- Department of Cardiovascular Medicine, The First Hospital of Jilin University, Changchun, China
Background: Pulmonary Artery in situ Thrombosis (PAIST) refers to a thrombus forming within the pulmonary arterial system, distinct from an embolus originating from elsewhere in the body (e.g., the deep veins of the lower extremities) and traveling to the lungs where it lodges and forms.
Case presentation: We present a case of PAIST caused by the arterial ductus arteriosus. The patient primarily presented with dyspnea, and the chest pain dichotomy Computed Tomography Angiography(CTA) suggested that a nodular low-density filling defect was seen in the lumen of the left pulmonary artery trunk. Initially, pulmonary embolism (PE) was suspected. However, upon reevaluation of the imaging, it became apparent that the patient's pulmonary artery obstruction was closely associated with the ductus arteriosus. After admission, the patient was treated with sodium ampicillin (2.0 g Q12H) for infection, heparin sodium (5,000 IU Q12H) for anticoagulation, and metoprolol succinate extended-release tablets (23.75 mg QD) to correct cardiac remodeling, among other treatments. Subsequently, the patient underwent a cardiac surgery involving the ligation of the arterial duct, resection of pulmonary artery lesions, and open-heart surgery with extracorporeal circulation support. Postoperative examination of the pulmonary artery mass indicated coagulation tissue. The final diagnosis was “PAIST”.
Conclusion: Both PAIST and PE manifest as low-density filling defects in the pulmonary arteries. However, due to the relative unfamiliarity with PAIST, such findings are often initially attributed to PE.
1 Background
PAIST typically arises amidst pathophysiologic lung changes, featuring thrombosis within the pulmonary arterial system. This condition often presents with thrombus propagation from the peripheral to the central pulmonary arteries, inducing hemodynamic alterations within the lungs. Consequently, patients may experience symptoms like dyspnea and chest pain, albeit right heart insufficiency is a rare occurrence (1, 2). Various factors, including structural lung changes, infections, trauma, and sickle cell disease, can precipitate focal inflammation and dysfunction of the pulmonary vascular endothelium, thereby fostering the development of PAIST. The precise mechanism underlying its formation remains elusive and may entail pulmonary artery endothelial cell damage, heightened blood viscosity, augmented blood flow, and inflammation (1, 2). A study on a clinical translational model of blunt chest trauma first demonstrated the occurrence of de novo pulmonary thrombosis, further supporting the formation of in situ pulmonary artery thrombi following severe trauma (3). Literature reports suggest that PAIST seems more likely to occur in patients with acute chest syndrome (ACS) who have elevated platelet counts and lower hemolysis rates (4). PAIST is relatively rare, and the exact incidence is unknown, However, there is growing evidence in the literature suggesting that the incidence of PAIST may have been underestimated, particularly in recent years, as seen in case review studies of COVID-19 patients with concurrent PE, Literature reports indicate that pulmonary artery thrombosis in COVID-19 is an immune-mediated inflammatory thrombosis, with an incidence of pulmonary artery thrombosis/embolism of 36% among COVID-19 patients (5–8). Knudson et al. recently reviewed injury data from 888,652 trauma patients in the National Trauma Data Bank (NTDB) and reported a significant increase in the incidence of PE, while the incidence of deep vein thrombosis (DVT) did not increase (9). Currently, there is no diagnostic gold standard for PAIST, and the absence of specific imaging or pathological manifestations to determine the etiology of thrombosis makes it primarily a diagnosis of exclusion. Imaging tests, however, can be relied upon to aid in the diagnosis (1), such as Computed tomography pulmonary angiography right (CTPA) or Magnetic resonance imaging (MRI).PAIST has a better prognosis and is treated similarly to PE with therapeutic measures such as anticoagulation, thrombolysis, and surgery (1, 2). A retrospective study involving 23 patients with PAT indicated that thrombolysis of in situ pulmonary artery thrombi occurs more slowly compared to pulmonary embolism (10). In this paper, we report a case of PAIST suspected to be PE and review the relevant literature.
2 Case presentation
The patient, a 59-year-old male, was admitted to the hospital due to “sudden dyspnea persisting for more than half a day”. He experienced a sudden onset of dyspnea without accompanying chest pain, cough, fever, or other discomforts. He had (6–8) previously been in good health. Physical examination on admission revealed the body temperature was 36.5℃, the pulse rate was 99 beats per minute, the breathing rate was 20 beats per minute, blood pressure was 151/100 mmHg (1 mmHg = 0.133 kPa), his spirit was clear and the speech is clear, there was no cyanosis of the lips or mouth, and there was no filling of the jugular veins. Breath sounds were coarse in both lungs, and no dry or wet rales were heard. The apical beat was located between the 5th intercostal space and 0.5 cm outside the left midclavicular line, the cardiac border was enlarged, and a continuous mechanical murmur could be heard in the second intercostal space at the left edge of the sternum, but no murmur was heard in the rest of the heart.
Auxiliary tests: blood gas analysis: pH: 7.41, pCO2: 28 mmHg, pO2: 112 mmHg, SO2 98%, D-dimer: 1,640.39 ng/ml, N-terminal cerebral natriuretic peptide precursor: 361.31 pg/ml; leukocytes: 16.02 × 10^9/L, neutrophil percentage 94%, ultrasensitive C-reactive protein 29.22 mg/L, calcitonin 1.31 ng/ml, creatinine: 208.0 umol/L; Electrocardiogram (ECG): sinus rhythm Abnormal EKG I, avl, V1\R\V6 leads T-wave is low and inverted. Cardiac ultrasound (Figure 1): EF 59%, the patient's arterial duct is not fully closed and there is a left-to-right blood shunt with a maximum velocity of the shunt of approximately 440 cm/s. There is a 21 × 14 mm bulge in the left pulmonary artery. Enlarged left atrium (39 mm anteroposterior diameter), hypodiastolic left ventricle. The right atrium and right ventricle had no abnormalities and mild mitral regurgitation. CTA of chest pain diathesis (Figure 2): nodular low-density filling defects are seen in the lumen of the left pulmonary arterial trunk in close relation to the ductus arteriosus. The left pulmonary artery trunk is altered, and PE is considered a high possibility. Lower extremity venous ultrasound: right calf intermuscular vein thrombosis (acute phase.) PET-CT (Figure 3) suggests: no abnormal hypermetabolic foci in the pulmonary artery tract. Blood culture (anaerobic and aerobic): human staphylococcus. Admission diagnosis: pulmonary artery occupational lesion to be investigated. After admission, the patient was treated with sodium ampicillin (2.0 g Q12H) for infection, heparin sodium (5,000 IU Q12H) for anticoagulation, and metoprolol succinate extended-release tablets (23.75 mg QD) to correct cardiac remodeling, among other treatments, and his condition was relieved. Later, to further clarify the diagnosis, on the 9th day after admission, the patient underwent cardiac surgery with pulmonary artery ligation and pulmonary artery resection and extracorporeal circulation-assisted open cardiac surgery, and the pulmonary artery organisms were sent to the pathology after the surgery, which suggested normal blood clots. After 16 days of hospitalization, the patient was discharged from the hospital after his condition improved. Upon discharge, the patient was prescribed rivaroxaban 10 mg daily and digoxin 0.25 mg daily. During telephone follow-ups one week and two weeks after discharge, the patient reported a significant relief of dyspnea. Twenty days later, during an outpatient follow-up, a cardiac ultrasound: EF 65%, after arterial catheter ligation, after resection of pulmonary valve redundancy, with mild tricuspid regurgitation. Regular follow-up of patients in outpatient clinics. Reviewing the patient's medical history again, the patient did not have chest pain, fever and other discomforts, cardiac ultrasound suggested arterial duct failure, chest pain dichotomy CTA showed that the nodular low-density filling defect in the trunk of the left pulmonary artery was closely related to the ductus arteriosus, and PET-CT did not show show significant hypermetabolism of the pulmonary arteries. The pulmonary artery occupying lesion was considered to be a PAIST formation due to arterial ductus arteriosus, endothelial injury due to sustained high flow and pressure at the main pulmonary artery junction, and increased vascular shear.
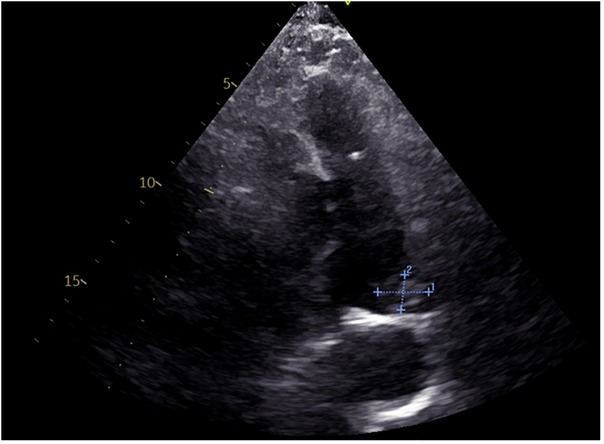
Figure 1. ECG: abnormal echoes of the left pulmonary artery, to be excluded superfluous (21 × 14 mm)?

Figure 2. CTA of chest pain diathesis: nodular low-density filling defect in the left pulmonary artery trunk in relation to the ductus arteriosus.
3 Discussion
PAIST refers to pulmonary arterial system ab initio thrombosis, meaning pulmonary artery (PA) occlusion occurs independently of peripheral thrombosis (1, 2, 11, 12). Hence, a case of isolated PE without deep vein thrombosis (DVT) is likely indicative of PAIST (1). However, the presence of peripheral thrombus does not rule out PAIST, as demonstrated in this case of PAIST with acute thrombosis of the deep veins in the right lower extremity.The specific etiology and pathogenesis of PAIST formation is unknown (2), and it has been reported that lung injury, infection, trauma, congenital heart disease, pulmonary hypertension(PH), radiotherapy, white stuffing syndrome, platelet activation, cytokine-mediated immunopathological response, and intravascular hemolysis can lead to the formation of PAIST (1, 2, 11–15), meanwhile, the thrombotic potential of Covid-19 has been recognized (8, 14, 15). PAIST originates in a hypoxic and inflammatory environment, leading to pulmonary vascular endothelial cell dysfunction in response to various factors such as disease, injury, and medications. This dysfunction results in an imbalance between thrombosis and fibrinolysis (1, 2). Endothelial dysfunction and platelet activation are key mechanisms involved in in situ thrombosis (13), along with age-dependent factors and neutrophil involvement (12, 16). In this case, the patient was a middle-aged male with a history of congenital heart disease in which arterial ductus arteriosus caused sustained high flow and pressure at the main pulmonary artery junction, and finally pulmonary artery endothelial cell damage, which impaired the antithrombotic function of the arterial wall by down-regulating the endothelial nitric oxide synthase (eNOS) or stimulating the expression of the adhesion receptor (8, 13), and, thus, formation of an in situ thrombus at the junction out of the main pulmonary artery.
The clinical manifestations of PAIST are nonspecific and similar to those of PE (Table 1), which may manifest as dyspnea, chest tightness, chest pain, palpitations, fever, hemoptysis, and other symptoms, and are less likely to cause right heart dysfunction, whereas PE often has right atrial and right ventricular changes, but it remains difficult to differentiate between the two in terms of symptoms (1–3). In this case, the patient presented with dyspnea, but without any right atrial or right ventricular changes or pulmonary hypertension (PH), which provides little support for the diagnosis of PAIST.
PAIST is a rare condition that lacks specific diagnostic criteria and imaging manifestations, often leading to misdiagnosis as PE (1). However, understanding its pathophysiological features and utilizing appropriate imaging techniques can aid in accurate diagnosis (1). In PE, the distribution of emboli is typically multiple rather than single, and they are commonly found in the lower lobes of the lungs rather than the upper lobes. In contrast, PAIST tends to manifest as a single lesion, frequently occurring at the site of an anomaly or lesion in the pulmonary artery (5, 10). The imaging manifestations of PAIST typically present in peripheral arteries as non-occlusive and eccentric lesions, often at an obtuse angle to the vessel wall and without vasodilation. In contrast, pulmonary embolism (PE) commonly exhibits centrally located emboli (proximal to segmental branches), frequently occlusive, with vasodilation, and at an acute angle to the vessel wall (4, 11, 17, 18). Solated pulmonary embolism: imaging often shows pulmonary artery obstruction. PAIST: may not have obvious features of acute obstruction, imaging may show that the thrombus in situ is more tightly bound to the wall of the pulmonary artery, and the morphology and density features may vary. Thrombosed pulmonary arteries are often located in lung tissue with underlying pathology, such as inflammation or tumour, and tend to be located in the distal branches, usually not involving the main trunk of the pulmonary artery. Alternatively, as in this case, the thrombus may form near the pulmonary artery connected to the ductus arteriosus (1, 10–13). In this case, the patient's imaging revealed a single filling defect in the left pulmonary artery trunk, located near the arterial conduit, with an unobstructed distal artery. Additionally, the lung parenchymal lesion was adjacent to the artery. Consequently, despite the presence of lower-extremity venous thrombosis, we attributed the low-density filling defect in the pulmonary artery to PAIST formation.
PAIST has received limited study, and its treatment parallels that of PE, including therapeutic options like anticoagulation, thrombolysis, and surgery. Hemoptysis is one of the most frequent complications associated with PAIST (1, 2, 11). It has also been reported that the treatment of in situ PAT may depend on the location of thrombosis in the pulmonary arterial tree and aspects of the specific clinical situation (1, 13). Inhibition of platelet activity through antiplatelet agents decreases the likelihood of recurrent PA thrombotic events following discontinuation of anticoagulation therapy (13). Inflammation is recognized as one of the causative mechanisms of in situ thrombosis, suggesting potential benefits for patients with in situ thrombosis from anti-inflammatory therapy (1). We should decide whether to go for anticoagulation only after considering the patient's symptoms, bleeding risk, and comorbidities (1, 11).
Our patient was previously in good health and had a low risk of bleeding, and was admitted to the hospital and treated with anti-infection and anticoagulation, and then underwent cardiac surgical operation of vein catheter ligation and resection of pulmonary artery lesion and extracorporeal circulation-assisted open heart surgery. Since the rate of thrombus regression in patients with PAIST is slower than that in patients with pulmonary embolism (10). The patient was then advised to continue taking oral anticoagulant rivaroxaban upon discharge from the hospital, with the decision for anticoagulation guided by the findings of outpatient follow-up visits. PAIST generally carries a favorable prognosis, but delayed diagnosis and treatment may lead to progression to chronic thromboembolic pulmonary hypertension (CTEPH) (13).
4 Conclusion
The clinical manifestations of PAIST lack specificity, and there is no definitive diagnostic gold standard. The incidence is often underestimated, and it is prone to misdiagnosis as PE (1, 2, 11–13). Hence, when encountering a solitary lesion within the pulmonary artery in clinical practice, differentiation from in situ thrombosis is necessary. In particular, the presence of underlying lesions of the pulmonary arteries, in this case thrombosis in the vicinity of the pulmonary artery connected to the ductus arteriosus, aided the diagnosis. Further research on PAIST is warranted to better understand its imaging characteristics, natural progression, and its significance in different disease contexts, ultimately informing treatment strategies and improving patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Validation, Writing – original draft, Writing – review & editing. CR: Data curation, Software, Writing – review & editing. ML: Conceptualization, Data curation, Writing – review & editing. WZ: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Clinical Research Program of Jilin Province (2022LC092).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baranga L, Khanuja S, Scott JA, Provancha L, Gosselin M, Walsh J, et al. In situ pulmonary arterial thrombosis: literature review and clinical significance of a distinct entity. AJR Am J Roentgenol. (2023) 221(1):57–68. doi: 10.2214/AJR.23.28996
2. Cao Y, Geng C, Li Y, Zhang Y. In situ pulmonary artery thrombosis: a previously overlooked disease. Front Pharmacol. (2021) 12:671589. doi: 10.3389/fphar.2021.671589
3. Brown IE, Rigor RR, Schutzman LM, Khosravi N, Chung K, Becker JA, et al. Pulmonary arterial thrombosis in a murine model of blunt thoracic trauma. Shock. (2018) 50(6):696–705. doi: 10.1097/SHK.0000000000001109
4. Mekontso Dessap A, Deux JF, Abidi N, Bombled CL, Melica G, Renaud B, et al. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. (2011) 184(9):1022–9. doi: 10.1164/rccm.201105-0783OC
5. Gabrielli M, Lamendola P, Esperide A, Valletta F, Franceschi F. COVID-19 and thrombotic complications: pulmonary thrombosis rather than embolism? Thromb Res. (2020) 193:98. doi: 10.1016/j.thromres.2020.06.014
6. Umairi RA, Adawi KA, Khoori MA, Lawati AA, Jose S. COVID-19-associated thrombotic complication: is it pulmonary embolism or in situ thrombosis? Radiol Res Pract. (2023) 2023:3844069. doi: 10.1155/2023/3844069
7. Babkina AS, Yadgarov MY, Volkov AV, Kuzovlev AN, Grechko AV, Golubev AM. Spectrum of thrombotic complications in fatal cases of COVID-19: focus on pulmonary artery thrombosis in situ. Viruses. (2023) 15(8):1681. doi: 10.3390/v15081681
8. Quartuccio L, Sonaglia A, Casarotto L, Mcgonagle D, Loreto CD, Pegolo E. Clinical, laboratory and immunohistochemical characterization of in situ pulmonary arterial thrombosis in fatal COVID-19. Thromb Res. (2022) 219:95–101. doi: 10.1016/j.thromres.2022.09.012
9. Ganie FA, Lone H, Lone GN, Wani ML, Singh A, Dar AM, et al. Lung contusion: a clinico-pathological entity with unpredictable clinical course. Bull Emerg Trauma. (2013) 1(1):7–16. doi: 10.1016/j.jpsychores.2023.110847
10. Cha SI, Choi KJ, Shin KM, Lim JK, Yoo SS, Lee J, et al. Clinical characteristics of in-situ pulmonary artery thrombosis in Korea. Blood Coagul Fibrinolysis. (2015) 26(8):903–7. doi: 10.1097/MBC.0000000000000343
11. Ahuja J, Shroff GS, Benveniste MF, Marom EM, Truong MT, Wu C. In situ pulmonary artery thrombosis: unrecognized complication of radiation therapy. AJR Am J Roentgenol. (2020) 215(6):1329–34. doi: 10.2214/AJR.19.22741
12. Porembskaya O, Zinserling V, Tomson V, Toropova Y, Starikova EA, Maslei VV, et al. Neutrophils mediate pulmonary artery thrombosis in situ. Int J Mol Sci. (2022) 23(10):5829. doi: 10.3390/ijms23105829
13. Porembskaya O, Toropova Y, Tomson V, Lobastov K, Laberko L, Kravchuk V, et al. Pulmonary artery thrombosis: a diagnosis that strives for its independence. Int J Mol Sci. (2020) 21(14):5086. doi: 10.3390/ijms21145086
14. Mandal AKJ, Kho J, Ioannou A, Abbeele KVD, Missouris CG. COVID-19 and in situ pulmonary artery thrombosis. Respir Med. (2021) 176:106176. doi: 10.1016/j.rmed.2020.106176
15. Mandal AKJ, Kho J, Ioannou A, Abbeele KVD, Missouris CG. In situ immune-mediated pulmonary artery thrombosis and COVID-19 pneumonitis. Thromb Res. (2021) 197:112–3. doi: 10.1016/j.thromres.2020.11.006
16. Caramuru LH, Maeda NY, Bydlowski SP, Lopes AA. Age-dependent likelihood of in situ thrombosis in secondary pulmonary hypertension. Clin Appl Thromb Hemost. (2004) 10(3):217–23. doi: 10.1177/107602960401000303
17. Liu ZB, Xie WM, Zhai ZG, Cao B, Wan J. Pulmonary aspergillosis with in situ pulmonary artery thrombosis: to anti-coagulate or not? Chin Med J (Engl). (2019) 132(14):1742–4. doi: 10.1097/CM9.0000000000000336
Keywords: pulmonary artery in situ thrombosis, congenital heart disease, patent ductus arteriosus (PDA), pulmonary embolism, PAIST
Citation: Wang Y, Rong C, Lu M and Zhang W (2024) Pulmonary artery in situ thrombosis due to patent ductus arteriosus: a case report. Front. Cardiovasc. Med. 11:1433847. doi: 10.3389/fcvm.2024.1433847
Received: 16 May 2024; Accepted: 19 August 2024;
Published: 10 September 2024.
Edited by:
Luca Spiezia, University of Padua, ItalyReviewed by:
James Horowitz, New York University, United StatesChiara Simion, University of Padua, Italy
Copyright: © 2024 Wang, Rong, Lu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihua Zhang, d2VpaHVhQGpsdS5lZHUuY24=
 Yin Wang
Yin Wang Weihua Zhang
Weihua Zhang