- 1Department of Vascular Anomaly, Fengcheng Hospital of Fengxian District, Shanghai, China
- 2Department of Interventional Therapy, Multidisciplinary Team of Vascular Anomalies, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Anesthesiology, Fengcheng Hospital of Fengxian District, Shanghai, China
Objective: This review aims to summarize the salvage experience of cardiopulmonary collapse occurring as a result of absolute ethanol sclerotherapy for vascular malformations.
Methods: In total, we reviewed three cases of cardiopulmonary collapse induced by ethanol sclerotherapy for vascular malformations and described the details of the salvage procedure. Saturation of pulse oxygen (SpO2), end-tidal CO2, and invasive arterial pressure were the routine monitors for ethanol injection patients. Cardiopulmonary resuscitation, epinephrine, norepinephrine, and deoxyepinephrine were mainly used to correct circulation parameters. Manually ventilated via endotracheal intubation with 100% O2, increased respiratory rate were mainly used to correct Respiratory parameters.
Results: All three cases were successfully salvaged without major complications. When cardiopulmonary collapse occurred, manual ventilation via endotracheal intubation with 100% O2, increased ventilation frequency and external cardiac compression were the emergency treatments. Epinephrine, norepinephrine, deoxyepinephrine infusion solely or combined were crucial to maintaining the basic vital signs.
Conclusion: Despite the severity of cardiopulmonary collapse caused by ethanol sclerotherapy, it can be detected by close observation and reversed with timely treatment.
Introduction
Vascular malformations are clinically problematic and often difficult lesions to treat. They display unique heterogeneous manifestations and can occur anywhere in the body. In the past decades, embolotherapy has been a mainstay of treatment, with many clinical studies demonstrating its varying degrees of efficacy. Nonetheless, although methods and materials for sclerotherapy are extensively varied and heavily studied, there is no clear consensus on the suitable embolic agents for various malformations (1–3). Yakes et al. first described the use of ethanol in malformation sclerotherapy in 1986 (4). Since then, ethanol has been increasingly used in high flows (AVMs) and lower flow venous, capillary, and lymphatic malformations (5).
Despite its high efficacy, ethanol is an extremely dangerous intravascular sclerotherapy agent, causing significant complications if it enters the systemic circulation. Ethanol sclerotherapy induces minor local complications including skin blistering, ulcerations, scar formation, and local nerve damage. Moreover, it may cause cardiopulmonary complications such as pulmonary embolism, pulmonary hypertension, and cardiac arrhythmias that potentially trigger cardiovascular collapse or even death (6–9). Thus, radiologists and anesthetists must be aware of the severity potential of these cardiopulmonary complications triggered by ethanol sclerotherapy, hence must be prepared to manage. Herein, we review three cases of cardiopulmonary collapse induced by ethanol sclerotherapy for vascular malformations and its periprocedural management.
Patients
The present work was approved by the Institutional Review Board of Shanghai Ninth People's Hospital, Shanghai Jiaotong University School of Medicine [No. SH9H-2019-T309-2]; All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Consent for publication was obtained for every individual person's data included in the study.
Case 1
A 32-year-old female patient with a height of 166 cm and weight of 58 kg was admitted to undergo ethanol sclerotherapy for a large venous malformation in the left waist. All preoperative examinations, laboratory tests, electrocardiography, and chest x-ray were normal. General anesthesia was intravenously induced using a bolus injection of 120 mg propofol and 40 mg rocuronium. Endotracheal intubation was inserted without difficulty and the patient was maintained using machine-controlled ventilation (tidal volume 300 ml, ventilation frequency 16 times per minute) on 68% nitrous oxide in oxygen with isoflurane at end-tidal concentrations of 1%–1.5%. Also, she was intravenously administered with 8 mg of atracurium benzenesulfonate. We applied routine patient monitors, including pulse oxygen saturation (SpO2), three-lead electrocardiogram (ECG), end-tidal CO2, and invasive arterial pressure. After venography from direct puncture, absolute ethanol injection was routinely performed (10). Immediately after ethanol injection, the blood pressure and heart rate of the patient slightly increased to 135/90 mmHg and 90 beats/min, respectively. Thereafter, blood pressure and heart rate became stable throughout the procedure. A 22 ml dose of 99.7% ethanol was injected into the lesion for over 15 min. Consequently, we noted an abrupt decrease in her invasive arterial pressure; 50–55 mmHg systolic and 20–22 mmHg diastolic blood pressure with a simultaneous decrease of heart rate to 35–38 beats/min; end-tidal CO2 decrease to 19–21 mmHg; SpO2 decrease to 55%. Within 60 s of onset, peripheral pulses were lost and invasive arterial pressure failed to yield a measurement. The ECG (lead II) showed that the heart rate first becomes slightly fast and then slows down, followed by severe sinus bradycardia with escape rhythm, and finally ventricular fibrillation until cardiac arrest. The anesthetic agents were discontinued then the oxygen concentration in ventilation was increased to under 100% and manually ventilated via endotracheal intubation 30 times per minute. External cardiac compression was started with resultant palpable femoral pulsation. Epinephrine 1 mg was given intravenously and chest compression continued for 3 min before the pulses were restored. Blood pressure increased to 50/30 mmHg, SpO2 to 65%, heart rate to 55 beats/min. Subsequently, norepinephrine infusion was administered at 20 μg/2 min and repeated twice; as a result, blood pressure increased to 75/45 mmHg; SpO2 to 86%; heart rate to 135 beats/min. Deoxyepinephrine infusion was administered at 10 μg/2 min and repeated twice; consequently, blood pressure increased to 93/55 mmHg; SpO2 to 96%; heart rate decreased to 82 beats/min. After 50 min in the operating theater, the patient regained consciousness, began to spontaneously breathe, and obeyed commands. After removing the endotracheal tube, the patient was transferred to the intensive care unit. Echocardiography follow-up during the next day showed a normal heart with efficient biventricular function. After 3 days of observation, the patient completely recovered and was discharged.
Case 2
A 28-year-old male patient with a height of 175 cm and weight of 68 kg was admitted to undergo ethanol sclerotherapy for venous malformation in the left back. Preoperative examinations, including endotracheal intubation, general anesthesia, absolute ethanol injection was routinely performed.
The cardiopulmonary collapse occurred in a total dose of 25 ml of 99.7% ethanol was injected into the lesion over 20 min. We observed an abrupt decrease in her invasive arterial pressure, 50–60 mmHg systolic and 18–20 mmHg diastolic blood pressure with a simultaneous decrease in her heart rate to 22–36 beats/min, end-tidal CO2 decrease to 20–23 mmHg, and SpO2 decrease to 56%. The anesthetic agents were discontinued then under and manually ventilated via endotracheal intubation with 100% O2 at 30 times per minute. Norepinephrine infusion was administered at 20 μg/2 min and repeated twice; consequently, the blood pressure increased to 94/65 mmHg, SpO2 to 92%, and heart rate to 125 beats/min. Then, deoxyepinephrine infusion was administered at 10 μg/2 min and repeated once; consequently, blood pressure increased to 105/68 mmHg, SpO2 to 98%, while heart rate decreased to 82 beats/min. After 35 min in the operating theater, the patient regained consciousness, began to spontaneously breathe, and obeyed commands. The endotracheal tube was removed and the patient completely recovered.
Case 3
A 12-year-old female patient with a height of 152 cm and weight of 46 kg was admitted to undergo ethanol sclerotherapy for venous malformation in the lateral chest. Endotracheal intubation general anesthesia and absolute ethanol injection were routinely performed.
The cardiopulmonary collapse occurred in a total dose of 20 ml of 99.7% ethanol was injected into the lesion over 18 min. We observed an abrupt decrease in her invasive arterial pressure, 45–52 mmHg systolic and 16–20 mmHg diastolic blood pressure with a simultaneous decrease in her heart rate to 25–35 beats/min, end-tidal CO2 decrease to 20–22 mmHg, and SpO2 decrease to 55%. The anesthetic agents were discontinued then the patient was manually ventilated via endotracheal intubation with 100% O2, 25 times per minute. Norepinephrine infusion was administered at 6 μg; consequently, blood pressure increased to 100/70 mmHg, SpO2 to 91%, heart rate to 145 beats/min. Deoxyepinephrine infusion was administered at 6 μg/2 min and repeated once; consequently, blood pressure increased to 115/75 mmHg, SpO2 to 96%, whereas heart rate decreased to 75 beats/min. After 45 min in the operating theater, the patient regained consciousness, began to spontaneously breathe, and obeyed commands. The endotracheal tube was removed and the patient completely recovered.
Clinical outcomes
All three cases were successfully salvaged without major complications. When cardiopulmonary collapse occurred, manual ventilation via endotracheal intubation with 100% O2, increased ventilation frequency and external cardiac compression were the emergency treatments. Epinephrine, norepinephrine, deoxyepinephrine infusion solely or combined were crucial to maintaining the basic vital signs. The clinical data and results of 3 Patients with cardiopulmonary collapse were summarized in Table 1. The steps in salvage of cardiopulmonary collapse induced by ethanol sclerotherapy for vascular malformations was described in Figure 1.
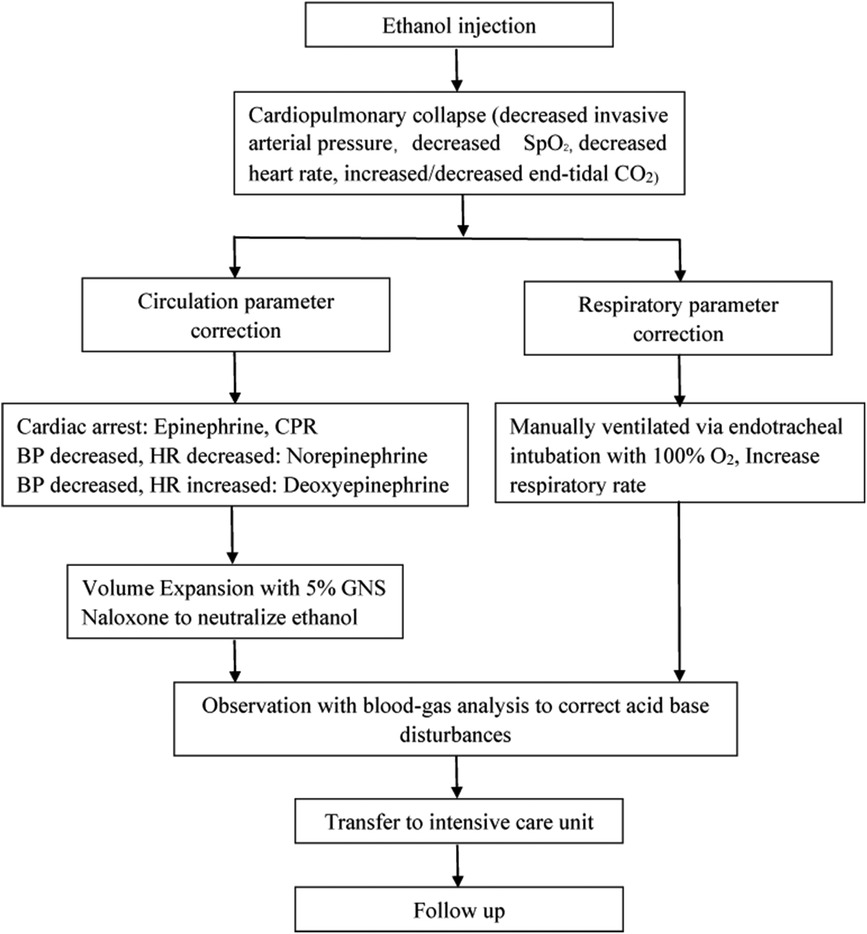
Figure 1. Steps in salvage of cardiopulmonary collapse induced by ethanol sclerotherapy for vascular malformations.
Discussion
Numerous sclerosants have been developed for the treatment of vascular malformations. Among these, absolute ethanol is preferred to other embolic agents due to its relatively desired outcomes and minimal rate of recanalization. The injected ethanol in vascular malformations causes direct tissue toxicity leading to endothelial damage, severe vascular spasm, proteins denudation. Despite its benefits, ethanol sclerotherapy often produces minor local complications, such as tissue necrosis, peripheral nerve injuries, skin blistering, and ulcers. Besides, if absorbed into the systemic circulation, although rare, ethanol may cause fatal complications, including pulmonary embolism and pulmonary vasospasm. Because of changes in the cardiac conduction system, these complications may trigger right heart failure, cardiac arrhythmias, and even death in severe cases (7–9, 11, 12).
Systemic contamination with ethanol occurring during percutaneous sclerotherapy is directly related to the dose injected and independent of the vascular malformation morphology, venous drainage, or injection technique (13, 14). Theoretically, with the fast flow rate of arteriovenous malformations, absolute ethanol enters the systemic circulation more easily through the fistula compared to that of venous malformations, thereby increasing the incidence of cardiopulmonary accidents. This phenomenon has not been reported in clinical practice. All three cases in this work were venous malformations. The reasons were speculated as follows: First, doctors were more cautious about the speed of absolute ethanol injection in the treatment of arteriovenous malformations due to its high flow characters. Secondly, dominant outflow vein embolization with coils or compression was often used to control the flow speed of arteriovenous malformations. The rate of absolute ethanol entering the systemic circulation was highly decreased. Thirdly, doctors experienced in using absolute ethanol to treat vascular malformations should observe the fast flow rate of arteriovenous malformations. Absolute ethanol is more likely to be washed into the systemic circulation and diluted during the injection of absolute ethanol into arteriovenous malformations. it is not as easy to produce coagulation and hemolysis when treated venous malformations. Thus, micro-thrombosis and hemolysis may have less stimulation to pulmonary vessels.
Although the theory of pulmonary hypertension is not fully clear, acute pulmonary hypertension causes cardiopulmonary collapse. Based on previous studies, ethanol injection induces severe pulmonary vasospasm, acutely increases thin-walled right ventricular afterload, and decreases the right ventricular cardiac output. Besides, these effects can be further aggravated by systemic effects of alcohol affecting right atrial and ventricular contractility, including chronotropic and inotropic functions (15, 16). Pulmonary emboli due to ethanol-induced thrombosis may have also contributed but appeared less likely considering the rapid response of the patient to resuscitation. Ethanol and blood interaction produces embolic debris comprising denatured protein and cellular fragments described as “Sludge” by Yata (17). The thrombus produced by absolute ethanol injection is mostly micro-embolism, which will not produce pulmonary embolism as severe as deep venous thrombosis of the lower extremity. Nevertheless, the influence of micro-embolism may be cumulative. This may be the reason there is a dose limit in ethanol injection therapy.
Furthermore, ethanol-induced hemolysis releases erythrocyte arginase, which hydrolyses L-arginine to L-ornithine. Notably, L-Arginine is the precursor for nitric oxide (NO) production via NO synthase in the vascular endothelium. The combination of decreased production and increased inactivation of NO tips the vascular balance toward vasoconstriction (18). Kielstein et al. investigated the relationship between percutaneous ethanol injection, pulmonary hypertension, and markers of altered NO metabolism. Specifically, ethanol, free hemoglobin, plasma nitrite, and L-arginine levels were analyzed before and after percutaneous ethanol injection treatment (19). NO is effective in both primary pulmonary hypertension and secondary pulmonary hypertension (SPH), of which ethanol-induced type is SPH (20). In Case #1, our anesthesiologist used 68% NO. The use of NO in anesthesia uses the secondary gas effect to increase the alveolar concentration of inhaled anesthetics and thus deepen the depth of anesthesia as quickly as possible. At the same time, NO can dilate pulmonary vessels and reduce pulmonary vascular resistance, so as to reduce pulmonary artery pressure, but has no effect on systemic pressure. However, if the concentration of NO in the inhaled gas is too high, it may lead to insufficient oxygen inhalation resulting in hypoxia, so the concentration of NO used clinically does not exceed 60%–70%. When rescuing patients, NO inhalation will be stopped, and 100% oxygen inhalation will be changed to improve the inhaled oxygen concentration and increase oxygen supply.
NO has little to zero effect on the peripheral vasculature and is very pulmonary specific so is the perfect agent in that cardiopulmonary collapse critical clinical situation and is administered quickly to act through the in-dwelling endotracheal tube.
In cardiopulmonary collapse the circulation is very sluggish (and may have zero cardiac output) and the circulatory system is severely compromised due to the significantly decreased left-heart filling and right heart overload. Also it could be stated that if any IV drugs are considered for vasodilation, the immediate placement of a catheter in the main Pulmonary Artery (PA) to administer drugs IV into the lungs would then facilitate drug placement to get where it was needed more expeditiously. However, placing a catheter in the main PA does take time to do and is a delay, whereas endotracheal NO is immediate without any additional catheter procedures. Further, placing a catheter in the main pulmonary artery can aggravate the cardiac conduction system and invoke an aggravating arrhythmia further complicating and adversely affecting this already critical situation.
Ethanol injection procedures are performed when the patient is intubated and under general anesthesia for numerous reasons, including severe pain during injection, the need for cessation of respiration during angiographic sequences, and the possibility of cardiovascular decompensation. Also, alcohol severely affects the heart. Studies have reported cardiopulmonary collapse among adults during ethanol sclerotherapy presumably related to the suppression of the myocardial conduction system with precapillary vasoconstriction and resultant elevation in pulmonary arterial pressures (21–24). In the present three cases, we observed an increase in airway resistance, a decrease in blood SpO2, and an increase in end-tidal carbon dioxide during the early stages of cardiopulmonary collapse. This implies that pulmonary factors may cause impairment of cardiopulmonary circulation. The proposed physiologic sequence for cardiovascular collapse as following steps: (1) A bolus of ethanol flows into the pulmonary vasculature. (2) If enough of a bolus, it causes diffuse severe pulmonary artery spasm. (3) This then restricts right heart outflow to the left heart, leading to right heart overload, then right heart failure. (4) This then results in decreased left heart filling with oxygenated blood, leading to blood pressure dropping. (5) Significantly decreased left heart filling and collapsing blood pressure, leads to coronary artery hypoperfusion, then cardiac ischemia, and then resultant arrhythmias, usually Electro-Mechanical Dissociation (EMD) which is organized electrical depolarization of the heart without synchronous myocardial fiber shortening (no myocardial contractures), and then there is no cardiac output. A similar phenomenon was also reported by Naik (6).
Invasive arterial pressure monitor provides accurate, reliable, and continuous arterial pressure value, and establishes the status of the blood volume or the heart. The slight increase in blood pressure among patients during ethanol sclerotherapy related to pain caused by sympathetic stimulation, even when patients are under general anesthesia. For the prevention of cardiopulmonary accidents, the observation of the decrease of invasive arterial pressure has more clinical significance. A rapid decrease in blood pressure indicates the possibility of cardiopulmonary impairment. This should be timely corrected to avoid cardiac arrest accompanied by a further decrease in blood pressure.
In the ethanol injection cases monitored using the Swan-Ganz catheter, nimodipine is administered once the pulmonary artery pressure has increased. This can quickly relieve the pulmonary artery pressure (25). Due to the high cost and the technique reason, Swan-Ganz catheter was not regularly be used by absolute ethanol sclerotherapy, especially for low flow vascular malformation. In our study, the three patients had a significant decrease in blood SpO2, peripheral blood pressure, and heart rate. Thus, the mechanisms of immediately improving the ventilation exchange function and maintaining the stability of basic vital signs are vital for cardiopulmonary resuscitation. Absolute ethanol injection was immediately stopped once blood SpO2 decreases and airway resistance increases. It should be noted that patients can be apneic during general anesthesia and minutes can pass before O2 levels drop. The critical factor to monitor is CO2 levels which are the very first to drop way before O2 levels drop. CO2 level is the first measurable parameter to drop physiologically because it measures the oxygen-carbon dioxide exchange at the alveolus and capillaries. O2 goes into the red blood cells to oxygenate the blood. CO2 is excreted into the alveolus and exhaled. The CO2 monitor reveals the CO2 level excreted. If there is no oxygen exchange occurring at the alveolus, there is no CO2 released for excretion and for measurement. Thus, once CO2 levels begin to fall, action must be taken then, not when O2 levels later drop which may be harder to correct. Besides, the anesthetic agents were discontinued and the patient was placed under 100% O2 and manually ventilated via endotracheal intubation to prevent further deterioration of cardiopulmonary impairment. Laryngeal Mask Airway was not recommended for general anesthesia for ethanol injection, which could not provide sufficient pressure when necessary.
Several types of drugs are used to increase blood pressure, including epinephrine, norepinephrine, deoxyepinephrine, dopamine, etc. Each drug has a different pharmacological effect and mechanism thus, requiring the guidance of professional anesthesiologists and physicians. Epinephrine is primarily used for the treatment of patients with acute allergy and cardiac arrest. On the other hand, norepinephrine is majorly used for the emergency treatment of patients with severe blood pressure reduction and heart rate reduction. Deoxyepinephrine is primarily used to raise blood pressure. Unlike norepinephrine, the effect of deoxyepinephrine in increasing heart rate is softer and used for blood pressure maintenance after the recovery of basic vital signs. A reasonable combination of various pressure-raising drugs to maintain the basic vital signs was crucial to the successful management of cardiopulmonary accidents caused by absolute ethanol sclerotherapy.
Conclusion
In conclusion, we reported three patients successfully salvaged from cardiovascular collapse potentially attributed to ethanol sclerotherapy. Despite its severity, the resulting complications can be reversed with timely treatment, which indicated our salvage techniques were effective in the adult and pediatric age groups. We recommend that anesthetists should continuously focus on the changes of airway pressure, SpO2, tidal carbon dioxide, and invasive arterial pressure during the procedure and in the recovery room to prevent severe cardiovascular complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by IRB of Shanghai Ninth People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LS: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YZ: Formal Analysis, Investigation, Resources, Writing – original draft. YS: Writing – review & editing. XF: Project administration, Supervision, Writing – review & editing. ZW: Validation, Visualization, Writing – review & editing. DW: Data curation, Methodology, Software, Writing – review & editing. QL: Conceptualization, Investigation, Methodology, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Health Clinical Research Project of Shanghai Municipal Health Commission [No. 202140425].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XL declared a shared affiliation with the author(s) to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yakes WF. Use of multiple sclerosant agents in vascular malformation management: a world in endovascular confusion and chaos. J Vasc Interv Radiol. (2015) 26(10):1494–6. doi: 10.1016/j.jvir.2015.06.002
2. Alsuwailem A, Myer CM, Chaudry G. Vascular anomalies of the head and neck. Semin Pediatr Surg. (2020) 29(5):150968. doi: 10.1016/j.sempedsurg.2020.150968
3. Cho SK, Do YS, Kim DI, Kim YW, Shin SW, Park KB, et al. Peripheral arteriovenous malformations with a dominant outflow vein: results of ethanol embolization. Korean J Radiol. (2008) 9(3):258–67. doi: 10.3348/kjr.2008.9.3.258
4. Yakes WF, Pevsner P, Reed M, Donohue HJ, Ghaed N. Serial embolizations of an extremity arteriovenous malformation with alcohol via direct percutaneous puncture. AJR Am J Roentgenol. (1986) 146(5):1038–40. doi: 10.2214/ajr.146.5.1038
5. Do YS, Yakes WF, Shin SW, Lee BB, Kim DI, Liu WC, et al. Ethanol embolization of arteriovenous malformations: interim results. Radiology. (2005) 235(2):674–82. doi: 10.1148/radiol.2352040449
6. Naik B, Matsumoto AH. Acute cor pulmonale and right heat failure complicating ethanol ablative therapy: anesthetic and radiologic considerations and management. Cardiovasc Intervent Radiol. (2013) 36(5):1213–20. doi: 10.1007/s00270-013-0549-7
7. Chapot R, Laurent A, Enjolras O, Payen D, Houdart E. Fatal cardiovascular collapse during ethanol sclerotherapy of a venous malformation. Interv Neuroradiol. (2002) 8(3):321–4. doi: 10.1177/159101990200800314
8. Mitchell SE, Shah AM, Schwengel D. Pulmonary artery pressure changes during ethanol embolization procedures to treat vascular malformations: can cardiovascular collapse be predicted? J Vasc Interv Radiol. (2006) 17(2 Pt 1):253–62. doi: 10.1097/01.RVI.0000196273.82991.03
9. Shin BS, Do YS, Cho HS, Hahm TS, Kim CS, Sim WS, et al. Cardiovascular effects and predictability of cardiovascular collapse after repeated intravenous bolus injections of absolute ethanol in anesthetized pigs. J Vasc Interv Radiol. (2010) 21(12):1867–72. doi: 10.1016/j.jvir.2010.08.007
10. Wang D, Su L, Han Y, Wang Z, Zheng L, Li J, et al. Direct intralesional ethanol sclerotherapy of extensive venous malformations with oropharyngeal involvement after a temporary tracheotomy in the head and neck: initial results. Head Neck. (2017) 39(2):288–96. doi: 10.1002/hed.24588
11. Cai MH, Su LX, Qiu L. Cardiovascular depression and intravascular hemolysis secondary to ethanol injection for arteriovenous malformation. Shanghai Kou Qiang Yi Xue. (2016) 25(4):511–2.27858083
12. Sidi A, Naik B, Muehlschlegel JD, Kirby DS, Lobato EB. Ethanol-induced acute pulmonary hypertension and right ventricular dysfunction in pigs. Br J Anaesth. (2008) 100(4):568–9. doi: 10.1093/bja/aen047
13. Mason KP, Michna E, Zurakowski D, Koka BV, Burrows PE. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology. (2000) 217(1):127–32. doi: 10.1148/radiology.217.1.r00se30127
14. Hammer FD, Boon LM, Mathurin P, Vanwijck RR. Ethanol sclerotherapy of venous malformations: evaluation of systemic ethanol contamination. J Vasc Interv Radiol. (2001) 12(5):595–600. doi: 10.1016/S1051-0443(07)61482-1
15. Yakes WF, Krauth L, Ecklund J, Swengle R, Dreisbach JN, Seibert CE, et al. Ethanol endovascular management of brain arteriovenous malformations: initial results. Neurosurgery. (1997) 40(6):1145–52. discussion 52-4. doi: 10.1097/00006123-199706000-00005
16. Kelly LF, Goldberg SJ, Donnerstein RL, Cardy MA, Palombo GM. Hemodynamic effects of acute ethanol in young adults. Am J Cardiol. (1996) 78(7):851–4. doi: 10.1016/S0002-9149(96)00439-0
17. Yata S, Hashimoto M, Kaminou T, Ohuchi Y, Sugiura K, Adachi A, et al. Influence of ethanol-induced pulmonary embolism on hemodynamics in pigs. Ann Card Anaesth. (2013) 16(4):245–9. doi: 10.4103/0971-9784.119164
18. Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. Jama. (2005) 293(13):1653–62. doi: 10.1001/jama.293.13.1653
19. Kielstein JT, Hesse G, Bahr MJ, Tsikas D, Terkamp C, Martens-Lobenhoffer J, et al. Procedure-related pulmonary hypertension in patients with hepatocellular carcinoma undergoing percutaneous ethanol injection–role of ethanol, hemolysis, asymmetric dimethylarginine, and the nitric oxide system. Crit Care Med. (2009) 37(4):1483–5. doi: 10.1097/CCM.0b013e31819c3a10
20. Krasuski RA, Warner JJ, Wang A, Harrison JK, Tapson VF, Bashore TM. Inhaled nitric oxide selectively dilates pulmonary vasculature in adult patients with pulmonary hypertension, irrespective of etiology. J Am Coll Cardiol. (2000) 36(7):2204–11. doi: 10.1016/S0735-1097(00)00994-3
21. El-Hakim I, Alyamani A. Management of palatal vascular malformation using absolute ethanol sclerotherapy. Clin Pract. (2011) 1(4):e86. doi: 10.4081/cp.2011.e86
22. Fan XD, Su LX, Zheng JW, Zheng LZ, Zhang ZY. Ethanol embolization of arteriovenous malformations of the mandible. AJNR Am J Neuroradiol. (2009) 30(6):1178–83. doi: 10.3174/ajnr.A1539
23. Wang YA, Zheng JW, Zhu HG, Ye WM, He Y, Zhang ZY. Sclerotherapy of voluminous venous malformation in head and neck with absolute ethanol under digital subtraction angiography guidance. Phlebology. (2010) 25(3):138–44. doi: 10.1258/phleb.2009.009019
24. Yakes WF, Luethke JM, Parker SH, Stavros AT, Rak KM, Hopper KD, et al. Ethanol embolization of vascular malformations. Radiographics. (1990) 10(5):787–96. doi: 10.1148/radiographics.10.5.2217971
25. Ko JS, Kim JA, Do YS, Kwon MA, Choi SJ, Gwak MS, et al. Prediction of the effect of injected ethanol on pulmonary arterial pressure during sclerotherapy of arteriovenous malformations: relationship with dose of ethanol. J Vasc Interv Radiol. (2009) 20(1):39–45. quiz. doi: 10.1016/j.jvir.2008.10.012
Keywords: cardiopulmonary collapse, ethanol, vascular malformations, sclerotherapy, emergency
Citation: Su L, Zhao Y, Shen Y, Fan X, Wang Z, Wang D and Li Q (2024) Salvage of cardiopulmonary collapse caused by ethanol sclerotherapy for vascular malformations: clinical experience at a single center and literature review. Front. Cardiovasc. Med. 11:1439839. doi: 10.3389/fcvm.2024.1439839
Received: 28 May 2024; Accepted: 24 October 2024;
Published: 20 November 2024.
Edited by:
Paul B. Yu, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Xiaobing Liu, Shanghai Jiao Tong University, ChinaWayne Yakes, The Yakes Vascular Malformation Center, United States
Chung Sim Lim, Royal Free London NHS Foundation Trust, United Kingdom
Copyright: © 2024 Su, Zhao, Shen, Fan, Wang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyang Li, MTg5MTYxMTE5NzBAMTg5LmNu
†These authors have contributed equally to this work
 Lixin Su
Lixin Su Yan Zhao3,†
Yan Zhao3,† Yuchen Shen
Yuchen Shen Xindong Fan
Xindong Fan Deming Wang
Deming Wang