Rim SJ, Kwon JW, et al. Echocardiographic and morphologic characteristics of left atrial myxoma and their relation to systemic embolism. Am J Cardiol. (1999) 83(11):1579–82. doi: 10.1016/s0002-9149(99)00156-3
2. Sogabe O, Inokawa H, Tanaka S, Yamamoto H, Hashimoto K. Left atrial myxoma with a coronary artery steal syndrome due to the coronary artery to left atrial fistula; report of a case. Kyobu Geka. (2013) 66(4):341–4.23575189
3. Zen K, Asai T, Tatsukawa H, Matsubara H. Coronary artery-left atrial fistula caused by feeding artery rupture in cardiac myxoma. Heart. (2007) 93(2):237. doi: 10.1136/hrt.2006.090753
4. Subash S, Thimmarayappa A, Patel GP, Dhananjaya M, Gopal D, Manjunatha N. A rare case of left atrial myxoma vascularity causing acquired coronary cameral Fistula: role of transesophageal echocardiography. Heart Views. (2018) 19(1):12–5. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_79_17
5. Demir M, Akpinar O, Acarturk E. Atrial myxoma: an unusual cause of myocardial infarction. Tex Heart Inst J. (2005) 32(3):445–7.16392241
6. Salyer WR, Salyer DC. Myxoma-like features of organizing thrombi in arteries and veins. Arch Pathol. (1975) 99(6):307–11.1147831
7. Van Cleemput J, Daenen W, De Geest H. Coronary angiography in cardiac myxomas: findings in 19 consecutive cases and review of the literature. Cathet Cardiovasc Diagn. (1993) 29(3):217–20. doi: 10.1002/ccd.1810290308
8. Fueredi GA, Knechtges TE, Czarnecki DJ. Coronary angiography in atrial myxoma: findings in nine cases. AJR Am J Roentgenol. (1989) 152(4):737–8. doi: 10.2214/ajr.152.4.737
9. Rahmanian PB, Castillo JG, Sanz J, Adams DH, Filsoufi F. Cardiac myxoma: preoperative diagnosis using a multimodal imaging approach and surgical outcome in a large contemporary series. Interact Cardiovasc Thorac Surg. (2007) 6(4):479–83. doi: 10.1510/icvts.2007.154096
10. Said SA, Schiphorst RH, Derksen R, Wagenaar LJ. Coronary-cameral fistulas in adults: acquired types (second of two parts). World J Cardiol. (2013) 5(12):484–94. doi: 10.4330/wjc.v5.i12.484
11. Ho HH, Cheung CW, Jim MH, Lam L. Images in cardiology: coronary-cameral fistula. Heart. (2005) 91(12):1540. doi: 10.1136/hrt.2005.064287
12. Achim A, Johnson NP, Liblik K, Burckhardt A, Krivoshei L, Leibundgut G. Coronary steal: how many thieves are out there? Eur Heart J. (2023) 44(30):2805–14. doi: 10.1093/eurheartj/ehad327
13. D'Aloia A, Bonadei I, Vizzardi E, Rovetta R, Sciatti E, Metra M. A case of a fistula between myxoma and a branch of right coronary artery. Echocardiography. (2014) 31(9):E289–90. doi: 10.1111/echo.12664.24975943
Keywords: left atrial myxoma, coronary artery fistula, feeding artery rupture, cardiac tumors, atrial fibrillation
Citation: Li J-Q, Dong P, Wang Q-L, Li X-X, Sha T-M, Zhang P, Zhao Z-Q and Liu C-L (2024) Complicated atrial myxoma with coro-cameral fistula arising from its feeding branch: a case report. Front. Cardiovasc. Med. 11:1445366. doi: 10.3389/fcvm.2024.1445366
Received: 7 June 2024; Accepted: 20 September 2024;
Published: 8 October 2024.
Edited by:
Enyi Shi, China Medical University, ChinaReviewed by:
Sivasankaran Sivasubramonian, Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), IndiaAlexandru Achim, Cantonal Hospital Baselland (KSBL), Switzerland
Copyright: © 2024 Li, Dong, Wang, Li, Sha, Zhang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao-Liang Liu, ZHJfbGNobEBzaW5hLmNvbQ==
 Jian-Qiang Li1
Jian-Qiang Li1 Tu-Min Sha
Tu-Min Sha Zhen-Qing Zhao
Zhen-Qing Zhao