- 1Cardiology Department, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain
- 2University of Murcia, Murcia, Spain
- 3Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain
- 4Cardiology Service, Hospital Universitari Germans Trias i Pujol, Badalona, Spain
The main goals of the pharmacological treatment of Heart failure with reduced ejection fraction (HFrEF) are the reduction of mortality and the prevention of hospitalizations. However, other outcomes such as improvements in cardiac remodeling and clinical status, functional capacity and quality of life, should be taken into account. Also, given the significant inter-individual and intra-individual variability of HF, and the fact that patients usually present with comorbidities, an appropriate treatment for HFrEF should exert a clinical benefit in most patient profiles irrespective of their characteristics or the presence of comorbidities, while providing organ protection beyond the cardiovascular system. The aim of this narrative review is to determine which are the proven effects of the guideline-directed treatments for HFrEF on five key clinical outcomes: cardiovascular mortality and hospitalization due to HF, sudden death, reverse cardiac remodeling, renal protection and evidence in hospitalized patients. Publications that fulfilled the pre-established selection criteria were selected and reviewed. Renin-angiotensin system (RAS) inhibitors, namely angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin II receptor blockers (ARBs) or angiotensin receptor-neprilysin inhibitors (ARNI), beta-blockers (BB), mineralocorticoid receptor antagonists (MRA), sodium-glucose co-transporter 2 inhibitors (SGLT2i) show a benefit in terms of mortality and hospitalization rates. ARNI, BB, and MRA have demonstrated a significant positive effect on the incidence of sudden death. ARB, ARNI, BB and SGLT2i have been associated with clear benefits in reverse cardiac remodeling. Additionally, there is consistent evidence of renal protection from ARB, ARNI, and SGLT2i in renal protection and of benefits for hospitalized patients from ARNI and SGLT2i. In conclusion, the combination of drugs that gather most beneficial effects in HFrEF, beyond cardiovascular mortality and hospitalization, would be ideally pursued.
Introduction
Hearth failure (HF) with reduced ejection fraction (HFrEF) is characterized by a left ventricular ejection fraction (LVEF) of ≤40% (1). HFrEF accounts for 66% of new-onset HF cases (2), and incidence rates are lower in women than in men (3). In a study of more than 2,300 ambulatory patients with HFrEF with an all-cause mortality rate of 33%, around 75% of patients died due to HF-related factors. This rate was lower in patients with LVEF over 40% (4). HF also contributes to the hospitalization burden: within 5 years after the diagnosis, 83% of patients with HF are hospitalized at least once, and 43% are hospitalized four or more times (5). Hospitalization, in turn, reduces survival rates vs. general population, and this effect is even greater in recurrently hospitalized patients (6). Additionally, hospitalization accounts for 76% of HF-associated costs (7). Also, the risk of sudden death (SD) among patients with HFrEF, although decreasing over time with the increasing use of evidence-based medications (8), still remains a major factor. In light of these data, two of the goals of the pharmacological treatment are the reduction of mortality and the prevention of recurrent hospitalizations (1, 9).
However, other outcomes should be taken into account when choosing the most appropriate drug in patients with HFrEF. Current guidelines state that improvement in clinical status, functional capacity, and quality of life of patients are the third goal of pharmacotherapy (1). HF is a dynamic condition and patients exhibit inter-individual and intra-individual variability, and differences are observed throughout the course of the disease (10). Consequently, the most appropriate treatment should exert a clinical benefit in most patient profiles and be efficient irrespective of patient characteristics (sex, age, etiology, LVEF, or the presence of comorbidities, among others). Patients with HF have a high comorbidity burden (11, 12), so the most appropriate treatment should provide organ protection beyond the cardiovascular system. Finally, adherence and appropriate dosing are crucial for achieving clinical benefit and protection and are directly related to survival and the reduction of HF-associated hospitalizations (13, 14).
In this article we review the suitability of each pharmacotherapeutic option for the treatment of patients with HFrEF, considering the four pillars based on guideline-directed medications: RAS blockers (ACE-I, ARB) or ARNI, BB, MR and SGLT2i.
Methods
For this narrative review we searched in the PubMed database merging our therapeutic classes of interest and the described clinical outcomes. The terms used for the search were ACEi, ARB, ARNI, BB, MRA and SGLT2i, in combination with cardiovascular mortality and HF, hospitalization and HF, SD and HF, reverse cardiac remodelling and HF, renal protection and HF or hospitalized patients and HF. We have analyzed a total of 48 studies of which 32/48 (66.6%) were double-blind randomized clinical trials (24 placebo-controlled, 8 of them controlled with an active comparator), 2/48 (4.2%) was an open-label randomized clinical trial, 9/48 (18.8%) were post hoc analysis of randomized clinical trials, 3/48 (6.3%) were cohort studies and 2/48 (4.2%) were meta-analysis.
Results
Effects of pharmacological treatment for heart failure on cardiovascular mortality and hospitalization due to heart failure
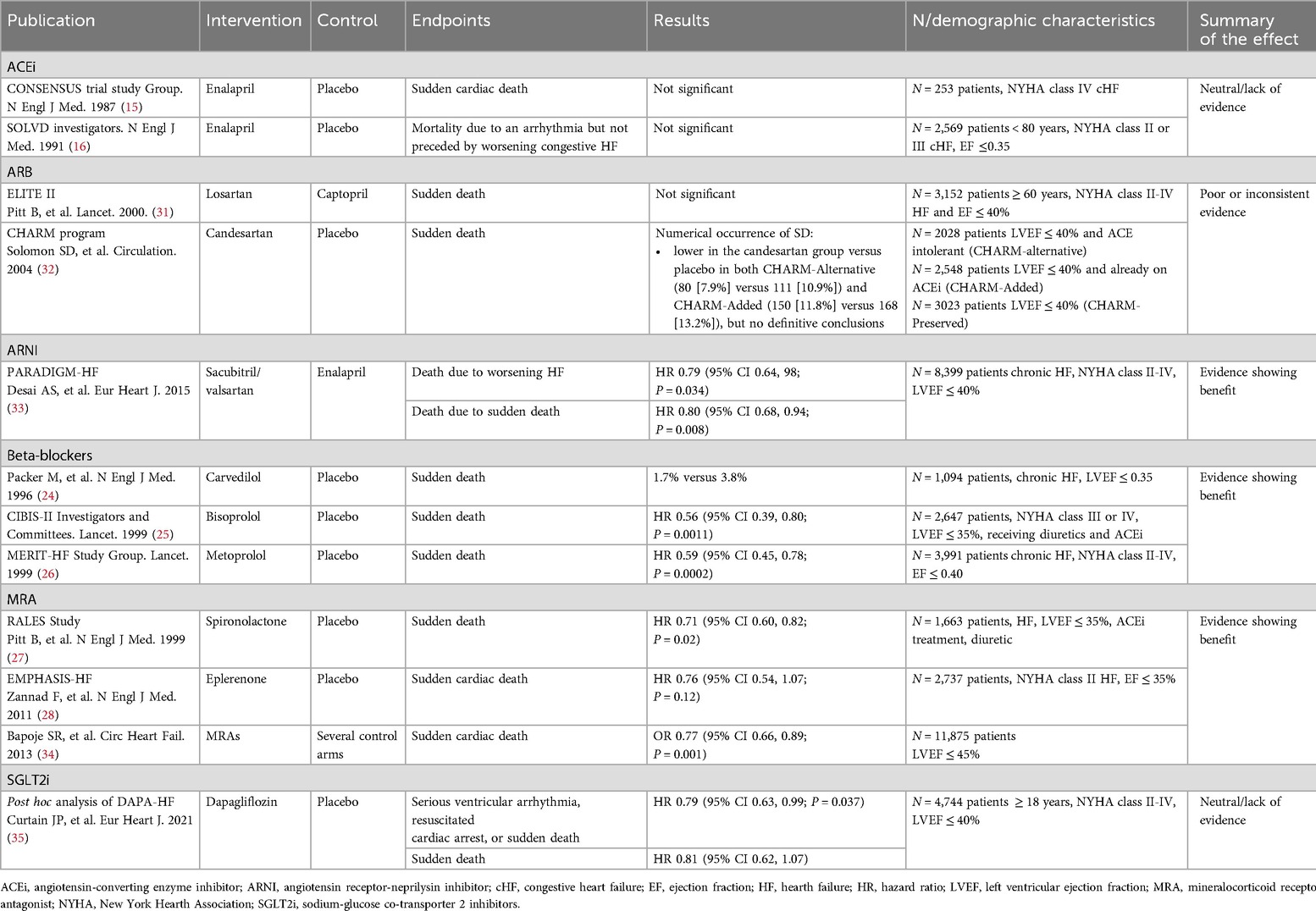
All approved therapeutic options for HFrEF exert a positive effect on cardiovascular mortality and hospitalization due to HF (Table 1). The CONSENSUS trial evaluated the effect of the ACEi enalapril compared to placebo on the prognosis of severe congestive HF. Conventional treatment for HF continued in both groups. After 6 months of treatment, a 40% reduction of total mortality was observed in the enalapril arm (P = 0.002). Mortality due to progression of HF also decreased by 50% in the enalapril arm (P < 0.001) (15). Regarding the effect of enalapril on hospitalization, the SOLVD trial showed that the addition of enalapril to conventional therapy produced a 26% reduction in the rate of events after 48 months, defined as either death or hospitalization of patients, compared to placebo (P < 0.0001) (16).
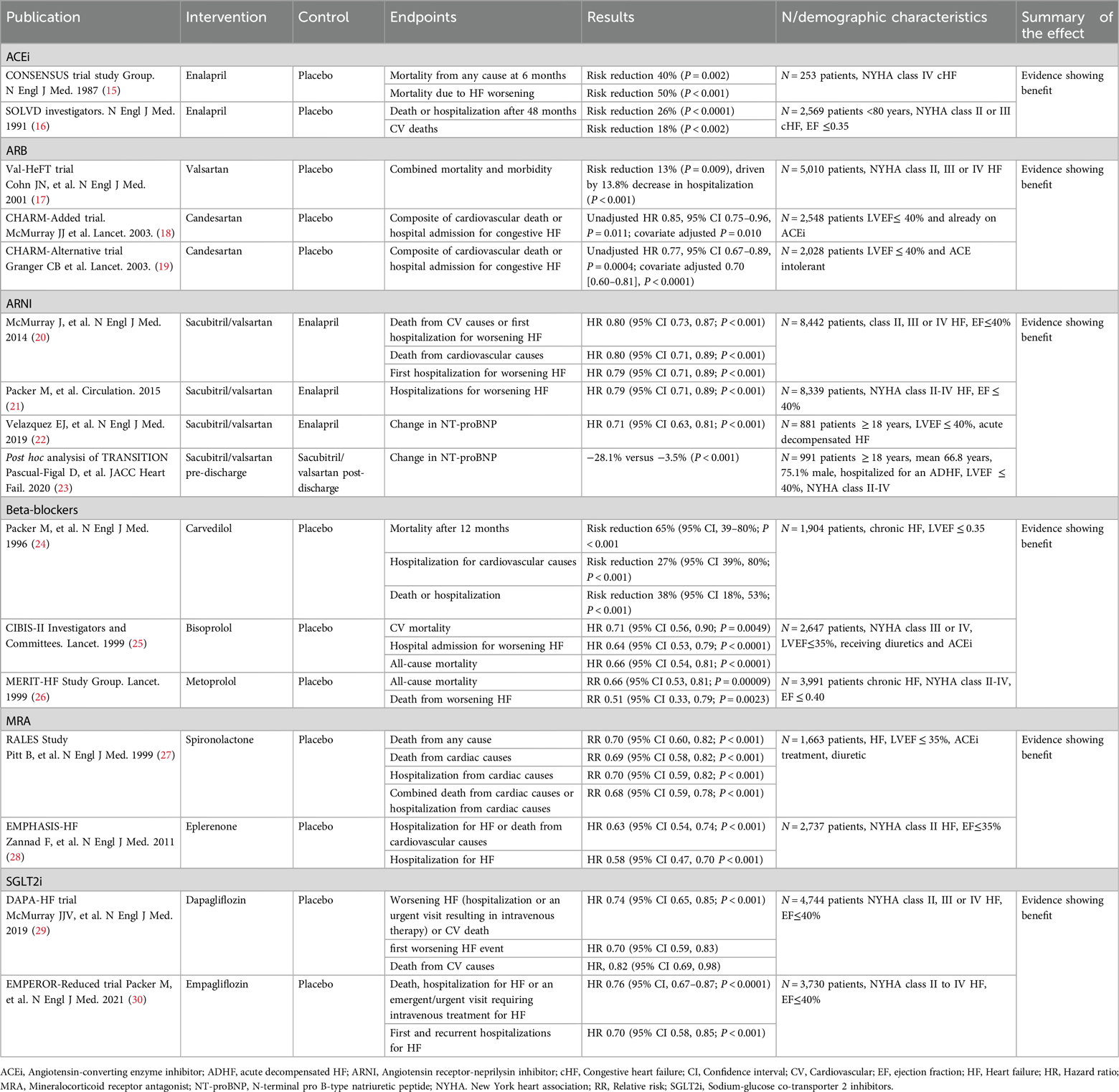
Table 1. Effect of pharmacological treatment on cardiovascular mortality and hospitalization due to heart failure.
ARB are used as alternative for HFrEF patients intolerant to ACEi. In the Val-HeFT trial, 5,010 patients were randomized to receive valsartan or placebo, with most on ACEi (93%), some on BB (35%), and few on spironolactone (5%), over a mean follow-up of 22.4 months (17). There was no significant difference in overall mortality (RR 1.02, P = 0.8), but a combined endpoint of mortality and morbidity showed a significant 13% reduction in the valsartan group (RR 0.87, P = 0.009), mainly due to fewer HF hospitalizations (P < 0.001) (17). In the CHARM Programme (18, 19), the CHARM-Alternative study (N = 2,028, median follow-up 33.7 months) showed candesartan significantly reduced cardiovascular death or HF hospitalization (HR 0.77, P = 0.0004), with cardiovascular death significant after adjustment (HR 0.80, P = 0.02) (19). The CHARM-Added study (N = 2,548, median follow-up 41 months) also found candesartan reduced cardiovascular death or HF hospitalization (HR 0.85, P = 0.011), with a significant reduction in cardiovascular deaths (adjusted P = 0.021) (18).
The PARADIGM-HF trial, a double-blind RCT, evaluated the effect of sacubitril/valsartan vs. enalapril added to recommended therapy on a composite endpoint of death from cardiovascular causes or hospitalization due to HF in 8,442 patients with HFrEF. Median follow-up was 27 months. Sacubitril/valsartan was superior to enalapril in reducing the risks of either cardiovascular death or HF-associated hospitalizations (P < 0.001), cardiovascular death (P < 0.001), and the risk of first hospitalization for HF (P < 0.001) (20). Another study examined the effect of sacubitril/valsartan on the risk of clinical progression compared to enalapril. Sacubitril/valsartan was associated with a 23% reduction in hospitalizations for worsening HF (P < 0.001). Sacubitril/valsartan also reduced the risk of other manifestations of disease progression, such as intensification of treatment, emergency room visits, cardiovascular-related hospitalizations, hospitalizations due to any cause, or need for intensive care. The 40% reduction in HF-associated hospitalization was significant within the first 30 days after randomization (21). A third study evaluated the time-averaged proportional change in N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration after 4 and 8 weeks of either sacubitril/valsartan or enalapril alone after hemodynamic stabilization in patients with HFrEF hospitalized due to acute decompensation. Sacubitril/valsartan therapy led to a greater reduction in the NT-proBNP concentration (P < 0.001) after 8 weeks of treatment (22). In the TRANSITION study, the reduction in NT-proBNP concentration was evaluated in patients with stabilized acute decompensated HFrEF who initiated sacubitril/valsartan either before or after discharge. Starting treatment in the hospital produced a greater reduction of NT-proBNP at discharge (23).
BB have also shown a clear benefit in terms of cardiovascular mortality and hospitalizations due to HF. In the CIBIS-II study, after 15 months of treatment, a 26% reduction in the risk of cardiovascular mortality (P = 0.0049) and a 31% reduction in hospital admission for worsening HF (P < 0.0001) were reported in the bisoprolol arm compared to placebo. All-cause mortality was also significantly lower in patients treated with bisoprolol (25). Another study comparing the effect of carvedilol to placebo showed a reduction in the mortality risk of 65% (P < 0.001) in the carvedilol group after 12 months. A 27% reduction in the risk of hospitalization due to cardiovascular causes (P = 0.036) and a 38% reduction in the risk of either hospitalization or death (P < 0.001) were also reported (24). A third double-blind randomized controlled study (MERIT-HF) enrolling almost 4,000 patients with chronic HFrEF showed that all-cause mortality was lower with metoprolol than with placebo (RR, 0.66, P = 0.00009) after 12 months of treatment; the risk of death from worsening HF was also lower with metoprolol (RR, 0.51, P = 0.0023) (26).
The risk of mortality and hospitalization has also been assessed in patients treated with MRAs. In an initial study of 1,663 patients (RALES) with severe HFrEF treated with ACEi, loop diuretics and digoxin showed a 30% reduction in death from any cause after the addition of spironolactone compared to placebo (P < 0.001) after 24 months. Death from cardiac causes was reduced by 21% in patients treated with spironolactone (P < 0.001), hospitalization from cardiac causes was reduced by 30% (P < 0.001), and the combination of both endpoints by 32% (P < 0.001) (27). In a second study with 2,737 patients with HFrEF treated with either eplerenone or placebo added to recommended therapy, the primary outcome was a composite of death from cardiovascular causes or hospitalization for HF: a reduction of 37% was observed in the intervention arm (HR, 0.63; 95% CI, 0.54–0.74; P < 0.001). The risk of death from any cause and the risk of hospitalization for HF were also significantly reduced (28).
SGLT2i have demonstrated a protective effect in patients with established HFrEF. The DAPA- HF study assessed the effect of dapagliflozin or placebo added to recommended therapy on the worsening of HF and cardiovascular death. A composite endpoint of worsening HF (including hospitalization or urgent visits requiring intravenous treatment) or cardiovascular death was significantly reduced in the dapagliflozin group (P < 0.001), as were each of those endpoints alone. The reduction in the primary event with dapagliflozin was rapidly statistically significant (28 days post-randomization), meaning that the benefit of the drug manifests promptly (29). Another study (EMPEROR-reduced trial) evaluated the efficacy of empagliflozin in patients with the same profile. Empagliflozin reduced by 24% the combined risk of death, hospitalization for HF or an emergent/urgent visit requiring intravenous treatment for HF (P < 0.0001). This benefit also reached early statistical significance (12 days after randomization) (30).
Effects of pharmacological treatment for heart failure on sudden death
Results obtained on the effect of the different therapeutic options on SD are summarized in Table 2. There were no differences in the incidence of SD between enalapril and placebo groups in either the CONSENSUS trial or SOLVD (15, 16).
The ELITE II trial, involving 3,152 patients over 60 years, found no significant differences between losartan and captopril in SD (HR 1.13, p = 0.16) or resuscitated arrests (HR 1.25, p = 0.08) (31). The CHARM program lacked the power to definitively assess candesartan's impact on causes of death. Although SD rates were numerically lower with candesartan in CHARM-Alternative (7.9% vs. 10.9%) and CHARM-Added (11.8% vs. 13.2%), no definitive conclusions were made (32).
The clinical evidence on ARNI shows a clear benefit in the reduction of the incidence of SD in patients with HFrEF. Mode of death was adjudicated by a blinded clinical endpoints committee and, at the end of a 42-month follow-up period (mean follow-up was 27 months), the risk of SD was reduced by 20% (P = 0.008) while the risk of death from worsening HF was also reduced by 21% (P = 0.034) (33).
Several studies have addressed the incidence of SD in patients with HFrEF treated with BB (36). The CIBIS-II study reported a 44% reduction in the risk of SD (P = 0.0011) with bisoprolol compared to placebo (25). In the MERIT-HF study, there was a 41% reduction in SD (P = 0.0002) in the metoprolol group compared to the placebo group (26). Results obtained with carvedilol were similar and a 55% reduction was reported (24).
An overall benefit for MRA has been observed in the reduction of SD. In the RALES study, a 29% reduction in the relative risk of SD (P = 0.02) was described in the arm treated with spironolactone compared to the arm treated with placebo (27). In contrast, in the EMPHASIS-HF study, the reduction in the relative risk of SD of 24% with eplerenone vs. placebo was not statistically significant (P = 0.12) (28). A subsequent metaanalysis evaluated the relative risk reduction of SD with any MRA compared to control arms. Eight randomized controled trials that included almost 12,000 patients met the inclusion criteria; five examined the role of spironolactone, two eplerenone, and one canrenone. MRAs were associated with a 23% reduction in sudden cardiac death compared to controls (P = 0.001). Similar reductions were observed in cardiovascular and total mortality (34).
The effect of SGLT2i on SD was examined in a post hoc analysis of the DAPA-HF study. Dapagliflozin significantly reduced a composite endpoint of serious ventricular arrhythmia, resuscitated cardiac arrest, and SD compared to placebo, but the effect on SD alone was not significant (35).
Effects of pharmacological treatment for heart failure on reverse cardiac remodeling
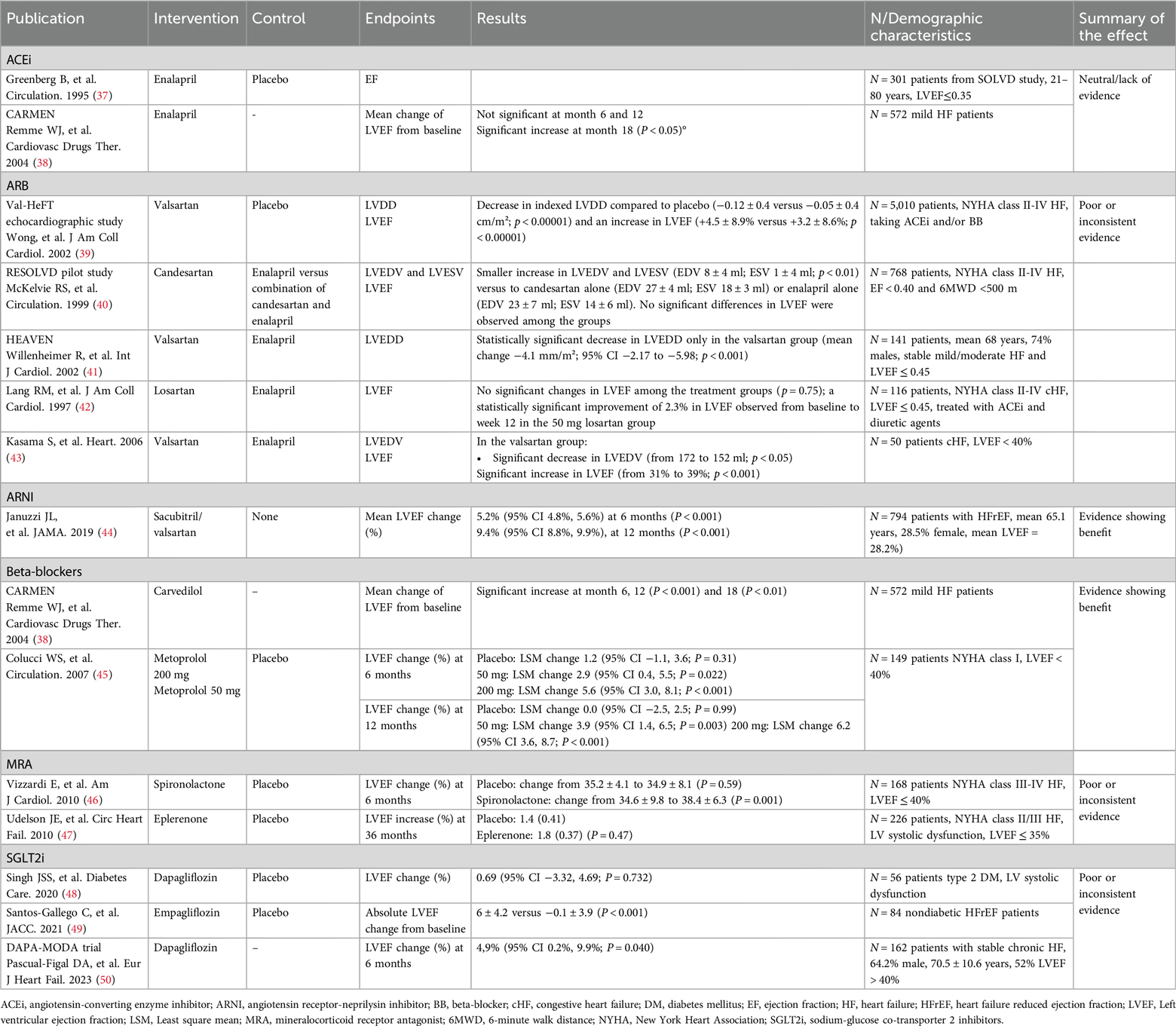
Some differences have been shown in the effect of the different therapeutic options on reverse cardiac remodeling (Table 3). A substudy from the SOLVD study was carried out to elucidate whether enalapril inhibited remodeling in the patients enrolled. A pool of 301 patients from 5 centers were examined using Doppler echocardiography before randomization and after 4 and 12 months of therapy with either enalapril or placebo. EF remained unchanged in both arms after 12 months (37). The CARMEN study evaluated the suitability of the combination of ACEi and BB for the treatment of chronic HFrEF, as well as the recommendation to initiate treatment with ACEi and only add BB in symptomatic patients. Regarding reverse cardiac remodeling, in patients treated with enalapril, changes in LVEF were not significant at 6 and 12 months and were statistically greater at 18 months (38).
The Val-HeFT substudy found valsartan significantly reduced LVDD (−0.12 vs. −0.05 cm/m2; p < 0.00001) and increased LVEF (+4.5% vs. + 3.2%; p < 0.00001) at 18 months (39). In the RESOLVD pilot, combination therapy (candersatan plus enalapril) led to smaller increases in LVEDV (8 vs. 27 ml) and LVESV (1 vs. 18 ml) than candesartan alone (40). An open-label study with 445 HFrEF patients showed similar LVEF, LVSD, and LVDD improvements for valsartan and enalapril (51). The HEAVEN study found a significant LVEDD decrease only with valsartan (−4.1 mm/m2; p < 0.001) (41). Another trial with 116 patients replacing ACEi with losartan showed a 2.3% LVEF improvement with high-dose losartan (50 mg) at week 12 (42). A trial with 50 HFrEF patients reported significant decreases in LVEDV (172 to 152 ml, p < 0.05) and increases in LVEF (31% to 39%; p < 0.001) only with valsartan (43).
Clinical evidence with ARNI has demonstrated also a clear benefit in terms of reverse cardiac remodeling. The PROVE-HF study enrolled 794 patients with HFrEF who were switched from ACEi/ARB to ARNI therapy. At 6 months, left ventricle and left atrial volume indexes, E/e’ ratio, LV mass index and LVEF were significantly improved compared to baseline. After 12 months, sacubitril/valsartan produced a mean LVEF increase of 9.4%. Moreover, 25% of patients experienced a LVEF increase of 13.4% or more. Patients with new-onset HF or who were ACEi/ARB naive at baseline showed mean improvements in LVEF of 12.8% (44).
In the aforementioned CARMEN study, patients receiving carvedilol monotherapy showed a significant increase in LVEF at 6, 12 and 18 months (38). The REVERT study elucidated the effect of therapy with the BB metaprolol on left ventricular remodeling in asymptomatic patients with left ventricular systolic dysfunction. A total of 149 patients were randomized to three treatment groups (200 mg, 50 mg, and placebo). LVEF increased significantly in patients treated with metoprolol after 6 months compared to placebo. After 12 months, LVEF remained unchanged in the placebo group, but increased significantly by 3.9% in patients receiving 50 mg metoprolol and by 6.2% in patients receiving 200 mg metoprolol. Changes were similar at 6 months (45).
A randomized control trial examined the effect of spironolactone on LV function and the functional capacity of patients with mild to moderate HF. LVEF increased from 35.2% to 39.1% ± 3.5% after 6 months of spironolactone treatment, while the placebo arm showed no significant differences (P = 0.003) (46). A second study evaluated the effect of eplerenone added to contemporary background therapy on ventricular remodeling in patients with HFrEF. After 36 weeks of treatment, no effects were observed in left heart remodeling parameters with eplerenone compared to placebo (47). Thus, the clinical evidence obtained with MRA in reverse cardiac remodeling is limited and inconsistent.
In the case of SGLT2i, the sample size of the studies addressing reverse cardiac remodeling to date is limited. The REFORM study only included 56 patients assigned to either dapagliflozin or placebo and no statistically significant effect was observed on LVEF changes at 12 months (48). Another study evaluating the role of empagliflozin on LV function and volumes in 84 nondiabetic HFrEF patients showed a statistically significant improvement in LV volumes, LV mass and LVEF at 6 months of treatment (49). Recently, the DAPA-MODA, a single-arm study including 162 patients (48% LVEF ≤40%, 52% LVEF >40%) showed a favorable and significant impact of dapagliflozin in both atrial and ventricular remodeling parameters including an increase of 5% in LVEF (p = 0.040), assessed by echocardiography and interpreted in a blinded manner (50).
Effects of pharmacological treatment for heart failure on renal protection
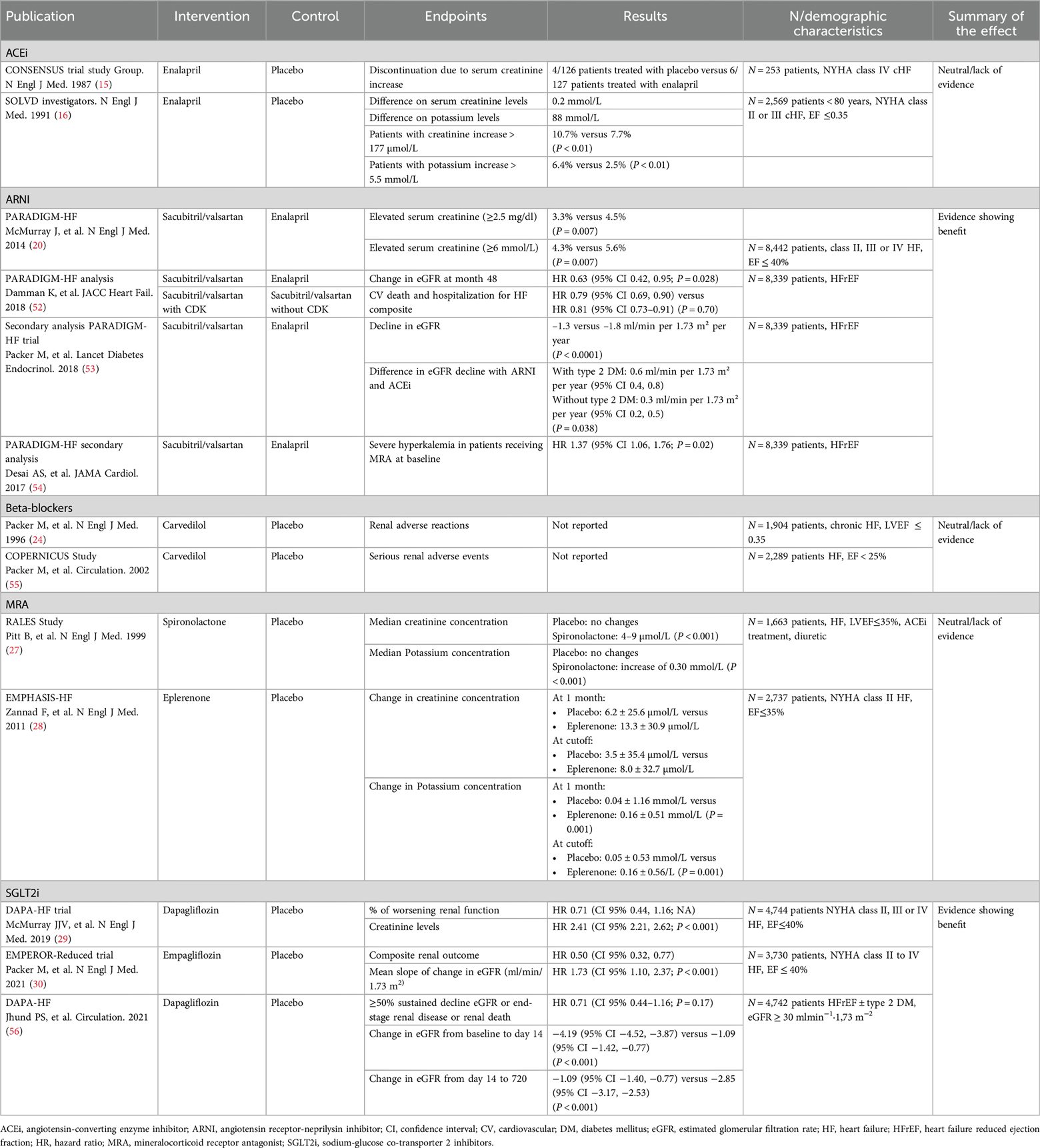
HF is associated with a greater decline in renal function over time. However, not all available HF treatments exert a positive effect on renal function (Table 4). In the SOLVD study, more patients receiving enalapril showed a serum creatinine increase of at least 2 mg/dl or a potassium increase of at least 5.5 mmol/L than in the placebo arm (P < 0.01) (16). In the CONSENSUS trial, however, the increase in serum creatinine levels did not lead to significant differences in treatment discontinuation between the enalapril and placebo arms (15). Clinical evidence suggests a beneficial clinical effect even in patients with HFrEF who suffer a decline in renal function after initiating treatment with ACEi (57). However, there is no clinical evidence to show that ACEi reduces the risk of lower eGFR. Similar data are available for ARBs (58). In addition, baseline chronic kidney disease (CKD) should not preclude ACEi/ARB use, as their efficacy persists in CKD, supported by trials like CONSENSUS, SOLVD, ValHeFt, and CHARM (59). However, specific studies on RAAS inhibitors in advanced CKD (GFR <15 ml/min/1.73 m2) are limited, and safety should be confirmed in long-term observational studies. KDIGO guidelines recommend ACEi and ARBs in CKD stage 5, with moderate evidence for use in dialysis and weak evidence for non-dialysis patients (60).
In the PARADIGM-HF trial, adverse events due to serum creatinine levels ≥ 2.5 mg/dl and serum potassium levels > 6.0 mmol/L were less frequent with sacubitril/valsartan than with enalapril (P < 0.05 for both comparisons) (20). A comprehensive analysis of the PARADIGM-HF trial illustrated a clear 37% reduction in renal function decline among patients treated with sacubitril/valsartan compared to patients treated with enalapril. Additionally, sacubitril/valsartan showed a consistent clinical benefit for cardiovascular death and hospitalization in the HF composite outcome, irrespective of the presence or absence of chronic kidney disease (52). Another study assessed differences in the effect of sacubitril/valsartan on renal function in patients with type 2 diabetes mellitus (T2DM). Patients treated with sacubitril/valsartan showed a slower decline in eGFR than those treated with enalapril. The difference between the sacubitril/valsartan and the enalapril arms observed in patients with T2DM was twice as high as that observed in patients without T2DM (53). A subsequent secondary analysis of the PARADIGM-HF trial described how sacubitril/valsartan reduced the risk of severe hyperkalemia compared to enalapril in patients treated with MRA at baseline (54).
Clinical evidence on the effect of BB on renal function is limited. Their use does not cause renal function to worsen over time, as is the case with ACEi. Several studies have reported that patients in the lowest GFR strata show a greater relative risk reduction with BB (57, 61, 62). No adverse events related to the worsening of renal function were reported in the randomized clinical trials addressing the effect of carvedilol on mortality and morbidity in patients with HFrEF (24, 55).
MRA does not seem to exert a protective effect on renal function. In fact, spironolactone was associated with significant but not clinically relevant increases in creatinine and potassium (27). In EMPHASIS study, eplerenone was associated with a significant but not clinically relevant serum potassium increase at one month and at the cutoff date, and with a non-significant increase in serum creatinine (28). In the EPHESUS study, eplerenone produced a decline in eGFR compared with placebo (P < 0.0001) that appeared within the first month and lasted throughout the 24-month follow-up (63). Thus, the initiation of treatment with MRA produces an acute decline in eGFR that is maintained throughout administration (57).
Two studies have corroborated the renal protective effect of SGLT2i. In the DAPA-HF trial, the percentage of patients with worsening renal function was similar in the dapagliflozin and placebo arms (29). In a post hoc analysis of the same study, a composite renal outcome (≥50% sustained decline eGFR or end-stage renal disease or renal death) was not reduced by dapagliflozin, although the rate of decline in eGFR between day 14 and 720 was less with dapagliflozin than with placebo (56). In the EMPEROR-REDUCED trial, a composite renal outcome was assessed in patients treated with either empagliflozin or placebo, and a statistically significant difference emerged in favor of empagliflozin (30). Both studies demonstrated a reduction in the risk of worsening renal function.
Effects of pharmacological treatment for heart failure on hospitalized patients
The effect of the different therapeutic options on hospitalized patients is summarized in Table 5. To date, no robust data are available from randomized controlled trials on the initiation of ACEi in hospitalized patients (71). The CONSENSUS study, for instance, only included 253 hospitalized patients from a total of 1,987 recruited patients (15).
An analysis of the GWTG-HF registry revealed that hospitalized patients who continued ACEi/ARB therapy experienced significantly lower mortality rates at 30 days, 90 days, and 1 year, as well as reduced 30-day readmission rates, compared to those who discontinued ACEi/ARB therapy (64).
ARNI have shown clinical benefit in hospitalized patients. In a subanalysis of the PIONEER-HF study, a 46% reduction was recorded in the combined risk of death, readmission for HF, need for LV assistance or inclusion in a heart transplantation waiting list (65). In another study (PIONEER-HF), patients with de novo HF had lower risk of either cardiovascular death or rehospitalization for HF than patients with previous history of HF. Likewise, patients not treated with ACEi or angiotensin receptor blockers at admission also showed a significantly lower incidence of the same composite endpoint (66).
The IMPACT-HF study, a prospective trial in 363 patients, showed a non-significant trend towards a benefit in terms of a lower composite of death or rehospitalization for the predischarge initiation of carvedilol in stabilized patients compared to initiation of any BB more than two weeks after discharge (67). A meta-analysis that included five observational studies and one randomized clinical trial showed that the discontinuation of BB in patients admitted to hospital was associated with increased in-hospital and short-term mortality (68).
No randomized clinical trials have addressed the role of MRA in hospitalized patients and available evidence is currently limited to a single-center observational study of 685 patients discharged after admission for acute HF. In this study, starting MRA in the hospital was associated with significantly lower mortality than late initiation (69). In contrast, the EMPULSE trial (empagliflozin) showed a clear clinical benefit in the composite endpoint (death from any cause, number of HF events and time to first HF event, or a 5-point or greater change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days) (70).
Discussion
This review highlights that the current guideline-directed medications (ACEi/ARB/ARNi + BB + MRA + SGLT2i) have a positive effect on the main components of disease progression in HFrEF patients. However, the extent of protection varies among these drugs (Graphical Abstract). All medications prevent cardiovascular mortality and HF hospitalization (15–30), but ACEi lack clear evidence for SD, renal protection, or benefits in hospitalized patients, possible due to older studies (15, 16, 71). ARNI (sacubitril-valsartan) extends benefits beyond ACEi/ARB, showing improvements in all disease progression components, and is increasingly recommended over ACEi (20–23, 33, 44, 52–54, 65, 66).
The benefit of BB is clear for decades, with numerous trials supporting the effect on cardiac protection and the reduction of related complications, with a neutral effect in renal function (24, 25, 55, 57, 61, 62). MRAs are limited by the risk of hyperkalemia in kidney disease (27). Finally, SGTL2i, the last pillar incorporated to HFrEF, significantly reduce the risk of HF decompensation and improve renal protection, with less evidence on cardiac remodeling and SD (29, 30, 35, 48–50, 56).
In conclusion, all currently recommended medications for HFrEF have a positive effect on mortality and hospitalization rates. Considering the relevance of preventing SD as mode of death, ARNI, BB, and MRA are particularly effective. In addition, organ protection should be considered. In this regard, ARNI, BB, and SGLT2i improve LVEF and reverse parameters of cardiac remodeling, with ARNI and SGLT2i also offering renal protection. Likewise, it is important to consider effective and safe medications within the early phase of HF hospitalizations, and ARNI and SGLT2i are the main options in this acute setting (Figure 1). Therefore, although we have not a single ideal medication, the optimal therapy combines ARNI, BB, MRA and SGLT2i to maximize benefits across all disease progression aspects.
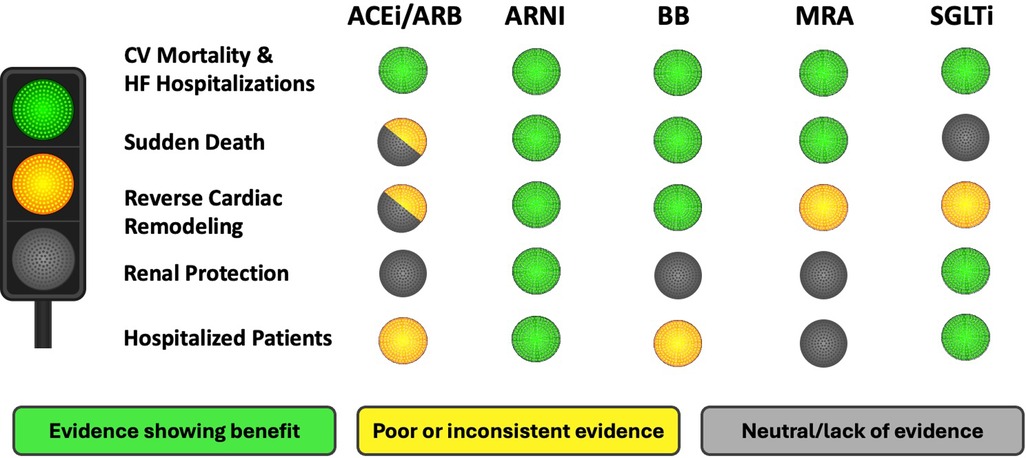
Figure 1. Suitability of therapeutic options for the treatment of HFrEF. HFrEF, heart failure with reduced ejection fraction; CV, cardiovascular; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta-blockers; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium-glucose co-transporter 2 inhibitor.
Author contributions
DP-F: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. AB-G: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work has been partially supported by a non-conditional grant from the Spanish Society of Cardiology (SEC) and Novartis.
Conflict of interest
DP-F reports educational and/or consultancy fees from AstraZeneca, Pfizer, Novartis, Vifor Pharma, Roche, Abbot, Servier and Rovi. AB-G has lectured and/or participated in board meetings for Abbott, AstraZeneca, Boehringer-Ingelheim, Bayer, Novartis, Roche Diagnostics and Vifor.
The reviewer JA-G declared a past co-authorship with the authors to the handling editor.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta-blockers; BP, blood pressure; CHF, chronic heart failure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LV, left ventricle; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RAS, renin-angiotensin system; RCT, randomized control trial; SGLT2i, sodium-glucose co-transporter 2 inhibitor; SD, sudden death; T2DM, Type 2 diabetes mellitus.
References
1. McDonagh TA, Metra M, Adamo M, Gardener RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
2. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gasenvoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. Reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. (2013) 34(19):1424–31. doi: 10.1093/eurheartj/eht066
3. Meyer S, Brouwers FP, Voors AA, Hillege HL, de Boer RA, Gasenvoort RT, et al. Sex differences in new-onset heart failure. Clin Res Cardiol. (2015) 104(4):342–50. doi: 10.1007/s00392-014-0788-x
4. Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I, Vazquez R, Delgado-Jimenez J, Alvarez Garcia J, et al. Mid-range left ventricular ejection fraction: clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol. (2017) 240:265–70. doi: 10.1016/j.ijcard.2017.03.032
5. Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long Kirsten, Shah ND, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. (2009) 54(18):1695–702. doi: 10.1016/j.jacc.2009.08.019
6. Fernández-Gassó L, Hernando-Arizaleta L, Palomar-Rodríguez JA, Abellán-Pérez MV, Hernández-Vicente Á, Pascual-Figal DA. Population-based study of first hospitalizations for heart failure and the interaction between readmissions and survival. Rev Esp Cardiol (Engl Ed). (2019) 72(9):740–8. doi: 10.1016/j.rec.2018.08.014
7. Escobar C, Varela L, Palacios B, Margarita C, Sicras A, Sicras A, et al. Costs and healthcare utilisation of patients with heart failure in Spain. BMC Health Serv Res. (2020) 20(1):964. doi: 10.1186/s12913-020-05828-9
8. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Chunxia T, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. (2020) 36(7):e3319. doi: 10.1002/dmrr.3319
9. Anker SD, Schroeder S, Atar D, Bax JJ, Ceconi C, Cowie MR, et al. Traditional and new composite endpoints in heart failure clinical trials: facilitating comprehensive efficacy assessments and improving trial efficiency. Eur J Heart Fail. (2016) 18(5):482–9. doi: 10.1002/ejhf.516
10. Pascual-Figal DA. Acute heart failure and biomarkers: time also matters, don't relax. Eur J Heart Fail. (2017) 19(8):1011–3. doi: 10.1002/ejhf.880
11. Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. (2003) 42(7):1226–33. doi: 10.1016/s0735-1097(03)00947-1
12. Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, et al. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol. (2018) 271:132–9. doi: 10.1016/j.ijcard.2018.04.001
13. Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. (2017) 38(24):1883–90. doi: 10.1093/eurheartj/ehx026
14. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS. Physicians’ guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail. (2017) 19(11):1414–23. doi: 10.1002/ejhf.887
15. The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the cooperative north scandinavian enalapril survival study (CONSENSUS). N Engl J Med. (1987) 316(23):1429–35. doi: 10.1056/nejm198706043162301
16. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. (1991) 325(5):293–302. doi: 10.1056/nejm199108013250501
17. Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. (2001) 345(23):1667–75. doi: 10.1056/NEJMoa010713
18. McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-added trial. Lancet. (2003) 362(9386):767–71. doi: 10.1016/S0140-6736(03)14283-3
19. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet. (2003) 362(9386):772–6. doi: 10.1016/S0140-6736(03)14284-5
20. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371(11):993–1004. doi: 10.1056/NEJMoa1409077
21. Packer M, McMurray JJ, Desai AS, Gong A, Lefkowitz MP, Rizkala AR, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. (2015) 131(1):54–61. doi: 10.1161/circulationaha.114.013748
22. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. (2019) 380(6):539–48. doi: 10.1056/NEJMoa1812851
23. Pascual-Figal D, Wachter R, Senni M, Bao W, Noè A, Schwende H, et al. NT-proBNP Response to sacubitril/valsartan in hospitalized heart failure patients with reduced ejection fraction: tRANSITION study. JACC Heart Fail. (2020) 8(10):822–33. doi: 10.1016/j.jchf.2020.05.012
24. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol heart failure study group. N Engl J Med. (1996) 334(21):1349–55. doi: 10.1056/nejm199605233342101
25. CIBIS-II Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet. (1999) 353(9146):9–13. doi: 10.1016/S0140-6736(98)11181-9
26. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. (1999) 353(9169):2001–7. doi: 10.1016/S0140-6736(99)04440-2
27. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. (1999) 341(10):709–17. doi: 10.1056/nejm199909023411001
28. Zannad F, McMurray JJ, Krum H, Van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. (2011) 364(1):11–21. doi: 10.1056/NEJMoa1009492
29. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
30. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-reduced trial. Circulation. (2021) 143(4):326–36. doi: 10.1161/circulationaha.120.051783
31. Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial–the losartan heart failure survival study ELITE II. Lancet. (2000) 355(9215):1582–7. doi: 10.1016/s0140-6736(00)02213-3
32. Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJV, et al. Effect of candesartan on cause-specific mortality in heart failure patients: the candesartan in heart failure assessment of reduction in mortality and morbidity (CHARM) program. Circulation. (2004) 110(15):2180–3. doi: 10.1161/01.CIR.0000144474.65922.AA Erratum in: Circulation. 2005 January 25;111(3):378.15466644
33. Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. (2015) 36(30):1990–7. doi: 10.1093/eurheartj/ehv186
34. Bapoje SR, Bahia A, Hokanson JE, Peterson PN, Heidenreich PA, Lindenfeld J, et al. Effects of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with left ventricular systolic dysfunction: a meta-analysis of randomized controlled trials. Circ Heart Fail. (2013) 6(2):166–73. doi: 10.1161/circheartfailure.112.000003
35. Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. (2021) 42(36):3727–38. doi: 10.1093/eurheartj/ehab560
36. Martínez-Milla J, Raposeiras-Roubín S, Pascual-Figal DA, Ibáñez B. Role of Beta-blockers in cardiovascular disease in 2019. Rev Esp Cardiol (Engl Ed). (2019) 72(10):844–52. doi: 10.1016/j.rec.2019.04.014
37. Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. (1995) 91(10):2573–81. doi: 10.1161/01.cir.91.10.2573
38. Remme WJ, Riegger G, Hildebrandt P, Komajda M, Jaarsma W, Bobbio M, et al. The benefits of early combination treatment of carvedilol and an ACE-inhibitor in mild heart failure and left ventricular systolic dysfunction. The carvedilol and ACE-inhibitor remodelling mild heart failure evaluation trial (CARMEN). Cardiovasc Drugs Ther. (2004) 18(1):57–66. doi: 10.1023/B:CARD.0000025756.32499.6f
39. Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, Chiang Y-T, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. (2002) 40(5):970–5. doi: 10.1016/s0735-1097(02)02063-6
40. McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD pilot study investigators. Circulation. (1999) 100(10):1056–64. doi: 10.1161/01.cir.100.10.1056
41. Willenheimer R, Helmers C, Pantev E, Rydberg E, Löfdahl P, Gordon A, et al. Safety and efficacy of valsartan versus enalapril in heart failure patients. Int J Cardiol. (2002) 85(2-3):261–70. doi: 10.1016/s0167-5273(02)00154-7
42. Lang RM, Elkayam U, Yellen LG, Krauss D, McKelvie RS, Vaughan DE, et al. Comparative effects of losartan and enalapril on exercise capacity and clinical status in patients with heart failure. The losartan pilot exercise study investigators. J Am Coll Cardiol. (1997) 30(4):983–91. doi: 10.1016/s0735-1097(97)00253-2
43. Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, et al. Comparative effects of valsartan and enalapril on cardiac sympathetic nerve activity and plasma brain natriuretic peptide in patients with congestive heart failure. Heart. (2006) 92(5):625–30. doi: 10.1136/hrt.2005.062463
44. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. (2019) 322(11):1085–95. doi: 10.1001/jama.2019.12821
45. Colucci WS, Kolias TJ, Adams KF, Armstrong WF, Ghali JK, Gottlieb SS, et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular remodeling with toprol-XL (REVERT) trial. Circulation. (2007) 116(1):49–56. doi: 10.1161/circulationaha.106.666016
46. Vizzardi E, D'Aloia A, Giubbini R, Bordonali T, Bugatti S, Pezzali N, et al. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol. (2010) 106(9):1292–6. doi: 10.1016/j.amjcard.2010.06.052
47. Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, et al. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. (2010) 3(3):347–53. doi: 10.1161/circheartfailure.109.906909
48. Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. (2020) 43(6):1356–9. doi: 10.2337/dc19-2187
49. Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2021) 77(3):243–55. doi: 10.1016/j.jacc.2020.11.008
50. Pascual-Figal DA, Zamorano JL, Domingo M, Morillas H, Nuñez J, Marcos MC, et al. Impact of dapagliflozin on cardiac remodelling in patients with chronic heart failure: the DAPA-MODA study. Eur J Heart Fail. (2023) 25(8):1352–60. doi: 10.1002/ejhf.2884
51. Lee YS, Kim KS, Lee JB, Ryu JK, Choi JY, Kim BK, et al. Effect of valsartan on N-terminal pro-brain natriuretic peptide in patient with stable chronic heart failure: comparison with enalapril. Korean Circ J. (2011) 41(2):61–7. doi: 10.4070/kcj.2011.41.2.61
52. Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz M, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. (2018) 6(6):489–98. doi: 10.1016/j.jchf.2018.02.004
53. Packer M, Claggett B, Lefkowitz MP, McMurray JJ, Rouleau JL, Solomon SD, et al. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. (2018) 6(7):547–54. doi: 10.1016/s2213-8587(18)30100-1
54. Desai AS, Vardeny O, Claggett B, McMurray JJV, Packer M, Swedberg K, et al. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. (2017) 2(1):79–85. doi: 10.1001/jamacardio.2016.4733
55. Packer M, Fowler MB, Roecker EB, Coats AJS, Katus HA, Krum H, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. (2002) 106(17):2194–9. doi: 10.1161/01.cir.0000035653.72855.bf
56. Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. (2021) 143(4):298–309. doi: 10.1161/circulationaha.120.050391
57. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory—a position statement from the heart failure association of the European Society of Cardiology. Eur J Heart Fail. (2020) 22(4):584–603. doi: 10.1002/ejhf.1697
58. Ahmed A, Fonarow GC, Zhang Y, Sanders PW, Allman RM, Arnett DK, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. (2012) 125(4):399–410. doi: 10.1016/j.amjmed.2011.10.013
59. Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, et al. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. (2014) 63(9):853–71. doi: 10.1016/j.jacc.2013.11.031
60. House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, et al. Heart failure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2019) 95(6):1304–17. doi: 10.1016/j.kint.2019.02.022
61. Cohen-Solal A, Kotecha D, van Veldhuisen DJ, Babalis D, Böhm M, Coats AJ, et al. Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. (2009) 11(9):872–80. doi: 10.1093/eurjhf/hfp104
62. Castagno D, Jhund PS, McMurray JJ, Lewsey JD, Erdmann E, Zannad F, et al. Improved survival with bisoprolol in patients with heart failure and renal impairment: an analysis of the cardiac insufficiency bisoprolol study II (CIBIS-II) trial. Eur J Heart Fail. (2010) 12(6):607–16. doi: 10.1093/eurjhf/hfq038
63. Rossignol P, Cleland JG, Bhandari S, Tala S, Gustafsson F, Fay R, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study. Circulation. (2012) 125(2):271–9. doi: 10.1161/circulationaha.111.028282
64. Krantz MJ, Ambardekar AV, Kaltenbach L, Hernandez AF, Heidenreich PA, Fonarow GC, et al. Patterns and predictors of evidence-based medication continuation among hospitalized heart failure patients (from get with the guidelines-heart failure). Am J Cardiol. (2011) 107(12):1818–23. doi: 10.1016/j.amjcard.2011.02.322
65. Late-breaking science abstracts from the American Heart Association’s scientific sessions 2018 and late-breaking abstracts in resuscitation science from the resuscitation science symposium 2018. Circulation. (2018) 138(25):e751–82. doi: 10.1161/cir.0000000000000636
66. Ambrosy AP, Braunwald E, Morrow DA, DeVore AD, McCague K, Meng X, et al. Angiotensin receptor-neprilysin inhibition based on history of heart failure and use of renin-angiotensin system antagonists. J Am Coll Cardiol. (2020) 76(9):1034–48. doi: 10.1016/j.jacc.2020.06.073
67. Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the initiation management predischarge: process for assessment of carvedilol therapy in heart failure (IMPACT-HF) trial. J Am Coll Cardiol. (2004) 43(9):1534–41. doi: 10.1016/j.jacc.2003.12.040
68. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of Beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail. (2015) 3(8):647–53. doi: 10.1016/j.jchf.2015.03.008
69. Rossi R, Crupi N, Coppi F, Monopoli D, Sgura F. Importance of the time of initiation of mineralocorticoid receptor antagonists on risk of mortality in patients with heart failure. J Renin Angiotensin Aldosterone Syst. (2015) 16(1):119–25. doi: 10.1177/1470320313482603
70. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. (2022) 28(3):568–74. doi: 10.1038/s41591-021-01659-1
Keywords: heart failure with reduced ejection fraction, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, sodium-glucose co-transporter 2 inhibitors, angiotensin receptor-neprilysin inhibitors
Citation: Pascual-Figal D and Bayes-Genis A (2024) Looking for the ideal medication for heart failure with reduced ejection fraction: a narrative review. Front. Cardiovasc. Med. 11:1439696. doi: 10.3389/fcvm.2024.1439696
Received: 28 May 2024; Accepted: 20 August 2024;
Published: 6 September 2024.
Edited by:
Eduardo Oliver, Margarita Salas Center for Biological Research, Spanish National Research Council (CSIC), SpainReviewed by:
Agustín Clemente Moragón, Spanish National Centre for Cardiovascular Research, SpainJesus Alvarez-Garcia, Ramón y Cajal University Hospital, Spain
Juan Gómez-Mesa, Valle del Lili Foundation, Colombia
Copyright: © 2024 Pascual-Figal and Bayes-Genis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence Domingo Pascual-Figal, ZHBhc2N1YWxAdW0uZXM=; Antoni Bayes-Genis, YWJheWVzZ2VuaXNAZ21haWwuY29t
 Domingo Pascual-Figal
Domingo Pascual-Figal Antoni Bayes-Genis
Antoni Bayes-Genis