
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 29 July 2024
Sec. Intensive Care Cardiovascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1435935
This article is part of the Research Topic Organ Support in Cardiac Intensive Care View all 9 articles
Background: Insufficient ventricular unloading is a serious complication during veno-arterial extracorporeal membrane oxygenation (VA-ECMO) that has a crucial impact on patient outcomes. The existing conservative treatment options are limited, while mechanical decompression techniques are challenging and restricted in terms of their adoption and application. Two patients with cardiogenic shock experienced insufficient left ventricular unloading with no pulsatile contraction and aortic valve closure during VA-ECMO support. Gentle chest compression was applied to establish an active left ventricular drainage mechanism, which prevented the formation of intracardiac thrombi. No life-threatening complications or technical problems occurred. Therefore, gentle chest compression was established as an effective and safe method for treating insufficient left ventricular unloading in VA-ECMO patients.
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is an advanced extracorporeal life support method that temporarily replaces the patient's heart pump function, allowing time for recovery of the damaged myocardium (1, 2). However, the arterial end of VA-ECMO typically involves cannulation via the femoral artery, which opposes the natural flow direction in the aorta. This reversed blood flow perfusion subsequently increases the heart's afterload, hindering the ejection of blood from the left ventricle. Consequently, this leads to an increase in left ventricular end-diastolic pressure, dilation of the left ventricle, and augmented stress on its wall. This condition is referred to as insufficient left ventricular unloading (3), which presents additional challenges for patients with cardiogenic shock (4) and might have a deleterious long-term effect (5, 6).
The current prevailing trend suggests that earlier or prophylactic combined left ventricular unloading is likely to yield greater benefits (5, 7). In recent years, mechanical left ventricular decompression methods have been widely used. These include left ventricular assist devices, such as an intra-aortic balloon pump, direct decompression through trans-thoracic left atrial and left ventricular catheterization, percutaneous trans-aortic valve left ventricular decompression, and other techniques (8, 9). Currently, there is no consensus on which unloading strategy should be adopted, with 65% of studies citing the risk of complications as the primary reason for not using mechanical left ventricular unloading (10). Additionally, mechanical decompression methods have extremely high requirements for patient selection, surgical techniques, and management. The present report described two cases where a gentle method of chest compressions was used as a replacement technique to decompress the left ventricle in VA-ECMO patients and resulted in successful resuscitation.
The first case describes a 39-year-old man who spent one day at a small hospital due to acute extensive myocardial infarction. ECMO organ support was indicated in the case of no improvement with routine treatment. During the ECMO treatment, the patient repeatedly experienced loss of arterial pulsation accompanied by a pulse pressure difference of <10–15 mmHg. Large amounts of watery secretions emerged from the artificial airway. Portable echocardiography revealed that the aortic valve was unable to open, the left ventricle was in a creeping state, and the right ventricular contractile function remained satisfactory. Pulmonary ultrasonography showed bilateral diffuse B3 lines. Based on these observations, a comprehensive diagnosis of excessive left ventricular loading was ultimately made.
At this juncture, an emergency percutaneous coronary intervention was promptly initiated to urgently reopen the right coronary artery and reestablish blood flow to the affected areas. Concurrently, measures to reduce the load on the left ventricle were implemented, including managing arrhythmias, administering dobutamine to strengthen myocardial contractility, and prescribing furosemide for diuretic therapy lasting for 2 h. None of these treatments were effective. In the absence of mechanical means for left ventricular decompression, chest compressions were used as an emergency measure and administered intermittently at a frequency of 5 min/h, with a depth of 5 cm, and a rate of 100 bpm. The patient's blood pressure began to fluctuate after 25 h of intermittent chest compressions supported by ECMO. A flowchart summarizing the treatment process of ECMO and intermittent chest compressions is presented in Figure 1. The improvement in all indicators led to the cessation of chest compressions (Figure 2).
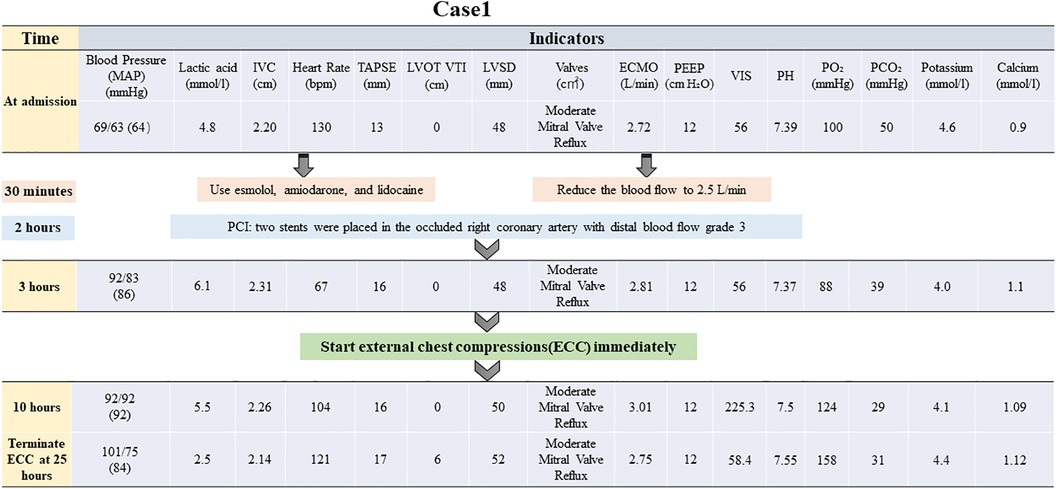
Figure 1. Brief timeline flowchart for patient treatment during chest compression (Case 1). MAP, mean arterial pressure; IVC, inferior vena cava; TAPSE, tricuspid annular plane systolic excursion; ECMO, extracorporeal membrane oxygenation; PEEP, positive end-expiratory pressure; PO₂, partial pressure of oxygen; LVOT VTI, left ventricular outflow tract velocity time integral; LVID, left ventricular internal diameter; VIS, vasoactive-inotropic score; PCI, percutaneous coronary intervention.
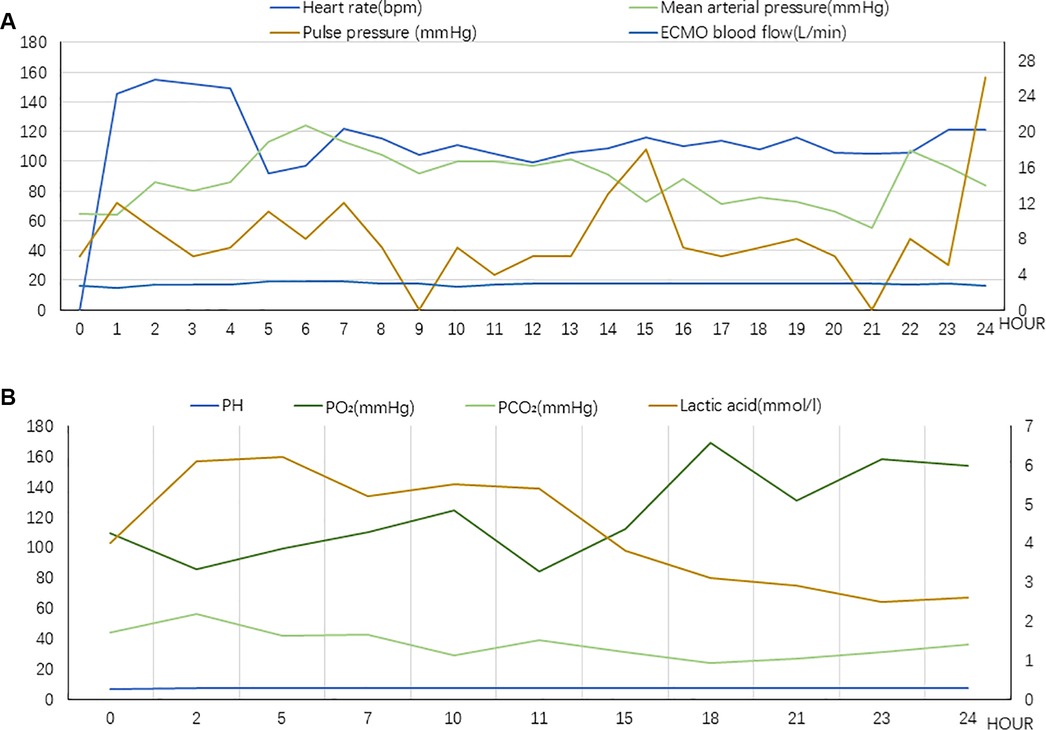
Figure 2. (A) Vital signs during chest compression (Case 1). The value of Pulse pressure and ECMO blood flow correspond to the secondary axis. (B) Laboratory tests indicators during chest compression (Case 1). The value of lactic acid corresponds to the secondary axis.
After withdrawing ECMO support, the echocardiography results revealed the formation of a left ventricular apical aneurysm accompanied by a mural thrombus measuring 33 mm × 9 mm. As a result, nadroparin calcium was prescribed for anticoagulation therapy and was subsequently replaced with warfarin treatment for three months. In summary, the patient received ECMO support for nine days and ventilator assistance for 13 days and remained at the intensive care unit (ICU) for 17 days. The total hospital stays lasted for 24 days. A follow-up examination 6 months later demonstrated that the patient recovered to an excellent state of health, retained full capacity for independent living, and displayed no residual signs of organ dysfunction.
A 50-year-old male patient with no prior significant medical history presented with symptoms of chest tightness and chest pain following an upper respiratory tract infection and was diagnosed with fulminant myocarditis. The patient's right radial artery exhibited no palpable pulsation during ECMO-assisted circulation support, leading to a pulse pressure difference of zero. Notably, large quantities of watery secretions emerging from the artificial airway were observed. Concurrent bedside echocardiography examination revealed that the aortic valve failed to open, with the left ventricle displaying a creeping motion and the right ventricle showing decreased contractile function. Furthermore, diffuse B3 lines were visible in both lung fields. After a thorough evaluation, it was determined that there was inadequate unloading of the left ventricle.
Prompt action to reduce the burden on the left heart was initiated by rapidly removing excess fluid using continuous renal replacement therapy (CRRT). Furthermore, dobutamine was prescribed to boost myocardial contractility, while beta-blockers were administered to regulate the heart rate. The patient exhibited signs of inadequate perfusion despite adjusting the ECMO flow rate to 2.65 L/min, with lactic acid levels continuously surging to reach a maximum of 9.9 mmol/L. The ECMO flow rate was subsequently increased once again to guarantee enough organ and tissue perfusion. Next, chest compressions were utilized as an emergency measure, maintaining a depth of 3 cm and a frequency of 80 bpm. After approximately 33 h of treatment, the patient's blood pressure began to fluctuate normally. A repeat bedside echocardiogram revealed that velocity time integral reached 3.4 cm and lactic acid level decreased to 1.6 mmol/L. Chest compressions were terminated following these improvements. A flowchart summarizing the treatment process and the important indicators were presented in Figures 3, 4.
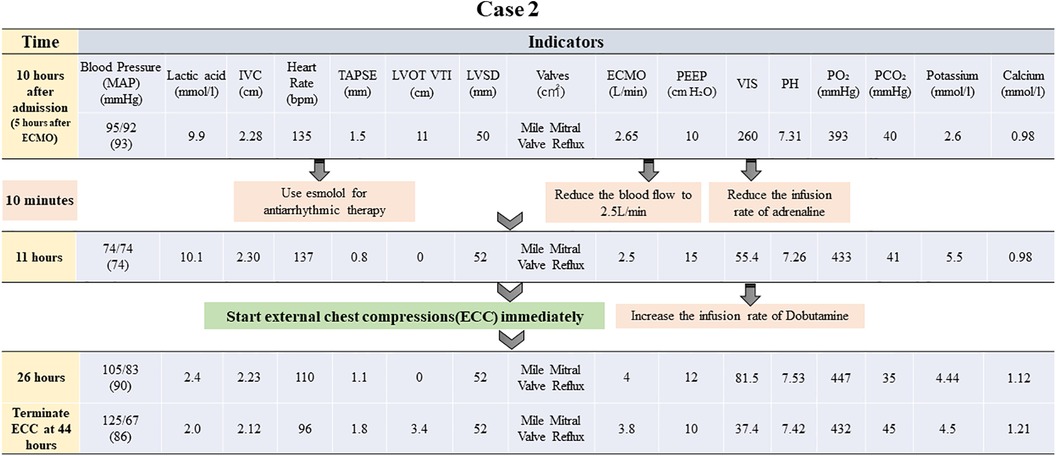
Figure 3. Brief timeline flowchart for patient treatment during chest compression (Case 2). MAP: mean arterial pressure, IVC: inferior vena cava, TAPSE: tricuspid annular plane systolic excursion, ECMO: extracorporeal membrane oxygenation, PEEP: positive end-expiratory pressure, PO2: partial pressure of oxygen, LVOT VTI: left ventricular outflow tract velocity time integral, LVID: left ventricular internal diameter, VIS: vasoactive-inotropic score.
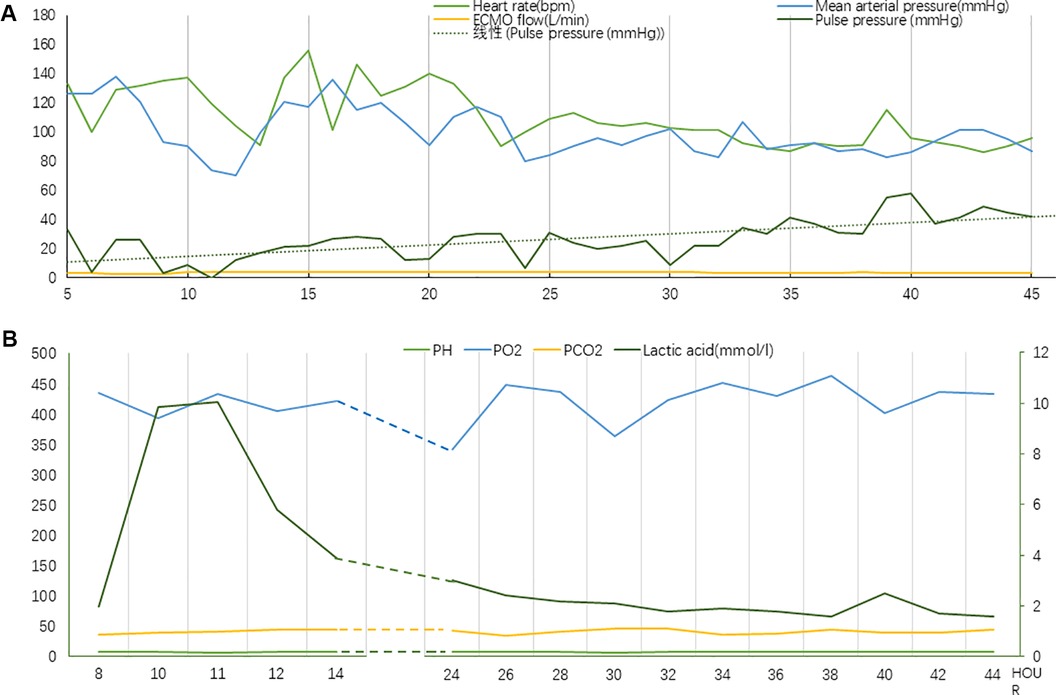
Figure 4. (A) Vital signs during chest compression (Case 2). (B) Laboratory tests indicators during chest compression (Case 2). The value of lactic acid corresponds to the secondary axis.
One week later, a repeat echocardiogram demonstrated mild enlargement of the left ventricle, along with improved motion of the left ventricular wall and enhanced left ventricular contractile function, resulting in an ejection fraction of 38%. Overall, the patient underwent ECMO support for nine days, received ventilator assistance for 12 days, and stayed at the ICU for 16 days. The overall hospital stays lasted for 21 days. A 6-month follow-up examination confirmed the patient's good health status and absence of any residual organ dysfunction.
The present study described the use of gentle chest compression to mitigate insufficient left heart unloading during VA-ECMO treatment after unsatisfactory conventional treatments. Notably, both patients recovered successfully and achieved positive outcomes. Moreover, compromised ECMO efficiency reduced oxygen supply, leading to insufficient tissue perfusion. This observation is consistent with those reported in a study by Myneni et al. (11). These treatment approaches, including CRRT, dobutamine, beta-blockers, and reducing ECMO flow rate, may be unfavorable for patients with severe heart failure. In 1960, Kouwenhoven et al. (12) first proposed that chest compressions produce antegrade blood flow as a result of directly compressing the heart between the sternum and the spine, which is known as the cardiac pump theory. According to this theory, chest compressions can be a rescue measure to prevent left chamber thrombosis and improve pulmonary circulation during left ventricular distension syndrome, in which an active left ventricular drainage mechanism is established (13). The first patient in the present study received chest compressions with a frequency and depth described in the 2015 International Guidelines for Cardiopulmonary Resuscitation (14). With the support of ECMO, only 15 pulse pressure differences were needed. Due to chest compression depth influences haemodynamic parameters during cardiopulmonary resuscitation (15), the 3 cm depth was implement to achieve carotid blood flow in the second patient. This change relieved left ventricular overload and achieved the desired effect of forward pulsatile blood flow ranging from 15 to 40 mmHg. If the difference in pulse pressure remained above 15 mmHg for more than 30 min, it was defined as the presence of natural pulsatile blood flow and chest compression was stopped. According to a study comparing the complications associated with chest compression depths of <5 cm, 5–6 cm, and >6 cm, the rate of iatrogenic injuries gradually increased with respective rates of 28%, 27%, and 49% (16). Remarkably, both patients in the present study were spared from any complications associated with chest compression lasting for more than 24 h. In the first patient, an intraventricular thrombus was observed after ECMO withdrawal. According to prior reports, the incidence of left ventricular thrombus with left ventricular aneurysm can reach 42% (17). Therefore, considering that this patient developed a left ventricular apical aneurysm and that the intramural thrombus was attached to the left apical wall, it is possible that the intramural thrombus was a complication of myocardial infarction rather than a result of insufficient chest compression. Although the duration of myocardial stunning in the second patient was relatively long and lasted for 45 h, the patient's cardiac function ultimately recovered similar to the results reported in previous studies (18, 19). Thus, myocardial stunning likely occurred, because the myocardial enzyme spectrum remained relatively unchanged throughout the patient's illness. Both patients received CRRT for 24 h and were able to resume spontaneous urine output by the time of discharge.
Although the expected effect of the gentle chest compression technique on patients with insufficient left ventricular unloading under ECMO support in the present study was encouraging, there were still some shortcomings associated with this method. First, the compression standards were inconsistent, including intermittent and continuous methods with significant differences in depth and frequency. Second, there was a lack of dynamic monitoring of intracoronary blood supply within the heart, such as transesophageal echocardiography, which can simultaneously assess the pressure in each atrium and ventricle.
The gentle chest compression technique yielded promising outcomes, making it an alternative diagnostic and therapeutic avenue for managing insufficient left ventricular unloading in VA-ECMO patients. In the future, more studies will focus on the gentle chest compression technique in the context of left ventricular unloading, aiming to provide more evidence-based medical data for clinical diagnosis and treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethical standards of the Ethics Committee of the People's Hospital of Guangxi Zhuang Autonomous Region (KY-KJT-2023-110). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LJ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. MH: Conceptualization, Data curation, Formal Analysis, Resources, Writing – original draft. SX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. BX: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing. GL: Data curation, Investigation, Validation, Writing – review & editing. YZ: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. LH: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The Guangxi Science and Technology Base and Talent Special Project (grant no. Guike AD22035101), the Youth Science Foundation Project of Guangxi (grant no. 2023GXNSFBA026096) and the Guangxi Health and Wellness Commission Self-funded Project (grant no. Z-A20220085) provided funding for this study.
We thank the patients for cooperating with our investigation and acknowledge the professionalism and compassion demonstrated by all the healthcare workers involved in patients' care. We also thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1435935/full#supplementary-material
1. Lim HS, Howell N, Ranasinghe A. Extracorporeal life support: physiological concepts and clinical outcomes. J Card Fail. (2017) 23(2):181–96. doi: 10.1016/j.cardfail.2016.10.012
2. Syed M, Khan MZ, Osman M, Sulaiman S, Agrawal P, Raina S, et al. Sixteen-year national trends in use and outcomes of VA-ECMO in cardiogenic shock. Cardiovasc Revasc Med. (2022) 44:1–7. doi: 10.1016/j.carrev.2022.06.267
3. Ezad SA-O, Ryan M, Donker DW, Pappalardo F, Barrett N, Camporota L, et al. Unloading the left ventricle in venoarterial ECMO: in whom, when, and how? Circulation. (2023) 147(16):1237–50. doi: 10.1161/CIRCULATIONAHA.122.062371
4. Cevasco M, Takayama H, Ando M, Garan AR, Naka Y, Takeda K. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J Thorac Dis. (2019) 11(4):1676–83. doi: 10.21037/jtd.2019.03.29
5. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation. Circulation. (2020) 142(22):2095–106. doi: 10.1161/CIRCULATIONAHA.120.048792
6. Kim D, Jang WJ, Park TK, Cho YH, Choi JO, Jeon ES, et al. Echocardiographic predictors of successful extracorporeal membrane oxygenation weaning after refractory cardiogenic shock. J Am Soc Echocardiogr. (2021) 34(4):414–22.e4. doi: 10.1016/j.echo.2020.12.002
7. Bernhardt AM, Schrage B, Westermann D, Reichenspurner H. Extracorporeal membrane oxygenation evolution: left ventricular unloading strategies. JTCVS Open. (2021) 8:85–9. doi: 10.1016/j.xjon.2021.10.042
8. Protti I, van Steenwijk MPJ, Meani P, Fresiello L, Meuwese CL, Donker DW. Left ventricular unloading in extracorporeal membrane oxygenation: a clinical perspective derived from basic cardiovascular physiology. Curr Cardiol Rep. (2024) 26(7):661–7. doi: 10.1007/s11886-024-02067-w
9. Bansal A, Verghese D, Vallabhajosyula S. Intra-aortic balloon pump for left ventricular unloading in veno-arterial extracorporeal membrane oxygenation: the last remaining indication in cardiogenic shock. J Am Heart Assoc. (2022) 11(7):e025274. doi: 10.1161/JAHA.122.025274
10. Ezad SM, Ryan M, Barrett N, Camporota L, Swol J, Antonini MV, et al. Left ventricular unloading in patients supported with veno-arterial extra corporeal membrane oxygenation; an international EuroELSO survey. Perfusion. (2024) 39(1_suppl):13S–22S. doi: 10.1177/02676591241229647
11. Myneni MA-O, Cheema FH, Rajagopal K. Alterations in coronary blood flow and the risk of left ventricular distension in venoarterial extracorporeal membrane oxygenation. ASAIO J. (2023) 69(6):552–60. doi: 10.1097/MAT.0000000000001905
12. Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed-chest cardiac massage. JAMA Oncol. (1960) 173:1064–7. doi: 10.1001/jama.1960.03020280004002
13. Manzur-Sandoval D, Salazar-Delgado JO, Jiménez-Rodríguez GM, Rojas-Velasco G. Chest compressions as a rescue maneuver to maintain aortic valve opening during left ventricular distention syndrome on venoarterial ECMO. Rev Esp Anestesiol Reanim (Engl Ed). (2024):S2341–1929(24)00050–7. doi: 10.1016/j.redare.2024.02.028
14. Perkins GD, Travers AH, Berg RA, Castren M, Considine J, Escalante R, et al. Part 3: adult basic life support and automated external defibrillation. Resuscitation. (2015) 95:e43–69. doi: 10.1016/j.resuscitation.2015.07.041
15. Bruckner M, O'Reilly M, Lee TF, Neset M, Cheung PY, Schmölzer GM. Effects of varying chest compression depths on carotid blood flow and blood pressure in asphyxiated piglets. Arch Dis Child Fetal Neonatal Ed. (2021) 106(5):553–6. doi: 10.1136/archdischild-2020-319473
16. Hellevuo H, Sainio M, Nevalainen R, Huhtala H, Olkkola KT, Tenhunen J, et al. Deeper chest compression—more complications for cardiac arrest patients? Resuscitation. (2013) 84(6):760–5. doi: 10.1016/j.resuscitation.2013.02.015
17. Brinza C, Cinteza M, Popa IV, Burlacu A. Safety and efficacy of less-invasive ventricular enhancement procedure with the transcatheter revivent TCTM system in patients with left ventricular aneurysm: a systematic review. Rev Cardiovasc Med. (2021) 22(2):445–52. doi: 10.31083/j.rcm2202050
18. Narain S, Paparcuri G, Fuhrman TM, Silverman RB, Peruzzi WT. Novel combination of impella and extra corporeal membrane oxygenation as a bridge to full recovery in fulminant myocarditis. Case Rep Crit Care. (2012) 2012:459296. doi: 10.1155/2012/459296
Keywords: gentle chest compression, VA-ECMO, left ventricular unloading, heart failure, pulsatile contraction
Citation: Jiang L, Huang M, Xiang S, Xiong B, Li G, Zhong Y and Han L (2024) Left ventricular unloading with gentle chest compressions for patients on veno-arterial extracorporeal membrane oxygenation: two case reports. Front. Cardiovasc. Med. 11: 1435935. doi: 10.3389/fcvm.2024.1435935
Received: 21 May 2024; Accepted: 16 July 2024;
Published: 29 July 2024.
Edited by:
Guo-wei Tu, Fudan University, ChinaReviewed by:
Linhao Ma, Shanghai Changzheng Hospital, China© 2024 Jiang, Huang, Xiang, Xiong, Li, Zhong and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonglong Zhong, eWwuemhvbmdAd2h1LmVkdQ==; Lin Han, eGhhbjA1MDdAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.