- 1Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2School of Management, Fudan University, Shanghai, China
- 3Emergency Department, Guangwai Hospital, Beijing, China
- 4Institute of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Objective: This network meta-analysis was to compare the efficacy of different drugs on cardiac function, renal function, and clinical outcomes in patients with acute heart failure (AHF) accompanied by renal dysfunction.
Methods: PubMed, EMBASE, Cochrane Library, and Web of Science were searched to screen all clinical trials of AHF between January 1st 2001 and March 31th 2024. The primary outcome measures were N-terminal pro-B type natriuretic peptide (NT-proBNP), B-type natriuretic peptide (BNP), glomerular filtration rate (GFR), blood urea nitrogen, serum creatinine, all-cause mortality within 60 days, and cardiovascular mortality.
Results: After screening 30,697 citations, 13 studies (21,745 patients) were included, and drugs including nesiritide, dopamine, tolvaptan, levosimendan, dobutamine, furosemide, and spirolactone, and high dose of diuretics (HDD, furosemide, and spirolactone) were estimated. The results indicated that HDD had the best efficacy in reducing NT-proBNP levels. In detail, HDD notably reduced NT-proBNP levels compared to conventional treatment or placebo (PLC) [MD = −950.24; 95% CrI (−1,832.21, −64.12)]. Levosimendan significantly increased GFR levels compared to PLC [MD = 14.46; 95% CrI (3.88, 25.97)] and tolvaptan [MD = 13.83; 95% CrI (2.31, 25.33)]. No significant difference was found in 60-day all-cause mortality and cardiovascular mortality across drugs.
Conclusion: HDD showed the best efficacy in reducing NT-proBNP levels compared with dopamine and nesiritide, and levosimendan could significantly improve GFR levels, with no marked difference in the effect of various drugs on 60-day all-cause mortality. Hence, HDD and levosimendan may be optimal agents in the treatment of AHF with renal dysfunction.
Systematic Review Registration: PROSPERO, identifier (CRD42023454616).
1 Introduction
Acute heart failure (AHF) is one of the most common acute cardiovascular diseases that appears or deteriorates rapidly after cardiac dysfunction, mainly characterized by dyspnea, pulmonary edema, pulmonary congestion, rapid heart rate, and elevated plasma natriuretic peptide levels (1). In AHF, myocardial contractility is reduced, resulting in a decrease in cardiac output (2) and renal blood perfusion insufficiency. Therefore, renal dysfunction is also a common complication of AHF.
The relationship between heart failure (HF) and renal function is complex since the disease itself, compensatory mechanisms, congestion, and medication can all impair renal function (3). A decline in renal function often leads to forced drug withdrawal (4) which accelerates HF progression and predetermines higher mortality and hospitalization rates in AHF patients (5, 6). One study (7) reported that 27%–45% of AHF patients experienced worsening renal function (WRF). Compared to those without, the mortality rate increased in such patients by 1.72–6.5 times, and the incidence of complications such as myocardial infarction or shock increased by 1.1 times. A meta-analysis also showed that renal protection therapy can greatly improve outcomes of HF patients (3). AHF patients combined with renal dysfunction are at risk for hyperemia and WRF, both of which can lead to unfavorable outcomes. Therefore, it is necessary to relieve congestion while protecting renal function when treating AHF (8–10).
Guideline-directed medical therapy significantly impacts mortality and morbidity in HF patients (11, 12), including diuretics, natriuretic peptides, positive inotropic drugs, vasoactive agents, β-receptor blockers, and sodium-glucose transporter 2 inhibitors (SGLT2i). Among them, diuretics are often considered the basis of HF treatment. AHF patients with renal dysfunction generally do not respond well to traditional diuretics and even develop diuretic resistance, which aggravates renal damage (13, 14). Although some emerging drugs such as nesiritide and tolvaptan have been approved for AHF treatment, their clinical efficacy on renal function remains controversial (15, 16). It is therefore necessary to compare the efficacy and safety of these drugs on cardiovascular outcomes and renal function in AHF patients with renal dysfunction to find a better therapeutic regimen.
However, to date, the efficacy of different agents in AHF patients with renal dysfunction has not been identified. A network meta-analysis (NMA) can simultaneously compare the effects of multiple interventions because of the lack of direct comparisons between these drugs (17). In addition, the NMA can rank interventions based on various outcomes. Thus, this study aims to compare the efficacy of different drugs on cardiac function, renal function, and clinical outcomes in patients with acute heart failure (AHF) combined with renal dysfunction, providing some references for clinicians to make evidence-based decisions.
2 Methods
2.1 Study design and registration
We designed and wrote this paper following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (18), and registered the protocol with the PROSPERO (CRD42023454616).
2.2 Eligibility criteria
Articles were included according to the following criteria: (1) Patients diagnosed with AHF combined with renal dysfunction of any age. The diagnosis of AHF was based on the Framingham criteria (19), accompanied by at least one of the following symptoms: pulmonary congestion, peripheral edema, pleural effusion, jugular venous distension, or orthopnea. Renal dysfunction was regarded as a glomerular filtration rate (GFR) of 15 to 60 ml/min per 1.73 m2 estimated by the Modification of Diet in Renal Disease equation (20, 21). (2) Patients in the intervention group took at least one type of the guidance-recommended medications for AHF, including tolvaptan, levosimendan, nesiritide, dopamine, dobutamine, and any kind of diuretics, regardless of the mode, dosage, and duration of administration. (3) Control individuals took either placebo, conventional treatment (PLC), or one of the drugs mentioned above. (4) Studies reported at least one of the following outcomes: (a) Serum creatinine (Cr); (b) Blood urea nitrogen (BUN); (c) Brain natriuretic peptide (BNP); (d) N-Terminal Pro-Brain Natriuretic Peptide (pro-BNP); (e) GFR; (f) Mortality (All-cause mortality or cardiovascular mortality within 60 days); (5) Randomized controlled trial (RCT) or retrospective study. (6) Studies published in English.
Exclusion criteria were as follows: (1) Unclear criteria for diagnosis and efficacy. (2) Animal studies, meta-analyses/reviews, conference abstracts, letters/responses to editors, guidelines, or case reports. (3) Incomplete data that could not be merged.
2.3 Search methods
PubMed, Embase, Cochrane Library, and Web of Science were searched independently by two researchers [Qianyu Lv (L.Q.Y.) and Qian Wu (W.Q)] up to March 2024, with no restrictions on document type, publication time, or publication status. The combination of Medical Subject Heading (MeSH) terms and their free words was used as the search strategy. MeSH terms included keywords, including “acute heart failure”, “acute decompensated heart failure”, “heart failure”, and recommended drugs: diuretics, vasopressin V2 receptor antagonists, ACEI, ARBs, natriuretic peptides administered alone, beta-blockers, Ivabradine, digitalis, levosimendan, phosphodiesterase III inhibitors, and SGLT2i.
2.4 Study selection
Two researchers (L.Q.Y. and W.Q) independently reviewed the titles, abstracts, and then the full texts of all searched articles. First of all, the possibly relevant research was imported into EndNote X9, and duplicates were removed automatically and manually. Then, titles and abstracts were reviewed to exclude unqualified literature, followed by full-text assessment. Any disagreement was resolved by discussion with a third researcher (LanLan.Li (L.L.L.)).
2.5 Data extraction and quality assessment
A Cochrane data extraction table was utilized to extract the following data: (1) General information of the article: title, first author, author's country, publication year; (2) Basic characteristics of the study population: age, sex, and included cases; (3) Details of the intervention: specific measures and intervention duration; (4) Levels of outcome indicators (including Cr, BUN, BNP, pro-BNP, GFR) before and after intervention; (5) Mortality rates. One researcher (L.Q.Y.) extracted data, while the other researcher (W.Q.) checked the data accuracy.
The study quality was independently assessed by two reviewers (L.Q.Y. and W.Q.) using the RoB 2 (22) in the following five bias domains: randomization process, allocation concealment, intervention blinding of participants and investigators, missing outcome data, outcome measurement and selection of reported results. An algorithm was employed to estimate the overall risk of bias, i.e., low risk, some concerns, or high risk. The above risk of bias assessment was conducted by two reviewers (L.Q.Y. and W.Q.), and any discrepancies were resolved by discussion with the third researcher (L.L.L.).
2.6 Statistical analysis
The statistical model based on the Bayesian framework was built using JAGS software 4.3.1 [gemtc package 0.8–2 and rjags package 4–10)] (Rstudio, Boston, MA, USA). Based on the clinical heterogeneity of the included trials (i.e., country, drug dose, and duration of administration), a random-effects model with four Markov chains was used for each outcome, with each chain producing 50,000 iterations (burn-in period of 20,000 iterations). The convergence of iterations was monitored using plots and the Gelman–Rubin–Brooks statistic (23). We also comparing the therapeutic effect of different drugs using a Surface Under the Cumulative Ranking curve (SUCRA), the closer SUCRA was to 100, the better the therapeutic effect. Model consistency was assessed using the deviation information criterion (DIC), with differences in DIC < 5 points indicating good consistency and consistency modeling was used (24). Heterogeneity was estimated using the I2 statistic with I2 values <25% indicating low heterogeneity, 25%–75%, moderate heterogeneity, and >75%, high heterogeneity (25). Publication bias was assessed by funnel plots. Network plots and funnel plots were drawn by Stata SE 15.0 (StataCorp, College Station, Texas, USA).
3 Results
3.1 Search outcomes
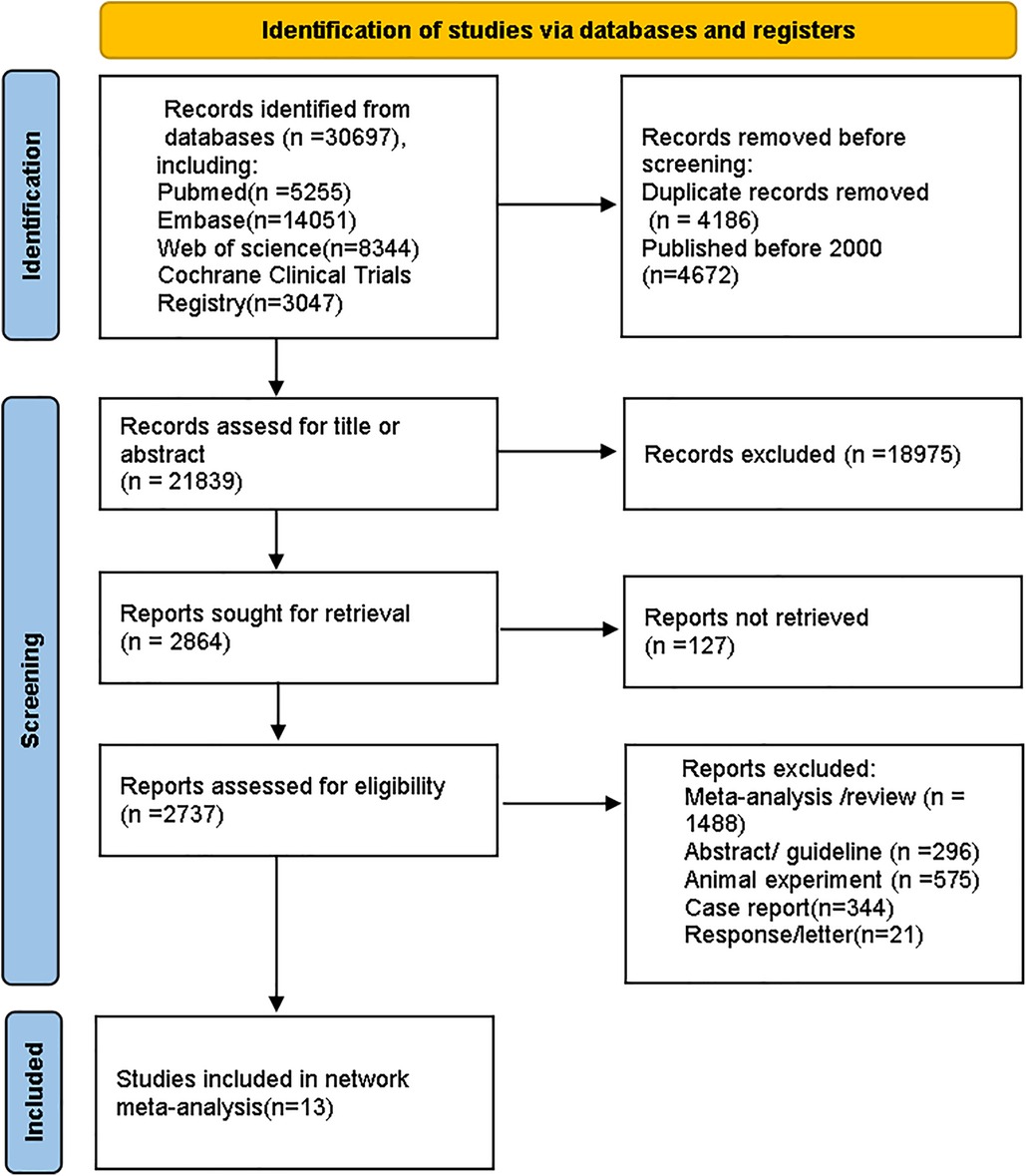
The process for selecting studies follows the updated PRISMA guidelines (26). A total of 30,697 records were identified from databases. After removing 4,186 duplicates and 4,672 studies that were published before 2,000, we reviewed the titles and abstracts of the remaining articles based on eligibility criteria. Ultimately, 13 studies were included. The screening process is shown in Figure 1.
3.2 Characteristics of included studies
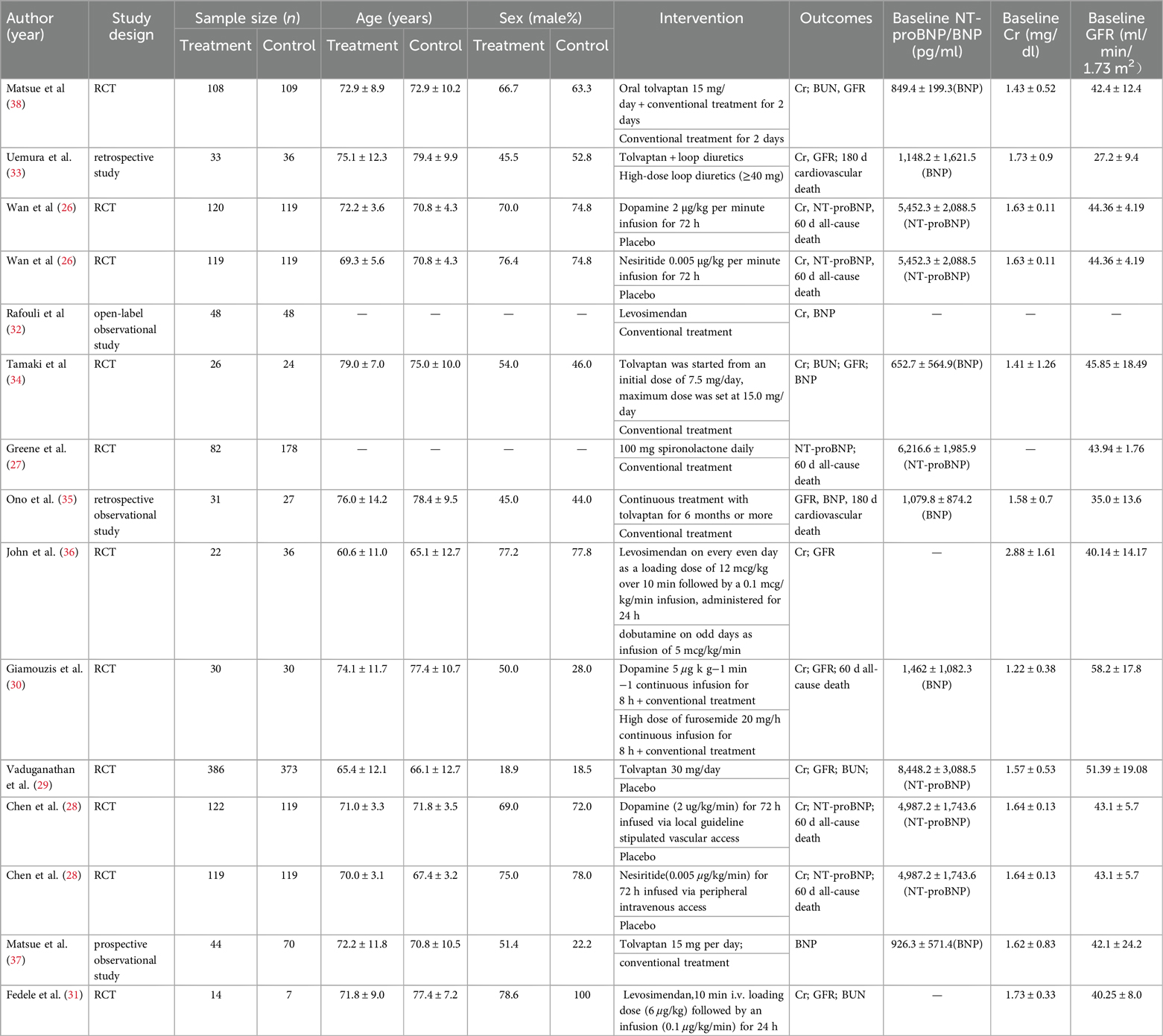
Of the 13 eligible studies published from 2001 January to March 2024, 4 studies were conducted in North America (27–30), 3 in Europe (31–33), and 6 studies (34–39) in Asia. A total of 21,745 AHF patients with renal dysfunction were enrolled. The sample size of each article ranged from 21 to 759 participants, and the mean age ranged from 60.6 to79.4. Seven drugs (nesiritide, dopamine, tolvaptan, levosimendan, dobutamine, and high doses of diuretics (HDD, including spirolactone and furosemide) were included. Since 3 studies used high doses of furosemide and spironolactone (28, 31, 34), unlike conventional treatment, we combined the two drugs into an HDD group). As for study design, our NMA included 9 RCTs (27–32, 35, 37, 39), 1 prospective observational study (38), and 3 retrospective studies (33, 34, 36). All treatments were completed during hospitalization (Table 1).
3.3 Quality assessment
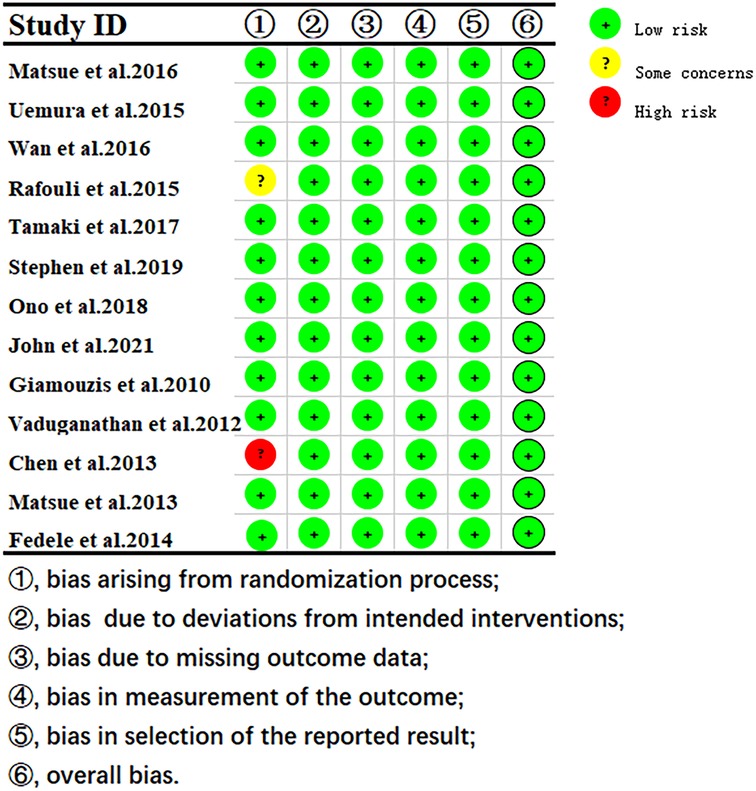
The quality assessment results showed that 1 study had some concerns about publication bias, and others showed a low risk of bias. (Figure 2).
3.4 NMA
3.4.1 Cardiac function indicator
3.4.1.1 NT-proBNP (pg/ml)
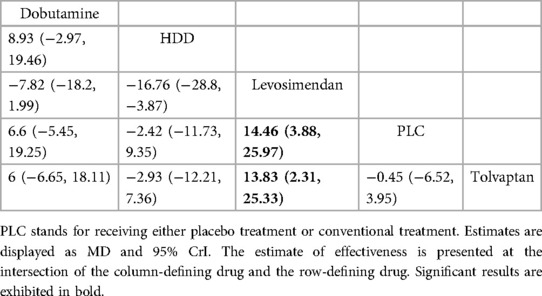
Three studies (27–29) compared the effect of drugs (diuretics, dopamine, and nesiritide) on NT-proBNP (Figure 3A, Table 2). DIC comparison results showed good agreement (DIC, 13.30 vs. 13.32). According to the NMA results, HDD was the most likely to be the optimal drug for reducing NT-proBNP levels, followed by dopamine, and nesiritide (SUCRA: HDD, 92%, dopamine, 67%, nesiritide, 34%). Among them, the pairwise comparison indicated that HDD significantly reduced NT-proBNP levels compared to PLC [MD = −950.24, 95% CrI (−1,832.21, −64.12)]. Besides, HDD reduced NT-proBNP levels compared to dopamine [MD = −342.31, 95% CrI (−1,424.03, 734.56)] and nesiritide [MD = −697.91, 95% CrI (−1,906.77, 498.05)], with no significant difference.
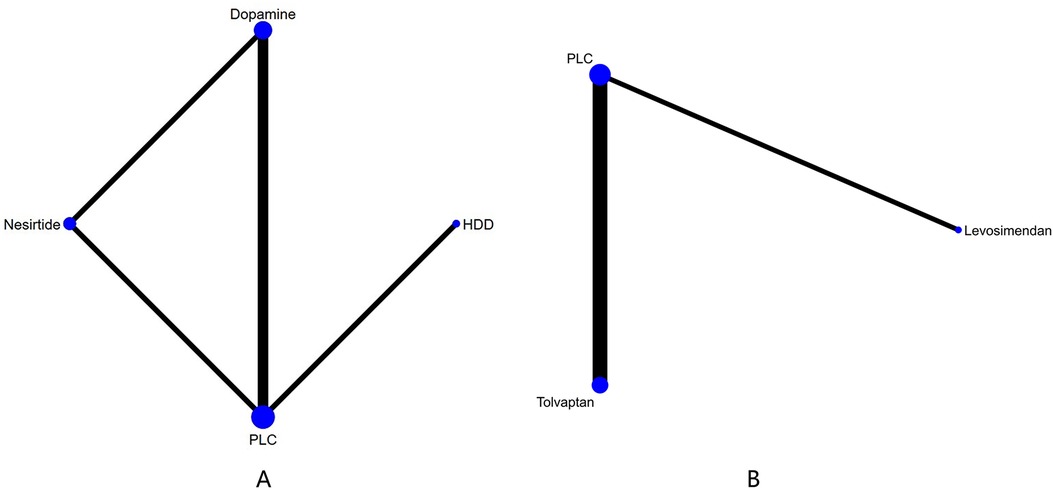
Figure 3. Network plots of cardiac function indexes. (A) Network plot for NT-proBNP. (B) Network plot for BNP. PLC stands for receiving either placebo treatment or conventional treatment. Nodes stand for the comparison between treatments and the size is proportional to the number of subjects. The width of the lines is proportional to the number of trials per pair of interventions.
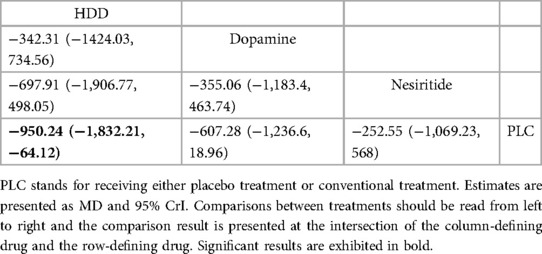
Table 2. Relative effects of different drugs on NT-proBNP (reported as odds ratios (OR) or weighted mean differences (MD) with 95% credible intervals (CrI).
3.4.1.2 BNP (pg/ml)
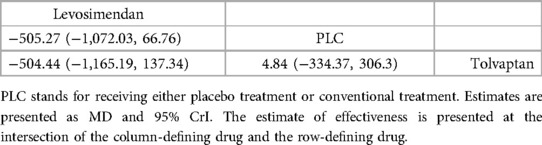
In total, four studies (33, 35, 36, 38) evaluated the effect of levosimendan and tolvaptan on BNP levels in AHF patients with renal dysfunction (Figure 3B). The main findings are shown in Table 3. DIC comparison results showed good agreement (15.34 vs. 15.36). Levosimendan showed the best efficacy in reducing BNP levels, followed by tolvaptan (SUCRA, levosimendan, 95%, tolvaptan, 28%). However, there were no significant differences among levosimendan, tolvaptan, and PLC.
3.4.2 Renal function indicators
3.4.2.1 GFR (ml/min 1.73 m2)
Five drugs across 8 studies (30–32, 34–37, 39) were analyzed to compare their effects on GFR levels (Figure 4A, Table 4). Comparison of the consistency model and the inconsistency model results showed good agreement (31.83 vs. 32.19). Levosimendan tended to be the most effective drug in increasing GFR levels, followed by dobutamine, tolvaptan, and HDD (SUCRA: levosimendan, 98%, dobutamine, 68%, tolvaptan, 38%, PLC, 31%, HDD, 14%). Levosimendan, dobutamine, and tolvaptan may have more positive effects than PLC, while HDD may have a more negative effect than PLC. Levosimendan significantly increased GFR levels compared to PLC [MD = 14.46; 95% CrI (3.88, 25.97)] and tolvaptan [MD = 13.83; 95% CrI (2.31, 25.33)].
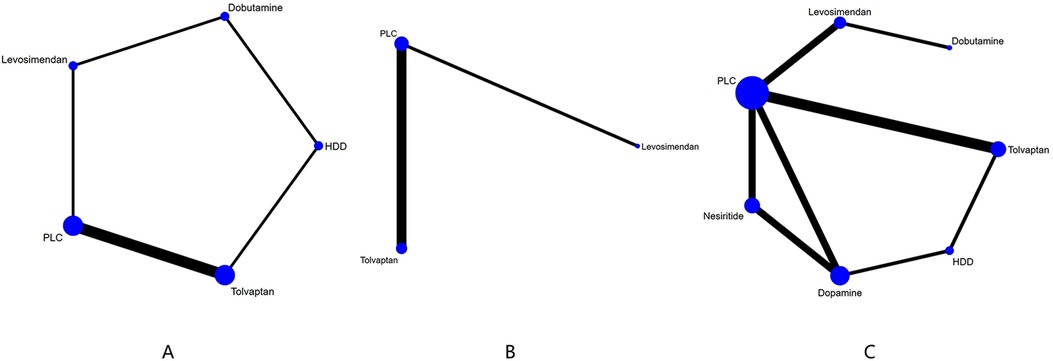
Figure 4. Network plot and results of NMA. (A) Network plot for GFR). (B) Network plot for BUN. (C) Network plot for Cr. PLC stands for receiving either placebo treatment or conventional treatment. Nodes stand for the comparison between treatments and the size is proportional to the number of participants. The width of the lines is proportional to the number of trials in each pair of interventions.
3.4.2.2 BUN (mg/dl)
The network plot of BUN is shown in Figure 4B, including 2 drugs (levosimendan, tolvaptan) from 4 studies (30, 32, 35, 39), and the main finding is shown in Table 5. DIC comparison results showed good agreement (17.01 vs. 16.81). Compared with placebo, both levosimendan and tolvaptan reduced BUN levels in patients. Additionally, levosimendan ranked first, followed by tolvaptan and placebo (SUCRA: levosimendan, 93%, tolvaptan, 41%, PLC, 14%), but the difference in the comparison between any two drugs was not significant.
3.4.2.3 Cr (mg/dl)
Ten studies (27, 29–35, 37, 39) analyzed the changes in Cr levels, with seven drugs included (Figure 4C, Table 6). DIC comparison results showed good agreement (45.33 vs. 46.01). NMA showed that each drug tended to reduce Cr compared with PLC. Although the differences of pairwise comparisons were not substantial, levosimendan was the most promising drug for reducing Cr levels, followed by dobutamine, dopamine, nesiritide, tolvaptan, and HDD (SUCRA: levosimendan 80%, dobutamine 67%, dopamine, 58%, nesiritide, 45%, PLC, 38%, tolvaptan, 33%, HDD, 26%). Among them, levosimendan, dobutamine, dopamine, and nesiritide may have better efficacy than PLC in reducing Cr levels, while tolvaptan and HDD may have worse efficacy than PLC.
3.4.3 Mortality
3.4.3.1 60-day all-cause mortality
In 60-day all-cause mortality, 4 trials (27–29, 31) with 3 drugs (HDD, nersirtide, dopamine) were involved (Figure 5A, Table 7). Comparison of the consistency model and the inconsistency model results showed good agreement (14.93 vs. 16.94). Pairwise comparisons did not show notable differences in the rate of 60-day all-cause mortality. Nesiritide was associated with the lowest rate of all-cause mortality within 60 days, followed by HDD and dopamine (SUCRA: nesiritide 75%, HDD, 55%, dopamine, 39%, PLC, 29%), all of which were safer than placebo.
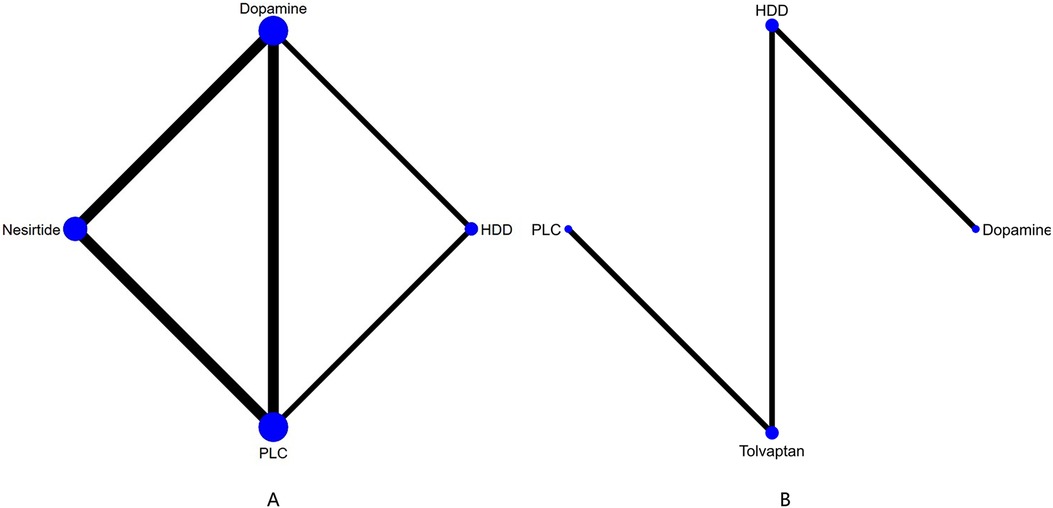
Figure 5. Network plot and results of NMA. (A) Network plot for 60-day all-cause mortality. (B) Network plot for 60-day cardiac-cause mortality. PLC stands for receiving either placebo treatment or conventional treatment. Nodes represent the comparison between treatments and the size is proportional to the number of subjects. The width of the lines is proportional to the number of trials in each pair of interventions.
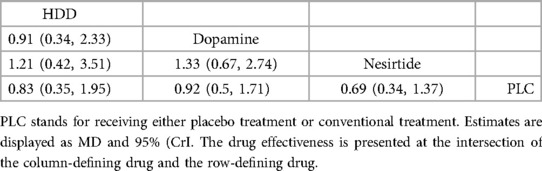
Table 7. Relative effects of different drugs on all-cause mortality within 60 days (reported as OR or weighted MD with 95% CrI).
3.4.3.2 Cardiovascular death
Three studies (31, 34, 36) analyzed the effect of different drugs on cardiovascular mortality (Figure 5B, Table 8). DIC comparison results showed good agreement (4.51 vs. 8.71). The pairwise comparisons did not show marked differences. Tolvaptan, dopamine, and HDD had the lowest to highest rate of cardiovascular death (SUCRA: tolvaptan 77%, dopamine, 50%, PLC, 38%, HDD, 33%). Among them, the effect of HDD on cardiovascular death within 60 days may be inferior to that of PLC, while the effect of dopamine and tolvaptan may be superior to that of PLC.
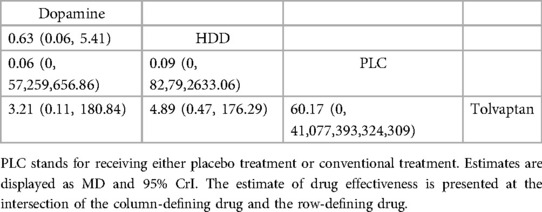
Table 8. Relative effects of different drugs on cardiovascular mortality within 60 days (reported as point estimates of OR or weighted MD with 95% CrI).
3.5 Consistency
The consistency model and inconsistency model were compared using DIC, with changes in DIC < 5 for all closed-loop models indicating good consistency.
4 Discussion
To date, the management of AHF with renal dysfunction remains challenging, and evidence is lacking in many areas. To analyze the efficacy of current therapies on cardiac function, renal function and clinical outcomes in patients with AHF with renal dysfunction, we performed this NMA. Our study is the first comparison of various drugs with either placebo or conventional treatment. Since conventional treatment mostly uses low-dose diuretics, we enrolled studies using high-dose diuretics, regardless of the type (furosemide and spironolactone here) as the High-Dose Diuretic group and compared with other drugs and PLC.
In terms of cardiac function indicators, NMA results indicated that diuretics had better effects than dopamine and nesiritide on reducing NT-proBNP, with a significant difference compared with PLC. As for BNP, the effect of levosimendan appeared to be the best, with no significant difference compared with PLC. NT-proBNP, released by cardiomyocytes against ventricular stress, can effectively regulate blood pressure and electrolyte balance, with strong associations with ventricular hypertrophy and systolic dysfunction (40, 41). NT-proBNP level can effectively evaluate the clinical efficacy in HF patients (42), and is also an independent risk factor for sudden cardiac death (41, 43). In addition, since NT-proBNP is excreted through glomerular filtration and is easily affected by renal function (44), it is therefore an important indicator for patients with AHF and renal dysfunction. Based on our NMA, HDD can significantly reduce NT-proBNP levels. The conventional treatment for AHF is diuretics, however, there are ongoing debates on the therapeutic effectiveness of high-dose diuretics in patients with AHF and renal dysfunction. Furosemide is a loop diuretic while spironolactone is an aldosterone antagonist. Some studies have found that high-dose diuretics are more effective in increasing urine volume in AHF patients than low-dose diuretics (45, 46). Therefore, high-dose diuretics may effectively reduce water and sodium retention, and alleviate cardiac preload, ventricular wall stress, and then, the secretion of NT-pro-BNP in cardiomyocytes is reduced.
BNP is a cardiac neuroendocrine hormone released by ventricular muscles during myocardial damage and has great value in the early prediction of death and other adverse events in AHF patients (47). One study showed that lower BNP levels at discharge were associated with lower mortality (48), and the WRF with higher BNP levels at discharge was associated with poor prognosis. Therefore, determining which drugs help reduce BNP levels has great prognostic implications. Although our NMA results showed that no included drugs significantly reduced BNP levels, levosimendan seems more effective than other drugs. In contrast to our findings, a meta-analysis demonstrated that levosimendan improved cardiac function and significantly reduced plasma BNP levels in patients with decompensated HF (49). These differences may be due to heterogeneity in the study population, including differences in baseline cardiac and renal function and therefore these results still warrant consideration. Levosimendan, a calcium sensitizer, is a novel positive inotropic drug (32) that specifically binds to troponin C in cardiomyocytes to enhance muscle strength without increasing myocardial oxygen consumption, and thus, has better safety (50, 51). The main mechanism of levosimendan in reducing BNP concentration is to enhance myocardial contractility, dilate coronary artery, and reduce cardiac load, ventricular wall stress, and BNP release by ventricular myocytes, without increasing myocardial oxygen consumption (52). Additionally, it inhibits the production of tumor necrosis factor-α, interleukin-6, leukocytin-8, and BNP (53).
As for renal function, levosimendan significantly increased GFR values. Although there was no significant difference in lowering BUN and Cr, levosimendan also showed better protective effects on the kidneys than other drugs. Similar to our findings, a meta-analysis of 529 patients suggested that levosimendan may reduce the incidence of perioperative acute kidney injury (54) by increasing renal blood flow through renal vasodilation (55). Levosimendan has been shown to increase GFR and protect the kidney in patients with cardiorenal syndrome (56–58). The mechanism may also include activation of renal K+ ATP channels in the bulbar arterioles, resulting in shortened duration of action potential in cardiomyocytes, hyperpolarization of vascular smooth muscle cells, reduced calcium flow, vasodilation, and increased blood perfusion (59). Furthermore, it can completely block angiotensinⅡ-induced mesangial cell contraction, thereby increasing the surface area of glomerular capillaries and protecting against acute renal failure (60, 61). In addition, other potential mechanisms of levosimendan involve anti-inflammatory and anti-apoptotic effects (62, 63). Mullens et al. (64) suggested that higher central venous pressure was related to higher renal venous pressure and WRF to a certain extent, resulting in decreased GFR and renal insufficiency. Levosimendan can improve renal function by reducing central venous pressure through right ventricular function. Notably, HDD may be inferior to placebo in improving GFR and Cr. Previous studies have shown that diuretics are an important method to reduce fluid retention in patients with acutely decompensated HF (ADHF), but they may lead to WRF (65–67). These results suggest that HDD should be used with caution in patients with AHF and renal insufficiency. Our results showed that tolvaptan may have a protective effect on kidney function, although there was no statistical difference. As a novel diuretic, tolvaptan increases urine output by inhibiting vasopressin receptors in the renal tubules. In a randomized controlled meta-analysis, tolvaptan significantly improved dyspnea symptoms compared with controls, but had little effect on kidney function (68). We hypothesize that tolvaptan may be beneficial in improving acute decompensated HF (ADHF) with renal insufficiency without reducing renal function, but large-scale clinical trials are still needed to confirm this. Besides, a new diuretic SGLT-2i has attracted our attention because of its potential applicability in AHF (69). SGLT-2i is believed to have some renal protection in the treatment of HF (70). A meta-analysis also showed that it can improve cardiovascular and renal outcomes in patients with chronic kidney disease (71). However, our search in the database did not find any clinical studies of SGLT-2i in the treatment of AHF with renal insufficiency, which is contrary to expectation. Therefore, we look forward to the clinical application of SGLT-2i in this field.
At last, we assessed the mortality of various drugs. There was no significant difference in the effect of included drugs on 60-day all-cause mortality and cardiovascular mortality. In terms of all-cause mortality, the efficacy of HDD, nersirtide, and dopamine was compared, and nesiritide had the best effect. Nesiritide is a recombinant B-type natriuretic peptide with vasodilatory effects (15, 72). Several meta-analyses have reported the effect of nesiritide on the mortality of AHF patients. Sackner Bernstein et al. (73) found that nesiritide may be associated with a higher risk of death after acute decompensated heart failure (ADHF) treatment, while Abraham (74) and Bin Yan (75) showed that nesiritide did not increase mortality of ADHF patients after short-term and long-term treatment. Another meta-analysis evaluated the effects of nesiritide on ADHF patients and found that high doses of nesiritide may increase the risk of WRF, however, standard and low doses of nesiritide may not affect renal function (76). Clinical trials of nesiritide in AHF with renal dysfunction are limited, and more studies are needed to validate its efficacy. In terms of reducing cardiovascular mortality, three regimens, including HDD, nesiritide, dopamine, and tolvaptan, were included, of which tolvaptan had the best effect. However, due to the small number of included studies and inconsistent follow-up time [2 studies reported cardiovascular death during 180 days (34, 36), and 1 study (31) reported 60 days], the results still need validation.
5 Strengths and limitations
To date, this is the first NMA to compare and rank the efficacy of different drugs on cardiac function, renal function, and clinical outcomes in patients with AHF with renal insufficiency. This NMA provides valuable references on the optimal drug for patients with AHF and renal insufficiency. The study did have some limitations, though. First, the limited studies and small sample size may impact the accuracy and applicability of the obtained results. Second, there were differences in the dosage, duration, and mode of administration across the included studies, which may lead to heterogeneity in the studies. This review focuses heavily on biochemical markers and lacks descriptions of some symptom improvements, which limits the practical application of the findings. In addition, newer therapies such as angiotensin receptor neprilysin inhibitors and SGLT2i are not included in this article, and we look forward to more relevant studies in the future.Finally, subgroup analysis was not performed due to the limited number of studies,reduces the specificity of the conclusions.
Therefore, more high-quality RCTs are needed to validate our findings.
6 Conclusions
We used a Bayesian NMA to analyze the advantages and disadvantages of nesiritide, dopamine, tolvaptan, levosimendan, dobutamine, and HDD (including furosemide and spirolactone) in the treatment of patients with AHF and renal insufficiency. However, no single drug can optimally improve all indicators in these patients. HDD had a significant effect in reducing NT-proBNP levels. No drug has a significant difference in reducing BNP. Levosimendan was the best at improving GFR levels, although the difference was not significant, it seems also to be better at reducing BUN and Cr levels. In terms of clinical outcomes, the rates of 60-day all-cause death and cardiovascular death were all similar across drugs. Due to limitations in clinical trials, future trials with larger sample sizes, longer follow-up period, and more rigorous design are warranted to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
QL: Writing – original draft, Writing – review & editing. QW: Writing – original draft, Writing – review & editing. YY: Formal Analysis, Investigation, Writing – review & editing. LL: Methodology, Writing – review & editing. XY: Resources, Writing – review & editing. SW: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. YL: Funding acquisition, Writing – original draft. MW: Supervision, Writing – review & editing. YL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Beijing Natural Science Foundation (7232311); Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (NO.CI2021A00921).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. Corrigendum to: 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42(48):4901. doi: 10.1093/eurheartj/ehab670
2. Fiuzat M, Wojdyla D, Pina I, Adams K, Whellan D, O'Connor CM. Heart rate or Beta-blocker dose? Association with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION trial. JACC Heart Fail. (2016) 4(2):109–15. doi: 10.1016/j.jchf.2015.09.002
3. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. (2014) 35(7):455–69. doi: 10.1093/eurheartj/eht386
4. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. (2015) 36(23):1437–44. doi: 10.1093/eurheartj/ehv010
5. Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. (2007) 13(8):599–608. doi: 10.1016/j.cardfail.2007.04.008
6. Ta-Shma A, Khan TN, Vivante A, Willer JR, Matak P, Jalas C, et al. Mutations in TMEM260 cause a pediatric neurodevelopmental, cardiac, and renal syndrome. Am J Hum Genet. (2017) 100(4):666–75. doi: 10.1016/j.ajhg.2017.02.007
7. Roik M, Starczewska MH, Stawicki S, Solarska-Półchłopek A, Warszawik O, Oreziak A, et al. The prognostic value of renal dysfunction in patients with chronic heart failure: 12-month follow-up. Kardiol Pol. (2006) 64(7):704–11. discussion 12.16886127
8. Correction to: 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. (2016) 134(13):e298. doi: 10.1161/CIR.0000000000000435
9. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. (2010) 31(6):703–11. doi: 10.1093/eurheartj/ehp507
10. Givertz MM, Teerlink JR, Albert NM, Westlake Canary CA, Collins SP, Colvin-Adams M, et al. Acute decompensated heart failure: update on new and emerging evidence and directions for future research. J Card Fail. (2013) 19(6):371–89. doi: 10.1016/j.cardfail.2013.04.002
11. Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. Br Med J. (2015) 351:h4937. doi: 10.1136/bmj.h4937
12. Correction to: 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2023) 147(14):e674. doi: 10.1161/CIR.0000000000001063
13. Emmens JE, Ter Maaten JM, Matsue Y, Figarska SM, Sama IE, Cotter G, et al. Worsening renal function in acute heart failure in the context of diuretic response. Eur J Heart Fail. (2022) 24(2):365–74. doi: 10.1002/ejhf.2384
14. Llauger L, Jacob J, Miró Ò. Renal function and acute heart failure outcome. Med Clin. (2018) 151(7):281–90. doi: 10.1016/j.medcli.2018.05.010
15. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. (2011) 365(1):32–43. doi: 10.1056/NEJMoa1100171
16. Nishikawa R, Kato T, Morimoto T, Yaku H, Inuzuka Y, Tamaki Y, et al. The characteristics and outcomes in patients with acute heart failure who used tolvaptan: from KCHF registry. ESC Heart Fail. (2023) 10(5):3141–51. doi: 10.1002/ehf2.14494
17. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. (2011) 14(4):429–37. doi: 10.1016/j.jval.2011.01.011
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
19. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. (1971) 285(26):1441–6. doi: 10.1056/NEJM197112232852601
20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. (1999) 130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002
21. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. (2003) 139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013
22. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
23. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. (1998) 7(4):434–55. doi: 10.1080/10618600.1998.10474787
24. Dempster AP. The direct use of likelihood for significance testing. Stat Comput. (1997) 7(4):247–52. doi: 10.1023/A:1018598421607
25. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10(10):Ed000142. doi: 10.1002/14651858.ED000142
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Declaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas. Rev Panam Salud Publica. (2022) 46:e112. doi: 10.26633/RPSP.2022.112
27. Wan SH, Stevens SR, Borlaug BA, Anstrom KJ, Deswal A, Felker GM, et al. Differential response to low-dose dopamine or low-dose nesiritide in acute heart failure with reduced or preserved ejection fraction: results from the ROSE AHF trial (renal optimization strategies evaluation in acute heart failure). Circ Heart Fail. (2016) 9(8):e002593. doi: 10.1161/CIRCHEARTFAILURE.115.002593
28. Greene SJ, Felker GM, Giczewska A, Kalogeropoulos AP, Ambrosy AP, Chakraborty H, et al. Spironolactone in acute heart failure patients with renal dysfunction and risk factors for diuretic resistance: from the ATHENA-HF trial. Can J Cardiol. (2019) 35(9):1097–105. doi: 10.1016/j.cjca.2019.01.022
29. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. (2013) 310(23):2533–43. doi: 10.1001/jama.2013.282190
30. Vaduganathan M, Gheorghiade M, Pang PS, Konstam MA, Zannad F, Swedberg K, et al. Efficacy of oral tolvaptan in acute heart failure patients with hypotension and renal impairment. J Cardiovasc Med. (2012) 13(7):415–22. doi: 10.2459/JCM.0b013e328355a740
31. Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail. (2010) 16(12):922–30. doi: 10.1016/j.cardfail.2010.07.246
32. Fedele F, Bruno N, Brasolin B, Caira C, D'Ambrosi A, Mancone M. Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail. (2014) 16(3):281–8. doi: 10.1002/ejhf.9
33. Rafouli-Stergiou P, Parissis JT, Farmakis D, Bistola V, Frogoudaki A, Vasiliadis K, et al. Effects of levosimendan on markers of kidney function in patients with acutely decompensated heart failure and renal impairment. J Cardiovasc Med. (2017) 18(10):771–3. doi: 10.2459/JCM.0000000000000244
34. Uemura Y, Shibata R, Takemoto K, Uchikawa T, Koyasu M, Ishikawa S, et al. Clinical benefit of tolvaptan in patients with acute decompensated heart failure and chronic kidney disease. Heart Vessels. (2016) 31(10):1643–9. doi: 10.1007/s00380-015-0775-9
35. Tamaki S, Sato Y, Yamada T, Morita T, Furukawa Y, Iwasaki Y, et al. Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure and preserved left ventricular ejection fraction- prospective randomized controlled study. Circ J. (2017) 81(5):740–7. doi: 10.1253/circj.CJ-16-1122
36. Ono Y, Takamatsu H, Inoue M, Mabuchi Y, Ueda T, Suzuki T, et al. Clinical effect of long-term administration of tolvaptan in patients with heart failure and chronic kidney disease. Drug Discov Ther. (2018) 12(3):154–60. doi: 10.5582/ddt.2018.01007
37. John B, Babu M, Shaji S, Abraham S, Abdullakutty J. Clinical outcomes of levosimendan versus dobutamine in patients with acute decompensated heart failure with reduced ejection fraction and impaired renal function. Indian Heart J. (2021) 73(3):372–5. doi: 10.1016/j.ihj.2021.02.010
38. Matsue Y, Suzuki M, Seya M, Iwatsuka R, Mizukami A, Nagahori W, et al. Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure in high-risk population. J Cardiol. (2013) 61(2):169–74. doi: 10.1016/j.jjcc.2012.08.020
39. Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, et al. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail. (2016) 22(6):423–32. doi: 10.1016/j.cardfail.2016.02.007
40. Nalcacioglu H, Ozkaya O, Kafali HC, Tekcan D, Avci B, Baysal K. Is N-terminal pro-brain natriuretic peptide a reliable marker for body fluid status in children with chronic kidney disease? Arch Med Sci. (2020) 16(4):802–10. doi: 10.5114/aoms.2019.85460
41. Boeddinghaus J, Nestelberger T, Twerenbold R, Koechlin L, Meier M, Troester V, et al. High-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin Chem. (2019) 65(7):893–904. doi: 10.1373/clinchem.2018.300061
42. Khand AU, Backus B, Campbell M, Frost F, Mullen L, Fisher M, et al. HEART score recalibration using higher sensitivity troponin T. Ann Emerg Med. (2023) 82(4):449–62. doi: 10.1016/j.annemergmed.2023.04.024
43. Kim SE, Cho DH, Son JW, Kim JY, Kang SM, Cho MC, et al. Impact of NT-proBNP on prognosis of acute decompensated chronic heart failure versus de novo heart failure. Int J Cardiol. (2022) 363:163–70. doi: 10.1016/j.ijcard.2022.06.055
44. Baba M, Yoshida K, Ieda M. Clinical applications of natriuretic peptides in heart failure and atrial fibrillation. Int J Mol Sci. (2019) 20(11):2824. doi: 10.3390/ijms20112824
45. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. (2011) 364(9):797–805. doi: 10.1056/NEJMoa1005419
46. Kapelios CJ, Bonou M, Vogiatzi P, Tzanis G, Mantzouratou P, Lund LH, et al. Association between high-dose spironolactone and decongestion in patients with acute heart failure: an observational retrospective study. Am J Cardiovasc Drugs. (2018) 18(5):415–22. doi: 10.1007/s40256-018-0290-3
47. Dass B, Beaver TM, Shimada M, Alquadan KF, Koratala A, Singhania G, et al. Natriuretic peptides in acute kidney injury—a sojourn on parallel tracks? Eur J Intern Med. (2020) 71:39–44. doi: 10.1016/j.ejim.2019.11.025
48. Zhao D, Gu L, Wei W, Peng D, Yang M, Yuan W, et al. Impact of the degree of worsening renal function and B-type natriuretic peptide on the prognosis of patients with acute heart failure. Front Cardiovasc Med. (2023) 10:1103813. doi: 10.3389/fcvm.2023.1103813
49. Cui D, Liao Y, Li G, Chen Y. Levosimendan can improve the level of B-type natriuretic peptide and the left ventricular ejection fraction of patients with advanced heart failure: a meta-analysis of randomized controlled trials. Am J Cardiovasc Drugs. (2021) 21(1):73–81. doi: 10.1007/s40256-020-00416-y
50. Heringlake M, Alvarez J, Bettex D, Bouchez S, Fruhwald S, Girardis M, et al. An update on levosimendan in acute cardiac care: applications and recommendations for optimal efficacy and safety. Expert Rev Cardiovasc Ther. (2021) 19(4):325–35. doi: 10.1080/14779072.2021.1905520
51. Agostoni P, Farmakis DT, García-Pinilla JM, Harjola VP, Karason K, von Lewinski D, et al. Haemodynamic balance in acute and advanced heart failure: an expert perspective on the role of levosimendan. Card Fail Rev. (2019) 5(3):155–61. doi: 10.15420/cfr.2019.01.R1
52. Robertson IM, Pineda-Sanabria SE, Yan Z, Kampourakis T, Sun YB, Sykes BD, et al. Reversible covalent binding to cardiac troponin C by the Ca(2+)-sensitizer levosimendan. Biochemistry. (2016) 55(43):6032–45. doi: 10.1021/acs.biochem.6b00758
53. Parissis JT, Adamopoulos S, Farmakis D, Filippatos G, Paraskevaidis I, Panou F, et al. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart. (2006) 92(12):1768–72. doi: 10.1136/hrt.2005.079707
54. Niu ZZ, Wu SM, Sun WY, Hou WM, Chi YF. Perioperative levosimendan therapy is associated with a lower incidence of acute kidney injury after cardiac surgery: a meta-analysis. J Cardiovasc Pharmacol. (2014) 63(2):107–12. doi: 10.1097/FJC.0000000000000028
55. Tholén M, Ricksten SE, Lannemyr L. Effects of levosimendan on renal blood flow and glomerular filtration in patients with acute kidney injury after cardiac surgery: a double blind, randomized placebo-controlled study. Crit Care. (2021) 25(1):207. doi: 10.1186/s13054-021-03628-z
56. Asif M. A review on role of the calcium sensitive inotropic agent, levosimendan and its metabolites. Mini Rev Med Chem. (2018) 18(16):1354–62. doi: 10.2174/1389557516666160905094721
57. Farmakis D, Alvarez J, Gal TB, Brito D, Fedele F, Fonseca C, et al. Levosimendan beyond inotropy and acute heart failure: evidence of pleiotropic effects on the heart and other organs: an expert panel position paper. Int J Cardiol. (2016) 222:303–12. doi: 10.1016/j.ijcard.2016.07.202
58. Qiang H, Luo X, Huo JH, Wang ZQ. Perioperative use of levosimendan improves clinical outcomes in patients after cardiac surgery: a systematic review and meta-analysis. J Cardiovasc Pharmacol. (2018) 72(1):11–8. doi: 10.1097/FJC.0000000000000584
59. McLachlan CS, Mossop P. Levosimendan and plasma BNP levels: do inflammatory cytokines regulate BNP in chronic decompensated heart failure? Eur J Heart Fail. (2006) 8(2):216–7. author reply 8. doi: 10.1016/j.ejheart.2006.01.004
60. Yilmaz MB, Grossini E, Silva Cardoso JC, Édes I, Fedele F, Pollesello P, et al. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther. (2013) 27(6):581–90. doi: 10.1007/s10557-013-6485-6
61. Zager RA, Johnson AC, Lund S, Hanson SY, Abrass CK. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol. (2006) 290(6):F1453–62. doi: 10.1152/ajprenal.00485.2005
62. Morelli A, De Castro S, Teboul JL, Singer M, Rocco M, Conti G, et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. (2005) 31(5):638–44. doi: 10.1007/s00134-005-2619-z
63. Papp Z, Édes I, Fruhwald S, De Hert SG, Salmenperä M, Leppikangas H, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. (2012) 159(2):82–7. doi: 10.1016/j.ijcard.2011.07.022
64. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. (2009) 53(7):589–96. doi: 10.1016/j.jacc.2008.05.068
65. Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. (2012) 60(12):1031–42. doi: 10.1016/j.jacc.2012.01.077
66. Abdel-Qadir HM, Tu JV, Yun L, Austin PC, Newton GE, Lee DS. Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. Am Heart J. (2010) 160(2):264–71.e1. doi: 10.1016/j.ahj.2010.05.032
67. Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. (2004) 147(2):331–8. doi: 10.1016/j.ahj.2003.08.012
68. Yin C, Li J, Yuan L. Comment on: benefits of tolvaptan on early dyspnea relief in patients with acute heart failure: a meta-analysis. Clin Cardiol. (2023) 46(5):584. doi: 10.1002/clc.23994
69. Patoulias D, Papadopoulos C, Zografou I, Doumas M. Acute heart failure, type 2 diabetes and loop diuretic use: any adjunct role for sodium-glucose cotransporter-2 inhibitors? J Cardiovasc Med. (2020) 21(4):343. doi: 10.2459/JCM.0000000000000938
70. Kosiborod MN, Vaduganathan M. SGLT-2 inhibitors in heart failure: volume or value? J Am Coll Cardiol. (2021) 77(11):1393–6. doi: 10.1016/j.jacc.2021.02.005
71. Mavrakanas TA, Tsoukas MA, Brophy JM, Sharma A, Gariani K. SGLT-2 inhibitors improve cardiovascular and renal outcomes in patients with CKD: a systematic review and meta-analysis. Sci Rep. (2023) 13(1):15922. doi: 10.1038/s41598-023-42989-z
72. van Deursen VM, Hernandez AF, Stebbins A, Hasselblad V, Ezekowitz JA, Califf RM, et al. Nesiritide, renal function, and associated outcomes during hospitalization for acute decompensated heart failure: results from the acute study of clinical effectiveness of nesiritide and decompensated heart failure (ASCEND-HF). Circulation. (2014) 130(12):958–65. doi: 10.1161/CIRCULATIONAHA.113.003046
73. Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. (2005) 293(15):1900–5. doi: 10.1001/jama.293.15.1900
74. Abraham WT, Trupp RJ, Jarjoura D. Nesiritide in acute decompensated heart failure: a pooled analysis of randomized controlled trials. Clin Cardiol. (2010) 33(8):484–9. doi: 10.1002/clc.20793
75. Yan B, Peng L, Zhao X, Chung H, Li L, Zeng L, et al. Nesiritide fails to reduce the mortality of patients with acute decompensated heart failure: an updated systematic review and cumulative meta-analysis. Int J Cardiol. (2014) 177(2):505–9. doi: 10.1016/j.ijcard.2014.08.078
76. Xiong B, Wang C, Yao Y, Huang Y, Tan J, Cao Y, et al. The dose-dependent effect of nesiritide on renal function in patients with acute decompensated heart failure: a systematic review and meta-analysis of randomized controlled trials. PLoS One. (2015) 10(6):e0131326. doi: 10.1371/journal.pone.0131326
Keywords: acute heart failure, renal insufficiency, diuretics, high dose of diuretics, network meta-analysis
Citation: Lv Q, Wu Q, Yang Y, Li L, Ye X, Wang S, Lv Y, Wang M and Li Y (2025) Comparative efficacy of different drugs in acute heart failure with renal dysfunction: a systematic review and network meta-analysis. Front. Cardiovasc. Med. 11:1444068. doi: 10.3389/fcvm.2024.1444068
Received: 23 July 2024; Accepted: 27 December 2024;
Published: 14 January 2025.
Edited by:
Xiaoyue Pan, New York University, United StatesReviewed by:
Heyue Du, TidalHealth, United StatesNikhil Agrawal, University of Texas Health Science Center at Houston, United States
Andreas J. Rieth, Kerckhoff Clinic, Germany
Copyright: © 2025 Lv, Wu, Yang, Li, Ye, Wang, Lv, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihan Wang, d2FuZ3NoaWhhbjkxQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Qianyu Lv
Qianyu Lv Qian Wu
Qian Wu Yingtian Yang
Yingtian Yang Lanlan Li
Lanlan Li Xuejiao Ye
Xuejiao Ye Shihan Wang
Shihan Wang Yanfei Lv2
Yanfei Lv2 Manshi Wang
Manshi Wang